Introduction
History—Initial Observations
Cerebellar ectopy may be acquired, e.g., due to mass effect or changes in cerebrospinal fluid pressures, or congenital.1 Chiari Malformations, which are now recognized as a spectrum of congenital anomalies involving the craniocervical junction, vary in age of onset, presenting symptoms, and etiology.1,2 The cause of these malformations has been long debated. In 1891, Hans Chiari, an Austrian pathologist, elaborated on the relationship between cerebellar ectopy and hydrocephalus.3,4 In this initial report, Chiari described three pathologies, which are now considered Chiari Malformations I through III (CMI-III).
As the title of his article suggested, the unifying theme of these pathologies was the displacement of the cerebellum through the craniocervical junction at the foramen magnum. In Type I (CMI), Chiari observed elongation and ectopia of the cerebellar tonsils and the medial portions of the inferior lobes of the cerebellum, which he described as cone-shaped.4 Of critical note, Chiari only found Type I cerebellar ectopy in cases of congenital hydrocephalus, not in cases of acute or late-onset hydrocephalus,4 suggesting a primary congenital etiology rather than an acquired pathology. In Type II (CMII), Chiari reported greater hindbrain and brainstem displacement through the craniocervical junction associated with a cervicothoracic syrinx, congenital lumbosacral dysraphism, and neural tube defect.4 Chiari described Type III (CMIII) based on one case of a 5-month-old child with what he called “hydrencephalocele cerebellaris cervicalis,” which included spina bifida, an enlarged skull, convergent strabismus, absence of the tentorium, cervical “hydromyelocele” communicating with the fourth ventricle, and complete herniation of the cerebellum into the spinal canal.4
While others described cerebellar ectopy in the setting of hydrocephalus before this report, Chiari was the first to investigate the origins of this relationship. In a more extensive cadaveric study several years later (1896), Chiari confirmed the above classification and added a fourth type of malformation (CMIV), which lacked cerebellar displacement through the craniocervical junction due to cerebellar hypoplasia.3,5 While this spectrum of pathologies was related to hydrocephalus, the severity of the hydrocephalus in this series did not correlate with the extent of the craniospinal malformation, which prompted Chiari to hypothesize another mechanism was responsible for obstruction of the flow of cerebrospinal fluid.3,5 He proposed that insufficient development of the cranial bones played a critical role in these pathologies. Supporting this hypothesis were observations by both Julius A. Arnold, who in 1894 published a case of cerebellar ectopy and spina bifida without hydrocephalus,3,6 and John Cleland, who described a similar case with hydrocephalus in 1883.3,7 These initial descriptions of CMI-IV made by Arnold, Chiari, and Cleland fueled debates and studies surrounding the acquired or congenital nature of cerebellar ectopy in Chiari Malformations and remain the basis of our ongoing investigations, diagnosis, and management.
Section Highlights.
Chiari Malformations I-IV are a spectrum of congenital malformations with varied age of onset, presenting symptoms, and etiologies that may be associated with syringomyelia
The unifying theme of CMI-III is displacement of the hindbrain through the cranio-cervical junction
Chiari and others proposed that insufficient development of the bony posterior fossa played a critical role in cerebellar ectopy in these malformations.
Discussion
Recent Insights—Syringomyelia and CMI
Early observations by Chiari and others suggested a shared mechanism of cerebellar ectopy and disorders of cerebrospinal fluid, i.e., hydrocephalus and syringomyelia. Critical animal studies by Dorcus Padget furthered our understanding of the embryologic underpinnings of CMI-IV and the relationship to dysraphism, such as occurs in the more severe Chiari Malformations and Dandy-Walker malformation.8 Further studies by Miguel Marín-Padilla supported the concept that these malformations—CMI-IV, Dandy-Walker malformations, and various forms of dysraphism—result from varying failures neuraxial induction of dorsal structures.9
The shared embryologic origin of these pathologies, though some nuances are still currently debated,1,2 provided a rationale for the investigations into the pathophysiologic relationship of cerebellar ectopy and syringomyelia. This original question led Chiari to report his findings. CMI, which in adults can have a presentation ranging from an asymptomatic MRI incidental finding to signs and symptoms of central myelopathy from syringomyelia, including suspended sensory loss, pain, upper extremity muscle atrophy, and lower extremity spasticity.1
Theories on the formation of a syrinx in CMI were proposed, including the “water-hammer” theory proposed by Gardner in the 1950s that postulated obstruction of the outlets of the fourth ventricle, enlarged CSF arterial pressure waves directed into the obex, and progressive dilation of the spinal cord central canal..1,12 Williams’ theorized that cranial-spinal pressure dissociation during the Valsalva maneuver caused by the ectopic tonsils obstructing the foramen magnum CSF pathway drove CSF into the obex and spinal cord central canal. Gardner’s and Williams’ theories required a patent communication of the obex and upper pole of the syrinx to transmit CSF and its pressure.1 Imaging and post-mortem studies did not demonstrate this patent communication in most cases, invalidating these theories.1 Oldfield and Heiss (current author) led further investigations to elucidate the pathophysiology of syringomyelia in CMI.1,10,11 Their studies involved CSF pressure measurements, phase-contrast magnetic resonance imaging (PC-MRI) CSF and syrinx flow studies, and intraoperative cardiac-gaited MRI in patients with Chiari I malformation and syringomyelia. The findings of these studies supported a theory in which the flow through the CSF spaces at the foramen magnum was impeded by the ectopic cerebellar tonsils, preventing the normal movement of CSF across the foramen magnum during the cardiac cycle. Instead of CSF, the ectopic cerebellar tonsils descended into the cervical spinal canal during the cardiac systole, acting on the enclosed spinal subarachnoid space, creating enlarged spinal subarachnoid pressure waves, and driving cerebrospinal fluid from the subarachnoid space into the perivascular and extracellular space of the spinal cord, resulting in syrinx formation. After forming the syrinx, these enlarged spinal subarachnoid pressure waves acted on the spinal cord surface, induced movement of syrinx fluid, and expanded the syrinx diameter and length (Figure 1).1,11 Surgical intervention involving suboccipital craniectomy, C-1 laminectomy, and duraplasty expanded the CSF space at the foramen magnum and resolved the syrinx. The cerebellar tonsils assumed a regular shape and ascended toward the foramen magnum after the surgical procedure.11
Figure 1.
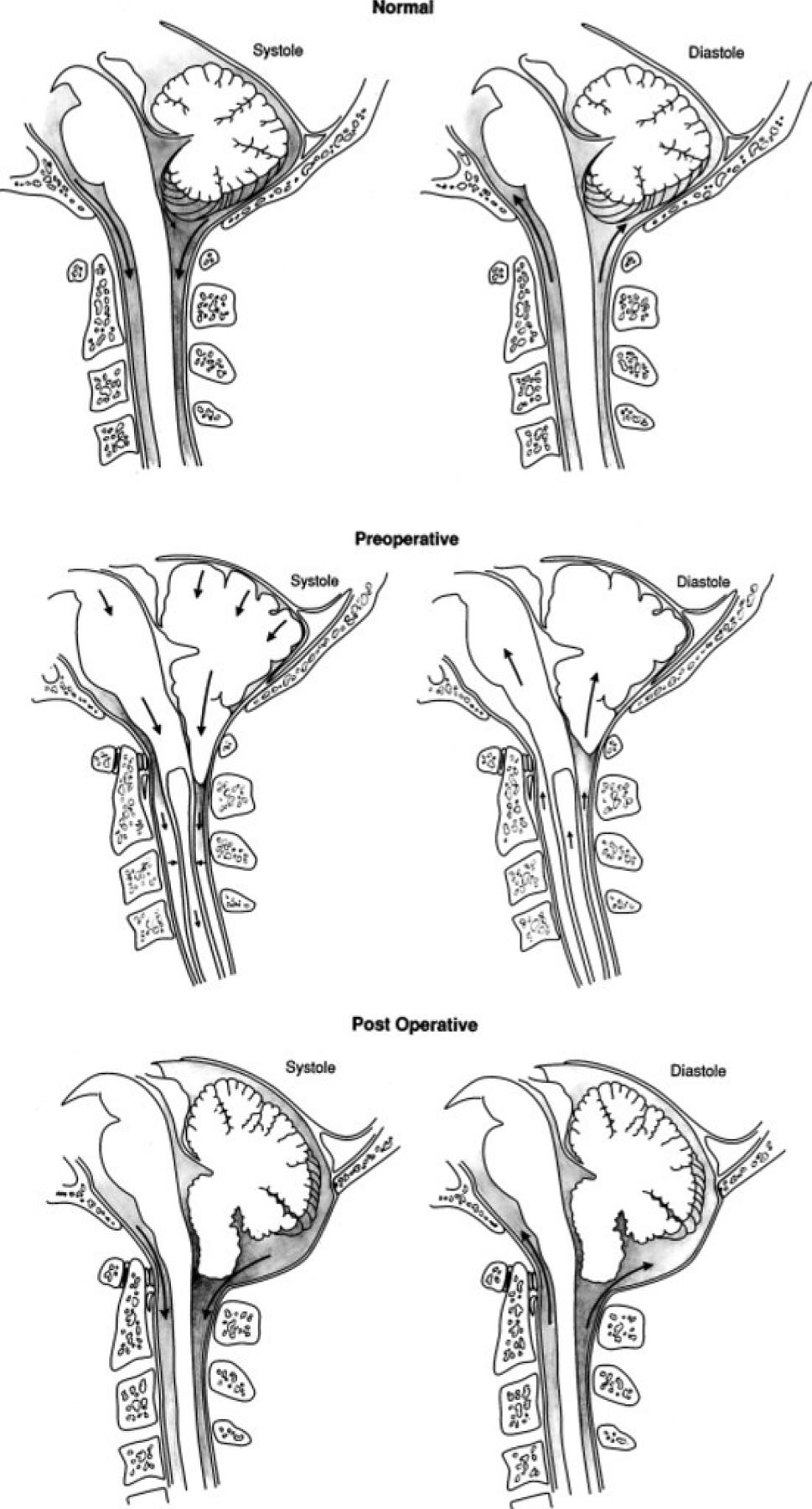
Illustrations showing normal anatomy and flow of CSF in the subarachnoid space at the foramen magnum during the cardiac cycle in a normal healthy volunteer (upper); obstructed flow of CSF in the subarachnoid space at the foramen magnum resulting in the cerebellar tonsils’ acting as a piston on the cervical subarachnoid space, creating cervical subarachnoid pressure waves that compress the spinal cord from without and propagating syrinx fluid movement (center); and relief of the obstruction of the subarachnoid space at the foramen magnum, eliminating the mechanism of progression of syringomyelia (lower).
(Adapted from Heiss JD, Patronas N, DeVroom HL, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91(4):553-562. doi:10.3171/jns.1999.91.4.0553; with permission)
CMI in most cases arises because the internal volume of the posterior fossa is inadequate to contain its neural contents and not from a primary maldevelopment of the cerebellum. Treatment of CM1 by surgically enlarging the posterior fossa results in the cerebellar tonsils ascending and assuming a regular shape, supporting this premise. Meticulous morphologic studies by Misao Nishikawa and embryologic studies by Charles Raybaud support the contention that CMI developed from a small posterior fossa. This conclusion has profound implications for the management of CMI because it directs treatment toward enlarging the posterior fossa rather than modifying a congenital brain abnormality.13,14
Section Highlights.
Several theories were developed to explain syringomyelia in CMI
The prevailing theory, supported by physiologic studies in patients with CMI using phase contrast MRI and ultrasound, proposes that occlusion of the subarachnoid spaces at foramen magnum leads to formation of the syrinx cavity by movement of cerebrospinal fluid through perivascular and extracellular spaces in the spinal cord
Surgical decompression of the posterior fossa results in return of the cerebellar tonsils to normal position and resolution of the syrinx, suggesting that, in most cases, cerebellar ectopy is secondary to an abnormally small volume of the bony posterior fossa
Controversies and Ongoing Investigations
Reduced posterior fossa size and crowding of neural structures is not the only cause of tonsillar ectopia in CM1.1,15 Oldfield described in 2016 CM1 cases in which the cerebellar tonsils extended below the foramen magnum in patients with normal posterior fossa volume.15 Based on this and other studies, Raybaud recently proposed that these cases of cerebellar ectopy resulted from an overgrowth of the cerebellum.16 This suggests that any developmental mismatch causing the posterior fossa contents to exceed that of the posterior fossa volume results in cerebellar tonsillar ectopy and CMI. Studies of syndromes with impaired bone development have further supported the concept that a mismatch between the growth of the bony posterior fossa and its contents may result in cerebellar ectopy. For example, CMI is often a feature of achondroplasia and fibrous dysplasia .17,18 We recently identified CMI and occult spinal dysraphism in patients with EPAS1-gain-of-function syndrome (Pacak-Zhuang syndrome) and the corresponding mouse model.19 This syndrome, originally described as a syndrome characterized by multiple paragangliomas, somatostatinoma, and polycythemia, is caused by early somatic mosaicism of gain-of-function mutations in EPAS1, encoding the protein hypoxia-inducible factor 2α (HIF-2α).19 Thus, while this syndrome does not represent isolated CMI, it is a sporadic disease that can be studied to understand CMI better. Despite occasional familial occurrences, it is most often sporadic and still has no known genetic cause.20
Section Highlights.
Recently, cerebellar tonsillar ectopy was described in patients with a spacious posterior fossa, suggesting that reduced posterior fossa size and crowding of neural structures is not the only cause of tonsillar ectopia and CMI
Tonsillar ectopia can arise in a normal bony posterior fossa if the combined volume of the posterior fossa neural contents and CSF is abnormally large.
Practical Considerations
Recent investigations into the etiology of Chiari Malformations have practical implications for diagnosing, managing, and treating these pathologies. Historically, the disease of CMI without syringomyelia has been challenging to define. Radiologists diagnose CM1 in symptomatic and asymptomatic individuals with an MRI finding of cerebellar tonsillar ectopy at least 5 millimeters below the foramen magnum.16 Further, there is still ongoing debate about the interplay between the extent of cerebellar ectopy and the development of a syrinx. There have been studies that have proposed additional classifications of Chiari Malformations such as CM0 for patients with less than 5 mm of tonsillar ectopia and associated cervical syringomyelia.2 It has been suggested that these cases arise from neural and CSF pathway crowding within the foramen magnum and diminished flow of cerebrospinal fluid through the subarachnoid spaces. This proposal, which agrees with the theory outlined above, raises the question of the best treatment for such patients.
While we now recognize that congenital Chiari Malformations broadly belong to a class of pathologies with a shared embryologic origin, ongoing imaging and physiologic studies continue to propose more nuanced definitions to reflect two primary considerations. First, if these pathologies have a common source, why are some more limited than others, e.g., CMI compared to CMII? Second, what is the best intervention in cases of CMI without syringomyelia and atypical clinical symptoms? From the studies discussed, the most practical implication is that the diagnosis of CMI and the decision to intervene to resolve CM1 symptoms or syringomyelia should not be based on cerebellar ectopy alone but evidence of the diminished flow of cerebrospinal fluid across the craniocervical junction assessed by phase-contrast magnetic resonance imaging.1,2 The aim of intervention thus should be the restoration of CSF flow across the craniocervical junction. While this was demonstrated in previous studies, this outcome has only recently been compared among the various surgical interventions for CM1.
Section Highlights.
Observations of cervical syringomyelia, a crowed posterior fossa, but less than 5 mm of tonsillar ectopiai.e. CM0, support diminished flow of CSF as the critical mechanism for syrinx formation.
Phase contrast MRI demonstrates constricted and obstructed CSF pathways in CM0 and in patients with a crowded posterior fossa, CM1 symptoms, but no tonsillar herniation or syringomyelia.
Controversies of Management
With our understanding of the natural history and etiologies of CMI evolving, management remains complex. We recently elaborated the history, importance of patient selection, and rationales for surgical treatments in symptomatic CMI with or without syringomyelia.21 The early surgical management of these patients by McConnell and Parker only involved a posterior fossa decompression.22 Later, several authors described craniocervical decompression in the treatment of CMI.23,24 Upon recognizing that syringomyelia was associated with CMI, Gardner at Cleveland Clinic aimed to eliminate the postulated “water-hammer” pulsation by removing the bone from the posterior aspect of the foramen magnum, opening the fourth ventricle, and plugging the obex, which Levy later found could permanently damage to the hypoglossal and vagal nuclei.25,26,27 Since these early procedures, several surgical procedures have been advocated, including extradural craniocervical decompression alone or with the following additional steps: scoring the dura, opening the dura, dissecting the arachnoid, exploring the fourth ventricle, and shrinking cerebellar tonsils, all with subsequent dural repair (duraplasty).21 These various procedures have similar outcomes in the medical literature.21 Recently, some investigators have advocated performing the less invasive interventions such as posterior fossa decompression alone or with dural scoring.28–32
As we previously elaborated, surgical intervention in CMI should provide enough enlargement of the subarachnoid space at the foramen magnum to relieve tonsillar impaction and reverse the signs and symptoms of CMI.21 The extent of expansion of the CSF spaces is more often reported in pediatric than adult studies.21 As investigations into the surgical management of CMI in both adults and children continue to search for the optimal intervention, standardization of patient selection, reporting, and measurements is required. The recent development of Common Data Elements for use in research regarding CMI by the National Institute of Neurologic Disorders and Stroke at NIH represents a significant advancement for investigations to determine the best or most effective treatments, interventions, and management for patients with CMI.33 This standardization of terminology and methods for studies, including case report forms, outcome measure recommendations, and data definitions assembled by a group of 30 experts, will serve to guide investigations regarding CMI.33 These guidelines are available on the NINDS CDE website (https://www.commondataelements.ninds.nih.gov/Chiari%20I%20Malformation).
Since the release of the Common Data Elements for CMI in 2016, several studies have evaluated surgical interventions for CMI. A single institutional study of 177 patients (97 with syringomyelia) assessed the effect of posterior fossa decompression (150 with opening the dura and intra-arachnoidal dissection; 135 with reduction of the cerebellar tonsils) on the postoperative resolution of signs and symptoms and size of the posterior fossa cisterns and syrinx.34 This study found that clinical improvement was strongly correlated with enlargement of the subarachnoid cisterns, which also strongly correlated with a reduction in the syrinx cavity.34
Several recent studies have investigated the success of these varied interventions in resolving specific pathologies associated with CMI. The Park-Reeves Syringomyelia Research Consortium recently evaluated whether extradural decompression of the posterior fossa alone or with duraplasty is superior at halting the progression of scoliosis in CMI.35 This large multicenter retrospective and prospective study of 1257 pediatric patients with CMI, as defined by cerebellar tonsillar descent greater than 5 mm below foramen magnum with an associated syrinx of greater than 3 mm in axial width, found that there was no difference in the occurrence of surgical correction of scoliosis between groups having undergone extradural decompression alone (n=51) or with duraplasty (n=346).35 However, patients treated with duraplasty were less likely to have curve progression; further, older age at the time of surgery and more significant preoperative curves were more strongly associated with subsequent fusion.35 The same group also evaluated complication rates with different grafts used for duraplasty within the 6-month postoperative period. They found higher rates of pseudomeningocele and meningitis in the group receiving nonautologous grafts (n=422), which included bovine pericardium, bovine collagen, synthetic, and human cadaveric allograft, than in the group receiving autografts (n=359).36
Cases of CMI with syringomyelia and complex pathology of the craniovertebral junction may require occipital-cervical fusion and ventral decompression as adjuncts to posterior fossa decompression.37 In another multicenter retrospective study by the Park-Reeves Syringomyelia Research Consortium, 12 of 637 patients who underwent posterior fossa decompression alone (n=132) or with duraplasty (n=505) subsequently required occipital-cervical fusion, 4 of which also needed ventral decompression.37 Of note, these patients had platybasia, Klippel-Feil syndrome, and/or basilar invagination.37
These recent studies highlight the need for continued investigation into the proper and optimal surgical management of CMI. The recent advances in the understanding of the pathogenesis of CMI may also impact our practical management regarding patient selection for surgery. Further, the recent development of Common Data Elements for CMI will fundamentally change future investigations.
Section Highlights.
There is still on-going debate regarding the optimal surgical intervention for resolution of symptoms and syrinx in CMI
Available options include posterior fossa decompression alone or with dural scoring, intra-arachnoidal dissection, exploration of the fourth ventricle, and/or reduction of the cerebellar tonsils
Recent studies have supported less invasive interventions such as posterior fossa decompression alone in selected cases of CM1 without syringomyelia.
The Common Data Elements for CMI developed by NINDS at NIH provide the framework for continued investigations in CMI
Summary
Congenital Chiari Malformations range in complexity from cerebellar tonsillar ectopy to complex neuraxial malformations with long-debated etiologies. Fundamental anatomic, physiologic, and embryologic studies over the past 50 years have deepened our understanding of the original question asked in early reports by Chiari: what is the relationship between tonsillar ectopy and syringomyelia or hydrocephalus? We are now better able to classify Chiari Malformations and make more informed decisions regarding surgical intervention based on our better understanding of the pathophysiology of these malformations. Continued investigations into the pathogenesis of Chiari Malformations through recently discovered sporadic diseases with associated Chiari Malformation pathologies will continue to inform our understanding of Chiari Malformations. Further, there remain practical questions in more nuanced presentations of CMI that warrant further investigation.
Key Points.
Chiari Malformation Type I is typically diagnosed by greater than 5 mm of cerebellar tonsillar ectopy below the foramen magnum, typically due to the reduced size of the bony posterior fossa
Recent investigations have found CMI with a spacious posterior fossa, suggesting there are additional mechanisms for cerebellar ectopy
Patient selection and proper choice of surgical intervention are critical for the successful resolution of signs and symptoms of CMI and syringomyelia
Clinical Care Points.
Chiari Malformation Type I (CMI) is typically defined by cerebellar tonsillar ectopy of at least 5 mm below the foramen magnum. The bony posterior fossa volume is small in most cases.
Syringomyelia in CM1 arises from the cerebellar tonsils impeding normal CSF flow across the foramen magnum during the cardiac cycle. Surgical relief of the obstruction to CSF flow results in syrinx resolution.
There are many options for surgical intervention, including posterior fossa decompression alone and with dural scoring, intra-arachnoidal dissection, exploration of the fourth ventricle, or reduction of the cerebellar tonsils. Their success in resolving syringomyelia depends on their ability to permanently establish a patent subarachnoid space at the foramen magnum.
Some cases of CMI have a spacious posterior fossa, suggesting that a reduced bony posterior fossa may not be the only mechanism for cerebellar ectopy. These patients have less constricted CSF pathways and are less likely to have an associated syrinx.
The recent development of the Common Data Elements for CMI provides a framework for continued investigation into CM1 pathogenesis, pathophysiology, and optimal surgical management.
Synopsis.
Chiari Malformation Type I (CMI) is a congenital malformation diagnosed by MRI findings of at least 5 mm of cerebellar ectopy below the foramen magnum. CM1 is frequently associated with syringomyelia. Herein, we discuss the history of CMI and syringomyelia, including early pathologic and surgical studies. We also describe recent investigations into the pathogenesis and pathophysiology of CMI and the practical implications on management and surgical intervention. We also highlight the recent development of the Common Data Elements for CMI, providing a framework for ongoing investigations. Finally, we discuss current controversies of surgical management in CMI.
Acknowledgments
The authors acknowledge support from the Intramural Research Program at the National Cancer Institute (J.R.) and the National Institute of Neurological Disorders and Stroke (I.J.P. and J.H.) at the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no relevant disclosures
References
- 1.Oldfield EH. Pathogenesis of Chiari I-Pathophysiology of Syringomyelia: Implications for Therapy: A Summary of 3 Decades of Clinical Research. Neurosurgery 2017; 64(CN_suppl_1):66–77 [DOI] [PubMed] [Google Scholar]
- 2.Hiremath SB, Fitsori A, Boto J, Torres C, Zakhari N, Dietemann J-L, Meling TR, Vargas MI. The Perplexity Surrounding Chiari Malformations—Are We Any Wiser Now? AJNR Am. J. Neuroradiol 2020; 41(11):1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehler PJ. Chiari’s description of cerebellar ectopy (1891): With a summary of Cleland’s and Arnold’s contributions and some early observations on neural-tube defects. J Neurosurg. 1991;75:823–826. [DOI] [PubMed] [Google Scholar]
- 4.Chiari H Concerning alterations in the cerebellum resulting from cerebral hydrocephalus. Pediatric Neurosci. 1891;13:3–8 [DOI] [PubMed] [Google Scholar]
- 5.Chiari H Uber Ver~inderungendes Kleinhirns, des Pons und der Medulla Oblongata infolge von congenitaler Hy- drocephalie des Grosshirns. Denksehriften der Kais Akad Wiss math-naturw. 1896;63:71–116 [Google Scholar]
- 6.Myelocyste Arnold J., Transposition von Gewebskeimen und Sympodie. Zieglers Beitr Pathol Anal. 1894;16:1–28 [Google Scholar]
- 7.Cleland J: Contribution to the study of spina bifida, encephalocele, and anencephalus. J Anat Physiol. 1883; 17: 257–292 [PMC free article] [PubMed] [Google Scholar]
- 8.Padget DH. Development of so-called dysraphism; with embryologic evidence of clinical Arnold-Chiari and Dandy-Walker malformations. Johns Hopkins Med J 1972; 130(3):127–65 [PubMed] [Google Scholar]
- 9.Marín-Padilla M Cephalic Axial Skeletal-Neural Dysraphic Disorders: embryology and Pathology. Can J Neurol Sci 1991; 18(2):153–169 [DOI] [PubMed] [Google Scholar]
- 10.Buell TJ, Heiss JD, Oldfield EH. Pathogenesis and Cerebrospinal Fluid Hydrodynamics of the Chiari I Malformation. Neurosurg Clin N Am 2015; 26(4):495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heiss JD, Patronas N, DeVroom HL, Shawker T, Ernis R, Kammerer W, Eidsath A, Talbot T, Morris J, Eskioglu E, Oldfield EH. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91(4):553–62 [DOI] [PubMed] [Google Scholar]
- 12.Gardner WJ, Angel J. The mechanism of syringomyelia and its surgical correction. Clin Neurosurg. 1958;6:131–140 [DOI] [PubMed] [Google Scholar]
- 13.Raybaud C and Jallo GI. Chapter 2: Chiari I deformity in children: etiopathogenesis and radiologic diagnosis. In: Ed(s) Manto M and Huisman TAGM. Handbook of Clinical Neurology Vol 155 (3rd series). Edinburgh, U.K. Elsevier;2018. 25–48 [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa M; Sakamoto H; Hakuba A; Nakanishi N; Inoue Y Pathogenesis of Chiari malformation: A morphometric study of the posterior cranial fossa. J Neurosurg 1997; 86: 40–47. [DOI] [PubMed] [Google Scholar]
- 15.Taylor DG, Mastorakos P, Jane JA, Oldfield EH. Two distinct populations of Chiari I malformation based on presence or absence of posterior fossa crowdedness on magnetic resonance imaging. J Neurosurg. 2016;126(6):1–7 [DOI] [PubMed] [Google Scholar]
- 16.Raybaud C Jallo GI. Chiari I deformity in children: etiopathogenesis and radiologic diagnosis. Handb Clin Neurol. 2018;155:25–48 [DOI] [PubMed] [Google Scholar]
- 17.Pan KS, Heiss JD, Brown SM, Collins MT, Boyce AM. Chiari I Malformation and Basilar Invagination in Fibrous Dysplasia: Prevalence, Mechanisms, and Clinical Implications. J Bone Miner Res 2018; 33(11): 1990–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbizu A; Khan TN; Ashley-Koch AE Genetic dissection of Chiari malformation type I using endophenotypes and stratification. J. Rare Dis. Res. Treat [Google Scholar]
- 19.Rosenblum JS, Cappadona AJ, Argersinger DP, Pang Y, Wang H, Nazari MA, Munasinghe JP, Donahuge DR, Jha A, Smirniotopoulos JG, Miettinen MM, Knutsen RH, Kozel BA, Zhuang Z, Pacak K, Heiss JD.. Neuraxial Dysraphism in EPAS1-Associated Syndrome due to Improper Mesenchymal Transition. Neurol Genet 2020; 6(3): e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kniffin CL; McKusick VA OMIM-Online Mendelian Inheritance in Man: Chiari Malformation Type, I. Published 5/12/1992. Updated 10/07/2016. Available online: https://www.omim.org/entry/118420#title (accessed on 27 July 2021). [Google Scholar]
- 21.Mastorakos P and Heiss JD. Chapter 38: Treatment of the Adult Chiari I Malformation. In: Tubbs RS et al. , eds. The Chiari Malformations. Springer Nature Switzerland; 2020: 443–457 [Google Scholar]
- 22.McConnell AA, Parker HL. A deformity of the hindbrain associated with internal hydrocephalus. Its relation to the Arnold-Chiari malformation. Brain. 1938;61:415–29. [Google Scholar]
- 23.Adams RD, Schatzki R, Scovill WB. The Arnold- Chiari malformation. Diagnosis, demonstration by intraspinal Lipiodol and successful surgical treatment. N Engl J Med. 1941;225:125–31. [Google Scholar]
- 24.Bucy PC, Lichtenstein BW. Arnold-Chiari defor- mity in an adult without obvious cause. J Neurosurg. 1945;2:245–50. [Google Scholar]
- 25.Gardner WJ, Goodall RJ. The surgical treatment of Arnold-Chiari malformation in adults; an explanation of its mechanism and importance of encephalography in diagnosis. J Neurosurg. 1950;7(3):199–206. [DOI] [PubMed] [Google Scholar]
- 26.Levy WJ, Mason L, Hahn JF. Chiari malformation presenting in adults: a surgical experience in 127 cases. Neurosurgery. 1983;12(4):377–90. [DOI] [PubMed] [Google Scholar]
- 27.Pillay PK. Thecoperitoneal shunting for syringomy- elia. J Neurosurg. 1991;75(5):835–6. [DOI] [PubMed] [Google Scholar]
- 28.Chauvet D, Carpentier A, George B. Dura splitting decompression in Chiari type 1 malformation: clinical experience and radiological findings. Neurosurg Rev. 2009;32(4):465–70. [DOI] [PubMed] [Google Scholar]
- 29.16. Durham SR, Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation Type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr. 2008;2(1):42–9. [DOI] [PubMed] [Google Scholar]
- 30.Isu T, Sasaki H, Takamura H, Kobayashi N. Foramen magnum decompression with removal of the outer layer of the dura as treatment for syringomyelia occurring with Chiari I malformation. Neurosurgery. 1993;33(5):844–9; discussion 9–50. [PubMed] [Google Scholar]
- 31.Kotil K, Ton T, Tari R, Savas Y. Delamination technique together with longitudinal incisions for treatment of Chiari I/syringomyelia complex: a prospective clinical study. Cerebrospinal Fluid Res. 2009;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero FR, Pereira CAdB. Suboccipital craniectomy with or without duraplasty: what is the best choice in patients with Chiari type 1 malformation? Arq Neuropsiquiatr. 2010;68(4):623–6. [DOI] [PubMed] [Google Scholar]
- 33.Luciano MG, Batzdorf U, Kula RW, Rocque BG, Maher CO, Heiss J, Martin BA, Bolognese PA, Ashley-Koch A, Limbrick D, Poppe DJ, Esposito KM, Odenkirchen J, Esterlitz JR, Ala’i S, Joseph K, Feldman RS, Riddle R; Chiari I Malformation Common Data Element Working Group. Development of Common Data Elements for Use in Chiari Malformation Type I Clinical Research: An NIH/NINDS Project. Neurosurgery. 2019. Dec 1;85(6):854–860. doi: 10.1093/neuros/nyy475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batzdorf U, McArthur DL, Bentson JR. Surgical treatment of Chiari malformation with and without syringomyelia: experience with 177 adult patients. J Neurosurg. 2013. Feb;118(2):232–42. doi: 10.3171/2012.10.JNS12305. Epub 2012 Nov 23. [DOI] [PubMed] [Google Scholar]
- 35.Sadler B, Skidmore A, Gewirtz J, Anderson RCE, Haller G, Ackerman LL, Adelson PD, Ahmed R, Albert GW, Aldana PR, Alden TD, Averill C, Baird LC, Bauer DF, Bethel-Anderson T, Bierbrauer KS, Bonfield CM, Brockmeyer DL, Chern JJ, Couture DE, Daniels DJ, Dlouhy BJ, Durham SR, Ellenbogen RG, Eskandari R, Fuchs HE, George TM, Grant GA, Graupman PC, Greene S, Greenfield JP, Gross NL, Guillaume DJ, Hankinson TC, Heuer GG, Iantosca M, Iskandar BJ, Jackson EM, Jea AH, Johnston JM, Keating RF, Khan N, Krieger MD, Leonard JR, Maher CO, Mangano FT, Mapstone TB, McComb JG, McEvoy SD, Meehan T, Menezes AH, Muhlbauer M, Oakes WJ, Olavarria G, O’Neill BR, Ragheb J, Selden NR, Shah MN, Shannon CN, Smith J, Smyth MD, Stone SSD, Tuite GF, Wait SD, Wellons JC, Whitehead WE, Park TS, Limbrick DD, Strahle JM. Extradural decompression versus duraplasty in Chiari malformation type I with syrinx: outcomes on scoliosis from the Park-Reeves Syringomyelia Research Consortium. J Neurosurg Pediatr. 2021. Jun 18:1–9. doi: 10.3171/2020.12.PEDS20552. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Yahanda AT, Adelson PD, Akbari SHA, Albert GW, Aldana PR, Alden TD, Anderson RCE, Bauer DF, Bethel-Anderson T, Brockmeyer DL, Chern JJ, Couture DE, Daniels DJ, Dlouhy BJ, Durham SR, Ellenbogen RG, Eskandari R, George TM, Grant GA, Graupman PC, Greene S, Greenfield JP, Gross NL, Guillaume DJ, Hankinson TC, Heuer GG, Iantosca M, Iskandar BJ, Jackson EM, Johnston JM, Keating RF, Krieger MD, Leonard JR, Maher CO, Mangano FT, McComb JG, McEvoy SD, Meehan T, Menezes AH, O’Neill BR, Olavarria G, Ragheb J, Selden NR, Shah MN, Shannon CN, Shimony JS, Smyth MD, Stone SSD, Strahle JM, Torner JC, Tuite GF, Wait SD, Wellons JC, Whitehead WE, Park TS, Limbrick DD. Dural augmentation approaches and complication rates after posterior fossa decompression for Chiari I malformation and syringomyelia: a Park-Reeves Syringomyelia Research Consortium study. J Neurosurg Pediatr. 2021. Feb 12:1–10. doi: 10.3171/2020.8.PEDS2087. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.CreveCoeur TS, Yahanda AT, Maher CO, Johnson GW, Ackerman LL, Adelson PD, Ahmed R, Albert GW, Aldana PR, Alden TD, Anderson RCE, Baird L, Bauer DF, Bierbrauer KS, Brockmeyer DL, Chern JJ, Couture DE, Daniels DJ, Dauser RC, Durham SR, Ellenbogen RG, Eskandari R, Fuchs HE, George TM, Grant GA, Graupman PC, Greene S, Greenfield JP, Gross NL, Guillaume DJ, Haller G, Hankinson TC, Heuer GG, Iantosca M, Iskandar BJ, Jackson EM, Jea AH, Johnston JM, Keating RF, Kelly MP, Khan N, Krieger MD, Leonard JR, Mangano FT, Mapstone TB, McComb JG, Menezes AH, Muhlbauer M, Oakes WJ, Olavarria G, O’Neill BR, Park TS, Ragheb J, Selden NR, Shah MN, Shannon C, Shimony JS, Smith J, Smyth MD, Stone SSD, Strahle JM, Tamber MS, Torner JC, Tuite GF, Wait SD, Wellons JC, Whitehead WE, Limbrick DD. Occipital-Cervical Fusion and Ventral Decompression in the Surgical Management of Chiari-1 Malformation and Syringomyelia: Analysis of Data From the Park-Reeves Syringomyelia Research Consortium. Neurosurgery. 2021. Jan 13;88(2):332–341. doi: 10.1093/neuros/nyaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hale AT, Adelson PD, Albert GW, Aldana PR, Alden TD, Anderson RCE, Bauer DF, Bonfield CM, Brockmeyer DL, Chern JJ, Couture DE, Daniels DJ, Durham SR, Ellenbogen RG, Eskandari R, George TM, Grant GA, Graupman PC, Greene S, Greenfield JP, Gross NL, Guillaume DJ, Heuer GG, Iantosca M, Iskandar BJ, Jackson EM, Johnston JM, Keating RF, Leonard JR, Maher CO, Mangano FT, McComb JG, Meehan T, Menezes AH, O’Neill B, Olavarria G, Park TS, Ragheb J, Selden NR, Shah MN, Smyth MD, Stone SSD, Strahle JM, Wait SD, Wellons JC, Whitehead WE, Shannon CN, Limbrick DD; Park-Reeves Syringomyelia Research Consortium Investigators. Factors associated with syrinx size in pediatric patients treated for Chiari malformation type I and syringomyelia: a study from the Park-Reeves Syringomyelia Research Consortium. J Neurosurg Pediatr. 2020. Mar 6:1–11. doi: 10.3171/2020.1.PEDS19493. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


