Abstract
Objective
To describe the development and validation of a novel patient reported outcome measure for symptom burden from long covid, the symptom burden questionnaire for long covid (SBQ-LC).
Design
Multiphase, prospective mixed methods study.
Setting
Remote data collection and social media channels in the United Kingdom, 14 April to 1 August 2021.
Participants
13 adults (aged ≥18 years) with self-reported long covid and 10 clinicians evaluated content validity. 274 adults with long covid field tested the draft questionnaire.
Main outcome measures
Published systematic reviews informed development of SBQ-LC’s conceptual framework and initial item pool. Thematic analysis of transcripts from cognitive debriefing interviews and online clinician surveys established content validity. Consensus discussions with the patient and public involvement group of the Therapies for Long COVID in non-hospitalised individuals: From symptoms, patient reported outcomes and immunology to targeted therapies (TLC Study) confirmed face validity. Rasch analysis of field test data guided item and scale refinement and provided initial evidence of the SBQ-LC’s measurement properties.
Results
SBQ-LC (version 1.0) is a modular instrument measuring patient reported outcomes and is composed of 17 independent scales with promising psychometric properties. Respondents rate their symptom burden during the past seven days using a dichotomous response or 4 point rating scale. Each scale provides coverage of a different symptom domain and returns a summed raw score that can be transformed to a linear (0-100) score. Higher scores represent higher symptom burden. After rating scale refinement and item reduction, all scales satisfied the Rasch model requirements for unidimensionality (principal component analysis of residuals: first residual contrast values <2.00 eigenvalue units) and item fit (outfit mean square values within 0.5 -1.5 logits). Rating scale categories were ordered with acceptable category fit statistics (outfit mean square values <2.0 logits). 14 item pairs had evidence of local dependency (residual correlation values >0.4). Across the 17 scales, person reliability ranged from 0.34 to 0.87, person separation ranged from 0.71 to 2.56, item separation ranged from 1.34 to 13.86, and internal consistency reliability (Cronbach’s alpha) ranged from 0.56 to 0.91.
Conclusions
SBQ-LC (version 1.0) is a comprehensive patient reported outcome instrument developed using modern psychometric methods. It measures symptoms of long covid important to people with lived experience of the condition and may be used to evaluate the impact of interventions and inform best practice in clinical management.
Introduction
Since the emergence of SARS-CoV-2 in 2019, the covid-19 pandemic has resulted in more than 450 million infections and more than six million deaths worldwide.1 Although infection is mild and short lived for many people, a proportion continue to experience or go on to develop symptoms that persist beyond the acute phase of infection. These persistent symptoms are known collectively as post-acute sequelae of covid-19, post-acute covid-19, post-covid-19 syndrome, post-covid-19 condition, or long covid.2 3
Symptom burden can be defined as the “subjective, quantifiable prevalence, frequency, and severity of symptoms placing a physiologic burden on patients and producing multiple negative, physical, and emotional patient responses.”4 The symptoms reported by those with long covid are heterogenous and can affect multiple organ systems, with fatigue, dyspnoea, and impaired concentration among the most prevalent symptoms.5 6 7 Symptoms may be persistent, cyclical, or episodic and can pose a substantial burden for affected individuals, with negative consequences for work capability, functioning, and quality of life.8 9 10 There is a growing body of research on the prevalence, incidence, co-occurrence, and persistence of the signs and symptoms of long covid.5 6 8 11 12 13 These data have largely been collected using bespoke, cross sectional survey tools due, in part, to the limited availability of condition specific, validated self-report instruments.14
Patient reported outcomes are measures of health reported directly by patients without amendment or interpretation by clinicians or anyone else.15 Validated instruments measuring patient reported outcomes developed specifically for long covid that address the complex, multifactorial nature of the condition are needed urgently to further the understanding of long covid symptoms and underlying pathophysiology, support best practice in the clinical management of patients, and evaluate the safety, effectiveness, acceptability, and tolerability of interventions.16 17 18 Validated instruments to measure patient reported outcomes have recently been developed to measure the global impact of long covid, and several unvalidated screening tools, surveys, and questionnaires are also available.19 20 However, individuals living with long covid have suggested that existing self-report measures fail to capture the breadth of experienced symptoms.10 21 22 To address the need for a comprehensive measure of self-reported symptom burden specific to long covid, we used Rasch analysis to develop and validate, in accordance with US Food and Drug Administration guidance, a novel instrument measuring patient reported outcomes, the symptom burden questionnaire for long covid (SBQ-LC).15 23
Methods
Setting and study design
This multiphase, prospective mixed methods study (fig 1) was nested within the Therapies for Long COVID in non-hospitalised individuals: From symptoms, patient reported outcomes and immunology to target therapies (TLC) Study.24 The study took place from 14 April to 1 August 2021.
Fig 1.
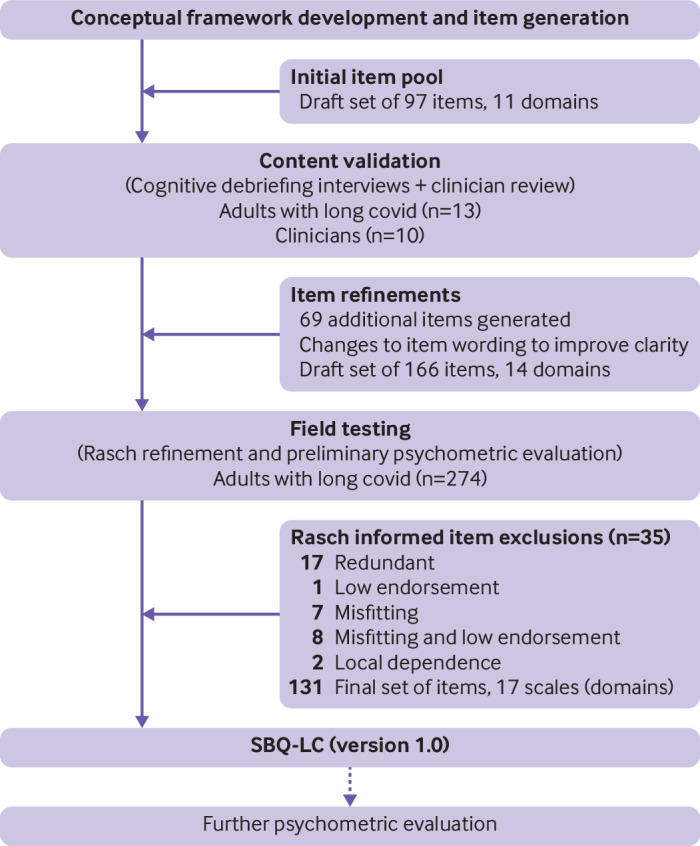
Development of symptom burden questionnaire for long covid (SBQ-LC)
Study population
Content validation was undertaken with adults with long covid recruited from the TLC study’s patient and public involvement (PPI) group and clinicians recruited from the TLC study and long covid research studies based in the UK. The field test population included adults with self-reported long covid. Participants were aged 18 years or older who could self-complete SBQ-LC in English. No exclusion criteria relating to duration of long covid symptoms, hospital admissions for SARS CoV-2 infection, or vaccination status were applied. A minimum sample size of 250 respondents was prespecified for field testing. In Rasch analysis, a sample of 250 respondents provides 99% confidence that item calibrations and person measures are stable within ±0.50 logits.25
Symptom coverage and existing patient reported outcomes
The conceptual framework underpinning SBQ-LC was developed from systematic literature reviews of long covid symptoms.5 26 Existing symptom measures (n=6) with good face validity in the context of long covid were reviewed to establish whether a new instrument for symptom burden that measured patient reported outcomes was needed.20 27 28 29 30 31 When mapped to the conceptual framework, symptom coverage of these instruments ranged from 27.0% to 60.3%: mean 34.5% (standard deviation 16.2%). Supplementary table S1 presents the concept coverage matrix mapping symptom coverage of the candidate instruments to the conceptual framework. The finding from this mapping suggested that complete coverage of long covid symptoms could not be guaranteed using existing measures, providing justification for the development of SBQ-LC.
Study procedures
Content validation
Content validation involved an online clinician survey to explore item relevance and clarity and identify symptoms of clinical concern, and cognitive debriefing interviews with adults with long covid to ascertain the relevance, comprehensiveness, comprehensibility, and acceptability of SBQ-LC’s items for the target population. The clinician survey (supplementary file S1) was administered using the survey software application SmartSurvey.32 A content validity index value was calculated for each item (item-content validity index) as the proportion of clinicians who rated the item as relevant adjusted for chance agreement (modified κ). A modified κ value in the range of 0.4-0.59 was considered as fair content validity, 0.60-0.74 as good, and ≥0.74 as excellent.33 We used the item-content validity index values to identify item candidates requiring in-depth exploration of relevance and comprehensibility with long covid patients.
Cognitive debriefing interviews took place through videoconferencing and were recorded. Verbatim transcripts of the interview recordings, field notes, and free text comments from the clinician survey were analysed qualitatively using thematic analysis and a prespecified framework to identify problems with the relevance, comprehensiveness, clarity, and acceptability of the items.34 35 Participants with lived experience of long covid were asked to identify additional symptoms not present in the initial item pool. Findings informed revisions, which were tracked for each item using an Excel spreadsheet. A draft version of SBQ-LC was constructed and sent forward for field testing.
Field testing
The Atom5 platform (Apartio, Wrexham, UK) is a regulated (ISO13485, ISO/IEC 27001:2013 Accreditation, FDA CFR21 Part 11 compliant) software platform that provides remote data collection and real time patient monitoring through a smartphone application and integration with wearable devices.36 The draft SBQ-LC, the EQ-5D-5L as a measure of health related quality of life, and a demographic questionnaire were programmed onto Atom5 for delivery.37 38 Participants were recruited through social media advertisements posted on Twitter and Facebook by the study team and through other support group platforms and website registrations in collaboration with long covid support groups based in the UK. Interested individuals connected via a URL to a study specific website where they could read detailed information about the study, provide informed consent, and download the Atom5 app to their mobile device (smartphone or tablet). Participants accessed the questionnaires by way of a unique QR code. Once the completed questionnaires were submitted, participants could delete the app from their phone. We securely downloaded the anonymised field test data from Atom5 for analysis.
Statistical analyses
STATA (version 16) was used to clean and prepare the data, and for descriptive data analyses. We conducted Rasch analysis on the field test data to refine SBQ-LC (ie, item reduction) and assess its scaling properties. A Rasch analysis is the formal evaluation of an instrument that measures patient reported outcomes against the Rasch measurement model. The Rasch model is a mathematical ideal that specifies a set of criteria for the construction of interval level measures from ordinal data.39 It is a probabilistic model, which specifies that an individual’s response to an item is only governed by the individual and the location of the item on a shared scale measuring the latent trait. The probability that a person will endorse an item is a logistic function of the difference between an individual’s trait level (expressed as person ability) and the amount of trait expressed by the item (expressed as item difficulty).40 41
Rasch analysis enabled SBQ-LC to be constructed as a modular instrument measuring patient reported outcomes (ie, a multi-domain item bank) with linear, interval level measurement properties. These properties render Rasch developed patient reported outcomes suitable for use with individual patients as well as for group level comparisons, permit direct comparisons of scores across domains, and facilitate the construction of alternative test formats (ie, short forms and computer adaptive tests).42
Rasch analyses were carried out using Winsteps software (version 5.0.5) and the partial credit model for polytomous data.43 We selected the partial credit model because the question wording and rating scale categories varied across items. Joint maximum likelihood estimation in Winsteps enabled parameter estimation when data were missing. Misfitting response patterns (eg, arising from respondents guessing or other unexpected behaviour) have been shown to result in biased item estimates with detrimental impacts for model fit.44 45 Therefore, as is customary in Rasch analysis, we appraised person fit statistics, iteratively removed individuals with misfitting response patterns (ie, outfit mean square values >2.0 logits), and re-estimated item parameters until evidence of item parameter stability was observed.40 44
Rating scale functioning for individual items was assessed against several criteria: all items oriented in the same direction as a check for data entry errors (ie, appraisal of point measure correlations); average category measures advance (ie, higher categories reflect higher measures); category outfit mean square values ≤2.0 logits (ie, as an indicator of unexpected randomness in the model); and each category endorsed by a minimum of 10 respondents.46 If an item’s rating scale failed to meet these criteria, we combined adjacent categories or removed the item. Category probability curves provided a graphical representation as further evidence of rating scale functioning.
To confirm model fit, we completed Rasch analyses (including appraisal of unidimensionality, local independence, and individual item fit statistics) iteratively as items were removed or grouped to create new scales. We also evaluated person reliability and separation indices and scale-to-sample targeting. Targeting examines the correspondence between items and individuals, and, for a well targeted scale, the items in a scale should be spaced evenly across a reasonable range of the scale and correspond to the range of the construct experienced by the sample.40 Person reliability examines the reproducibility of relative measure location, and person separation provides a measure of the number of distinct levels of person ability (symptom burden) that can be distinguished by a scale.47 For each scale we computed Cronbach’s alpha as a measure of internal consistency reliability.48 Box 1 describes the parameters evaluated in the development and validation of SBQ-LC, along with acceptability criteria. EQ-5D-5L values were generated following guidance from the National Institute for Health and Care Excellence.49 Valuation was undertaken using the crosswalk method to the EQ-5D-3L value set.50 We compared against published data on population norms. This analysis was, however, exploratory only and therefore a representative study would be needed to comprehensively analyse the effects on health related quality of life associated with long covid.
Box 1. Rasch measurement properties, definition or aim of evaluation, and acceptability criteria.
Valid measurement model
To identify a set of items that effectively measure the target construct of symptom burden in people with long covid (ie, fulfil the axioms of fundamental measurement permitting the construction of interval level scales)
Acceptability criteria for fit
Unidimensionality: Principal component analysis of residuals, highest eigenvalue of first residual contrast <2.0; disattenuated correlations >0.70
Local item independence: Residual correlation <0.40
Fit statistics: Outfit/infit mean square values within 0.5-1.5 logits
Point measure correlations >0.40
Rating scales: Scale oriented with latent variable, categories advance monotonically, category fit statistics: Outfit mean square values <2.0 logits, uniform category endorsement
Rasch reliability
Rasch based reliability is the share of true variance of the total observed variance of the measure; person separation index is the number of distinct levels of person ability (symptom burden) that can be identified by the measure
Acceptability criteria for fit
Person reliability: r≥0.70 acceptable; r≥0.80 good; r≥0.90 excellent
Person separation index: 1.5-2.0 acceptable level; 2.0-3.0 good level; ≥3.0 excellent level
Internal consistency reliability
Extent to which items comprising a scale measure the same construct (degree of homogeneity or relatedness among items of a scale)
Acceptability criteria for fit
Cronbach’s alpha range ≥0.70
Targeting
Extent to which the range of the variable measured by the scale matches the range of that variable in the study sample
Acceptability criteria for fit
Item person map
Acceptable targeting shown by close correspondence of the person mean with the item mean for a scale (±1.0 logits from the mean of zero)
Patient and public involvement
The TLC study PPI group was established in line with guidance from the National Institute for Health Research improving inclusion of under-served groups in clinical research (INCLUDE) project.51 Members of the TLC study PPI group and representatives from UK based long covid support groups were involved from the outset in the development of the study design, recruitment strategy, and all participant facing materials. Field test participants were recruited from long covid patient support groups identified on social media channels. PPI members reviewed and provided critical feedback during the drafting of the manuscript. We will work with PPI members to disseminate the study results to relevant patient and public communities.
Results
Item development and content validation
An initial pool of 97 items was constructed, guided by the conceptual framework developed from the published literature. The clinicians’ review (n=10) of the item pool informed changes to the wording of items to improve clarity. Content validity indices were calculated for each item (item-content validity index) based on clinician ratings of relevance and used to identify items requiring further investigation of relevancy during cognitive debriefing. Item-content validity index values ranged from 0.4 to 1.0, with 115 (94%) of the draft items rated as good or as excellent (supplementary table S2). Content validity was confirmed by 13 people with lived experience of long covid in two rounds of cognitive debriefing interviews. All participants were white and ranged in age from 20 to 60 years. Ten participants (77%) were women. Cognitive debriefing identified gaps in symptom coverage, resulting in the generation of 69 new items. Findings also guided the design of the rating scale layout in Atom5 and confirmed patient preferences for response category labels. Thematic analysis classified problems with draft items’ relevance, comprehensiveness, clarity, and acceptability. Supplementary table S3 presents key themes, together with exemplar quotations, from the thematic analysis.
The draft SBQ-LC included 166 items (155 symptoms and 11 interference items) and an a priori theoretical domain classification comprised of 14 domains, each constructed as an independent scale. Items utilised a seven day recall period, and burden was measured using a dichotomous response (yes or no) or a 5 point rating scale measuring either severity, frequency, or interference. Higher scores represented greater symptom burden. Commonly experienced symptoms were presented earlier in each scale, and potentially sensitive items (eg, self-harm) were positioned in the middle or at the end of a scale. Neutral wording ensured items were not phrased as leading questions. Response scales with empirical evidence of their use in validated instruments measuring patient reported outcomes reinforced the rigor of SBQ-LC’s design.52 To confirm face validity, we held a virtual meeting with the TLC Study’s PPI group in May 2021 to obtain consensus on the utility, acceptability, and format of SBQ-LC.
Readability
Readability, measured using the Flesch-Kincaid reading grade level test and the Simple Measure of Gobbledygook (SMOG) index score, was calculated using the web based application Readable.53 54 55 The American Medical Association and the National Institutes of Health recommend that the readability of patient materials should not exceed a sixth grade reading level (Flesh-Kincaid score 6.0).56 SBQ-LC’s Flesch-Kincaid reading grade level score was 5.33 and SMOG index score was 8.27. Text with a SMOG index score ranging between 7 and 9 would be understood by 93% of adults in the UK.57
Field testing
Participants
Over a two week period in June 2021, 906 questionnaires were delivered and 330 responses were received (response rate 36%). Fifty six submissions were incomplete and excluded from the analyses. The final sample included 274 complete responses (completion rate 83%). The age of respondents ranged from 21 to 70 years, with a mean age of 45.0 (standard deviation 10.0) years. The sample included 240 (88%) female respondents, and 253 (92%) respondents were white. All respondents (100%) had self-reported covid-19, with half the field test sample (n=150, 55%) being infected during the first wave of the pandemic, defined as March to May 2020 in England.58 One hundred and twenty nine (47%) respondents reported having a positive polymerase chain reaction test result for SARS CoV-2, and 22 (8%) reported having a positive lateral flow test result. Owing to limited availability of testing early in the pandemic, not all participants were tested for SARS-CoV-2. Eleven respondents (4%) had been admitted to hospital with covid-19. Seventy (25%) respondents had received one dose of a covid-19 vaccine and 187 (68%) had received two doses. In total, 153 (56%) respondents were in either full time or part time employment (table 1). Exploratory analysis showed that EQ-5D-5L values (mean score 0.490 (standard deviation 0.253)) were lower than UK population norms reported in published studies and suggested that further research is needed to evaluate the impacts of long covid on health related quality of life—for example, the following EQ-5D values were reported in a recent study for the indicated age groups in England, 25-34: 0.919; 35-44: 0.893; 45-54: 0.855; 55-64: 0.810; 65-74: 0.773; and ≥75: 0.703).59 Overall, 214 respondents (78%) reported one or more comorbidities (table 2).
Table 1.
Personal characteristics of field test sample. Values are numbers (percentages) of participants unless stated otherwise
| Characteristics | Respondents (n=274) |
|---|---|
| Age (years): | |
| Mean (SD)*; range | 45.0 (10.0); 21-70 |
| Sex: | |
| Female | 240 (88) |
| Male | 34 (12) |
| Ethnicity: | |
| White | 253 (92) |
| Asian or Asian British | 7 (3) |
| Black, African, Caribbean, or black British | 3 (1) |
| Mixed or multiple ethnic groups | 11 (4) |
| Other ethnic group | 0 (0) |
| Occupational status: | |
| Employed full time | 114 (42) |
| Employed but currently not working | 51 (19) |
| Employed part time | 39 (14) |
| Furloughed | 7 (3) |
| Retired | 6 (2) |
| Caregiver | 3 (1) |
| In full time education | 3 (1) |
| Voluntary work | 3 (1) |
| Unemployed | 20 (7) |
| Other | 28 (10) |
| Month and year of SARS-Co-V-2 infection: | |
| December 2019 | 3 (1) |
| January-December 2020 | 233 (85) |
| January-May 2021 | 38 (14) |
| Positive PCR test result for SARS-Co-V-2 infection†: | |
| Yes | 129 (47) |
| No | 137 (50) |
| Do not know | 8 (3) |
| Positive lateral flow test result for SARS-Co-V-2 infection†: | |
| Yes | 22 (8) |
| No | 227 (83) |
| Do not know | 25 (9) |
| Admitted to hospital for SAR-Co-V-2 infection: | |
| Yes | 11 (4) |
| No | 263 (96) |
| Admitted to ICU for SARS-Co-V-2 infection: | |
| Yes | 0 (0) |
| No | 274 (100) |
| Attended hospital emergency department for SARS-Co-V-2 infection: | |
| Yes | 111 (41) |
| No | 163 (59) |
| Vaccine status: | |
| Two doses | 187 (68) |
| One dose | 70 (25) |
| No dose | 17 (6) |
| Received shielding letter from UK government‡: | |
| Yes | 12 (4) |
| No | 262 (96) |
| Care home resident: | |
| Yes | 3 (1) |
| No | 271 (99) |
| Mean (SD) EQ-5D-5L utility score | 0.490 (0.253) |
SD=standard deviation; PCR=polymerase chain reaction; ICU=intensive care unit; EQ-5D-5L=health related quality of life instrument.
n=263 respondents.
Owing to difficulties accessing PCR and lateral flow tests in the early weeks of the pandemic, not all participants had access to testing.
Letter to indicate clinical vulnerability requiring enhanced social distancing.
Table 2.
Number and types of self-reported comorbidities in field test sample
| Comorbidities | No (%) of respondents (n=274) |
|---|---|
| No of comorbidities: | |
| 0 | 60 (22) |
| 1 | 77 (28) |
| 2 | 54 (20) |
| 3 | 38 (14) |
| ≥4 | 45 (16) |
| Comorbidities: | |
| Anxiety | 93 (34) |
| Asthma | 65 (24) |
| Depression | 61 (22) |
| Irritable bowel syndrome | 55 (20) |
| Other | 53 (19) |
| Back or neck pain, or both | 49 (18) |
| Hypertension | 30 (11) |
| Chronic fatigue syndrome | 29 (11) |
| Osteoarthritis | 18 (7) |
| Polycystic ovary syndrome | 11 (4) |
| Diabetes | 8 (2.9) |
| Rheumatoid arthritis | 5 (1.8) |
| Coeliac disease | 4 (1.5) |
| Chronic obstructive pulmonary disease | 3 (1) |
| Inflammatory bowel disease | 3 (1) |
| Spleen | 3 (1) |
| Stroke or transient ischaemic attack | 3 (1) |
| Cancer | 2 (0.7) |
| Epilepsy | 2 (0.7) |
| Heart disease | 2 (0.7) |
| Immunosuppression treatment | 2 (0.7) |
| Osteoporosis | 2 (0.7) |
| Cystic fibrosis | 1 (0.4) |
| Kidney disease | 1 (0.4) |
| Liver disease | 1 (0.4) |
| Severe combined immunodeficiency | 1 (0.4) |
| Sickle cell anaemia | 1 (0.4) |
| Atrial fibrillation | 0 (0) |
| Dementia | 0 (0) |
| Down’s syndrome | 0 (0) |
| Multiple sclerosis | 0 (0) |
| Parkinson’s disease | 0 (0) |
| Transplant recipient | 0 (0) |
Assessment of rating scale functioning, item fit, and scale refinement
To assess rating scale functioning we examined the relevant Winsteps output tables and item category probability curves for 166 items. Appraisal of category endorsement revealed the presence of floor effects and positively skewed scoring distributions. To deal with non-uniform category distribution, we collapsed the 5 point rating scale to either a dichotomous response or a 4 point rating scale (0=none, 1=mild, 2=moderate, 3=severe; 0=never, 1=rarely, 2=sometimes, 3=always; 0=not at all, 1=very little, 2=somewhat, 3=severely). After adjustment of the rating scale and removal of 35 items (figure 1 shows the iterative process of item reduction together with reasons for removal), point-measure correlations were positive (range 0.23-0.92), categories were ordered, and category fit statistics were productive for measurement (outfit mean square values <2.0 logits) for all items. Category distribution remained positively skewed overall (mean skewness 1.44, mean standard error 0.15 (range −2.30-11.64)) and disordered thresholds were observed for 52 (40%) items (supplementary figure S1). Threshold disordering, indicative of low category endorsement, is only considered a cause for concern when category disordering is also observed.43 Consequently, no further items were removed. We systematically grouped the remaining 131 items to construct scales that were clinically sensible and satisfied the Rasch model requirements of unidimensionality, item fit, and local independence.
Calibration of final SBQ-LC
After optimisation of the response scale, we performed Rasch analyses to report the psychometric properties of the finalised SBQ-LC (version 1.0). SBQ-LC (version 1.0) is composed of 17 independent scales (fig 2). Supplementary table S4 presents the items included in each of SBQ-LC’s scales. To illustrate the respondent instructions, item wording, and response scales, in supplementary file S2 we present the SBQ-LC breathing scale as an exemplar. Table 3 presents the Rasch based statistics for each of SBQ-LC’s scales. All scales met the Rasch model criteria for unidimensionality and item fit. The first residual contrast values from principal component analyses of the residuals ranged from 1.46 to 2.03 eigenvalues. No serious misfit was identified. Item infit mean square values ranged from 0.67 to 1.32 logits and outfit mean square values ranged from 0.44 to 1.53 logits. Fourteen item pairs across eight scales showed local item dependency, with residual correlation values >0.4 (range 0.44-0.88). In practical terms, a degree of local dependency is always observed in empirical data; therefore, it is necessary to consider the implications for content validity before proceeding with item removal.60 After qualitative appraisal of the 14 dependent pairs, we retained all items to ensure comprehensive symptom coverage.
Fig 2.

Conceptual framework showing scales (domains) of symptom burden questionnaire for long covid (SBQ-LC, version 1.0)
Table 3.
Summary of scale level Rasch based psychometric properties for symptom burden questionnaire for long covid (SBQ-LC, version 1.0) in 274 adults with long covid
| Scale (symptom domain) | No of items | Misfitting items | Misfitting persons removed (%) | Mean person ability (logits) | PCA eigenvalue (first contrast)* | Dependent item pairs | Item separation | Item reliability | Person separation† | Person reliability‡ | Internal consistency reliability§ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Breathing | 7 | 0 | 31 (11.3) | −1.95 (SE 0.99) | 1.72 | 0 | 7.68 | 0.98 | 1.8 | 0.76 | 0.84 |
| Circulation | 5 | 0 | 17 (6.2) | −1.16 (SE 0.98) | 1.58 | 0 | 7.43 | 0.98 | 1.1 | 0.55 | 0.63 |
| Fatigue | 4 | 0 | 33 (12.0) | 3.98 (SE 1.45) | 1.46 | 1 | 1.34 | 0.64 | 1.78 | 0.76 | 0.91 |
| Memory, thinking, and communication | 10 | 0 | 12 (4.38) | −1.04 (SE 0.54) | 1.72 | 0 | 13.86 | 0.99 | 2.56 | 0.87 | 0.9 |
| Sleep | 4 | 0 | 31(11.3) | −0.10 (SE 0.78) | 1.62 | 2 | 13.44 | 0.99 | 1.67 | 0.74 | 0.56 |
| Movement | 3 | 0 | 31 (11.3) | −6.27 (SE 2.06) | 1.57 | 3 | 11.23 | 0.99 | 2.08 | 0.81 | 0.86 |
| Muscles and joints | 9 | 0 | 23 (8.39) | −0.85 (SE 0.54) | 1.68 | 0 | 7.38 | 0.98 | 1.68 | 0.73 | 0.84 |
| Skin and hair | 8 | 0 | 26 (9.49) | −2.38 (SE 0.92) | 1.55 | 0 | 4.6 | 0.95 | 0.71 | 0.34 | 0.68 |
| Eyes | 10 | 0 | 12 (4.38) | −1.21 (SE 0.72) | 1.85 | 0 | 4.28 | 0.95 | 1.08 | 0.54 | 0.72 |
| Ears, nose, and throat | 14 | 0 | 11 (4.01) | −1.25 (SE 0.49) | 1.96 | 1 | 3.39 | 0.92 | 1.22 | 0.6 | 0.8 |
| Stomach and digestion | 8 | 1 | 23 (8.39) | −1.52 (SE 0.70) | 1.6 | 0 | 6.19 | 0.97 | 0.95 | 0.48 | 0.7 |
| Mental health and wellbeing | 9 | 0 | 25 (9.12) | −0.70 (SE 0.52) | 1.66 | 0 | 8.71 | 0.99 | 1.78 | 0.76 | 0.82 |
| Female reproductive and sexual health | 7 | 0 | 27 (9.85) | −2.07 (SE 1.05) | 1.99 | 2 | 5.52 | 0.97 | 0.99 | 0.5 | 0.59 |
| Male reproductive and sexual health | 3 | 0 | 1 (3.03) | −1.48 (SE 1.98) | 2.03 | 2 | 2.27 | 0.84 | 0.79 | 0.38 | 0.6 |
| Pain | 4 | 0 | 25 (9.12) | −1.53 (SE 1.05) | 1.75 | 2 | 13.06 | 0.99 | 1.62 | 0.72 | 0.77 |
| Other symptoms | 18 | 0 | 22 (8.03) | −1.53 (SE 0.43) | 1.67 | 1 | 5.5 | 0.97 | 1.33 | 0.64 | 0.79 |
| Interference | 8 | 0 | 21 (7.66) | 2.35 (SE 0.89) | 1.95 | 0 | 11.21 | 0.99 | 2.13 | 0.82 | 0.89 |
SE=standard error.
Unidimensionality: principal component analyses (PCA) of residuals eigenvalue of first residual contrast <2.0.
Person separation: ≥1.50 acceptable, ≥2.00 good, ≥3.00 excellent.
Rasch based reliability: r≥0.70 acceptable, r≥0.80 good, r≥0.90 excellent.
Cronbach’s alpha ≥0.70 acceptable.
Lastly, we evaluated scale-to sample targeting, item separation, person reliability and separation, and internal consistency reliability. Four scales had mean person ability values within ±1.0 logits of mean item difficulty. Mean person ability ranged from −6.27 to 3.98 logits (standard error range 0.43-2.06 logits). Supplementary figure S2 shows the item person maps for SBQ-LC’s scales. Item separation values ranged from 1.34 to 13.86 across the 17 scales. Person reliability ranged from 0.34 to 0.87 and person separation indices ranged from 0.71 to 2.56. Values for Cronbach’s alpha, as further evidence of internal consistency reliability, ranged from 0.56 to 0.91.
Discussion
In this study we developed and validated SBQ-LC, a Rasch developed multi-domain item bank and modular instrument measuring symptom burden in people with long covid. SBQ-LC was developed in accordance with international, consensus based standards and regulatory guidance and can be used to evaluate the impact of interventions and to inform clinical care.15 23 61 We used the findings from published systematic reviews to construct a conceptual framework and generate an initial item pool. Rigorous content validity testing provided evidence of SBQ-LC’s relevance, comprehensiveness, comprehensibility, and acceptability. Rasch analysis guided optimisation of SBQ-LC’s items and response scales to construct an interval level instrument ready for psychometric evaluation using traditional indicators.
SBQ-LC was developed with the extensive involvement of adults with lived experience of long covid, and patient input is a strength of this study. Involvement of the target population is regarded as ideal in the development of instruments to measure patient reported outcomes and may be considered of particular importance in the context of long covid where the evidence base is rapidly evolving and affected individuals have reported experiences of stigma and a lack of acknowledgement from the medical community about the breadth and nature of their symptoms.10 Involvement of adults with long covid in all phases of the study (development, refinement, and validation) ensured patients’ voices were embodied in SBQ-LC’s items.
Rasch analysis of SBQ-LC guided reduction in the number of items and refinement of the rating scale, optimising the scales’ measurement accuracy and minimising respondent burden. Despite evidence of model fit, several of SBQ-LC’s scales were off-target, with low reliability values (measured by person separation and reliability and Cronbach’s alpha). Poor scale-to sample targeting is indicative of items within a scale failing to provide full coverage of person locations (ie, range of symptom burden experienced by the sample).62 Negative mean person measures (>1.0 logits), floor effects, and positively skewed distributions of response categories suggested SBQ-LC might be targeting individuals with higher levels of symptom burden than the level of burden represented by the field test sample. Highly skewed scoring distributions and poor targeting can produce low reliability coefficients even if an instrument is functioning as intended, providing a possible explanation for the low person reliability and alpha values observed for some of SBQ-LC’s scales.62 63 In the first instance, a further Rasch analysis conducted in a representative clinical sample is required to confirm these findings. Scales remaining off-target will require critical review and further refinements (eg, creation of additional items to improve coverage of person locations) considered.
As a Rasch developed instrument, SBQ-LC’s ordinal raw scales may be converted to linear scales, with each 1 point change in a scale score being equidistant across the entire scale. Linear scores will enable the direct comparison of scores across SBQ-LC’s scales for a comprehensive assessment of symptom burden. As a multi-domain item bank, the modular construction of SBQ-LC means researchers and clinicians have the option of selecting only those scales required to provide targeted assessment of a particular symptom domain, thereby reducing respondent burden by removing the need to complete SBQ-LC in its entirety. Moreover, the Rasch model makes it possible to compare data from the SBQ-LC with other instruments measuring patient reported outcomes through co-calibration studies. As each item of a Rasch derived scale functions independently from others on that scale, SBQ-LC can be adapted to construct short forms, profile tools, or computer adaptive tests.42 A computer adaptive test is administered via a computer, which adapts to the respondent’s ability in real time by selecting different questions from an item bank to provide a more accurate measure of the respondent’s ability without the need to administer a large number of items.64 These tests can reduce respondent burden, improve accuracy, and provide individualised assessment—instrument characteristics that are attractive when assessing a health condition with heterogeneous, relapsing, and remitting symptoms such as long covid.
The burden of long covid on healthcare systems continues to grow as more people become infected with SARS-CoV-2.65 To meet this growing demand, services require cost effective resources to support safe, effective clinical management. The use of SBQ-LC in the TLC study will provide early evidence of SBQ-LC’s feasibility for use in remote patient monitoring. A previous randomised controlled trial has shown that remote symptom monitoring using patient reported outcomes can result in fewer attendances to emergency departments, reduce hospital admissions, prompt earlier intervention, and improve patients’ health related quality of life.66 If SBQ-LC is used in a clinical trial, symptom data collected remotely could provide valuable information on the safety, efficacy, and tolerability of new interventions for long covid.16 If used within routine care, SBQ-LC has potential to facilitate patient-clinician conversations, guide treatment decision making, and facilitate referrals to specialist services.67 68 69
Limitations of this study
Sample representativeness is a limitation of this study. The personal characteristics of the content validation study sample were highly skewed and the use of social media for recruitment meant it was not possible to confirm the representativeness of the field test sample, including clinical evidence of covid-19 infection. The personal characteristics of the study sample (respondents were mostly female, of white ethnicity, older, with several comorbidities) were, however, consistent with large, UK based epidemiological studies reporting on the prevalence of long covid symptoms.70 71 Findings from the REACT-2 (Real-time Assessment of Community Transmission 2) study, a cross sectional observational study of a community based sample, found that the persistence of one or more SARS-CoV-2 symptoms for 12 or more weeks was higher in women and increased linearly with age. Asian ethnicity was associated with lower risk of persistent symptoms compared with people of white ethnicity.71 The UK Office for National Statistics reported the prevalence of self-reported long covid to be highest in people aged 35 to 69 years, females, and those with another activity limiting health conditions or disabilities.70 A large retrospective cohort study on the incidence and co-occurrence of long covid features found white and non-white ethnicities to be affected equally.11 These studies suggest the field test sample in our study is broadly consistent with prevalence trends for long covid in the UK and that symptom reporting through SBQ-LC should not be substantially different for people of white versus non-white ethnicity. Nonetheless, further psychometric evaluation of SBQ-LC undertaken in a clinically confirmed, representative sample (with oversampling of underserved groups) remains a priority. Validation in patients not admitted to hospital will be undertaken as part of the TLC study, where potential participants will be identified from UK primary care practices to recruit a representative sample. Further work to validate SBQ-LC in a cohort of patients with long covid who were admitted to hospital with SARS-CoV-2 infection is planned.24
The relatively low response rate (37%), although within the typical range for electronic surveys, suggested potential field test participants (ie, possibly people experiencing higher levels of symptom burden) may have been deterred by the consenting and onboarding process or lacked sufficient incentive to participate.72 Personal information was not collected for people who opted not to participate, precluding analysis of the personal characteristics of non-respondents. The high completion rate (83%) suggested that most participants, once onboarded to Atom5, were able to complete the full SBQ-LC.
Validation of SBQ-LC is planned as part of the TLC study to confirm the study findings. Further Rasch analysis and an evaluation of SBQ-LC using traditional psychometric indicators (test-retest reliability, construct validity, responsiveness, and measurement error) will be undertaken. Studies to explore the feasibility and acceptability of SBQ-LC for use in health and social care settings are also needed and will help to inform guidance on the use of SBQ-LC in routine care. SBQ-LC is currently available in UK English as an electronic patient reported outcome and in paper form. Linguistic and cross cultural validation studies will ensure SBQ-LC is suitable for use in a range of health and social care settings in the UK and in other countries, including low and middle income countries.73
Conclusions
The presence of symptoms of covid-19 persisting beyond the acute phase of infection in a considerable number of patients represents an ongoing challenge for healthcare systems globally. High quality instruments to measure patient reported outcomes are required to better understand the signs, symptoms, and underlying pathophysiology of long covid, to develop safe and effective interventions, and to meet the day-to-day needs of this growing patient group. SBQ-LC was developed as a comprehensive measure of the symptom burden from long covid. With promising psychometric properties, SBQ-LC is available for use in long covid research studies and in the delivery of clinical care.
What is already known on this topic
As of December 2021, 1.3 million people in the United Kingdom and an estimated >100 million worldwide are currently living with long covid or post-covid-19 syndrome; this figure will continue to rise as more people are affected with SARS-CoV-2 infection
Studies have shown that long covid is a novel, multisystem condition with considerable symptom burden and negative impacts on work capability and quality of life
Owing to a lack of patient reported outcome measures specific to long covid, researchers and clinicians are using bespoke surveys, generic patient reported outcome measures, or symptom burden measures validated in other disease groups to assess the symptom burden from long covid
What this study adds
With extensive patient involvement, this mixed methods study developed and validated the symptom burden questionnaire for long covid
This novel questionnaire has the potential to benefit international clinical trials and inform best practice in clinical management
Acknowledgments
We gratefully acknowledge the contributions of the clinicians who participated in the online survey. We thank Anita Slade for their support with interpretation of the Rasch analyses, and LongCovidSOS, Long Covid Scotland, Long Covid Support, and Asthma UK and the British Lung Foundation for their help with field test recruitment. The SBQ-LC (version 1.0) is available under license. For more information about the SBQ-LC, to view a review copy, or obtain a license for use, please visit the SBQ-LC website at www.birmingham.ac.uk/sbq.
Web extra.
Extra material supplied by authors
Supplementary information: Additional tables S1-S4, files S1 and S2, and figures S1 and S2
Contributors: MJC, SH, SEH, and OLA developed the concept and design of the SBQ-LC. EHD and CF conceptualised and designed delivery of the SBQ-LC in Atom5. MJC, SH, SEH, AS, CM, OLA, GMT, LJ, EHD, and GP obtained funding. SH and MJC supervised the study. SEH, SH, OLA, GMT, CM, and MJC developed the study design and methodology. MJC, SH, and SEH were responsible for project management. AW and GM provided administrative and project management support. OLA, GP, KM, JO, JC, SEH, CM, and CF were responsible for data acquisition. SEH, AS, LJ, and CF were responsible for data curation and validation. SEH, SH, LJ, and MJC did the analyses. SEH wrote the original manuscript. SEH, SH, and MC are the guarantors. All authors provided critical revisions and approved the final manuscript. The corresponding author attests that all authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work is independent research jointly funded by the National Institute for Health Research (NIHR) and UK Research and Innovation (UKRI) (Therapies for Long COVID in non-hospitalized individuals: From symptoms, patient reported outcomes and immunology to targeted therapies (TLC Study), (COV-LT-0013). The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, the Department of Health and Social Care, or UKRI. The funders had no role in the design and conduct of the study, including the collection, management, analysis, and interpretation of the data, and preparation and review of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: SEH receives funding from the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) West Midlands and UK Research and Innovation (UKRI) and declares personal fees from Aparito and Cochlear outside the submitted work. MJC is director of the Birmingham Health Partners Centre for Regulatory Science and Innovation and director of the Centre for Patient Reported Outcomes Research and is an NIHR senior investigator. MJC receives funding from the NIHR, UKRI, NIHR Birmingham Biomedical Research Centre, NIHR Surgical Reconstruction and Microbiology Research Centre, NIHR ARC West Midlands, UK SPINE, European Regional Development Fund-Demand Hub and Health Data Research UK at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Innovate UK (part of UKRI), Macmillan Cancer Support, UCB Pharma, Janssen, GlaxoSmithKline, and Gilead. MJC has received personal fees from Astellas, Aparito, CIS Oncology, Takeda, Merck, Daiichi Sankyo, Glaukos, GlaxoSmithKline, and the Patient-Centered Outcomes Research Institute outside the submitted work. In addition, a family member owns shares in GlaxoSmithKline. OLA receives funding from the NIHR Birmingham Biomedical Research Centre, NIHR ARC West Midlands, UKRI, Health Foundation, Janssen, Gilead, and GlaxoSmithKline. He declares personal fees from Gilead Sciences, Merck, and GlaxoSmithKline outside the submitted work. JC is a lay member on the UK National Institute for Health and Care Excellence COVID expert panel, a citizen partner to the COVID-END Evidence Synthesis Network, patient and public involvement lead on the NIHR CICADA ME Study, patient representative at the EAN European Neurology Autonomic Nervous Systems Disorders Working Group, a member of the Medical Research Council/UKRI Advanced Pain Discovery Platform, and external board member of Plymouth Institute of Health. She also reports contracts with GlaxoSmithKline and Medable. CM receives funding from NIHR Surgical Reconstruction and Microbiology Research Centre, UKRI, and declares personal fees from Aparito. KM is employed by the NIHR. SH receives funding from NIHR and UKRI.
The manuscript’s guarantors (SEH, SH, and MC) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Dissemination to participants and related patient and public communities: We will distribute the article to clinicians and long covid support groups. We will distribute findings on social media, and a plain language summary on the Therapies for Long COVID Study website (www.birmingham.ac.uk/research/applied-health/research/long-covid/index.aspx). We will share findings through conference presentations, including invited talks and webinars. Information on the symptom burden questionnaire for long covid (SBQ-LC) and obtaining a license for use is available at www.birmingham.ac.uk/sbq.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the University of Birmingham Research Ethics Committee (ERN_21-0191).
Data availability statement
Data for this project are not currently available for access outside the Therapies for Long COVID Study research team. The dataset may be shared when finalised, but this will require an application to the data controllers. The data may then be released to a specific research team for a specific project dependent on the independent approvals being in place.
References
- 1.COVID-19 situation update worldwide, as of week 44, updated 11 November 2021. www.nice.org.uk/guidance/ng188.
- 2.National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. https://app.magicapp.org/#/guideline/EQpzKn/section/n3vwoL. [PubMed]
- 3.World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
- 4. Gapstur RL. Symptom burden: a concept analysis and implications for oncology nurses. Oncol Nurs Forum 2007;34:673-80. 10.1188/07.ONF.673-680. [DOI] [PubMed] [Google Scholar]
- 5. Aiyegbusi OL, Hughes SE, Turner G, et al. TLC Study Group . Symptoms, complications and management of long COVID: a review. J R Soc Med 2021;114:428-42. 10.1177/01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int J Clin Pract 2021;75:e14357. 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Post-COVID Conditions. 2020. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
- 8. Amin-Chowdhury Z, Harris RJ, Aiano F, et al. Characterising post-COVID syndrome more than 6 months after acute infection in adults; prospective longitudinal cohort study, England. medRxiv 2021;2021.03.18.21253633. 10.1101/2021.03.18.21253633 [DOI]
- 9. Parker AM, Brigham E, Connolly B, et al. Addressing the post-acute sequelae of SARS-CoV-2 infection: a multidisciplinary model of care. Lancet Respir Med 2021;9:1328-41. 10.1016/S2213-2600(21)00385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res 2020;20:1144. 10.1186/s12913-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773. 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID [Erratum in: Nat Med. 2021;27:1116. PMID: 33692530]. Nat Med 2021;27:626-31. 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with covid-19: a systematic review. JAMA Netw Open 2021;4:e2111417. 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sivan M, Wright S, Hughes S, Calvert M. Using condition specific patient reported outcome measures for long covid. BMJ 2022;376:o257. 10.1136/bmj.o257. [DOI] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration . Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims. 2009:1-39. www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aiyegbusi OL, Calvert MJ. Patient-reported outcomes: central to the management of COVID-19. Lancet 2020;396:531. 10.1016/S0140-6736(20)31724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berry P. Use patient reported outcome measures (PROMs) in treatment of long covid. BMJ 2021;373:n1260. 10.1136/bmj.n1260. [DOI] [PubMed] [Google Scholar]
- 18. Calvert M, Brundage M, Jacobsen PB, Schünemann HJ, Efficace F. The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health Qual Life Outcomes 2013;11:184. 10.1186/1477-7525-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tran V-T, Riveros C, Clepier B, et al. Development and validation of the Long Coronavirus Disease (COVID) Symptom and Impact Tools: a set of patient-reported instruments constructed from patients’ lived experience. Clin Infect Dis 2022;74:278-87. 10.1093/cid/ciab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Connor RJ, Preston N, Parkin A, et al. The COVID-19 Yorkshire Rehabilitation Scale (C19-YRS): Application and psychometric analysis in a post-COVID-19 syndrome cohort. J Med Virol 2021. 10.1002/jmv.27415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alwan NA. The teachings of Long COVID. Commun Med 2021;1:1-3. 10.1038/s43856-021-00016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med 2021;268:113426. 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. Principles for selecting, developing, modifying, and adapting patient-reported outcome instruments for use in medical device evaluation: draft guidance for industry and food and drug administration staff, and other stakeholders. 2020. www.fda.gov/regulatory-information/search-fda-guidance-documents/principles-selecting-developing-modifying-and-adapting-patient-reported-outcome-instruments-use
- 24. Haroon S, Nirantharakumar K, Hughes SE, et al. Therapies for Long COVID in non-hospitalised individuals - from symptoms, patient-reported outcomes, and immunology to targeted therapies (The TLC Study): study protocol. medRxiv 2021;2021.12.20.21268098. 10.1101/2021.12.20.21268098 [DOI] [PMC free article] [PubMed]
- 25. Linacre JM. Sample Size and Item Calibration or Person Measure Stability. Rasch Meas Trans 1994;7:328. [Google Scholar]
- 26. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021;11:16144. 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans RA, McAuley H, Harrison EM, et al. PHOSP-COVID Collaborative Group . Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study [Erratum in: Lancet Respir Med 2022;10:e9. PMID: 34627560]. Lancet Respir Med 2021;9:1275-87. 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Haes JC, van Knippenberg FCE, Neijt JP. Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer 1990;62:1034-8. 10.1038/bjc.1990.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 2000;89:1634-46. . [DOI] [PubMed] [Google Scholar]
- 30. Powers JH, Guerrero ML, Leidy NK, et al. Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis 2016;16:1. 10.1186/s12879-015-1330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014;106:dju244. 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smart Survey. www.smartsurvey.co.uk/
- 33. Polit DF, Beck CT, Owen SV. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health 2007;30:459-67. 10.1002/nur.20199. [DOI] [PubMed] [Google Scholar]
- 34. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 35. Willis GB. Analysis of the Cognitive Interview in Questionnaire Design. Oxford University Press, 2015. [Google Scholar]
- 36. Aparito Limited . www.aparito.com/
- 37. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 39. Rasch G. Probabilistic Models for Some Intelligence and Attainment Tests. Nielson and Lydiche, 1960, 10.1016/S0019-9958(61)80061-2. [DOI] [Google Scholar]
- 40. Bond TG, Yan Z, Heene M. Applying the Rasch Model: Fundamental Measurement in the Human Sciences. 4th ed. Routledge, 2020. 10.4324/9780429030499. [DOI] [Google Scholar]
- 41. Hobart J, Cano S. Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods. Health Technol Assess 2009;13:iii , ix-x, 1-177. 10.3310/hta13120. [DOI] [PubMed] [Google Scholar]
- 42. Tennant A, McKenna SP, Hagell P. Application of Rasch analysis in the development and application of quality of life instruments. Value Health 2004;7(Suppl 1):S22-6. 10.1111/j.1524-4733.2004.7s106.x. [DOI] [PubMed] [Google Scholar]
- 43.Linacre JM. A User’s Guide to WINSTEPS MINISTEPS Rasch-Model Computer Programs. 2020. [Google Scholar]
- 44. Mousavi A, Cui Y. The effect of person misfit on item parameter estimation and classification accuracy: a simulation study. Educ Sci (Basel) 2020;10:324. 10.3390/educsci10110324. [DOI] [Google Scholar]
- 45. Tennant A, Conaghan PG. The Rasch measurement model in rheumatology: what is it and why use it? When should it be applied, and what should one look for in a Rasch paper? Arthritis Rheum 2007;57:1358-62. 10.1002/art.23108. [DOI] [PubMed] [Google Scholar]
- 46. Linacre JM. Optimizing rating scale category effectiveness. J Appl Meas 2002;3:85-106. [PubMed] [Google Scholar]
- 47. Duncan PW, Bode RK, Min Lai S, Perera S, Glycine Antagonist in Neuroprotection Americans Investigators . Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil 2003;84:950-63. 10.1016/S0003-9993(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 48. Streiner DL, Norman GR, Cairney J. Health Measurement Scales: A practical guide to their development and use. Oxford University Press, 2015. 10.1093/med/9780199685219.001.0001. [DOI] [Google Scholar]
- 49.Position statement on use of the EQ-5D-5L value set for England (updated October 2019). www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l.
- 50. van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 51.National Institute for Health Research. Improving inclusion of under-served groups in clinical research: guidance from the NIHR-INCLUDE project. UK: National Institute for Health Research. 2020. www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435.
- 52.Krosnick JA, Presser S. Question and Questionnaire Design. Handbook of Survey Research 2010;94305:886. 10.1111/j.1432-1033.1976.tb10115.x [DOI] [Google Scholar]
- 53. Readable . www.readable.io.
- 54. Flesch R. A new readability yardstick. J Appl Psychol 1948;32:221-33. 10.1037/h0057532. [DOI] [PubMed] [Google Scholar]
- 55. McLaughlin GH. SMOG grading: a new readability formula. J Read 1969;12:639-46. [Google Scholar]
- 56. Weiss BD. Help patients understand: manual for clinicians. American Medical Association Foundation and American Medical Association, 2007. [Google Scholar]
- 57.NHS Digital Service Manual. Use a readability tool to prioritise content - NHS digital service manual. nhs.uk. 2019. https://service-manual.nhs.uk.
- 58.Office for National Statistics. Coronavirus (COVID-19) Infection Survey technical article: waves and lags of COVID-19 in England, June 2021. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsurveytechnicalarticle/wavesandlagsofcovid19inenglandjune2021
- 59. Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ 2019;20:205-16. 10.1007/s10198-018-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Local Dependency and Rasch Measures . https://www.rasch.org/rmt/rmt213b.htm.
- 61.Mokkink LB, Prinsen AC, Patrick DL, et al. COSMIN manual for systematic reviews of PROMs COSMIN methodology for systematic reviews of Patient-Reported Outcome Measures (PROMs) user manual. 2018. www.cosmin.nl.
- 62.Separation, Reliability and skewed distributions: statistically different levels of performance. https://www.rasch.org/rmt/rmt144k.htm.
- 63. Anselmi P, Colledani D, Robusto E. A comparison of classical and modern measures of internal consistency. Front Psychol 2019;10:2714. 10.3389/fpsyg.2019.02714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Harrison CJ, Geerards D, Ottenhof MJ, et al. Computerised adaptive testing accurately predicts CLEFT-Q scores by selecting fewer, more patient-focused questions. J Plast Reconstr Aesthet Surg 2019;72:1819-24. 10.1016/j.bjps.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 65. Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS One 2021;16:e0254523. 10.1371/journal.pone.0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016;34:557-65. 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Greenhalgh J, Gooding K, Gibbons E, et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J Patient Rep Outcomes 2018;2:42. 10.1186/s41687-018-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Calvert M, Kyte D, Price G, Valderas JM, Hjollund NH. Maximising the impact of patient reported outcome assessment for patients and society. BMJ 2019;364:k5267. 10.1136/bmj.k5267. [DOI] [PubMed] [Google Scholar]
- 69. Turner GM, Litchfield I, Finnikin S, Aiyegbusi OL, Calvert M. General practitioners’ views on use of patient reported outcome measures in primary care: a cross-sectional survey and qualitative study. BMC Fam Pract 2020;21:14. 10.1186/s12875-019-1077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Office for National Statistics. Technical article: Updated estimates of the prevalence of post-acute symptoms among people with coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021
- 71. Whitaker M, Elliott J. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people | medRxiv. 10.1101/2021.06.28.21259452. [DOI]
- 72. Menon V, Muraleedharan A. Internet-based surveys: relevance, methodological considerations and troubleshooting strategies. Gen Psychiatr 2020;33:e100264. 10.1136/gpsych-2020-100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hughes S, Aiyegbusi OL, Lasserson D, Collis P, Glasby J, Calvert M. Patient-reported outcome measurement: a bridge between health and social care? J R Soc Med 2021;114:381-8. 10.1177/01410768211014048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional tables S1-S4, files S1 and S2, and figures S1 and S2
Data Availability Statement
Data for this project are not currently available for access outside the Therapies for Long COVID Study research team. The dataset may be shared when finalised, but this will require an application to the data controllers. The data may then be released to a specific research team for a specific project dependent on the independent approvals being in place.


