Abstract
In the face of a growing human footprint, understanding interactions among threatened large carnivores is fundamental to effectively mitigating anthropogenic threats and managing species. Using data from a large-scale camera trap survey, we investigated the effects of environmental and anthropogenic variables on the interspecific interaction of a carnivore guild comprising of tiger, leopard and dhole in Bhutan. We demonstrate the complex effects of human settlement density on large carnivore interactions. Specifically, we demonstrate that leopard–dhole co-occupancy probability was higher in areas with higher human settlement density. The opposite was true for tiger–leopard co-occupancy probability, but it was positively affected by large prey (gaur) abundance. These findings suggest that multi-carnivore communities across land-use gradients are spatially structured and mediated also by human presence and/or the availability of natural prey. Our findings show that space-use patterns are driven by a combination of the behavioural mechanism of each species and its interactions with competing species. The duality of the effect of settlement density on species interactions suggests that the benefits of exploiting anthropogenic environments are a trade-off between ecological opportunity (food subsidies or easy prey) and the risk of escalating conflict with humans.
Keywords: eastern Himalaya, human settlement, interspecific interaction, large carnivores, multi-species occupancy model
1. Introduction
Interactions among species are the foundations of the structure and integrity of ecological communities [1]. The carnivore guild is mainly shaped by competition and intraguild predation [2–4]. Large carnivores regulate trophic levels by controlling prey populations and by moderating mesocarnivore populations and their effects, thus indirectly affecting both herbivore and plant communities [5,6]. However, their ecological roles are compromised by anthropogenic-induced habitat loss and fragmentation, prey depletion, and mortality due to direct persecution [7,8]. Studies have shown that extirpation of apex predators from an ecosystem leads to an increase in ungulate populations, which in turn results in overgrazing and suppression of plant growth and ultimately degradation of habitat for a range of species [9]. Assessing the impact of anthropogenic and environmental changes on large carnivore communities is key to understanding broader community structure and resilience across the human land-use gradient. Yet, the most recent studies on the interaction among threatened large carnivores have been predominantly confined to small, protected reserves (a few 100 km2) [10–12]. Inferring about carnivore community interactions, beyond protected areas, at a landscape scale to better understand carnivore community structure and stability is surprisingly rare.
According to coexistence theory, competing species must segregate at least along one or more dimensions of their ecological niches in order to coexist [13,14]. When such niche differentiation is achieved, interspecific competition is reduced and a greater number of species can coexist [15]. Resource competition intensifies when two or more species occupy a similar ecological niche and hence vie to exclude each other [16]. According to intraguild predation theory, the distribution of dominant predators is influenced by food availability, whereas that of subordinate species is determined by food availability and safety from predation [4]. Hence, from this theory, it is apparent that resource productivity underpins large carnivore interactions. While coexistence stabilizes ecosystem structure, lack of it leads to the population decline or extirpation of one of the interacting species, which can have rippling effects across the trophic level [9]. Antagonistic interactions among carnivores (i.e. competition and predation) change the species' spatial and temporal ecology [17,18]. One of the ways that large carnivores share prey and habitat is by spatial and temporal partitioning [19,20]. Food availability, it has been argued, is a principal driver of carnivore spatial organization and thus guild structure [21]. Where resources are rich and dispersed, sharing among carnivores is possible without additional cost to each species’ fitness [22].
One of the central tenets of community ecology is to understand interactions among species and their abiotic environments, as a way of predicting species' geographical distributions and abundances [23]. Anthropogenic influences alter species interactions by benefiting some and disadvantaging others [24]. Studies have demonstrated that a decrease in large/apex carnivore presence benefits mesopredators [25]—a phenomenon known as mesocarnivore release—resulting in the decline of smaller prey populations [26] and the further intensification of competition among smaller carnivores. Although species sensitive to humans avoid encounters by altering their spatio-temporal behaviour or habitat use [27], human tolerant species can modify their behaviour to increase overlap with humans in order to reduce intraguild competition [28]. However, such behaviour comes at a heightened risk of conflict with humans [29]. Furthermore, when human disturbances result in reduced carnivore diversity, it may lead to loss of ecological services if the prospering species are unable to perform the ecological functions of the extirpated ones [30], with detrimental consequences for ecosystem resilience [31].
The tigers (Panthera tigris), leopards (Panthera pardus) and dholes (Cuon alpinus) are syntopic across most of their range in the south and southeastern Asia and play an important role in regulating prey populations and balancing trophic levels. They are all categorized as ‘Threatened’ on the International Union for Conservation of Nature (IUCN) Red List due to decreasing population trends and are therefore considered high-priority conservation species across their ranges [32]. They prefer prey of a similar size range and hence intraguild competition among them is likely high [33]. This study aims to assess the patterns of coexistence and evaluate factors mediating sympatry among the large carnivore guilds, beyond what has been already studied within protected areas. Our primary objectives were: (1) to investigate the effects of anthropogenic and environmental variables on the occurrence of large carnivores across human land-use gradient and (2) to examine the influence of human disturbance on spatial interaction of threatened large carnivores at site and landscape level. Given the previously reported sensitivity of many large carnivores to human disturbance, we predicted that all three species will be negatively affected by human disturbance and that interaction between species pairs (tiger–leopard, tiger–dhole and leopard–dhole) will be negatively affected by settlement density and disturbance at the site. We also tested the hypothesis that prey choice and prey abundance would be able to support multiple carnivores and facilitate sympatry [12]. Our study is specifically aimed at unravelling the spatial partitioning mechanism of large carnivores across human land-use gradient and its implications on the management of threatened large carnivores.
2. Material and methods
(a) . Study area
Our study area was Bhutan, a small country of 38 394 km2 area, landlocked between the Tibetan Autonomous Region (China) to the north and India to the east, west and south (electronic supplementary material, appendix S1). About 70% of the country is covered by forests. The elevational gradient increases northwards from 100 m to 7000 m, mediating the vegetation composition: subtropical southern foothills are characterized by broadleaved forests, the temperate zone by cool-broadleaved and conifer mix forests, and the alpine and subalpine zones by rhododendron-scrub mix forests and stunted shrubs. The topography is typically rugged terrain with deep gorges, narrow valleys and steep slopes. The mean annual temperature ranges between 10 and 24°C and annual precipitation between 300 and 6000 mm. Human population density is low: 20 people km−2 [34]. Bhutan is entirely within the eastern Himalayan biodiversity hotspot [35] and harbours a rich spectrum of wildlife, including charismatic megafauna such as tigers, leopards, dholes, snow leopards (Panthera uncia), clouded leopards (Neofelis nebulosa) and Asian elephants (Elephas maximas) among others. More than half of the country's area is part of the national protected area network.
(b) . Camera trap survey
We conducted a camera trap survey following a regular square-grid design based on a putative female tiger home range of 25 km2 to guide the placement of camera traps [36]. We placed a pair of infrared motion-triggered camera traps in each grid cell along human or animal trails (except in the absence of trails, when cameras were placed randomly) at a height of approximately 45 cm above the ground with a 1 s reset time and burst image mode (for maximum captures). A total of 1129 unbaited camera stations were established. The camera models used were Bushnell, Cuddeback, HCO-Scoutguard, Reconyx, U-Way and Panthera. The mean distance between camera traps was 2.9 km (s.d. = 1.2 km) but this distance was highly variable depending on the terrain and site accessibility. We omitted grid cells that contained dense urban areas (more than 70% of the grid space), and those above 4500 m altitude due to the low probability of capturing tigers, which was the primary objective of the survey. For logistical convenience, the country was divided into two blocks, and camera traps were deployed during the dry season when most sites were easier to access. We deployed camera traps in the south block for 141 days (between March 2014 and June 2014) and in the north for 157 days (between October 2014 and March 2015). We monitored camera stations every 30th day to retrieve data, change batteries, replace memory cards and clear the camera's field of view of vegetation.
(c) . Model covariates
We modelled variation in occupancy probability using covariates that addressed our hypotheses: human settlement density, forest cover, disturbance at the camera trap site, river density, slope and prey abundance. We calculated settlement density using a point shapefile of all known households obtained from the Forest Department. After rasterizing the number of households at 90 m pixel resolution, we calculated the mean number of households among all pixels within a 4 km radius from each camera site in ArcGIS [37]. This variable reflects the density of houses per pixel (90 m) and when averaged across the 4 km radius characterizes human-related influence at the landscape scale. Moreover, as this variable is highly correlated with other infrastructures such as farm roads and highways, it also serves as a proxy for built-up areas. We used global tree cover data (rescaled to 90 m resolution) and averaged over a 4 km radius [38] for each camera site. For river density, we rasterized the river shapefile (obtained from the forest department of Bhutan) at a pixel resolution of 90 m and calculated the per cent of river pixels within a 4 km radius of each camera station. The radius of 4 km was selected to represent the mean home range of the smallest species of the three carnivores (i.e. leopard's average home range size of approx. 50 km2) [39]. Studies have shown that large prey species were the most preferred prey of tigers, leopards and dholes, accounting for a significant portion of the total prey biomass [33,40–42]. We used muntjac (Muntiacus muntjak), gaur (Bos gaurus) and serow (Capricornis thar) relative abundance as prey covariates in all large carnivore models. First, we used a hierarchical N-mixture model [43] to estimate the relative abundance of prey from camera trap data as a function of forest cover, elevation (extracted from digital elevation model) [44] and settlement density while accounting for imperfect detection probability using the ‘unmarked’ package [45] in R [46]. We then used site-level abundance as prey covariates in occupancy models of each species. The mean daily encounter rates (independent captures 30 min apart) of humans and livestock (dogs, cattle and horses) were used as a covariate to represent the effect of disturbance (hereafter human disturbance) at the site level (camera trap site). The detection covariates were trail (coded as 1 for cameras placed on-trail and 0 otherwise), human disturbance and trap effort (number of days a camera trap was functional during the survey) to account for unequal sampling effort (83.3[1–156]). For details on the mean and variation of covariates, see electronic supplementary material, appendix S2. We controlled for the geographical variation of north and south blocks on occupancy by including blocks as a random effect in the marginal occupancy model.
(d) . Multispecies interaction model
We used the multispecies species occupancy model [47] to examine the interaction among large carnivores. This model extends the interaction model of [48] but accommodates the effects of covariates and does not need a priori assumption of asymmetric interactions (i.e. need not consider one species dominant over the other). It models the interaction between two or more species using a multivariate Bernoulli (MVB) distribution, where Zi is a three-dimensional vector of binary detection/non-detection data denoting latent occupancy state of all three study species and ψi is a 23-dimensional vector that denotes the probability of all possible states Zi can take [47]. For the three species, we modelled Z as, . Here, the latent occupancy state for all species present is represented as ψ111, when all are absent as ψ000, when either two are present as ψ110, ψ011, ψ101 and only one is present as ψ001, ψ100, ψ010. As in any occupancy model, ψ can be modelled as a function of covariates and it describes the probability a site is occupied by only one (first order), two (second order) or more (higher order) interacting species. The latent states for S species have 2S−1 possible combinations described by natural parameters (f) (sensu [47]) which describe the log-odds a species occupies a site. For three species, the natural parameters are f1, f2, f3, f12, f13, f23, f123. Fixing f12, f13, f23 and f123 to zero assumes independence in species occurrence. We modelled marginal occupancy (i.e. f1, f2, f3 or no interaction) as a function of settlement density, forest cover, human disturbance, river density, slope, and prey abundance and conditional occupancy (i.e. f12, f13, f23 or pairwise interaction) as a function of settlement density, forest cover, disturbance, prey and interaction between prey and settlement density. We did not model higher-order interaction (i.e. f123 = 0) because we assumed the probability that three species occurred together was purely a function of species-specific and pairwise interaction parameters.
We binned detection/non-detection data for each camera station into 15-day per sampling occasion replicates to increase temporal independence of detections and reduce overdispersion. We built a set of 19 candidate models to test our hypotheses regarding the effects of anthropogenic and environmental variables on the occupancy of each species and interspecific interactions (table 1). We compared and ranked the models based on the Watanabe–Akaike information criterion (WAIC) [49] and selected the model with the lowest WAIC value for interpretation. We assessed model fit by comparing observed data to simulated data using Freeman–Tukey discrepancy and computed Bayesian p-value as a summary of posterior predictive check [50]. All covariates were z-standardized prior to analysis. We fitted models in JAGS [51] called through R using the package jagsUI [52]. We used uniform priors on first-order occupancy and detection intercepts and weakly informative normal priors on first- and second-order occupancy and detection slopes (see Data availability statement). We ran the models with three parallel chains of 100 000 Markov chain Monte Carlo (MCMC) iterations each, discarding 50 000 iterations during adaptation and 30 000 in burn-in phases and retaining every 50th (thinning) posterior sample for inference. Model convergence was diagnosed using the Gelman–Rubin statistic () for all parameters and visual inspection of trace plots [53]. All parameters in our model achieved convergence (). The top-rank model adequately fitted our data (Bayesian p-value = 0.13; electronic supplementary material, appendix S4).
Table 1.
Model selection results. β0 = marginal occupancy intercept; βx = marginal occupancy slopes; γ0 = two-way interaction intercept; γx = two-way interaction slopes. 0 = no interaction; 1 = constant two-way interaction (intercept only). WAIC = Watanabe–Akaike information criterion. ΔWAIC = delta WAIC (difference between WAIC of the top and subsequent models). Prey = gaur, muntjac, and serow.
| marginal occupancy | conditional occupancy | detection | WAIC | ΔWAIC |
|---|---|---|---|---|
| β0 + β1 settlement + β2 disturbance + β3 forest + β4 river + β5 slope + prey | γ0 + γ1 settlement + γ2 disturbance + prey | α0 + α1 trail + α2 effort + α3 disturbance | 3585.2 | 0 |
| β0 + β1 settlement + β2 disturbance + β3 forest + β4 river + β5 slope + prey | 1 | α0 + α1 trail + α2 effort + α3 disturbance | 3590.8 | 5.6 |
| β0 + β1 settlement + β2 disturbance + β3 forest + β4 river + β5 slope + prey | 0 | α0 + α1 trail + α2 effort + α3 disturbance | 3592.6 | 7.4 |
| β0 + β1 settlement + β2 disturbance + β3 forest + β4 river + β5 slope + prey | γ0 + γ1 settlement + γ2 disturbance + prey + γi prey × settlement | α0 + α1 trail + α2 effort + α3 disturbance | 3596.2 | 11 |
| β0 + β1 settlement + β2 disturbance + β3 forest + β4 river + β5 slope + prey + βi prey × settlement | γ0 + γ1 settlement + γ2 disturbance + prey + γi prey × settlement | α0 + α1 trail + α2 effort + α3 disturbance | 3617.8 | 32.6 |
| β0 + β1 settlement + β2 disturbance + β3 forest + β4 river + β5 slope + prey | γ0 + γ1 settlement + γ2 disturbance | α0 + α1 trail + α2 effort + α3 disturbance | 3621.9 | 36.7 |
| β0 + β1 settlement + β2 forest + prey | γ0 + γ1 settlement | α0 + α1 trail + α2 disturbance | 4221.1 | 635.9 |
| β0 + β1 forest + prey | γ0 + γ1 settlement | α0 + α1 trail + α2 disturbance | 4226.6 | 641.4 |
| β0 + β1 settlement + β2 forest + prey | 1 | α0 + α1 trail + α2 disturbance | 4229.7 | 644.5 |
| β0 + β1 settlement + prey | γ0 + γ1 settlement | α0 + α1 trail + α2 disturbance | 4237.7 | 652.5 |
| β0 + β1 settlement + β2 forest + prey | 0 | α0 + α1 trail + α2 disturbance | 4238.7 | 653.5 |
| β0 + β1 forest + prey | 1 | α0 + α1 trail + α2 disturbance | 4240.8 | 655.6 |
| β0 + β1 settlement + prey | 1 | α0 + α1 trail + α2 disturbance | 4245.2 | 660 |
| β0 + β1 forest + prey | 0 | α0 + α1 trail + α2 disturbance | 4247.1 | 661.9 |
| β0 + β1 settlement + prey | 0 | α0 + α1 trail + α2 disturbance | 4256.8 | 671.6 |
| β0 + β1 settlement + β2 forest | γ0 + γ1 settlement | α0 + α1 trail + α2 disturbance | 4283.6 | 698.4 |
| β0 + β1 settlement + β2 forest | 1 | α0 + α1 trail + α2 disturbance | 4294.2 | 709 |
| β0 + β1 settlement + β2 forest | 0 | α0 + α1 trail + α2 disturbance | 4306.6 | 721.4 |
| β0 | 0 | 0 | 4344.8 | 759.6 |
3. Results
Across the total survey effort of 73 259 sampling (trap) days at 849 stations (out of 1129, the rest were lost to animal vandalism, theft and malfunction) in 2014 and 2015, we obtained 323, 497 and 421 detections of tigers, leopards and dholes, respectively, at 151, 197 and 210 sites, respectively. The top model consisted of all pairwise interactions between species as a function of settlement density, human disturbance, and prey, and performed significantly better than models with constant (intercept only) pairwise interaction or independent occurrence (no interaction, table 1). The marginal occupancy parameters in the top model were settlement density, human disturbance, forest cover, river density, slope and prey (gaur, muntjac and serow) (table 1).
The detection probability of all species was positively associated with cameras placed on-trail than off-trail and trap effort. Human disturbance at the camera site had a strong negative effect on the detection probability of all three species (electronic supplementary material, appendices S11–S13).
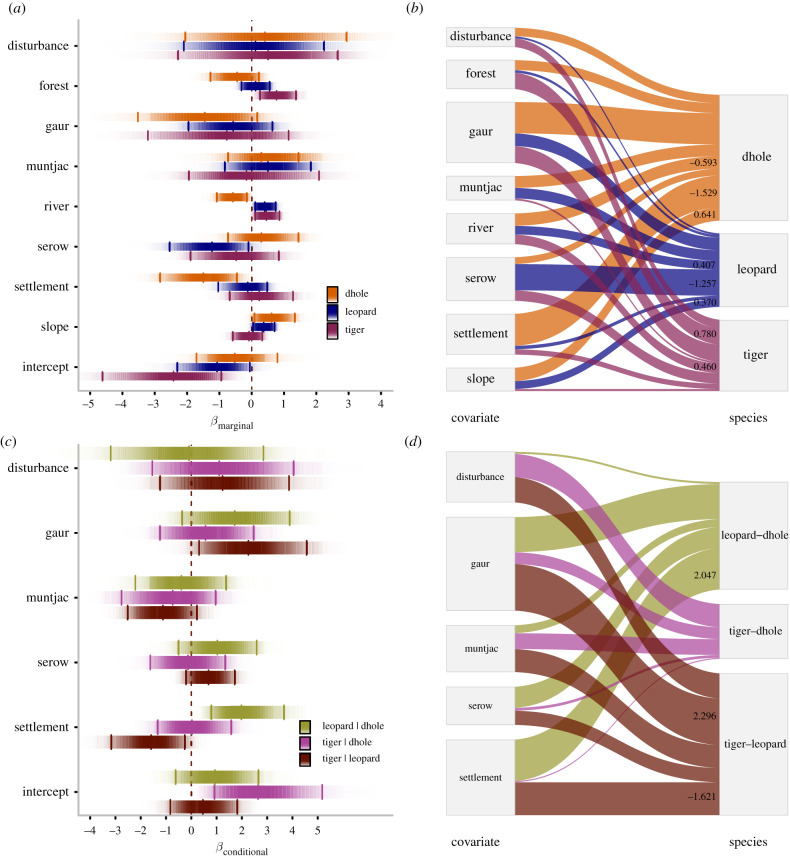
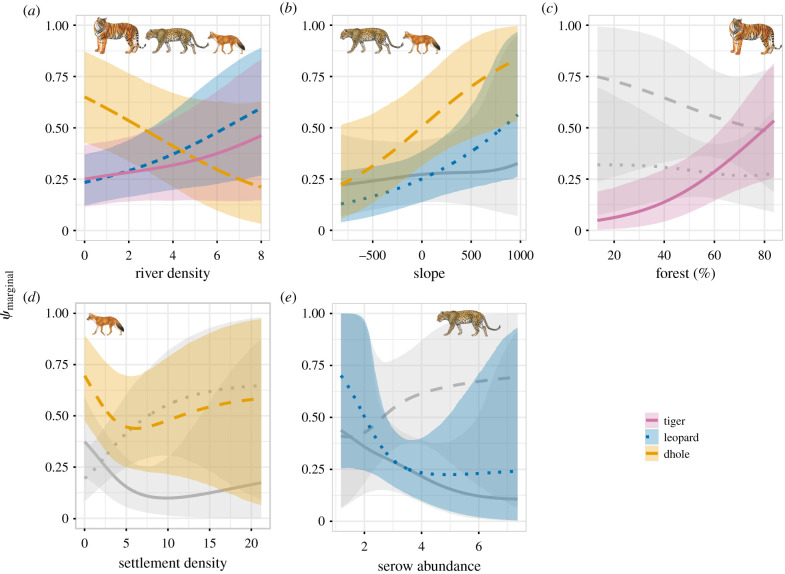
The density of river within 4 km buffer was strongly and positively associated with marginal occupancy probability of tiger (β [95% credible intervals] = 0.46[0.11 – 0.86]) and leopard (0.41[0.11 – 0.74]) but significantly negatively with that of dhole (−0.59[−1.08 − –0.15]) (figure 1). Slope had a strong positive effect on marginal occupancy of leopard (0.37[0.04 – 0.71]) and dhole (0.64[0.09 – 1.33]) (figure 2). Only tiger showed a strong positive association with forest cover (0.78[0.26 – 1.37]) (figure 2). Only dhole and leopard were strongly and negatively associated with settlement density (−1.53[−2.83 − –0.46]) and serow abundance (−1.26[−2.54 − –0.10]), respectively (electronic supplementary material, appendices S5–S7).
Figure 1.
The effects of anthropogenic and environmental variables on individual species (a,b) and pairwise interactions (c,d). The posterior density plots (a,c) show -coefficients shaded in proportion to the posterior probability density (dark shade = high density). Effect sizes are on the logit scale and represent the effect of 1 s.d. change in covariate value. Lines indicate median and 95% credible intervals. (b,d) The relationship between covariates and species occupancy probability. Line width corresponds to the strength of the relationship. Significant relationships are illustrated with coefficient estimates. (Online version in colour.)
Figure 2.
Marginal occupancy probability of the tiger (orchid solid line), leopard (blue dotted line) and dhole (gold dashed line) as a function of river density, slope, forest cover, settlement density and prey (serow) abundance, mean response and associated 95% credible interval are represented by lines and shaded ribbons, respectively. Light grey colour indicates a non-significant relationship (95% CRI straddles zero). (Online version in colour.)
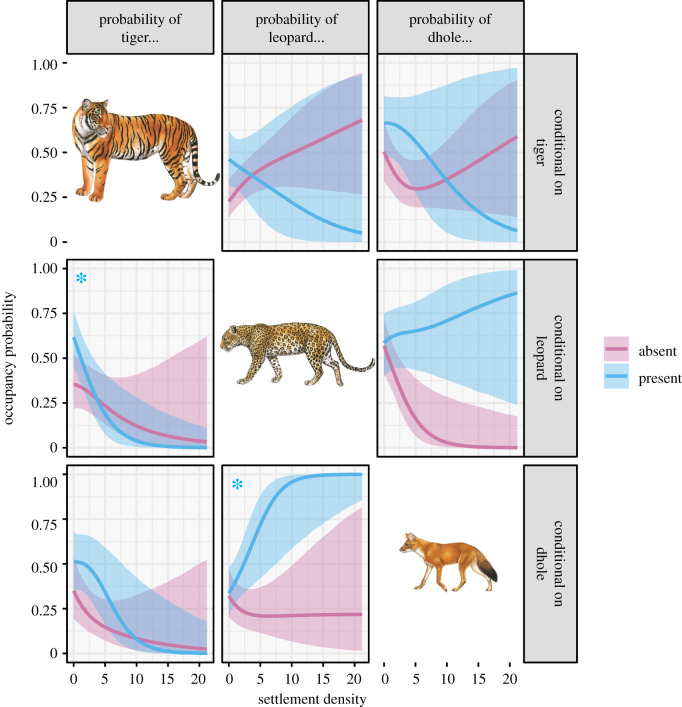
Evidence that the occupancy probability of one species varied in the presence and absence of another species was apparent for only one species pair: tiger and dhole indicated by the exclusion of zero from the credible intervals of intercept parameters in the linear models (; electronic supplementary material, appendices S8–S10). We found strong statistical support that the probability that two species occurred together varied as a function of the human settlement density within a 4 km radius of a camera station in two species pairs: tiger and leopard and leopard and dhole. The probability of occupancy of leopard as a function of settlement density varied markedly depending on whether tigers and dholes were present. At low levels of human settlement density, tigers were more likely to occupy sites if leopards were present () and occurred largely independent of dholes (figure 3, panel 7). At a higher level of human settlement density, leopards were more likely to occupy sites where dholes were also present () and occurred largely independent of tigers (figure 3, panel 2). We found strong evidence that tigers and leopards were likely to occupy sites together when gaur was abundant (). However, there was no evidence that other prey species and human disturbance affected the interaction between species pairs (95% credible intervals straddled zero).
Figure 3.
Occupancy probability of tiger, leopard and dhole conditional on the presence and absence of each of the other species along a human settlement density gradient around each camera trap. The probability of the species in each column is conditional on the presence and absence of the species in each row. The posterior means are represented by lines and 95% credible intervals by shaded ribbons. Asterisks indicate a significant relationship. Image courtesy [54]. (Online version in colour.)
The probability of occupancy of dhole was highest (ψdhole = 0.38[0.15–0.69]) followed by leopard (ψleopard = 0.26[0.09–0.49]) and tiger (ψtiger = 0.1[0.01–0.28]). Similarly, the probability of detecting a leopard was higher (pleopard = 0.08[0.05–0.12]) compared with tiger (ptiger = 0.04[0.02–0.07]) and dhole (pdhole = 0.04[0.02–0.06]).
4. Discussion
Our study revealed that patterns of sympatry among large carnivores in a heterogeneous landscape is mediated by settlement density and prey abundance. We provide evidence that human settlement modifies the direction and strength of interaction among carnivores [8]. We show that the multi-carnivore system is negatively affected by human settlement density at the landscape level. Spatial overlap between tigers and leopards was lower in areas with high human settlement density, whereas it was higher between leopards and dholes in similar settings. Likewise, gaur abundance positively influenced tiger and leopard interaction. Our results improve our understanding of how human activity at the site level and human infrastructure at the landscape level affect large carnivore behaviour. The above findings demonstrate that space-use patterns are likely driven by a combination of the behavioural mechanism of each species (i.e. response to environmental factors such as habitat, prey, and disturbance) and interaction with competing species [19]. This study examined interactions among globally threatened large carnivores in an understudied Himalayan landscape and provides important insights into the human–carnivore interface.
Human settlements are fragmenting natural habitats, increasingly forcing spatial interactions among large carnivores [55]. We demonstrate a clear difference in the effects of settlement density on these interactions. The strong positive interaction between the leopard–dhole pair in areas with high settlement density could be due to both species being attracted to anthropogenic resources (e.g. livestock, garbage), and that the benefits of accessing them are higher than any ensuing intraguild competition. Another plausible reason is that wild ungulates are attracted to crop fields—typically located near human settlements, which in turn attract carnivores [56]. However, large predators that share the same space and prey must, according to theory, segregate along the temporal axis to coexist [11]. Such temporal segregation seems likely given that dholes are diurnal hunters, whereas leopards are nocturnal [57].
On a cautionary note, increasing utilization of areas with high human settlement density by leopards and dholes might be an ecological trap. Leopards and dholes are notorious livestock predators [58–60] and could perhaps face fatal retaliation from affected farmers. Poignant evidence to the effect of such persecution is that dholes in Bhutan were almost entirely extirpated in the early 1980s due to widespread poisoning of livestock carcasses in retaliation to predation [61]. Consequently, an explosion of wild pig (Sus scrofa) populations, in turn, increased damage to crops [61]. Such trophic cascade effects due to the removal of top predators from an ecosystem are widely recognized [7]. Perhaps a reminder of the toil suffered by dholes was partially reflected in our study by the negative response (marginal occupancy) to human settlement density. Lax livestock husbandry coupled with free-range grazing fuels the growing conflict between humans and large carnivores in Bhutan [58]. Studies showed that livestock depredation by large carnivores mostly occurred in forests near the settlement where livestock was left unattended [58,60]. Thus, our finding implies that the ubiquity of human presence may benefit carnivore interaction but must be traded off against the risk of escalating conflict with humans.
Settlement density, however, had a strong negative effect on tiger–leopard interaction. This suggests that tigers and leopards may compete more directly in resource-limited areas [20] than leopards and dholes. This may result in spatial segregation [11] and/or displacement of leopards to sub-optimal habitats [62]. Studies have documented that in the presence of tigers, leopards used habitats near human settlement, consequently increasing predation on domestic livestock and ultimately conflict [58,63]. In a multi-carnivore system, our findings demonstrate the negative effect of human settlement and its consequences on interaction and habitat use. Congruent with our hypothesis, gaur abundance positively mediated tiger–leopard interaction suggesting the possibility of co-occurrence in the presence of abundant large prey. Although leopards may not directly hunt adult gaur, they are known to target calves and weaklings or scavenge on tiger kill carcasses [64]. Furthermore, it is possible that in a prey-rich environment, tigers may prefer large-bodied prey sparing smaller prey species for leopards thus segregating along the diet niche [65,66]. Our findings highlight that abundant large prey is critical for supporting large carnivore communities [67].
Although we did not find any concrete evidence of human disturbance (measured by the daily encounter rates of humans and livestock) on carnivore occupancy probability at a camera station level, growing evidence from other studies (both observational and experimental studies) suggests that large carnivores exhibit a strong fear response to humans [68–70]. Our findings rather show that human disturbance at the camera station level was negatively associated with the detection probability of all three species. Studies elsewhere show mixed results, ranging from the negative effect on carnivore detections to neutral or positive effect on habitat use [8,68,69,71]. These differences may partly be due to the spatial distribution of human presence on the landscape. For example, the human population density in western Bhutan is higher than in central, south or eastern Bhutan where the spatial interaction between the two species was low (electronic supplementary material, appendix S16).
(a) . Limitations
Observational studies such as camera trap surveys are often inadequate to infer the true processes underpinning carnivore coexistence, and hence the causal effects. Future studies could undertake studies across multiple years to infer dynamics of interspecific interactions—a pattern worth exploring in changing landscapes [72]. We acknowledge that temporal segregation might facilitate syntopy [11] but also allow coexistence with humans [73]. Moreover, study species abundance may induce heterogeneity in detection probability [74,75]. Therefore, investigating the effect of abundance on interaction could help better understand the mechanistic underpinnings of co-occupancy. Direct observation of feeding behaviour and prey choice is impossible with a camera trap survey. Our results could be better interpreted if dietary information using the scat-analysis method was available [76]. Furthermore, underlying correlates of human settlement such as poaching, roadkill and land expansion that affect carnivore co-occurrences warrant further research.
(b) . Management implications
The coexistence between humans and large carnivores is contentious [77] and in most instances, humans purportedly perceive carnivores as a threat to their lives and livelihoods resulting in conflictual responses. The polarity of human perceptions about coexisting with large carnivores has produced a mixed response and stymied conservation efforts. Management intervention aimed at protecting a single apex carnivore may need to consider the nature of interaction with other carnivores and its surrounding environments. For example, in India's Rajaji National Park, recovery of tiger population spatially displaced leopards to human-dominated habitats, the latter increased depredation on livestock and consequently suffered a decline in their numbers due to conflict and retaliation [63]. Such imbalance may jeopardize conservation efforts and induce antagonism and aversion towards large carnivores.
Conservation of multi-carnivore system would benefit from limiting the conversion of forests to other land-use types and the protection of natural habitats (irrespective of topography, e.g. rugged terrain, river network) and prey. However, as the human footprint expands rapidly, human–carnivore interaction is inevitable. Land-sharing models (where humans and wildlife share the same landscape) may need to be adopted and tailored in the context of a working landscape mosaic. Our study suggests the importance of protecting habitats adjacent to human settlements and therefore highlights the potential for conservation prioritization outside protected forest reserves. Further amending livestock husbandry practice to reduce spatial overlap between livestock and carnivores by employing guard dogs, corralling animals at night, securing overnight shelters, and supervising and guarding while grazing would be crucial to minimizing conflict [78]. Protecting crop fields by employing strategies such as electric fencing, biophysical barriers and sound repellents may help deter wild ungulates and minimize crop raids. Hunting wild ungulates for meat or in retaliation to crop damage may sever the natural food supply for carnivores [79] and inadequate wild prey may drive carnivores to increase predation of livestock as an alternative [80]. Therefore, implementing ‘collateral conservation’ measures (i.e. protecting habitat, removing snares) to protect a broad prey base may enhance carnivore conservation efforts [67].
Acknowledgements
We thank Mike Meredith for his help with codes and fruitful discussions. We appreciate the Department of Forests and Park Services, Bhutan for sharing the carnivore data.
Data accessibility
Data available via the Dryad Digital Repository: https://doi.org/10.5061/dryad.w6m905qrp [81] and sample R script via GitHub: https://github.com/ugyenpenjor1/Multispecies-Interaction-Model.
Authors' contributions
U.P.: conceptualization, data curation, formal analysis, methodology, validation, visualization, writing—original draft, writing—review and editing; C.A.: validation, writing—review and editing; S.A.C.: writing—review and editing; Ż.K.: writing—review and editing; D.W.M.: funding acquisition, project administration, resources, supervision, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was funded by the Wildlife Conservation Research Unit, Oxford University, Robertson Foundation, WWF-EFN Russell E. Train, University of Oxford QR GCRF and LMH College, Oxford University. The camera trap survey was funded by the Royal Government of Bhutan, IDA-World Bank, WWF-Bhutan and the Bhutan Foundation.
References
- 1.Polis GA, Myers CA, Holt RD. 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst. 20, 297-330. ( 10.1146/annurev.es.20.110189.001501) [DOI] [Google Scholar]
- 2.Caro T, Stoner C. 2003. The potential for interspecific competition among African carnivores. Biol. Conserv. 110, 67-75. ( 10.1016/S0006-3207(02)00177-5) [DOI] [Google Scholar]
- 3.Swanson A, Arnold T, Kosmala M, Forester J, Packer C. 2016. In the absence of a ‘landscape of fear’: how lions, hyenas, and cheetahs coexist. Ecol. Evol. 6, 8534-8545. ( 10.1002/ece3.2569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polis GA, Holt RD. 1992. Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol. Evol. 7, 151-154. ( 10.1016/0169-5347(92)90208-S) [DOI] [PubMed] [Google Scholar]
- 5.Ritchie EG, Martin JK, Johnson CN, Fox BJ. 2009. Separating the influences of environment and species interactions on patterns of distribution and abundance: competition between large herbivores. J. Anim. Ecol. 78, 724-731. ( 10.1111/j.1365-2656.2008.01520.x) [DOI] [PubMed] [Google Scholar]
- 6.Ford AT, Goheen JR. 2015. Trophic cascades by large carnivores: a case for strong inference and mechanism. Trends Ecol. Evol. 30, 725-735. ( 10.1016/j.tree.2015.09.012) [DOI] [PubMed] [Google Scholar]
- 7.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 1241484. ( 10.1126/science.1241484) [DOI] [PubMed] [Google Scholar]
- 8.Sévêque A, Gentle LK, López-Bao JV, Yarnell RW, Uzal A. 2020. Human disturbance has contrasting effects on niche partitioning within carnivore communities. Biol. Rev. 95, 1689-1705. ( 10.1111/brv.12635) [DOI] [PubMed] [Google Scholar]
- 9.Atkins JL, Long RA, Pansu J, Daskin JH, Potter AB, Stalmans ME, Tarnita CE, Pringle RM. 2019. Cascading impacts of large-carnivore extirpation in an African ecosystem. Science 364, 173-177. ( 10.1126/science.aau3561) [DOI] [PubMed] [Google Scholar]
- 10.Steinmetz R, Seuaturien N, Chutipong W. 2013. Tigers, leopards, and dholes in a half-empty forest: assessing species interactions in a guild of threatened carnivores. Biol. Conserv. 163, 68-78. ( 10.1016/j.biocon.2012.12.016) [DOI] [Google Scholar]
- 11.Karanth KU, Srivathsa A, Vasudev D, Puri M, Parameshwaran R, Kumar NS. 2017. Spatio-temporal interactions facilitate large carnivore sympatry across a resource gradient. Proc. R. Soc. B 284, 20161860. ( 10.1098/rspb.2016.1860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahkar D, Ahmed M, Begum R, Das S, Harihar A. 2021. Inferring patterns of sympatry among large carnivores in Manas National Park—a prey-rich habitat influenced by anthropogenic disturbances. Anim. Conserv. 24, 589-601. ( 10.1111/acv.12662) [DOI] [Google Scholar]
- 13.Macarthur R, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377-385. ( 10.1086/282505) [DOI] [Google Scholar]
- 14.Schoener TW. 1974. Resource partitioning in ecological communities. Science 185, 27-39. ( 10.1126/science.185.4145.27) [DOI] [PubMed] [Google Scholar]
- 15.Loreau M. 2010. Linking biodiversity and ecosystems: towards a unifying ecological theory. Phil. Trans. R. Soc. B 365, 49-60. ( 10.1098/rstb.2009.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchinson GE. 1957. Concluding remarks. Cold Spring Harbor Symp. Quantit. Biol. 22, 415-427. ( 10.1101/SQB.1957.022.01.039) [DOI] [Google Scholar]
- 17.Hersteinsson P, Macdonald DW. 1992. Interspecific competition and the geographical distribution of red and arctic foxes Vulpes vulpes and Alopex lagopus. Oikos 64, 505-515. ( 10.2307/3545168) [DOI] [Google Scholar]
- 18.Palomares F, Caro TM. 1999. Interspecific killing among mammalian carnivores. Am. Nat. 153, 492-508. ( 10.1086/303189) [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez Curras M, Donadio E, Middleton AD, Pauli JN. 2021. Carnivore niche partitioning in a human landscape. Am. Nat. 199, 496-509. ( 10.1086/718472) [DOI] [PubMed] [Google Scholar]
- 20.Vanak AT, Fortin D, Thaker M, Ogden M, Owen C, Greatwood S, Slotow R. 2013. Moving to stay in place: behavioural mechanisms for coexistence of African large carnivores. Ecology 94, 2619-2631. ( 10.1890/13-0217.1) [DOI] [PubMed] [Google Scholar]
- 21.Macdonald DW. 1983. The ecology of carnivore social behaviour. Nature 301, 379-384. ( 10.1038/301379a0) [DOI] [Google Scholar]
- 22.Macdonald D, Johnson D. 2015. Patchwork planet: the resource dispersion hypothesis, society, and the ecology of life. J. Zool. 295, 75-107. ( 10.1111/jzo.12202) [DOI] [Google Scholar]
- 23.Horn HS, Macarthur RH. 1972. Competition among fugitive species in a harlequin environment. Ecology 53, 749-752. ( 10.2307/1934797) [DOI] [Google Scholar]
- 24.Alberti M, Marzluff JM, Shulenberger E, Bradley G, Ryan C, Zumbrunnen C. 2003. Integrating humans into ecology: opportunities and challenges for studying urban ecosystems. BioScience 53, 1169-1179. ( 10.1641/0006-3568(2003)053[1169:IHIEOA]2.0.CO;2) [DOI] [Google Scholar]
- 25.Wang Y, Allen ML, Wilmers CC. 2015. Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biol. Conserv. 190, 23-33. ( 10.1016/j.biocon.2015.05.007) [DOI] [Google Scholar]
- 26.Crooks KR, Soulé ME. 1999. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563-566. ( 10.1038/23028) [DOI] [Google Scholar]
- 27.Llaneza L, Sazatornil V, López-Bao JV. 2018. The importance of fine-scale breeding site selection patterns under a landscape-sharing approach for wolf conservation. Biodivers. Conserv. 27, 1239-1256. ( 10.1007/s10531-017-1491-9) [DOI] [Google Scholar]
- 28.Deuel NR, Conner LM, Miller KV, Chamberlain MJ, Cherry MJ, Tannenbaum LV. 2017. Gray fox home range, spatial overlap, mated pair interactions and extra-territorial forays in southwestern Georgia, USA. Wildlife Biol. 2017, 1-10. ( 10.2981/wlb.00326) [DOI] [Google Scholar]
- 29.Ordeñana MA, et al. 2010. Effects of urbanization on carnivore species distribution and richness. J. Mammalogy 91, 1322-1331. ( 10.1644/09-MAMM-A-312.1) [DOI] [Google Scholar]
- 30.Flynn DF, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, Simpson N, Mayfield MM, Declerck F. 2009. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 12, 22-33. ( 10.1111/j.1461-0248.2008.01255.x) [DOI] [PubMed] [Google Scholar]
- 31.Mori AS, Furukawa T, Sasaki T. 2013. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 88, 349-364. ( 10.1111/brv.12004) [DOI] [PubMed] [Google Scholar]
- 32.IUCN. 2021. The IUCN Red List of Threatened Species. In: 2021-2, V. (ed.). See https://www.iucnredlist.org.
- 33.Karanth KU, Sunquist ME. 1995. Prey selection by tiger, leopard and dhole in tropical forests. J. Anim. Ecol. 64, 439-450. ( 10.2307/5647) [DOI] [Google Scholar]
- 34.NSB. 2020. Statistical yearbook of Bhutan 2020, Thimphu, Bhutan: National Statistics Bureau. [Google Scholar]
- 35.Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853-858. ( 10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 36.Karanth KU, Nichols JD. 2002. Monitoring tigers and their prey: a manual for wildlife researchers, managers and conservationists in tropical Asia. Bangalore, India: Centre for Wildlife Studies. [Google Scholar]
- 37.ESRI. 2011. ArcGIS desktop: release 10.2. Redlands, CA: Environmental Systems Research Institute. [Google Scholar]
- 38.Hansen MC, et al. 2013. High-resolution global maps of 21st-century forest cover change. Science 342, 850. ( 10.1126/science.1244693) [DOI] [PubMed] [Google Scholar]
- 39.Snider MH, et al. 2021. Home range variation in leopards living across the human density gradient. J. Mammalogy 102, 1138-1148. ( 10.1093/jmammal/gyab068) [DOI] [Google Scholar]
- 40.Wang S, Macdonald D. 2009. Feeding habits and niche partitioning in a predator guild composed of tigers, leopards and dholes in a temperate ecosystem in central Bhutan. J. Zool. 277, 275-283. ( 10.1111/j.1469-7998.2008.00537.x) [DOI] [Google Scholar]
- 41.Kamler JF, Johnson A, Vongkhamheng C, Bousa A. 2012. The diet, prey selection, and activity of dholes (Cuon alpinus) in northern Laos. J. Mammalogy 93, 627-633. ( 10.1644/11-MAMM-A-241.1) [DOI] [Google Scholar]
- 42.Hayward MW, Lyngdoh S, Habib B. 2014. Diet and prey preferences of dholes (Cuon alpinus): dietary competition within Asia's apex predator guild. J. Zool. 294, 255-266. ( 10.1111/jzo.12171) [DOI] [Google Scholar]
- 43.Royle JA. 2004. N-Mixture models for estimating population size from spatially replicated counts. Biometrics 60, 108-115. ( 10.1111/j.0006-341X.2004.00142.x) [DOI] [PubMed] [Google Scholar]
- 44.Jarvis A, Reuter HI, Nelson A, Guevara E. 2008. Hole-field seamless SRTM data V4, International Centre for Tropical Agriculture (CIAT). See http://srtm.csi.cgiar.org.
- 45.Fiske I, Chandler R. 2011. Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 43, 1-23. ( 10.18637/jss.v043.i10) [DOI] [Google Scholar]
- 46.R Core Team. 2021. R: a language and environment for statistical computing (version 4.1.2). Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 47.Rota CT, Wikle CK, Kays RW, Forrester TD, Mcshea WJ, Parsons AW, Millspaugh JJ. 2016. A two-species occupancy model accommodating simultaneous spatial and interspecific dependence. Ecology 97, 48-53. ( 10.1890/15-1193.1) [DOI] [PubMed] [Google Scholar]
- 48.Mackenzie DI, Bailey LL, Nichols JD. 2004. Investigating species co-occurrence patterns when species are detected imperfectly. J. Anim. Ecol. 73, 546-555. ( 10.1111/j.0021-8790.2004.00828.x) [DOI] [Google Scholar]
- 49.Hooten MB, Hobbs NT. 2015. A guide to Bayesian model selection for ecologists. Ecol. Monogr. 85, 3-28. ( 10.1890/14-0661.1) [DOI] [Google Scholar]
- 50.Kéry M, Royle J. 2016. Applied hierarchical modeling in ecology: analysis of distribution, abundance and species richness in R and BUGS, 1st edn. London, UK: Academic Press. [Google Scholar]
- 51.Plummer M. 2003. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. In Proc. of the 3rd Int. Workshop on Distributed Statistical Computing, 2003. Vienna, Austria, 1–10. [Google Scholar]
- 52.Kellner K. 2019. JagsUI: A wrapper around ‘rjags’ to streamline ‘JAGS’ analyses. v.1.4.9.
- 53.Gelman A, Hwang J, Vehtari A. 2014. Understanding predictive information criteria for Bayesian models. Stat. Comput. 24, 997-1016. ( 10.1007/s11222-013-9416-2) [DOI] [Google Scholar]
- 54.Francis C. 2009. A guide to the mammals of Southeast Asia. Princeton, NJ: Princeton University Press. [Google Scholar]
- 55.Parsons AW, Rota CT, Forrester T, Baker-Whatton MC, Mcshea WJ, Schuttler SG, Millspaugh JJ, Kays R. 2019. Urbanization focuses carnivore activity in remaining natural habitats, increasing species interactions. J. Appl. Ecol. 56, 1894-1904. ( 10.1111/1365-2664.13385) [DOI] [Google Scholar]
- 56.Thinley P, et al. 2018. The ecological benefit of tigers (Panthera tigris) to farmers in reducing crop and livestock losses in the eastern Himalayas: implications for conservation of large apex predators. Biol. Conserv. 219, 119-125. ( 10.1016/j.biocon.2018.01.015) [DOI] [Google Scholar]
- 57.Venkataraman AB, Arumugam R, Sukumar R. 1995. The foraging ecology of dhole (Cuon alpinus) in Mudumalai Sanctuary, southern India. J. Zool. 237, 543-561. ( 10.1111/j.1469-7998.1995.tb05014.x) [DOI] [Google Scholar]
- 58.Wang SW, Macdonald D. 2006. Livestock predation by carnivores in Jigme Singye Wangchuck National Park, Bhutan. Biol. Conserv. 129, 558-565. ( 10.1016/j.biocon.2005.11.024) [DOI] [Google Scholar]
- 59.Sangay T, Vernes K. 2008. Human–wildlife conflict in the Kingdom of Bhutan: patterns of livestock predation by large mammalian carnivores. Biol. Conserv. 141, 1272-1282. ( 10.1016/j.biocon.2008.02.027) [DOI] [Google Scholar]
- 60.Katel ON, Pradhan S, Schmidt-Vogt D. 2014. A survey of livestock losses caused by Asiatic wild dogs, leopards and tigers, and of the impact of predation on the livelihood of farmers in Bhutan. Wildlife Res. 41, 300-310. ( 10.1071/WR14013) [DOI] [Google Scholar]
- 61.Wangchuk T. 2004. Predator–prey dynamics: the role of predators in the control of problem species. J. Bhutan Stud. 10, 68-89. [Google Scholar]
- 62.Odden M, Wegge P, Fredriksen T. 2010. Do tigers displace leopards? If so, why? Ecol. Res. 25, 875-881. ( 10.1007/s11284-010-0723-1) [DOI] [Google Scholar]
- 63.Harihar A, Pandav B, Goyal SP. 2011. Responses of leopard Panthera pardus to the recovery of a tiger Panthera tigris population. J. Appl. Ecol. 48, 806-814. ( 10.1111/j.1365-2664.2011.01981.x) [DOI] [Google Scholar]
- 64.Hayward M, Henschel P, O'Brien J, Hofmeyr M, Balme G, Kerley GI. 2006. Prey preferences of the leopard (Panthera pardus). J. Zool. 270, 298-313. ( 10.1111/j.1469-7998.2006.00139.x) [DOI] [Google Scholar]
- 65.Andheria A, Karanth K, Kumar N. 2007. Diet and prey profiles of three sympatric large carnivores in Bandipur Tiger Reserve, India. J. Zool. 273, 169-175. ( 10.1111/j.1469-7998.2007.00310.x) [DOI] [Google Scholar]
- 66.Hayward M, Jędrzejewski W, Jedrzejewska B. 2012. Prey preferences of the tiger Panthera tigris. J. Zool. 286, 221-231. ( 10.1111/j.1469-7998.2011.00871.x) [DOI] [Google Scholar]
- 67.Wolf C, Ripple WJ. 2016. Prey depletion as a threat to the world's large carnivores. R. Soc. Open Sci. 3, 160252. ( 10.1098/rsos.160252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kays R, Parsons AW, Baker MC, Kalies EL, Forrester T, Costello R, Rota CT, Millspaugh JJ, Mcshea WJ. 2017. Does hunting or hiking affect wildlife communities in protected areas? J. Appl. Ecol. 54, 242-252. ( 10.1111/1365-2664.12700) [DOI] [Google Scholar]
- 69.Nickel BA, Suraci JP, Allen ML, Wilmers CC. 2020. Human presence and human footprint have non-equivalent effects on wildlife spatiotemporal habitat use. Biol. Conserv. 241, 108383. ( 10.1016/j.biocon.2019.108383) [DOI] [Google Scholar]
- 70.Suraci JP, Clinchy M, Zanette LY, Wilmers CC. 2019. Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol. Lett. 22, 1578-1586. ( 10.1111/ele.13344) [DOI] [PubMed] [Google Scholar]
- 71.Reilly M, Tobler MW, Sonderegger D, Beier P. 2017. Spatial and temporal response of wildlife to recreational activities in the San Francisco Bay ecoregion. Biol. Conserv. 207, 117-126. ( 10.1016/j.biocon.2016.11.003) [DOI] [Google Scholar]
- 72.Fidino M, Simonis JL, Magle SB. 2019. A multistate dynamic occupancy model to estimate local colonization–extinction rates and patterns of co-occurrence between two or more interacting species. Methods Ecol. Evol. 10, 233-244. ( 10.1111/2041-210X.13117) [DOI] [Google Scholar]
- 73.Lamb CT, Ford AT, Mclellan BN, Proctor MF, Mowat G, Ciarniello L, Nielsen SE, Boutin S. 2020. The ecology of human–carnivore coexistence. Proc. Natl Acad. Sci. USA 117, 17 876-17 883. ( 10.1073/pnas.1922097117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Royle JA, Nichols JD. 2003. Estimating abundance from repeated presence–absence data or point counts. Ecology 84, 777-790. ( 10.1890/0012-9658(2003)084[0777:EAFRPA]2.0.CO;2) [DOI] [Google Scholar]
- 75.Cubaynes S, et al. 2010. Importance of accounting for detection heterogeneity when estimating abundance: the case of French wolves. Conserv. Biol. 24, 621-626. ( 10.1111/j.1523-1739.2009.01431.x) [DOI] [PubMed] [Google Scholar]
- 76.Klare U, Kamler JF, Macdonald DW. 2011. A comparison and critique of different scat-analysis methods for determining carnivore diet. Mammal Rev. 41, 294-312. ( 10.1111/j.1365-2907.2011.00183.x) [DOI] [Google Scholar]
- 77.Lute ML, Carter NH. 2020. Are we coexisting with carnivores in the American West? Front. Ecol. Evol. 8, 48. ( 10.3389/fevo.2020.00048) [DOI] [Google Scholar]
- 78.Spencer K, Sambrook M, Bremner-Harrison S, Cilliers D, Yarnell RW, Brummer R, Whitehouse-Tedd K. 2020. Livestock guarding dogs enable human–carnivore coexistence: first evidence of equivalent carnivore occupancy on guarded and unguarded farms. Biol. Conserv. 241, 108256. ( 10.1016/j.biocon.2019.108256) [DOI] [Google Scholar]
- 79.Velho N, Karanth KK, Laurance WF. 2012. Hunting: a serious and understudied threat in India, a globally significant conservation region. Biol. Conserv. 148, 210-215. ( 10.1016/j.biocon.2012.01.022) [DOI] [Google Scholar]
- 80.Puri M, Srivathsa A, Karanth KK, Patel I, Kumar NS. 2020. The balancing act: maintaining leopard–wild prey equilibrium could offer economic benefits to people in a shared forest landscape of central India. Ecol. Indic. 110, 105931. ( 10.1016/j.ecolind.2019.105931) [DOI] [Google Scholar]
- 81.Penjor U, Astaras C, Cushman SA, Kaszta Ż, Macdonald DW. 2022. Data from: Contrasting effects of human settlement on the interaction among sympatric apex carnivores. Dryad Digital Repository. ( 10.5061/dryad.w6m905qrp) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Penjor U, Astaras C, Cushman SA, Kaszta Ż, Macdonald DW. 2022. Data from: Contrasting effects of human settlement on the interaction among sympatric apex carnivores. Dryad Digital Repository. ( 10.5061/dryad.w6m905qrp) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data available via the Dryad Digital Repository: https://doi.org/10.5061/dryad.w6m905qrp [81] and sample R script via GitHub: https://github.com/ugyenpenjor1/Multispecies-Interaction-Model.