Highlights
-
•
A regression-based model used 7T MRI imaging metrics to predict brain age.
-
•
Mean absolute error of 6.6 years with 0.0 years brain-PAD in healthy controls.
-
•
Brain-PAD of +5.0 years in non-lesional epilepsy cohort, despite similar accuracy.
-
•
Significantly greater brain-PAD epilepsy patients compared to controls (p = 0.0007).
-
•
Differences in brain-PAD greater in patients with high seizure frequency.
Keywords: Brain age, Epilepsy, Ultrahigh field, Magnetic resonance imaging, 7T
Abstract
Purpose
Epilepsy patients exhibit morphological differences on neuroimaging compared to age-matched healthy controls, including cortical and sub-cortical volume loss and altered gray-white matter ratios. The objective was to develop a model of normal aging using the 7T MRIs of healthy controls. This model can then be used to determine if the changes in epilepsy patients resemble the changes seen in aging, and potentially give a marker for the severity of those changes.
Methods
Sixty-nine healthy controls (24F/45M, mean age 36.5 ± 10.5 years) and forty-four epilepsy patients (24F/20M, 33.2 ± 9.9 years) non-lesional at 3T were scanned with volumetric T1-MPRAGE at 7T. These images were segmented and quantified using FreeSurfer. A linear regression-based model trained on healthy controls was developed to predict ages using derived imaging features among the epilepsy patient cohort. The model used 114 features with significant linear correlation with age.
Results
The regression-based model estimated brain age with mean absolute error (MAE) of 6.6 years among controls. Comparable prediction accuracy of 6.9 years MAE was seen epilepsy patients. T-test of mean absolute error showed no difference in the prediction accuracy with controls and epilepsy patients (p = 0.68). However, average signed error showed elevated (+5.0 years, p = 0.0007) predicted age differences (PAD; brain-PAD=, predicted minus biological age) among epilepsy patients. Morphological metrics in the medial temporal lobe were major contributors to PAD. Additionally, patients with seizure frequency greater than once a week showed significantly elevated brain-PAD (+8.2 ± 5.3 years, n = 13) compared to patients with lower seizure frequency (3.7 ± 6.5 years, n = 31, p = 0.033).
Major conclusions
Morphological patterns suggestive of premature aging were observed in non-lesional epilepsy patients vs. controls and in high seizure frequency patients vs. low frequency patients. Modeling brain age with 7T MRI may provide a sensitive imaging marker to assess the differential effects of the aging process in diseases such as epilepsy.
Introduction
Voxel-based brain morphometry has generated extensive interest in characterization of neurological disorders such as epilepsy. This technique has elucidated structural abnormalities observed in the hippocampus [1], [2], amygdala [3], white matter [4], [5] and other brain regions [6], [7]. Previous studies have suggested that these volumetric changes may be dependent on age or disease duration [8]. In the normal brain, aging-related changes may include atrophy of gray and white matter, thinning of cortical volumes and a consequential increase in ventricular cerebrospinal fluid volume [9], [10], [11], [12]. A model capable of predicting brain age through these morphological features may help determine if the pathological changes in epilepsy resemble premature aging.
There is a large body of research suggesting potential pathophysiological effects of epilepsy such as white and gray matter atrophy in cortical regions [2], [3], [13], [14] and atrophy of subcortical structures such as thalamus and hippocampus [15], [16], [17]. Cumulatively, these changes may resemble or be suggestive of premature brain aging. Indeed, estimations of gray and white matter volume with structural MRI have shown signs which resemble premature aging in medically refractory epilepsy [18].
By exploiting the higher resolution and improved image contrast provided by ultrahigh field scanning, anatomical T1-weighted MRI combined with automatic segmentation and quantification techniques can generate imaging markers that more precisely define brain morphology than is possible with traditional high-field imaging. Previous research has described the development of predictive models for brain-age based on these features [19], [20], [21] and it is anticipated that modeling of imaging-derived features may yield sensitive markers for brain-age. These models may potentially be used to identify pathological or premature brain aging in epilepsy and other neurological and neuropsychiatric disorders [22].
In this study, a linear regression model for age-correlation was developed using brain morphology data derived from a cohort of healthy volunteers scanned at ultrahigh field. The model was then applied to a cohort of patients diagnosed with non-lesional epilepsy to probe the differential effects of the disease on the age relationship of these imaging markers. This disease model was studied because previous research has shown changes which resemble premature brain aging at lower field strengths in epilepsy patients [18], [23], [24], [25]. The higher spatial resolution and signal quality using ultrahigh field imaging may facilitate comparable reliability in these techniques with smaller training cohorts and ultimately lead to more reliable models.
Materials & methods
Image acquisition
Sixty-nine healthy controls (24 female, 45 male, age 36.5 ± 10.5 years) were scanned on a Siemens Magnetom 7T whole-body MRI scanner (Siemens Healthineers, Erlangen, Germany) with a 32Rx/1Tx channel Nova head coil (Nova Medical, Wilmington, MA) using a T1-weighted magnetization rapid gradient-echo (MPRAGE) sequence and the following parameters: echo time (TE) = 3.62 s, repetition time (TR) = 6000 ms, field-of-view (FOV) = 224 × 168 mm2, array size = 320 × 240, slices = 240, voxel size = 0.7 mm3 isotropic, acquisition time = 7:26 minutes. Forty-four patients diagnosed with non-lesional epilepsy (24 female, 20 male, age 33.2 ± 9.9 years) were scanned with the same MR protocol. All scans were performed under a protocol approved by the local institutional review board (IRB). Epilepsy patients were recruited among a cohort of patients suspected of temporal lobe epilepsy, but showing no apparent lesions under clinical MRI at 3T. Patients were diagnosed by collaborating epileptologists and diagnosed based on history, physical exam, electroencephalogram (EEG) data and clinical imaging. Diagnosis of TLE was based on seizure semiology, scalp EEG data and, when available, intracranial EEG data. Self-reported seizure frequency was recorded for all epilepsy patients. Table 1 is a chart summarizing the demographic characteristics of the included cohorts.
Table 1.
Demographics of healthy control and epilepsy patient cohorts included in the study. Epilepsy patient subgroups are also shown and indicated with “EP_” in their label. MTLE and nMTLE indicate patients with and without mesial temporal lobe epilepsy, respectively. EP_High and EP_Low indicate patients with high (>1 a week) and low (<1 week) seizure frequency.
| Controls | Epilepsy | EP_Male | EP_Female | EP_MTLE | EP_nMTLE | EP_High | EP_Low | |
|---|---|---|---|---|---|---|---|---|
| Number | 69 | 44 | 20 | 24 | 14 | 30 | 13 | 31 |
| Age Mean | 36.5 | 33.2 | 33.7 | 32.8 | 30.3 | 34.6 | 29.7 | 34.7 |
| Age S.D. | 10.5 | 9.9 | 11.3 | 9.1 | 7.7 | 10.7 | 10.5 | 7.8 |
| Brain-PAD | 0.0 | 5.0 | 5.7 | 4.4 | 5.1 | 5.0 | 8.2 | 3.7 |
| Brain-PAD S.D. | 7.9 | 6.4 | 5.8 | 7.0 | 6.1 | 6.7 | 5.3 | 6.5 |
| Male | 45 | 20 | 20 | 0 | 6 | 14 | 5 | 15 |
| Female | 24 | 24 | 0 | 24 | 8 | 16 | 8 | 16 |
Brain age modeling
All scan data was post-processed using FreeSurfer version 6.0 [26] to perform automatically segment and cortical & subcortical voxel-based volumetrics. These imaging parameters were separated into five categories: 1) whole brain measures including white/gray matter volume and ratios, 2) cortical thickness, 3) cortical gray-white ratios and 4) cortical volume normalized to whole brain volume and 5) subcortical volumes also normalized to whole brain volume. In all, 258 imaging features were selected, representing an structural imaging-based subset of the features considered in a previous brain-age study of the UK BioBank data [19]. Single linear regressions were performed on each feature, and slopes, intercepts and p-values for each regression were computed in Matlab (Mathworks, Natick, MA). The use of single linear regressions in place of a multiple regression or non-linear or machine learning based approaches was motivated by the desire to avoid over-fitting the large number of imaging features (258) compared to training cohort size (69).
Imaging features showing a significant linear relationship with age (p < 0.05) each produced individual predictions for brain age. The significance threshold of p < 0.05 was not adjusted for multiple comparisons to allow the broadest possible range of imaging criteria in the model. For example, left and right inferior parietal lobe cortical thickness measures produced two distinct and independent age predictions, in years. The availability of region of interest (ROI)-based predictions for age allowed for the generation of a brain-aging volumetric maps showing which regions in particular contributed to the brain predicted age difference (brain-PADs) observed in the patient cohort. The final predicted age was computed by taking a geometric mean of age predictions from all imaging metrics significantly correlated with age. Brain-PAD was computed as the predicted brain age minus the biological age, so positive brain-PAD suggests features are older than reported age, and vice versa. To correct for systemic bias in age prediction [27], a compensation technique analogous to that performed by Han et al. [28] was implemented by scaling the predicted age distribution to match the age distribution of the training set while minimizing mean absolute error.
This model, trained on a cohort of 69 healthy controls, was subsequently applied to analogously-processed T1-imaging data features from epilepsy patients. Two-sided student’s t-tests were performed to evaluate whether brain-PADs were significantly different between the control and epilepsy cohorts.
Results
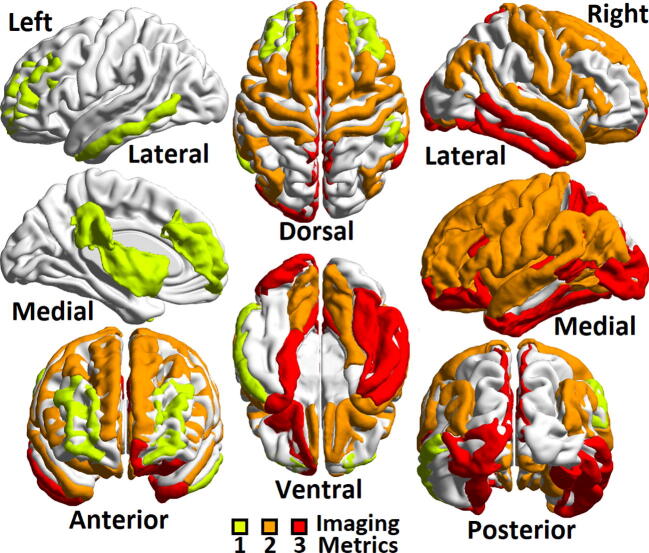
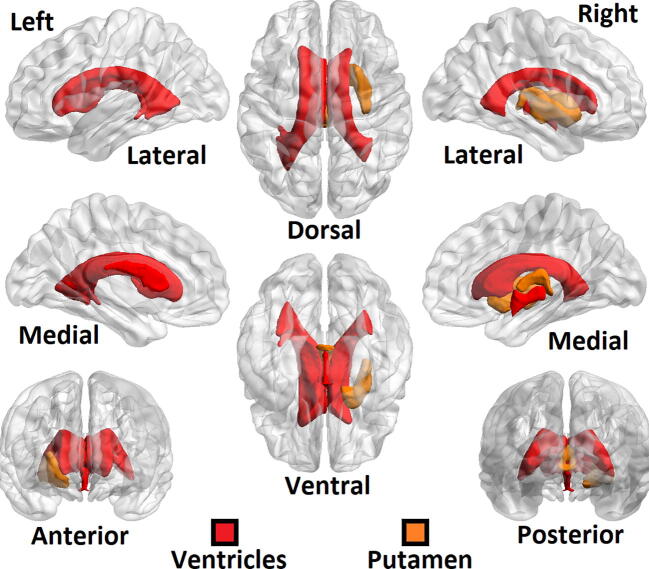
Of the 258 imaging features initially considered, 114 were determined to have a significantly linear correlation with patient age at the p < 0.05 level. Among the imaging features that showed significant linear correlation with age were the whole-brain gray matter volumes, total cortical volume, normalized precentral, parietal and middle temporal lobe volumes and gray/white matter ratios of the precuneus and pars triangularis. Each of these 114 features along with the associated p-values from their linear regressions are shown in supplemental table S1. Fig. 1, Fig. 2 [29] show brain regions as identified on the ICBM152 atlas in the Montreal Neurological Institute (MNI) standardized space with significant linear correlations with age. Among cortical regions (Fig. 1), the frontal lobe showed high linear correlation with age, whereas the occipital lobes showed no significant correlations. Among subcortical regions (Fig. 2), cerebrospinal fluid containing ventricles showed linear correlation between volume and age, along with right Putamen.
Fig. 1.
Three quantitative metrics were considered in the development of the brain age model: cortical thickness, normalized volume and gray-white ratio, and those with significant linear correlation with age (p < 0.05) were given non-zero weight in the model. This ROI map of cortical brain regions shows areas with high (n = 3 metrics, meaning cortical thickness, normalized volume and gray-white ratio, greenish yellow), medium (n = 2 of the three metrics, orange), low (n = 1 metric, red) and no correlation with age (n = 0 metrics) among the control cohort. Left column shows, from top, left lateral, left medial, and anterior view. Central column shows, from top, dorsal and ventral view. Right column shows, from top, right lateral, right medial and posterior view. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Fig. 2.
ROI map of subcortical areas showing correlation with age among the control cohort. Of the subcortical regions, only the ventricles and right putamen showed significant correlation between age and volume. Ventral/dorsal, medial/lateral and posterior/anterior views are the same as those shown in Fig. 1.
The regression model showed a mean absolute error (MAE) of 6.6 years among the control subjects, which in this cohort represents an error rate of 18%. Applying the brain age model to the epilepsy patients showed a MAE of 6.9 years and a brain-PAD (equivalent to mean signed error) of +5.0 ± 6.4 years (38.2 years predicted age vs. 33.2 years chronological age) with a SD of 6.4 years. Healthy controls showed a brain-PAD of 0.0 ± 7.9 years. These controls were the same 69 subjects who used to train the model, so a brain-PAD of 0.0 was expected. A two-sided t-test comparing the control (n = 69) and epilepsy cohort (n = 44) showed a significant between-group difference in brain-PAD with a p-value of 0.0007. The Cohen’s d for this comparison was 0.64. Fig. 3 shows a scatterplot showing the relationship between actual age and predicted age among the healthy control and epilepsy cohorts. A cross-validation was performed by randomly splitting the 69 controls subjects into groups of 34 and 35. These two groups were compared to each other and also to the epilepsy cohort. In 1 million iterations, the two healthy control cohorts showed a significant difference in brain-PADs 5.01% of the time, as would be expected by random chance.
Fig. 3.
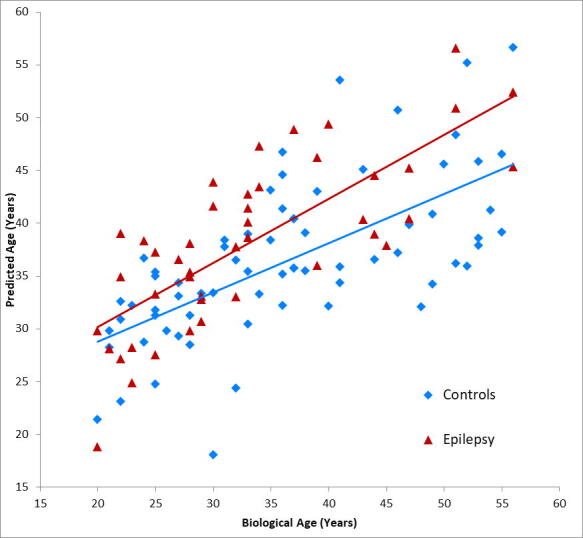
Plots showing biological age versus predicted age of the brain age model along with linear regression trendlines for control (blue) and epilepsy (red) cohorts. Correlation between predicted age and biological age (using Pearson’s r) was 0.7 for the controls and 0.8 for the epilepsy patients. For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.
Subgroup analysis showed brain-PAD was comparable among male (+5.7 ± 5.8 years, n = 20, p = 0.004 when compared to controls) and female patients (+4.4 ± 7.0 years, n = 24, p = 0.018 when compared to controls). No significant between-group differences were seen when male and female epilepsy patients were compared to each other (p = 0.25). Mesial temporal lobe epilepsy (MTLE) patients (+5.1 ± 6.1 years, n = 14, p = 0.026 when compared to controls) and non-MTLE patients (+5.0 ± 6.7 years, n = 30, p = 0.004 when compared to controls) likewise both showed comparable brain-PAD. These two patient subgroups likewise showed no significant differences when compared to each other (p = 0.491 comparing MTLE vs. non-MTLE). When patients were subdivided into high seizure frequency (once a week or more) or lower seizure frequency (fewer than once a week) both cohorts also showed significantly elevated brain-PAD: +3.7 ± 6.5 years, n = 31, p = 0.026 when compared to controls for low-frequency patients and +8.2 ± 5.3 years, n = 13, p = 0.001 when compared to controls for high-frequency patients. However, when these two groups were compared to each other, there was a significant difference between the brain-PAD of low seizure frequency and high seizure frequency patients (p = 0.033). Brain-PAD showed a significant linear correlation with reported seizure frequency, but this significance disappeared when two outlier subjects (both reporting multiple seizures per day) were removed from the analysis.
Fig. 4 shows brain-PAD as predicted by individual cortical regions among epilepsy patients. Patients showed most prominent positive brain-PAD in left middle temporal lobe and rostral middle frontal lobe, generally positive brain-PAD was observed throughout the cerebral cortex.
Fig. 4.
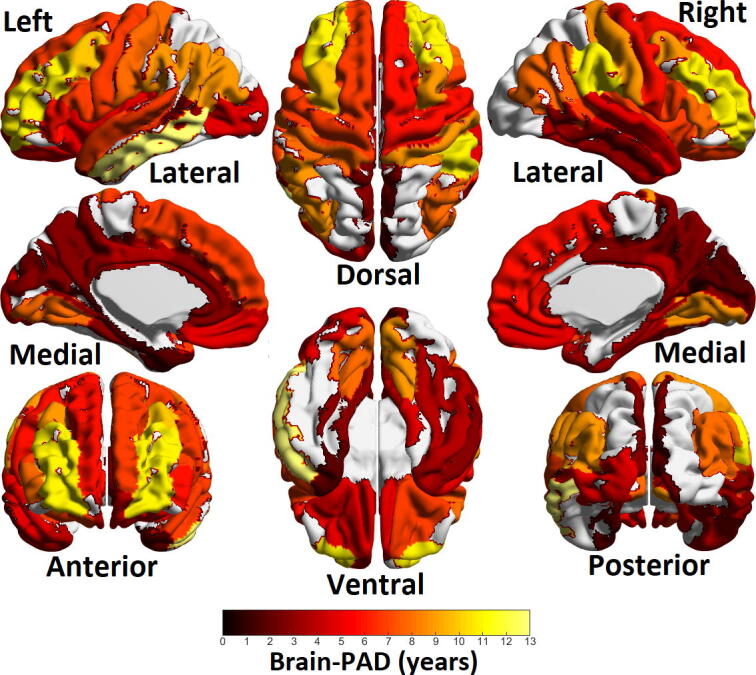
Heatmap of brain-PAD (in years) among cortical regions of epilepsy patients. Areas in yellow to red range show positive brain-PAD while areas in blue show negative brain-PAD. The areas of maximum brain-PAD were left temporal lobe and left and right rostral middle frontal regions. Ventral/dorsal, medial/lateral and posterior/anterior views are the same as those shown in Fig. 1.
Discussion
Based on literature review, this represents the first study using 7T structural MRI to find evidence for premature brain aging in patients with epilepsy. Furthermore, more frequent seizures (i.e., once a week or more) were associated with significantly greater brain-PAD in patients with epilepsy. No significant differences in brain age were noted based either on gender or MTLE vs. non-MTLE status. The cohort of epilepsy patients showed positive brain-PAD, suggesting brain pathology in epilepsy may produce morphological changes resembling or exacerbating the physiological effects of brain aging. Two previous studies of brain age in epilepsy at lower field strength showed a comparable brain-PAD of +5.8 years in a cohort of MRI-negative epilepsy patients [24] and +4.5 years among refractory focal epilepsy patients. Those studies trained healthy cohorts of 1196 and 2001, respectively, developing aging models with MAE of 5.3 and 5.0 years. In comparison, the present study used significantly smaller cohorts of controls subjects (n = 69) and MRI-negative epilepsy patients (n = 44), yet arrived at comparable findings. At the time of this study, a literature search yielded no research studies attempting to quantify brain aging using ultrahigh field MRI or attempting to assess brain aging in epilepsy patients using ultrahigh field MRI.
Because the brain aging model was trained on the cohort of healthy controls, the predicted age in this cohort was expected to show zero bias and hence zero brain-PAD. Therefore, the healthy control cohort did show a non-zero mean absolute error despite this lack of bias. The model was also built using single regressions to avoid over-fitting the large number of imaging features to the smaller number of controls. To utilize the broadest possible range of imaging metrics, correlations with age were not adjusted for multiple comparisons in developing the model. However, doing so wouldn’t substantially alter the final model, as 88 of the 114 selected features would survive correction for multiple comparisons using the Benjamini-Hochberg method [30]. Mapping aging hotspots using brain-PAD predicted by regional features showed the diffuse nature of epilepsy, with none of the probed regions showing negative or zero brain-PAD. Though regions like frontal and temporal lobe showed the highest brain-PAD, signs of greater-than-actual age were observed throughout the brain of epilepsy patients.
There is great clinical interest in the possible connection between temporal lobe epilepsy and neuropsychiatric and cognitive effects [31], [32], [33]. Recent research has also suggested an association between epilepsy and Alzheimer’s disease [34]. These studies suggest epilepsy is not a localized process, but instead has diffuse and far-reaching effects throughout the brain. These effects may be exacerbated by the progression, frequency or severity of seizures, as the studied cohort suggests the stresses associated with more frequent seizures may manifest in more severe aging-like effects. Future research should attempt to stratify a larger patient cohort along these dimensions to test if aging effects are more severe in patients with greater seizure severity or frequency. In particular, longitudinal studies of epilepsy patients and the inclusion of neuropsychiatric measures may further clarify the relationship between epilepsy and the morphological differences identified by the brain-PAD measures.
Premature brain aging has also been studied in other disease models such as major depressive disorder (MDD), with mixed results [28], [35] suggesting the sensitivity of the technique may be dependent on effect size, cohort size and MAE. A study of the ENIGMA working group involving 2675 depression patients and 2151 healthy controls showed mean absolute errors of 6.5–7.2 years [28], in line with those presented in this study with much smaller cohorts. Recent studies have suggested brain-PAD may be an early predictor of all-cause mortality with elevated predicted ages showing correlation with increased mortality and reduced life expectancy [36], [37]. The largest study by cohort size probing the relationship between imaging features and brain age used data from over 2000 people scanned as part of the UK Biobank [19], [36], [38]. That model also considered data derived with other imaging modalities including diffusion MRI and functional MRI, and produced a more accurate prediction than the model presented here (mean absolute error = 3.55 years).
As ultrahigh field scanners become more widespread, enough high-resolution imaging data of healthy controls may become available to facilitate these non-linear modeling approaches and more accurate models. Likewise, more widespread scanning of epilepsy patients may facilitate better sub-analysis of the disease, including by the presence and location of seizure onset zones, or by the severity and frequency of seizures.
Because the imaging features considered are based on morphological features rather than radiomics or contrast derived features, it may be possible to expand the dataset using images from other scanners, including those acquired at low field strengths. Previous studies modeling brain age have used large databases of subjects scanned at MR field strengths used for clinical purposes, such as the ENIGMA working group and the UK BioBank.
Conclusion
Brain-age models based on imaging features show the potential to estimate the morphological effects of aging, and show signs of premature aging in non-lesional epilepsy patients. Refining these models with more sophisticated fitting and larger datasets may facilitate their ability to probe the subtle effects of neurological disorders and potentially stratify epilepsy patients by seizure severity or frequency. A non-linear regression or machine learning based approach may yield more precise estimation of brain age, though over-fitting may remain a concern in this limited dataset.
Conflict of interest statement
Dr. Priti Balchandani is a named inventor on patents relating to magnetic resonance imaging (MRI) and RF pulse design. This intellectual property has been licensed to GE Healthcare, Siemens AG, and Philips international. Dr. Balchandani received one-time royalty payments for this intellectual property. Dr. Jha has received contract research grants from Acadia Pharmaceuticals and Janssen Research & Development, educational grant to serve as Section Editor of the Psychiatry & Behavioral Health Learning Network, consultant fees from Eleusis Therapeutics US, Inc and Guidepoint, and honoraria for CME presentations from North American Center for Continuing Medical Education and Global Medical Education.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgements
The authors would like to thank Ameen Al-Qadi and Hung-Mo Lin of the Icahn School of Medicine at Mount Sinai for their technical contributions to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebr.2022.100530.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Coan A.C., et al. Distinct functional and structural MRI abnormalities in mesial temporal lobe epilepsy with and without hippocampal sclerosis. Epilepsia. 2014;55(8):1187–1196. doi: 10.1111/epi.12670. [DOI] [PubMed] [Google Scholar]
- 2.Bernasconi N., et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126(2):462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 3.Cendes F., et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43(4):719. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 4.Kemmotsu N., et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011;52(12):2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatton S.N., et al. White matter abnormalities across different epilepsy syndromes in adults: an ENIGMA-Epilepsy study. Brain. 2020;143(8):2454–2473. doi: 10.1093/brain/awaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woermann F.G., et al. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122(11):2101–2108. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- 7.Bernasconi N., Natsume J., Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005;65(2):223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- 8.Fuerst D., et al. Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology. 2001;57(2):184–188. doi: 10.1212/wnl.57.2.184. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre H., et al. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33(3):617.e1–617.e9. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z.J., et al. Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. Neuroimage. 2011;56(1):235–245. doi: 10.1016/j.neuroimage.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 11.McGinnis S.M., et al. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011;24(3):279–291. doi: 10.1007/s10548-011-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salat D.H., et al. Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. Neuroimage. 2009;48(1):21–28. doi: 10.1016/j.neuroimage.2009.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briellmann R.S., et al. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol. 2002;51(5):641–644. doi: 10.1002/ana.10171. [DOI] [PubMed] [Google Scholar]
- 14.Dreifuss S., et al. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57(9):1636–1641. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- 15.Natsume J., et al. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60(8):1296–1300. doi: 10.1212/01.wnl.0000058764.34968.c2. [DOI] [PubMed] [Google Scholar]
- 16.Lee H.-J., Seo S.A., Park K.M. Quantification of thalamic nuclei in patients diagnosed with temporal lobe epilepsy and hippocampal sclerosis. Neuroradiology. 2019:185–195. doi: 10.1007/s00234-019-02299-6. [DOI] [PubMed] [Google Scholar]
- 17.Park K.M., et al. Reduction of ipsilateral thalamic volume in temporal lobe epilepsy with hippocampal sclerosis. J Clin Neurosci. 2018;55:76–81. doi: 10.1016/j.jocn.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Pardoe H.R., et al. Structural brain changes in medically refractory focal epilepsy resemble premature brain aging. Epilepsy Res. 2017;133:28–32. doi: 10.1016/j.eplepsyres.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Miller K.L., et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole J.H., et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage. 2017;163:115–124. doi: 10.1016/j.neuroimage.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 21.Franke K., et al. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50(3):883–892. doi: 10.1016/j.neuroimage.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Wisse L.E.M., et al. Hippocampal subfield volumes at 7T in early Alzheimer's disease and normal aging. Neurobiol Aging. 2014;35(9):2039–2045. doi: 10.1016/j.neurobiolaging.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Hwang G., et al. Brain aging in temporal lobe epilepsy: chronological, structural, and functional. NeuroImage: Clinical. 2020;25 doi: 10.1016/j.nicl.2020.102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sone D., et al. Neuroimaging-based brain-age prediction in diverse forms of epilepsy: a signature of psychosis and beyond. Mol Psychiatry. 2019:1–10. doi: 10.1038/s41380-019-0446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C.L., et al. Premature white matter aging in patients with right mesial temporal lobe epilepsy: a machine learning approach based on diffusion MRI data. NeuroImage: Clinical. 2019;24 doi: 10.1016/j.nicl.2019.102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang H., Zhang F., Niu X. Wiley Online Library; 2019. Investigating Systematic Bias in Brain Age Estimation with Application to Post‐traumatic Stress Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han L.K., et al. Brain aging in major depressive disorder: results from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2020:1–16. doi: 10.1038/s41380-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia M., Wang J., He Y., Csermely P. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS one. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 1995;57(1):289–300. [Google Scholar]
- 31.McCagh J., Fisk J.E., Baker G.A. Epilepsy, psychosocial and cognitive functioning. Epilepsy Res. 2009;86(1):1–14. doi: 10.1016/j.eplepsyres.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Seidenberg M., Pulsipher D.T., Hermann B. Cognitive progression in epilepsy. Neuropsychol Rev. 2007;17(4):445–454. doi: 10.1007/s11065-007-9042-x. [DOI] [PubMed] [Google Scholar]
- 33.Thompson P.J., Duncan J.S. Cognitive decline in severe intractable epilepsy. Epilepsia. 2005;46(11):1780–1787. doi: 10.1111/j.1528-1167.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 34.Tombini M., et al. Temporal lobe epilepsy and Alzheimer’s disease: from preclinical to clinical evidence of a strong association. J Alzheimer's Dis Rep. 2021(Preprint):243–261. doi: 10.3233/ADR-200286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besteher B., Gaser C., Nenadić I. Machine-learning based brain age estimation in major depression showing no evidence of accelerated aging. Psychiatry Res: Neuroimaging. 2019;290:1–4. doi: 10.1016/j.pscychresns.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Cole J.H., et al. Brain age predicts mortality. Mol Psychiatry. 2018;23(5):1385–1392. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paixao L., et al. Excess brain age in the sleep electroencephalogram predicts reduced life expectancy. Neurobiol Aging. 2020;88:150–155. doi: 10.1016/j.neurobiolaging.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole J.H. Multimodality neuroimaging brain-age in UK biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol Aging. 2020;92:34–42. doi: 10.1016/j.neurobiolaging.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.