Abstract
We report herein an efficient protocol for the synthesis of 4-vinylphenols by a catalyst-free decarboxylation of trans-4-hydroxycinnamic acids. A variety of 4-vinylphenols has been synthesized in moderate to excellent yields. This protocol also features no polymerization.
Keywords: catalyst-free, decarboxylation, 4-hydroxycinnamic acids, 4-vinylphenols
1. Introduction
4-Vinylphenols are of both natural and biological interest. They are part of a large number of significant natural products such as 4-vinylphenol, 4-vinylcatechol, 2,6-dimethoxy-4-vinylphenol and many others [1–5]. Most of these compounds display varied bioactivities such as anti-oxidant [6–10], anti-mutagenic [9], anti-fungal [11] and anti-cancer [12–14] properties (figure 1). They are also building blocks in the synthesis of bioactive compounds [15–17]. In addition, they are widely used in industry [18]. Therefore, the synthesis of 4-vinylphenols has gained widespread attention.
Figure 1.
Selected bioactive 4-vinylphenols.
However, the susceptibility of the hydroxy function toward polymerization often results in the formation of polymers [19]. To overcome this barrier chemical [20–27] and biological [28–32] protocols have been developed, but most of them suffer from narrow substrate scope. To the best of our knowledge, the most efficient synthetic routes reported for the preparation of 4-vinylphenols involve (i) piperidine-catalysed Knoevenagel–Doebner [20] and Knoevenagel reaction [21] from 4-hydroxybenzaldeydes and malonic acid (scheme 1a); (ii) decarboxylation of 4-hydroxycinnamic acids using DBU [22], [C2C1Im][OAc] [23] or Bacillus subtilis [28] (scheme 1b). But these methods only test 4-hydroxycinnamic acids bearing electron-donating groups (EDG) [20–23,28] and suffer some disadvantages such as the use of a base catalyst (Stamford/Joshi/Setti/Singh's work), the addition of polymerization inhibitor (Setti's work), need of microwave-assisted (Joshi/Setti's work) and long reaction times (Stamford/Kourist's work). Several catalyst-free decarboxylation methods of cinnamic acids have been reported, however, they need using some sort of biocatalysts [29,33,34].
Scheme 1.
The most efficient approaches for the synthesis of 4-vinylphenols.
Herein, we report an efficient procedure for the preparation of 4-vinylphenols from trans-4-hydroxycinnamic acids by a catalyst-free decarboxylative reaction without any additive (scheme 1c). This approach tolerates 4-hydroxycinnamic acids bearing electron-donating/withdrawing groups and containing substituents on the double bond. Additionally, it can effectively inhibit the polymerization in the absence of inhibitor.
2. Results and discussion
Our initial studies were carried out with readily available 4-hydroxycinnamic acid 1a as a test substrate. 1a in DMF was stirred at 200°C for 60 min under an air atmosphere to give the desired product 2a in 15% yield (table 1, entry 1). To further improve the reaction yield, control experiment was performed. To our delight, we found that decreasing the reaction time to 30 or 40 min had obvious effect on the reaction (table 1, entries 2–3). However, a decrease in the yield was observed upon further decreasing the reaction time (table 1, entry 4). Subsequently, we investigated the effect of solvents on the efficiency of the decarboxylation. Unfortunately, the reaction performed in DMA, ethylene glycol, DMSO, 1,4-dioxane or DCE exerted detrimental effect on the yield (table 1, entries 5–9). We tried to lower the temperature but failed. The reaction was conducted under microwave irradiation at 100°C, yielding 2a only in 45% yield (table 1, entry 10). Therefore, the optimized reaction conditions were determined as 1a (0.2 mmol) in DMF (1 ml) at 200°C for 30 min under an air atmosphere (table 1, entry 3). With the optimized reaction conditions in hand, a gram scale reaction was carried out and provided the product 2a in 87% yield (table 1, entry 11).
Table 1.
Optimization of the reaction conditions.a
| entry | time (min) | solvent | yield 2a (%)b |
|---|---|---|---|
| 1 | 60 | DMF | 15 |
| 2 | 40 | DMF | 85 |
| 3 | 30 | DMF | 89 |
| 4 | 20 | DMF | 77 |
| 5 | 30 | DMA | 23 |
| 6 | 30 | ethylene glycol | 61 |
| 7 | 30 | DMSO | 30 |
| 8 | 30 | 1,4-dioxane | / |
| 9 | 30 | DCE | / |
| 10c | 30 | DMF | 45 |
| 11d | 30 | DMF | 87 |
aReaction conditions: 1a (0.2 mmol), solvent (1 ml).
bIsolated yield.
cReaction conditions: 1a (0.2 mmol), solvent (1 ml), under microwave irradiation at 100°C.
dReaction conditions: 1a (7 mmol), solvent (25 ml).
Under the optimized conditions, the scope of this decarboxylation was then examined by varying 4-hydroxycinnamic acids 1 (table 2). It is apparent from table 2 that 4-vinylphenols bearing electron-donating/withdrawing groups were prepared in excellent yields (86–96%) and no polymers were detected under the given conditions. Specifically, we investigated the decarboxylation of some natural products such as p-coumaric acid (1a), caffeic acid (1b), ferulic acid (1c) and sinapinic acid (1d), achieving corresponding 4-vinylphenols in excellent yields (2a–2d). Meanwhile, employing methyl- or ethoxy-substituted 4-hydroxycinnamic acids (1e–1g) also afforded the products in high yields (2e–2g). However, electron-withdrawing group (NO2, F, Cl or Br) gave lower yields (2 h–2l). Moreover, 4-hydroxycinnamic acids containing methyl on the double bond (1p and 1q) were also compatible with the current conditions, providing the corresponding products 2p and 2q in moderate yields (68% and 63%). The products 2p and 2q exist in the E forms, based on their NMR spectra and in accordance with literature report [35]. Next, we tested several cinnamic acids without 4-hydroxyl substituent under the optimized reaction conditions. Unfortunately, no corresponding vinylbenzenes were detected (scheme 2).
Table 2.
Evaluation of substrate scope.a
| entry | substrates 1 | temp. (oC) | products 2 | yield (%)b |
|---|---|---|---|---|
| 1 |  |
200 |  |
89 |
| 2 |  |
200 |  |
87 |
| 3 | 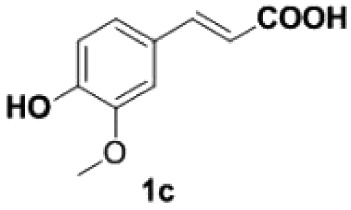 |
200 |  |
94 |
| 4 |  |
170 |  |
94 |
| 5 |  |
180 |  |
90 |
| 6 |  |
130 |  |
93 |
| 7 |  |
200 |  |
96 |
| 8 |  |
160 |  |
86 |
| 9 |  |
140 |  |
87 |
| 10 |  |
180 |  |
86 |
| 11 |  |
140 |  |
89 |
| 12 |  |
140 |  |
87 |
| 13 |  |
200 |  |
68 |
| 14 |  |
200 |  |
63 |
aReaction conditions: 1 (0.2 mmol), DMF (1 ml).
bIsolated yield.
Scheme 2.

Decarboxylation of cinnamic acids without 4-hydroxyl substituent.
In addition, we found that the polymerization product 2aa was isolated in 48% yield when the reaction time and temperature were increased (table 3, 2aa). Subsequently, we tested another two substrates bearing electron-donating/withdrawing group (table 3, 1f and 1l). Moderate yields were obtained. Obviously, for electron-withdrawing group (Br) substituted substrate, related polymer was got in higher yield (table 3, 2ff versus 2ll).
Table 3.
Synthesis of 4-vinylphenol dimers.a
| entry | substrates | temp. (oC) | dimers | yield (%)b |
|---|---|---|---|---|
| 1 |  |
220 |  |
48 |
| 2 | 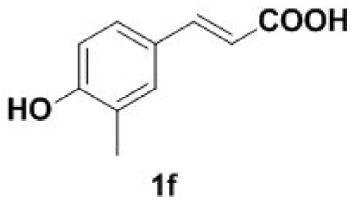 |
200 |  |
50 |
| 3 |  |
170 |  |
67 |
aReaction conditions: 1a/1f/1 l (0.2 mmol), DMF (1 ml).
bIsolated yield.
Plausible mechanisms for the decarboxylation and polymerization of 4-hydroxycinnamic acids are depicted in scheme 3. Species 3 is formed under elevated reaction temperature. Finally, hydrogen transfer followed by release of a molecule of carbon dioxide ensures the formation of 4-vinylphenol 2a. Radical polymerization of styrene initiated at higher reaction temperature, yielding intermediate 6 followed by expelling hydrogen radical leads to the polymerization product 2aa.
Scheme 3.
Plausible mechanism for the decarboxylation and polymerization.
3. Conclusion
In summary, we have developed an efficient method for the preparation of 4-vinylphenols via a catalyst-free decarboxylation of 4-hydroxycinnamic acids. This method features good functional group tolerance and no polymerization. However, corresponding polymers were obtained in moderate yields when under harsher reaction conditions.
4. Experimental section
4.1. General information
Unless otherwise noted, all reagents, catalysts and solvents were purchased from commercial suppliers and used without further purification. Column chromatography was performed with silica gel (200–300 mesh). Melting points were determined using a X-4 melting point apparatus with microscope. The IR spectra were recorded with Mattson FTIR spectrometer 5000. Absorption maxima were measured in cm−1. 1H and 13C NMR spectra were achieved on a Bruker Avance 600 MHz spectrometer (1H 600 MHz; 13C 151 MHz; 19F 565 MHz) in CDCl3, CD3OD, DMF-d7, DMSO-d6. High-resolution mass spectra were measured on a ThermoFish QE Focus facility. Thin-layer chromatographies were done on pre-coated silica gel 60F254 plates (Merck).
4.2. General procedure for the synthesis of 4-vinylphenols (2a–2l and 2p–2q)
4.2.1. Procedure for 4-vinylphenols bearing electron-donating groups (2a–2g and 2p)
To a stirred solution of DMF (1 ml) was added 4-hydroxycinnamic acids (1a–1g and 1p) (0.2 mmol) in 5 ml pressure-resistant reaction bottle. The reaction mixture was stirred at 130–200°C until the completion of the starting materials as monitored by TLC (30 min). The reaction mixture was quenched with water and extracted with ethyl acetate. The organic layer was dried over Na2SO4 and evaporated under reduced pressure. The resulting crude compound was purified by silica gel column chromatography to yield the pure product (2a–2g and 2p), which was dissolved immediately in methanol for storage.
4.2.1.1. 4-Vinylphenol 2a
Light yellow solid, yield 89%, mp 72–74°C. IR (KBr plate): νmax 3328, 3019, 2924, 2850, 1609, 1228, 834.1 H NMR (600 MHz, DMSO-d6) δ 7.29–7.23 (m, 2H), 6.72 (d, J = 8.5 Hz, 2H), 6.60 (dd, J = 17.6, 10.9 Hz, 1H), 5.57 (dd, J = 17.6, 0.9 Hz, 1H), 5.03 (dd, J = 10.9, 0.8 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 157.78, 136.86, 128.77, 127.89, 115.79, 111.12. HRMS-ESI (m/z): [M + H]+ calcd. for C8H9O: 121.06479; found, 121.06487.
4.2.1.2. 2-Hydroxy-4-vinylphenol 2b
Light yellow oil, yield 87%. IR (KBr plate): νmax 3346, 2929, 1604, 1524, 1281, 1111, 815. 1H NMR (600 MHz, CD3OD) δ 6.94 (d, J = 1.8 Hz, 1H), 6.78 (dd, J = 8.1, 1.8 Hz, 1H), 6.75 (d, J = 8.1 Hz, 1H), 6.61 (dd, J = 17.6, 10.9 Hz, 1H), 5.56 (dd, J = 17.6, 0.9 Hz, 1H), 5.06 (dd, J = 10.9, 0.8 Hz, 1H). 13C NMR (151 MHz, CD3OD) δ 145.16, 144.98, 136.75, 130.03, 118.31, 114.82, 112.21, 109.34. HRMS-ESI (m/z): [M + H]+ calcd. for C8H9O2: 137.05971; found, 137.05946.
4.2.1.3. 2-Methoxy-4-vinylphenol 2c
Colourless oil, yield 94%. IR (KBr plate): νmax 3411, 2924, 2852, 1603, 15 141 463, 1269, 817. 1H NMR (600 MHz, CDCl3) δ 6.95–6.91 (m, 2H), 6.87 (d, J = 8.1 Hz, 1H), 6.64 (dd, J = 17.5, 10.8 Hz, 1H), 5.65 (s, 1H), 5.59 (d, J = 17.5 Hz, 1H), 5.13 (d, J = 10.9 Hz, 1H), 3.91 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 146.59, 145.64, 136.63, 130.28, 120.08, 114.35, 111.47, 108.01, 55.89. HRMS-ESI (m/z): [M + H]+ calcd. for C9H11O2: 151.07536; found, 151.07515.
4.2.1.4. 2,6-Dimethoxy-4-vinylphenol 2d
Yellow oil, yield 94%. IR (KBr plate): νmax 3144, 2938, 2844, 1605, 1462, 1213, 1115, 837. 1H NMR (600 MHz, CDCl3) δ 6.65 (s, 2H), 6.61 (dd, J = 17.5, 10.9 Hz, 1H), 5.60 (d, J = 17.5 Hz, 1H), 5.56 (s, 1H), 5.15 (d, J = 10.8 Hz, 1H), 3.90 (s, 6H). 13C NMR (151 MHz, CDCl3) δ 147.06, 136.83, 134.76, 129.18, 111.87, 102.9, 56.26. HRMS-ESI (m/z): [M + H]+ calcd. for C10H13O3: 181.08592; found, 181.08562.
4.2.1.5. 2-Ethoxy-4-vinylphenol 2e
White solid, yield 90%, mp 125–127°C. IR (KBr plate): νmax 3436, 2979, 2929, 1606, 1513, 1237, 1122, 823. 1H NMR (600 MHz, CD3OD) δ 6.99 (d, J = 1.7 Hz, 1H), 6.85 (dd, J = 8.1, 1.7 Hz, 1H), 6.74 (d, J = 8.1 Hz, 1H), 6.61 (dd, J = 17.6, 10.9 Hz, 1H), 5.56 (d, J = 17.6 Hz, 1H), 5.04 (d, J = 10.9 Hz, 1H), 4.10 (q, J = 7.0 Hz, 2H), 1.42 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CD3OD) δ 146.77, 146.59, 136.73, 129.95, 119.41, 114.84, 110.25, 109.67, 64.19, 13.76. HRMS-ESI (m/z): [M + H]+ calcd. for C10H13O2: 165.09101; found, 165.09073.
4.2.1.6. 2-Methyl-4-vinylphenol 2f
Yellow oil, yield 93%. IR (KBr plate): νmax 3375, 2922, 2850, 1599, 1461, 1267, 822. 1H NMR (600 MHz, DMF-d7) δ 7.24 (d, J = 1.5 Hz, 1H), 7.14 (dd, J = 8.2, 2.1 Hz, 1H), 6.87 (d, J = 8.2 Hz, 1H), 6.63 (dd, J = 17.6, 10.9 Hz, 1H), 5.60 (dd, J = 17.6, 1.0 Hz, 1H), 5.01 (dd, J = 10.9, 1.0 Hz, 1H), 2.18 (s, 3H). 13C NMR (151 MHz, DMF-d7) δ 158.04, 141.10, 133.70, 133.70, 130.49, 129.88, 121.53, 116.99, 34.39. HRMS-ESI (m/z): [M-H]− calcd. for C9H9O: 133.06479; found, 133.06454.
4.2.1.7. 2,6-Dimethyl-4-vinylphenol 2g
Yellow oil, yield 96%. IR (KBr plate): νmax 3436, 2924, 2853, 1600, 1202, 1148, 871. 1H NMR (600 MHz, CDCl3) δ 7.05 (s, 2H), 6.59 (dd, J = 17.6, 10.9 Hz, 1H), 5.58 (d, J = 17.6 Hz, 1H), 5.08 (d, J = 10.9 Hz, 1H), 4.65 (s, 1H), 2.25 (s, 6H). 13C NMR (151 MHz, CDCl3) δ 152.09, 136.46, 129.90, 126.59, 122.97, 111.16, 15.91. HRMS-ESI (m/z): [M + H]+ calcd. for C10H13O: 149.09609; found, 149.09592.
4.2.1.8. (E)-1-(4-hydroxyphenyl)propene 2p
Colourless oil, yield 68%. IR (KBr plate): νmax 3388, 2964, 2927, 1615, 1558, 1507, 1457, 1239, 853.03, 688. 1H NMR (600 MHz, CDCl3) δ 7.24–7.20 (m, 2H), 6.80–6.77 (m, 2H), 6.35 (dd, J = 15.7, 1.6 Hz, 1H), 6.09 (dq, J = 15.7, 6.6 Hz, 1H), 1.87 (dd, J = 6.6, 1.7 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 154.77, 130.75, 130.32, 127.04, 123.36, 115.39, 18.39. HRMS-ESI (m/z): [M-H]− calcd. for C9H9O: 133.06479; found, 133.06425.
4.2.2. Procedure for 4-vinylphenols bearing electron-withdrawing groups (2h–2l and 2q)
To a stirred solution of DMF (1 ml) was added 4-hydroxycinnamic acids (1h–1l and 1q) (0.2 mmol) in 5 ml pressure-resistant reaction bottle. The reaction mixture was stirred at 140–200°C until the completion of the starting materials as monitored by TLC (30 min). The reaction mixture was quenched with water and extracted with dichloromethane. The dichloromethane layer was washed with pure water 2–3 times. The combined extract was dried over Na2SO4. The filtrate was evaporated under reduced pressure to yield the pure product (2h–2l and 2q), which was dissolved immediately in methanol for storage.
4.2.2.1. 2-Nitro-4-vinylphenol 2h
Yellow oil, yield 86%. IR (KBr plate): νmax 3418, 2925, 2853, 1627, 1536, 1322, 1260, 802. 1H NMR (600 MHz, CDCl3) δ 10.57 (s, 1H), 8.09 (d, J = 2.0 Hz, 1H), 7.67 (dd, J = 8.7, 2.1 Hz, 1H), 7.13 (d, J = 8.7 Hz, 1H), 6.65 (dd, J = 17.6, 10.9 Hz, 1H), 5.73 (d, J = 17.5 Hz, 1H), 5.32 (d, J = 10.9 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 154.59, 134.85, 134.11, 130.53, 122.42, 120.16, 114.96. HRMS-ESI (m/z): [M + H]+ calcd. for C8H8O3N: 166.04987; found, 166.04968.
4.2.2.2. 2-Fluoro-4-vinylphenol 2i
Colourless oil, yield 87%. IR (KBr plate): νmax 3436, 2924, 2852, 1612, 1094. 1H NMR (600 MHz, CDCl3) δ 7.15 (dd, J = 11.8, 2.0 Hz, 1H), 7.06 (d, J = 8.3 Hz, 1H), 6.96–6.93 (m, 1H), 6.59 (dd, J = 17.5, 10.9 Hz, 1H), 5.61–5.58 (m, 1H), 5.56 (s, 1H), 5.17 (d, J = 10.8 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 151.15(237.72), 143.30(14.33), 135.46(2.19), 131.14(6.21), 123.03(2.74), 117.18, 112.80(18.79). HRMS-ESI (m/z): [M-H]− calcd. for C8H6OF: 137.03972; found, 137.03989.
4.2.2.3. 2-Chloro-4-vinylphenol 2j
Yellow oil, yield 86%. IR (KBr plate): νmax 3498, 2923, 2850, 1619, 1261, 1099, 804. 1H NMR (600 MHz, CDCl3) δ 7.38 (d, J = 2.0 Hz, 1H), 7.23 (dd, J = 8.4, 2.0 Hz, 1H), 6.98–6.96 (m, 1H), 6.59 (dd, J = 17.5, 10.9 Hz, 1H), 5.61 (d, J = 17.5 Hz, 1H), 5.58 (s, 1H), 5.18 (d, J = 10.9 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 150.92, 135.10, 131.60, 126.61, 126.36, 120.08, 116.21, 113.03. HRMS-ESI (m/z): [M-H]− calcd. for C8H6OCl: 153.01017; found, 153.01038.
4.2.2.4. 2-Bromo-4-vinylphenol 2k
Colourless oil, yield 89%. IR (KBr plate): νmax 3425, 2919, 2850, 1602, 1126, 618. 1H NMR (600 MHz, CDCl3) δ 7.52 (s, 1H), 7.28 (s, 1H), 6.97 (d, J = 8.4 Hz, 1H), 6.58 (dd, J = 17.5, 10.9 Hz, 1H), 5.61 (d, J = 17.5 Hz, 1H), 5.53 (s, 1H), 5.17 (d, J = 10.9 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 151.85, 134.94, 132.01, 129.65, 127.08, 116.03, 113.06, 110.44. HRMS-ESI (m/z): [M + H]+ calcd. for C8H8OBr: 198.97530; found, 198.97460.
4.2.2.5. 3-Bromo-4-vinylphenol 2l
Colourless oil, yield 87%. IR (KBr plate): νmax 3405, 2925, 2850, 1605, 1228, 873, 596. 1H NMR (600 MHz, CDCl3) δ 7.44 (d, J = 8.5 Hz, 1H), 7.06 (d, J = 2.5 Hz, 1H), 6.97 (dd, J = 17.4, 10.9 Hz, 1H), 6.78 (dd, J = 8.5, 2.5 Hz, 1H), 5.57 (d, J = 17.4 Hz, 1H), 5.30 (s, 1H), 5.24 (d, J = 10.9 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 155.74, 135.03, 130.23, 127.48, 123.87, 119.45, 115.08, 114.62. HRMS-ESI (m/z): [M + H]+ calcd. for C8H8OBr: 198.97530; found, 198.97508.
4.2.2.6. (E)-1-(2-chloro-4-hydroxyphenyl)propene 2q
Colourless oil, yield 63%. IR (KBr plate): νmax 3390, 2962, 2931, 2848, 1605, 1493, 1435, 1252, 1222, 1041, 963, 903, 854, 824, 691. 1H NMR (600 MHz, CDCl3) δ 7.38 (d, J = 8.5 Hz, 1H), 6.87 (d, J = 2.6 Hz, 1H), 6.73–6.68 (m, 2H), 6.11 (dq, J = 15.7, 6.6 Hz, 1H), 1.92 (dd, J = 6.6, 1.8 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 154.89, 132.80, 128.83, 127.44, 126.66, 126.60, 116.23, 114.48, 18.70. HRMS-ESI (m/z): [M-H]− calcd. for C9H8OCl: 106.02582; found, 168.02541.
4.3. General procedure for the synthesis of 4-vinylphenol dimers (2aa/2ff/2ll)
To a stirred solution of DMF (1 ml) were added 4-hydroxycinnamic acids 1a/1f/1l (0.2 mmol) in 5 ml pressure-resistant reaction bottle. The reaction mixture was stirred at 170–220°C until the completion of the starting materials as monitored by TLC (2 h). The reaction mixture was quenched with water and extracted with ethyl acetate. The organic layer was washed with saturated sodium bicarbonate solution, dried over Na2SO4 and evaporated under reduced pressure. The resulting crude compound was purified by silica gel column chromatography and dried by vacuum freeze-drying, affording the pure dimer (2aa/2ff/2ll). The pure product was dissolved immediately in methanol for storage.
4.3.1. Dimer 2aa
Light yellow oil, yield 48%. IR (KBr plate): νmax 3328, 3021, 2962, 1610, 15 121 233,1171, 834. 1H NMR (600 MHz, CDCl3) δ 7.23 (d, J = 8.6 Hz, 2H), 7.13 (d, J = 8.5 Hz, 2H), 6.81–6.74 (m, 4H), 6.31 (d, J = 15.9 Hz, 1H), 6.20 (dd, J = 15.9, 6.8 Hz, 1H), 4.82 (s, 1H), 4.74 (s, 1H), 3.55 (p, J = 6.7 Hz, 1H), 1.41 (d, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 154.69, 153.82, 138.13, 133.54, 130.63, 128.42, 127.56, 127.43, 115.37, 115.23, 41.64, 21.40. HRMS-ESI (m/z): [M + H]+ calcd. for C16H17O2: 241.12231; found, 241.12279.
4.3.2. Dimer 2ff
Light yellow oil, yield 50%. IR (KBr plate): νmax 3416, 2960, 2921, 1611, 15 021 263,1114, 814. 1H NMR (600 MHz, CD3OD) δ 7.09 (s, 1H), 7.01 (dd, J = 8.2, 2.1 Hz, 1H), 6.97 (s, 1H), 6.90 (dd, J = 8.2, 2.1 Hz, 1H), 6.69 (dd, J = 15.1, 8.2 Hz, 2H), 6.25 (d, J = 15.9 Hz, 1H), 6.16 (dd, J = 15.8, 6.8 Hz, 1H), 4.62 (s, 2H), 3.46 (p, J = 7.2 Hz, 1H), 2.19 (s, 3H), 2.18 (s, 3H), 1.39 (d, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CD3OD) δ 142.81, 141.78, 127.48, 123.49, 120.83, 120.65, 119.76, 119.27, 116.87, 116.30, 116.17, 116.06, 107.50, 107.46, 43.85, 25.47, 20.29, 20.21. HRMS-ESI (m/z): [M-H]− calcd. for C18H19O2: 267.13796; found, 267.13870.
4.3.3. Dimer 2ll
Light yellow oil, yield 67%. IR (KBr plate): νmax 3405, 2965, 2923, 1603, 1485, 1228, 1209, 875. 1H NMR (600 MHz, CD3OD) δ 7.40 (d, J = 8.6 Hz, 1H), 7.16 (d, J = 8.5 Hz, 1H), 7.04 (d, J = 2.5 Hz, 1H), 7.00 (d, J = 2.5 Hz, 1H), 6.80 (dd, J = 8.5, 2.5 Hz, 1H), 6.75 (dd, J = 8.6, 2.4 Hz, 1H), 6.69–6.64 (m, 1H), 6.17 (dd, J = 15.8, 6.3 Hz, 1H), 4.62 (s, 2H), 4.10–4.00 (m, 2H), 1.41 (d, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CD3OD) δ 157.24, 156.27, 134.86, 134.08, 128.48, 128.34, 127.15, 127.09, 123.58, 123.02, 118.98, 118.68, 114.85, 114.80, 40.22, 19.49. HRMS-ESI (m/z): [M-H]− calcd. for C16H13Br2O2: 394.92768; found, 394.92844.
Acknowledgements
We are grateful to the staff at the Key Laboratory of Chemistry for Natural Products of Guizhou Province and the Chinese Academy of Sciences.
Contributor Information
Lishou Yang, Email: 1039160204@qq.com.
Xiaosheng Yang, Email: gzcnp@sina.cn.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material [36].
Authors' contributions
Q.Y.: investigation, methodology; Y.L.: investigation, methodology; H.L.: methodology; E.W.: data curation; M.P.: data curation, formal analysis; T.D.: data curation; X.P.: data curation; Z.L.: writing—original draft; Y.Y.: writing—original draft; L.Y.: funding acquisition, project administration, writing—original draft, writing—review and editing; X.Y.: funding acquisition, project administration, resources, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors have no competing interests.
Funding
The work here was supported by the Guizhou Provincial Natural Science Foundation (no. QKHJC[2019]1213), the Project of NSFC-Guizhou Karst Science Research Center (no. U1812403-3), the Project of Guizhou Science and Technology Platform and Talent Team (no. QKHZC[2021] General 424), the National Natural Science Foundation of China (no. 81860609) and the Project of Engineering Center of Guizhou Provincial Development and Reform Commission (no. QCJ[2019]303).
References
- 1.Ames JM, Macleod G. 1990. Volatile components of okra. Phytochemistry 29, 1201-1207. ( 10.1016/0031-9422(90)85429-J) [DOI] [Google Scholar]
- 2.Das B, Kashinatham A, Srinivas K. 1996. Alkamides and other constituents of Piper longum. Planta Med. 62, 582. ( 10.1055/s-2006-957982) [DOI] [PubMed] [Google Scholar]
- 3.Lasekan OO, Teixeira JPF, Salva TJG. 2001. Volatile flavour compounds of cooked acha (Digitaria exilis Stapf). Food Chem. 75, 333-337. ( 10.1016/S0308-8146(01)00210-2) [DOI] [Google Scholar]
- 4.Wakamatsu D, Morimura S, Sawa T, Kida K, Nakai C, Maeda H. 2005. Isolation, identification, and structure of a potent alkyl-peroxyl radical scavenger in crude canola oil, canolol. Biosci. Biotech. Bioch. 69, 1568-1574. ( 10.1271/bbb.69.1568) [DOI] [PubMed] [Google Scholar]
- 5.Koski A, Pekkarinen S, Hopia A, Wahala K, Heinonen M. 2003. Processing of rapeseed oil: effects on sinapic acid derivative content and oxidative stability. Eur. Food Technol. 217, 110-114. ( 10.1007/s00217-003-0721-4) [DOI] [Google Scholar]
- 6.Dong X, Li Z, Wang W, Zhang W, Liu S, Zhang C, Fang J, Maeda H, Matsukura M. 2011. Protective effect of canolol from oxidative stress-induced cell damage in ARPE-19 cells via an ERK-mediated antioxidative pathway. Mol. Vis. 17, 2040-2048. ( 10.1186/1476-4598-10-91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amarowicz R, Naczk M, Shahidi F. 2000. Antioxidant activity of various fractions of non-tannin phenolics of canola hulls. J. Agr. Food Chem. 48, 2755-2759. ( 10.1021/jf9911601) [DOI] [PubMed] [Google Scholar]
- 8.Xia X, Xiang X, Huang F, Zheng M, Cong R, Han L, Zhang Z. 2018. Dietary polyphenol canolol from rapeseed oil attenuates oxidative stress-induced cell damage through the modulation of the p38 signaling pathway. RSC Adv. 8, 24 338-24 345. ( 10.1039/C8RA04130J) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwahara H, Kanazawa A, Wakamatu D, Morimura S, Kida K, Akaike T, Maeda H. 2004. Antioxidative and antimutagenic activities of 4-vinyl-2, 6-dimethoxyphenol (canolol) isolated from canola oil. J. Agr. Food Chem. 52, 4380-4387. ( 10.1021/jf040045+) [DOI] [PubMed] [Google Scholar]
- 10.Han L, Xia X, Xiang X, Huang F, Zhang Z. 2017. Protective effects of canolol against hydrogen peroxide-induced oxidative stress in AGS cells. RSC Adv. 7, 42 826-42 832. ( 10.1039/C7RA08524A) [DOI] [Google Scholar]
- 11.Ayer WA, Muir DJ, Chakravarty P. 1996. Phenolic and other metabolites of Phellinus pini, a fungus pathogenic to pine. Phytochemistry 42, 1321-1324. ( 10.1016/0031-9422(96)00125-2) [DOI] [Google Scholar]
- 12.Cao D, Jiang J, Tsukamoto T, Liu R, Ma L, Jia Z, Kong F, Oshima M, Cao X. 2015. Canolol inhibits gastric tumors initiation and progression through COX-2/PGE2 pathway in K19-C2mE transgenic mice. PLoS ONE 10, e0120938. ( 10.1371/journal.pone.0120938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J, Cao D, Tsukamoto T, Wang G, Jia Z, Suo J, Cao X. 2013. Anticancer effects of 4-vinyl-2, 6-dimethoxyphenol (canolol) against SGC-7901 human gastric carcinoma cells. Oncol. Lett. 5, 1562-1566. ( 10.3892/ol.2013.1230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao X, et al. 2007. 4-Vinyl-2,6-dimethoxyphenol (canolol) suppresses oxidative stress and gastric carcinogenesis in Helicobacter pylori-infected carcinogen-treated Mongolian gerbils. Int. J. Cancer 122, 1445-1454. ( 10.1002/ijc.23245) [DOI] [PubMed] [Google Scholar]
- 15.Namboodiri VV, Varma RS, Sahle-Demessie E, Pillai UR. 2002. Selective oxidation of styrene to acetophenone in the presence of ionic liquids. Green Chem. 4, 170-173. ( 10.1039/B109534J) [DOI] [Google Scholar]
- 16.Aslam SN, Stevenson PC, Phythian SJ, Veitch NC, Hall DR. 2006. Synthesis of cicerfuran, an antifungal benzofuran, and some related analogues. Tetrahedron 62, 4214-4226. ( 10.1016/j.tet.2006.02.015) [DOI] [Google Scholar]
- 17.Campos PJ, García B, Rodríguez MA. 2000. One-pot selective synthesis of β-nitrostyrenes from styrenes, promoted by Cu(II). Tetrahedron Lett. 41, 979-982. ( 10.1016/S0040-4039(99)02186-3) [DOI] [Google Scholar]
- 18.Lee SM, Frechet JMJ, Willson CG. 1994. Photocrosslinking of poly(4-hydroxystyrene) via electrophilic aromatic substitution: use of polyfunctional benzylic alcohols in the design of chemically amplified resist materials with tunable sensitivities. Macromolecules 27, 5154-5159. ( 10.1021/ma00096a044) [DOI] [Google Scholar]
- 19.Cohen LA, Jones WM. 1960. A study of pH dependence in the decarboxylation of p-hydroxycinnamic acid. J. Am. Chem. Soc. 82, 1907-1911. ( 10.1021/ja01493a019) [DOI] [Google Scholar]
- 20.Sinha AK, Sharma A, Joshi BP. 2007. One-pot two-step synthesis of 4-vinylphenols from 4-hydroxy substituted benzaldehydes under microwave irradiation: a new perspective on the classical Knoevenagel–Doebner reaction. Tetrahedron 63, 960-965. ( 10.1016/j.tet.2006.11.023) [DOI] [Google Scholar]
- 21.Simpson CJ, Fitzhenry MJ, Stamford NPJ. 2005. Preparation of vinylphenols from 2- and 4-hydroxybenzaldehydes. Tetrahedron Lett. 46, 6893-6896. ( 10.1016/j.tetlet.2005.08.011) [DOI] [Google Scholar]
- 22.Bernini R, Mincione E, Barontini M, Provenzano G, Setti L. 2007. Obtaining 4-vinylphenols by decarboxylation of natural 4-hydroxycinnamic acids under microwave irradiation. Tetrahedron 63, 9663-9667. ( 10.1016/j.tet.2007.07.035) [DOI] [Google Scholar]
- 23.Liu D, Sun J, Simmons BA, Singh S. 2018. N-Heterocyclic carbene promoted decarboxylation of lignin-derived aromatic acids. ACS Sustain. Chem. Eng. 6, 7232-7238. ( 10.1021/acssuschemeng.7b03612) [DOI] [Google Scholar]
- 24.Sinha AK, Kumar V, Sharma A, Sharma A, Sinha AK. 2007. An unusual, mild and convenient one-pot two-step access to (E)-stilbenes from hydroxy-substituted benzaldehydes and phenylacetic acids under microwave activation: a new facet of the classical Perkin reaction. Tetrahedron 63, 11 070-11 077. ( 10.1016/j.tet.2007.08.034) [DOI] [Google Scholar]
- 25.Sheng W, Sumera Y, Chen C, Zhang M, Lei Y, Peng C, Li B, Jian Y, Wang W. 2020. Metal-free and base-free decarboxylation of substituted cinnamic acids in a deep eutectic solvent. Synthetic Commun. 50, 558-563. ( 10.1080/00397911.2019.1708945) [DOI] [Google Scholar]
- 26.Zajac WW Jr, Nowicki RB. 1966. On the mechanism of cinnamic acid decarboxylation in an acid medium. J. Org. Chem. 31, 2712-2713. ( 10.1021/jo01346a529) [DOI] [Google Scholar]
- 27.Cadot S, Rameau N, Mangematin S, Pinel C, Djakovitch L. 2014. Preparation of functional styrenes from biosourced carboxylic acids by copper catalyzed decarboxylation in PEG. Green Chem. 16, 3089-3097. ( 10.1039/c4gc00256c) [DOI] [Google Scholar]
- 28.Schweiger AK, Ríos-Lombardía N, Winkler CK, Schmidt S, Morís F, Kroutil W, González-Sabín J, Kourist R. 2019. Using deep eutectic solvents to overcome limited substrate solubility in the enzymatic decarboxylation of bio-based phenolic acids. ACS Sustain. Chem. Eng. 7, 16 364-16 370. ( 10.1021/acssuschemeng.9b03455) [DOI] [Google Scholar]
- 29.Sharma UK, Sharma N, Salwan R, Kumar R, Kasana RC, Sinha AK. 2012. Efficient synthesis of hydroxystyrenes via biocatalytic decarboxylation/deacetylation of substituted cinnamic acids by newly isolated Pantoea agglomerans strains. J. Sci. Food Agr. 92, 610-617. ( 10.1002/jsfa.4616) [DOI] [PubMed] [Google Scholar]
- 30.Takemoto M, Achiwa K. 2001. Synthesis decarboxylation of trans-cinnamic acids by plant cell cultures. Chem. Pharm. Bull. 49, of styrenes through the biocatalytic 639-641. ( 10.1248/cpb.49.639) [DOI] [PubMed] [Google Scholar]
- 31.Coghe S, Benoot K, Delvaux F, Vanderhaegen B, Delvaux FR. 2004. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agr. Food Chem. 52, 602-608. ( 10.1021/jf0346556) [DOI] [PubMed] [Google Scholar]
- 32.Morley KL, Grosse S, Leisch H, Lau PCK. 2013. Antioxidant canolol production from a renewable feedstock via an engineered decarboxylase. Green Chem. 15, 3312-3317. ( 10.1039/C3GC40748A) [DOI] [Google Scholar]
- 33.Misra K, Maity HS, Chanda S, Nag A. 2012. New greener alternatives for bioreduction of aromatic aldehydes and decarboxylation of aromatic acids using juice of fruits. J. Mol. Catal. B-Enzym. 82, 92-95. ( 10.1016/j.molcatb.2012.06.004) [DOI] [Google Scholar]
- 34.Fujiwara R, Noda S, Kawai Y, Tanaka T, Kondo A. 2016. 4-Vinylphenol production from glucose using recombinant Streptomyces mobaraense expressing a tyrosine ammonia lyase from Rhodobacter sphaeroides. Biotechnol. Lett. 38, 1543-1549. ( 10.1007/s10529-016-2126-z) [DOI] [PubMed] [Google Scholar]
- 35.Nakayama K, Maeta N, Horiguchi G, Kamiya H, Okada Y. 2019. Radical cation Diels–Alder reactions by TiO2 photocatalysis. Org. Lett. 21, 2246-2250. ( 10.1021/acs.orglett.9b00526) [DOI] [PubMed] [Google Scholar]
- 36.Yang Q, et al. 2022. Catalyst-free decarboxylation of 4-hydroxycinnamic acids: efficient synthesis of 4-vinylphenols. Figshare. ( 10.6084/m9.figshare.c.5953384) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Yang Q, et al. 2022. Catalyst-free decarboxylation of 4-hydroxycinnamic acids: efficient synthesis of 4-vinylphenols. Figshare. ( 10.6084/m9.figshare.c.5953384) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material [36].





