Key Findings.
-
▪
The Withings Scanwatch (Withings SA, Issy les Moulineaux, France) is a novel smartwatch that can record an intelligent electrocardiogram (iECG) with automated detection of atrial fibrillation (AF).
-
▪
In this prospective cohort of patients with suspected cardiac arrhythmias, 1 in 7 tracings was deemed as unreadable by the smartwatch. This rate could be lowered to 4.1% by cardiologist review of tracings.
-
▪
Automatic rhythm classification by the smartwatch was inferior to manual interpretation of iECGs. Cardiologist interpretation of the iECG interpretation was highly reliable, with diagnostic accuracy of 98% (95% confidence interval 96%–100%).
-
▪
The iECG algorithm of this novel smartwatch for differentiating sinus rhythm from AF is of limited clinical value alone, and physician overview with manual interpretation is required to achieve clinically sufficient diagnostic accuracy for detection of AF.
Wearable smart devices capable of screening for atrial fibrillation (AF) presently are available, and more are expected to enter the market soon. The Withings Scanwatch (Withings SA, Issy les Moulineaux, France) is a novel smartwatch that can record an intelligent electrocardiogram (iECG) with automated detection of AF. Although the iECG function from 3 major manufacturers have been investigated extensively1, 2, 3 and are approved by the Food and Drug Administration, there is a paucity of data regarding the diagnostic performance of the iECG function of the Withings Scanwatch. Whereas previous studies assessed smart device–based photoplethysmography technology mainly in healthy smartwatch users and reported AF prevalence as low as <1%,4,5 we sought to assess the diagnostic accuracy of the iECG function of a novel smart device in patients with suspected cardiac arrhythmias.
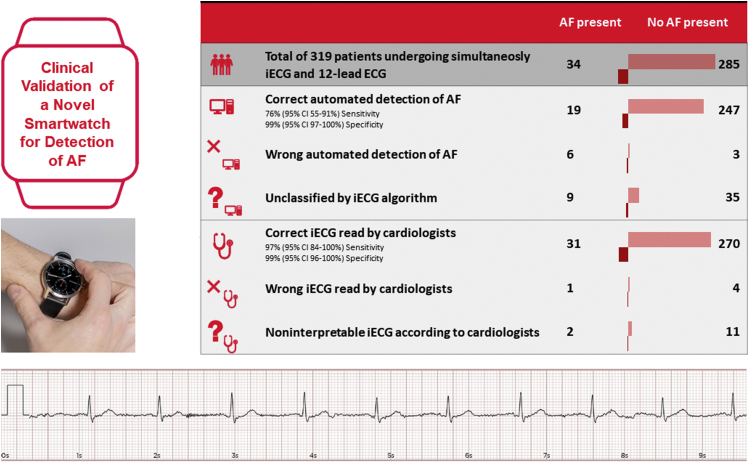
This prospective, observational study enrolled consecutive patients who presented to the cardiology department of the University Hospital Basel. The study was approved by the local ethics committee and was performed according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients. The aim of this study was to assess the diagnostic performance of the iECG function of the Withings Scanwatch in detecting AF compared to a simultaneously acquired cardiologist-interpreted 12-lead ECG. To obtain an iECG, patients were instructed to hold the stainless steel ring on the top case of the smartwatch for 30 seconds. The reading from the automated algorithm (sinus rhythm, AF, or unclassified) was recorded, and a PDF file of the iECG waveform was saved. Incomplete (<30 seconds) recordings were repeated immediately, but unclassified tracings by the algorithm were not. All iECG rhythm strips and 12-lead ECGs were anonymized and distributed to 2 blinded cardiologists who independently interpreted each tracing and assigned a diagnosis of sinus rhythm, AF, or unclassified. Sensitivities and specificities were directly calculated after tabulating values in a 2×2 table. iECGs and 12-lead ECGs were simultaneously recorded in 319 patients (median 67 years; interquartile range 54–76 years; 48% female). The clinical reasons for obtaining an ECG were assessment of cardiac rhythm in 80.2%, signs of ischemia in 1.3%, QT-interval measurements in 5%, and unknown in 13.5%. Baseline intervals such as HR, PR, QRS, and QT intervals were automatically calculated by the smartwatch in 297 (93%), 227 (71%), 250 (78%), and 179 (56%) patients, respectively. Using the automated algorithm, rhythm was deemed inconclusive in 44 patients (14%). Among these patients, 17 tracings (5.3%) were due to high or low heart rate, and 27 tracings (8.4%) were due to motion artifacts. Overall, AF was present in 34 patients (11%). Of the tracings for which the algorithm provided a diagnosis, the algorithm correctly identified AF with sensitivity of 76% (95% confidence interval [CI] 55%–91%), specificity of 99% (95% CI 97%–100%), and a kappa (κ) coefficient of 0.72 compared with cardiologist-interpreted 12-lead ECGs. Of the patients in sinus rhythm, 3 were labeled as AF (false-positive). The 44 unclassified recordings were interpreted by blinded cardiologists to determine whether these tracings were clinically useful. The interpreting cardiologists were able to correctly diagnose AF with sensitivity of 100% (95% CI 59%–100%), specificity of 93% (95% CI 77%–99%), and κ coefficient of 0.49. To assess the quality of the iECG tracings produced by the smartwatch, cardiologist-interpreted iECGs were compared to corresponding 12-lead ECGs. A total of 13 iECG recordings (4.1%) were determined to be noninterpretable by the cardiologists due to low quality. Of the remaining 306 patients with simultaneous recordings, cardiologist interpretation of the iECG tracings demonstrated sensitivity of 97% (95% CI 84%–100%), specificity of 99% (95% CI 96%–100%), and κ coefficient of 0.75 for the detection of AF. A general overview is shown in Figure 1.
Figure 1.
Automated atrial fibrillation (AF) detection algorithm using novel smartwatch technology. Right box: Cardiologist diagnosis of 12-lead electrocardiogram (ECG) (top); overview of interpretation of the smartwatch algorithm by diagnosis (middle); and overview of interpretation of intelligent electrocardiogram (iECG) by cardiologists (bottom). Left, middle: Position to obtain a smartwatch ECG. Bottom: Example of a single-lead tracing created by the smartwatch. CI = confidence interval.
We performed a clinical validation study of the iECG function of a novel smartwatch for the detection of AF. The main findings of this study were as follows. (1) The unreadable (inconclusive) rate of the iECG algorithm in this study was 14%. With cardiologist review of tracings, this rate could be lowered to 4.1%. This relevant rate of unreadable recordings is in line with other studies that assessed different smart devices, with rates ranging between 6% and 52%.1, 2, 3 (2) Automatic rhythm classification was inferior to manual interpretation of iECGs. We found a lower sensitivity for the detection of AF using the Withings iECG function compared to data reported for other smart devices.1, 2, 3 However, cardiologist interpretation of the iECG interpretation was highly reliable, with diagnostic accuracy of 98% (95% CI 96%–100%) compared to the gold standard. These findings extend and corroborate previous studies of other smart devices and help to determine the most appropriate clinical use of any smart device and associated iECG function for arrhythmia management. Although automated iECG interpretation by the iECG algorithm is still found to be wanting and clinical interpretation by a cardiologist is strongly encouraged, the iECG might be a promising option for use as a mobile event monitor for documentation of AF. The use of such devices by patients and healthy individuals is growing exponentially. Thus, well-organized programs to incorporate these smart devices and handle the data (overload) are needed.
The limitations of this study are as follows. We tested only a single device with no simultaneous comparison with other commonly used monitors. We did not assess patient perception. This was a single-center study in which patients were instructed by a trained professional and assisted in recording the single-lead ECG, thus possibly challenging the application of the findings in an at-home setting. Recordings deemed unclassified by the algorithm were excluded from the calculation, implying that in an intention-to-diagnose analysis, sensitivity and specificity would be considerably lower.
In conclusion, the iECG algorithm of this novel smartwatch for differentiating sinus rhythm from AF is of limited clinical value alone, and physician overview with manual interpretation is required to achieve clinically sufficient diagnostic accuracy for detection of AF. The clinical utility of personalized wearable technologies and the resources to integrate these devices need to be defined.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Dr Badertscher has received research funding from the University of Basel, Stiftung für Herzschrittmacher und Elektrophysiologie, Freiwillige Akademische Gesellschaft Basel, and Johnson & Johnson, all outside the submitted work. Dr Sven Knecht has received funding from the Stiftung für Herzschrittmacher und Elektrophysiologie. Dr Christian Sticherling is a member of the Medtronic Advisory Board Europe and Boston Scientific Advisory Board Europe; and received educational grants from Biosense Webster and Biotronik, a research grant from the European Union’s FP7 program and Biosense Webster, and lecture and consulting fees from Abbott, Medtronic, Biosense Webster, Boston Scientific, MicroPort, and Biotronik, all outside the submitted work. Dr Michael Kühne reports personal fees from Bayer, Böhringer Ingelheim, Pfizer BMS, Daiichi Sankyo, Medtronic, Biotronik, Boston Scientific, Johnson & Johnson, and Roche; grants from Bayer, Pfizer, Boston Scientific, BMS, Biotronik, and Daiichi Sankyo, all outside the submitted work. Dr Beat Schaer reports speaker’s bureau for Medtronic. Others all authors have nothing to declare.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed consent was obtained from all patients.
Ethics Statement
The study was approved by the local ethics committee and carried out according to the principles of the Declaration of Helsinki.
References
- 1.Seshadri D.R., Bittel B., Browsky D., et al. Accuracy of Apple watch for detection of atrial fibrillation. Circulation. 2020;141:702–703. doi: 10.1161/CIRCULATIONAHA.119.044126. [DOI] [PubMed] [Google Scholar]
- 2.Bumgarner J.M., Lambert C.T., Hussein A.A., et al. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol. 2018;71:2381–2388. doi: 10.1016/j.jacc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Avram R., Ramsis M., Cristal A.D., et al. Validation of an algorithm for continuous monitoring of atrial fibrillation using a consumer smartwatch. Heart Rhythm. 2021;18:1482–1490. doi: 10.1016/j.hrthm.2021.03.044. [DOI] [PubMed] [Google Scholar]
- 4.Perez M.V., Mahaffey K.W., Hedlin H., et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Y., Wang H., Zhang H., et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. doi: 10.1016/j.jacc.2019.08.019. [DOI] [PubMed] [Google Scholar]