Abstract
Mesoporous core–shell structure Ag@SiO2 nanospheres are constructed to prevent Ag nanoparticles from aggregation during the hydrogenation reaction. The prepared catalyst shows superior catalytic performance for hydrogenation of nitro compounds with 100% conversion and selectivity without any by-products, which also indicates good recycling performance for several times use.
Mesoporous core–shell structure Ag@SiO2 nanospheres are constructed to prevent Ag nanoparticles from aggregation during the hydrogenation reaction.
Introduction
In recent years, selective hydrogenation reaction of aromatic nitro compounds has been an active area in chemistry because aromatic amino compounds are important industrial intermediates for pharmaceuticals, organic synthesis, agrochemicals, dyestuffs, urethanes and other important fine chemicals.1–5 Although various commercial catalysts have been developed, the selective reduction of the nitro group from reducible functional groups needs stoichiometric reducing agents, such as iron, zinc, tin, or metal sulfide, which cause severe environmental problems and generate by-products.6–12 Supported noble metal catalysts such as Pd/SiO2,13 Pt/C,14,15 Au (Pd, Pt)/Fe(OH)x,16 Au/TiO2,17–20 Ni–Au21 and Pt-rGO@mSiO2 22 have good catalytic performance, but the noble metal is quite rare and expensive. Silver-loaded catalysts have attracted scientists' attention and several works have been reported.23–27 But, as comparison with gold catalysts, the study on catalytic hydrogenation of silver nanoparticles is relatively rare despite its' excellent catalytic performance. We are interested in the study on Ag nanocatalysis because it is a promise and undeveloped area. As we and other groups reported,23–28 the high activity and selectivity for hydrogenation of the aromatic nitro compounds on the silver-loaded heterogeneous catalyst have been achieved, such as chemoselective hydrogenation of chloronitrobenzenes on Ag/Y2O3 nanobelt catalyst26 and Ag/C60 catalyst27 to corresponding chloroanilines without any hydrodehalogenation.
But we found that Ag nanoparticles (NPs) are easy to grow up. In order to prevent Ag NPs from growth, we have constructed Ag/Y2O3 nanobelts,26 in which Ag NPs are partially embedded in Y2O3 nanobelts to stop Ag NPs from aggregation, but it failed. Then functionalized C60 supports were synthesized to fix Ag NPs,27 it also failed to block the aggregation of Ag NPs. Finally, we constructed an Ag NPs@mesoporous SiO2 core–shell nanostructure to solve this problem. The mesoporous shells benefit for the catalytic reaction because it permits molecules or ions pass through the pore freely and also prevents Ag NPs from aggregation. Although, up to now, several works about the preparation of core–shell nanostructures have been reported,29–34 few of them investigate the hydrogenation reaction for core–shell structure sliver catalysts. Here, novel Ag@mesoporous silica spheres (A-MSS) catalyst was prepared and exhibited excellent catalytic performance for exclusive hydrogenation of nitro-group without any accumulation of hydroxylamine intermediates and formation of by-products.
Results and discussion
The core–shell structured A-MSS catalyst was prepared as follows (Scheme 1): Ag NPs were synthesized first by reducing AgNO3 in nonaqueous system, which dispersed in chloroform under the assistant of oleic acid and octadecylamine. Then, Ag NPs were transferred to aqueous phase from chloroform using CTAB as a phase transfer agent (also acted as a making hole-agent for the formation of the mesoporous silica). Finally, CTAB was removed by extraction using acetone.
Scheme 1. Synthetic process of Ag@mesoporous silica spheres (A-MSS). (a) The transferation of Ag NPs from chloroform to aqueous phase using CTAB as phase transfer agent ( ). (b) The formation process of silica sol–gel on the surface of the Ag NPs in aqueous phase. (c) The formation of A-MSS by extraction using acetone at 70 °C.
). (b) The formation process of silica sol–gel on the surface of the Ag NPs in aqueous phase. (c) The formation of A-MSS by extraction using acetone at 70 °C.
Fig. 1a is the wide-angle XRD pattern of A-MSS at the 2θ degree range from 10° to 80°. A broad peak at 20–30° (2θ) corresponds to the amorphous SiO2 and others are good agreement with the standard XRD pattern of f.c.c Ag (JCPDS card No. 04-0783). The broad reflection peak in small-angle XRD pattern of A-MSS (Fig. 1b) indicates the presence of a mesoporous structure without the long-range ordering.
Fig. 1. (a) Wide-angle and (b) small-angle X-ray diffraction (XRD) patterns of A-MSS.
TEM and HRTEM images in Fig. 2 provided an insight into the microstructure of product. TEM image in Fig. 2a shows that synthesized Ag NPs have very uniform size distribution with average diameter 7 nm. HRTEM image (Fig. 2b) shows interplanar crystal spacing of 0.245 nm attributed to the (111) lattice plane of fcc Ag NPs. The TEM image of A-MSS (Fig. 2c) reveals Ag NPs are wrapped by mesoporous silica. The scanning electron microscopy (SEM) image in Fig. 2d shows the quite uniform size with about 50 nm diameter, which is agreement with the results of Fig. 2c. The energy dispersive spectrum (EDS) in Fig. S1† shows that the catalyst contains 18.5 wt% of silver.
Fig. 2. TEM (a) and HRTEM (b) images of Ag NPs. TEM (c) and SEM (d) images of A-MSS (the enlarged A-MSS image in inset of c).
To gain insights on the specific surface area and pore size distribution of the catalyst, Brunauer–Emmett–Teller (BET) N2 adsorption–desorption measurement was conducted. As shown in Fig. 3, the A-MSS exhibits the typical type IV isotherm plot with an apparent hysteresis loop, indicative of a mesoporous microstructure. The BET analysis outputs a specific surface area value of ∼742 m2 g−1. Meanwhile, the pore size distribution plot presents a sharp peak at ∼2.2 nm, which demonstrates their mesoporous characteristics. The pore size calculated by the BJH (Barrett–Joyner–Halenda) method is about 2.5 nm, which corresponds to the result in the inset of Fig. 3.
Fig. 3. Nitrogen adsorption/desorption isotherm and Barrett–Joyner–Halenda (BJH) pore size distribution plot (inset) of A-MSS.
To demonstrate the prepared A-MSS have high catalytic activity for hydrogenation reactions, we investigated this reaction system in details. Different reaction times and temperatures were screened (Table 1, entries 1–4) and it was found that the optimum reaction condition is 3 hours and 120 °C. The conversion and selectivity of the A-MSS were up to 100%, which will not need to prolong the reaction time or increase the reaction temperature. The solvent has a remarkable influence on the reaction. Water is not suitable for the reaction with a low conversion of 51.2% (Table 1, entry 5).
Control experiments for hydrogenation of nitrobenzene to aniline on A-MSSa.

| ||||
|---|---|---|---|---|
| Entry | T (oC) | Time (h) | Conversion (%) | Selectivity (%) |
| 1 | 100 | 2 | 25.2 | 100 |
| 2 | 120 | 2 | 78.5 | 100 |
| 3 | 120 | 2.5 | 85.6 | 100 |
| 4 | 120 | 3 | 100 | 100 |
| 5b | 120 | 3 | 51.2 | 100 |
Reaction conditions: 0.05 g catalyst, 0.5 g substrate and 30 mL ethanol, H2 pressure 2.0 MPa.
The same conditions as a except 30 mL H2O
Under the optimum reaction conditions the catalytic activity of A-MSS for the unsaturated substrates was investigated and the results were summarized in Table 2, which showed that A-MSS catalyzed the hydrogenation of nitrobenzene to aniline with 100% conversion and selectivity (Table 2, entry 1). Otherwise, A-MSS showed no or very low activity for the hydrogenation of styrene, benzaldehyde, acetophenone, benzonitrile, phenylacetonitrile, benzamide and acetanilide under the same condition (Table 2, entries 2–8). It is not easy to disclose the exact reason of the different hydrogenation activity for A-MSS, which maybe attribute to the exclusive hydrogenation selectivity of the catalyst for nitro-groups of the nitrobenzene derivatives and the different bond strength in the different groups.
Hydrogenation of unsaturated substrates over A-MSSa.
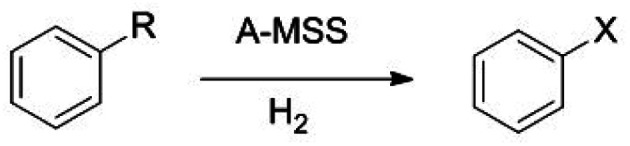
| ||||||
|---|---|---|---|---|---|---|
| Entry | R | X | Conversion (%) | Selectivity (%) | ||
| 120 (oC) | 140 (oC) | 120 (oC) | 140 (oC) | |||
| 1 | –NO2 | –NH2 | 100 | 100 | 100 | 100 |
| 2 | –CHCH2 | –CH2CH3 | 0 | 16.8 | 0 | 100 |
| 3 | –CHO | –CH2OH | 0 | 13.5 | 0 | 42.6b |
| 4 | –COCH3 | –CH(OH)CH3 | 0 | 6.7 | 0 | 100 |
| 5 | –CN | — | 0 | 0 | — | — |
| 6 | –CH2CN | — | 0 | 0 | — | — |
| 7 | –CONH2 | — | 0 | 0 | — | — |
| 8 | –NHCOCH3 | — | 0 | 0 | — | — |
Reaction conditions: 0.05 g catalyst, 0.5 g substrate and 30 mL ethanol, H2 pressure 3.0 MPa, and reaction time 3 h.
The only by-product is 2-hydroxypropiophenone.
In order to further evaluate the catalytic activity of A-MSS, various nitrobenzene derivatives were used as the starting materials and the results were listed in Table 3. Among them, the nitrobenzene derivatives containing electron-donating groups, such as chloro-, hydroxy- and methyl- groups, gave the complete conversion and selectivity without corresponding by-product to be detected (Table 3, entries 1–10). In case of nitrobenzaldehyde, nitroacetophenone, nitroacetanilide and nitrobenzonitrile, the conversion has an obvious decrease under the same mild reaction conditions (entries 11–14), which indicates that the existence of electron withdraw-groups such as nitrile-, aldehyde- and acyl- groups decrease the reactivity of the nitro-groups. But excellent conversion and selectivity can be achieved under serious conditions such as higher temperature, larger amount of catalyst and longer reaction time (entries 11–14). The high isolated yield can be achieved for nitrobenzene derivatives containing electron-donating groups (Table 3, entries 1–10), while low yield for other substrates (entries 11–14), which attributes to the low conversion and the separation loss of products.
Hydrogenation of substituted nitrobenzene over A-MSSaa.

| |||||
|---|---|---|---|---|---|
| Entry | R | Product | Conversion (%) | Selectivity (%) | Isolated yield (%) |
| 1 | H |

|
100 | 100 | 90 |
| 2 | 2-Cl |

|
100 | 100 | 88 |
| 3 | 3-Cl |

|
100 | 100 | 86 |
| 4 | 4-Cl |

|
100 | 100 | 91 |
| 5 | 2-OH |

|
100 | 100 | 87 |
| 6 | 3-OH |

|
100 | 100 | 82 |
| 7 | 4-OH |

|
100 | 100 | 85 |
| 8 | 2-CH3 |

|
100 | 100 | 90 |
| 9 | 3-CH3 |

|
100 | 100 | 86 |
| 10 | 4-CH3 |
|
100 | 100 | 90 |
| 11 | 4-CHO |

|
71 (100b) | 100 | 53 |
| 12 | 4-COCH3 |

|
65 (100b) | 100 | 45 |
| 13 | 4-CN |

|
62 (93.5b) | 100 | 35 |
| 14 | 4-CONH2 |

|
67 (100a) | 100 | 41 |
Reaction conditions: 0.05 g catalyst, 0.5 g substrate and 30 mL ethanol, H2 pressure 2.0 MPa; reaction temperature 120 °C; reaction time 3 h.
Reaction conditions: 0.1 g catalyst, 0.5 g substrate and 30 mL ethanol, H2 pressure 3.0 MPa; reaction temperature 140 °C; reaction time 4 h.
The reuse performance of A-MSS was also investigated, which demonstrated the conversion and selectivity of the catalyst reused for five times have no obvious change (Table S1†). For comparison, we also prepared free Ag NPs and Ag/SiO2 NPs by NaBH4 reduction, which demonstrated the A-MSS catalyst has better catalytic activity and recycle performance (Table S2†). The superior catalytic performance of A-MSS can be attributed to the stable porous structure with the large surface area, which not only contributes reaction molecules to pass through the pores freely, but also makes the single Ag NPs isolate from other particles and avoid aggregating.
Conclusions
In this work, the size uniform A-MSS catalyst with Ag core and mesoporous silica shell was synthesized successfully, which has the large surface area and fine pore structure. Hydrogenation reaction of nitrobenzene derivatives was adopted as model reaction to evaluate the catalytic performance of the prepared catalyst. The results show that the A-MSS catalyst has the superior catalytic performance of 100% conversion and selectivity for the hydrogenation of nitro compounds with electron-donating or electron withdraw-groups under different reaction conditions. The catalyst can be reused for several times without an obvious change, which shows good recycle performance. The excellent catalytic activity attributes to the special mesoporous core–shell structure. The approach of synthesis can also be applied to achieve other similar structure catalysts for the hydrogenation of aromatic nitro compounds. The A-MSS catalyst can also be expected to replace the expensive noble metal catalysts and have more wide applications.
Experimental section
Synthesis of Ag NPs
Ag NPs were prepared by the pyrolysis of AgNO3 in nonaqueous system. In a typical sample, 0.25 g AgNO3 was added to 100 mL flask containing 15 mL oleic acid and 10 mL octadecylamine, heated to 280 °C at the rate of 7 °C min−1 and kept at this temperature for 30 minutes under slowly stirring. The product was isolated and washed for several times with mixed solution of ethanol and n-heptane.
Synthesis of core–shell structured Ag NPs@mesoporous silica spheres
The transparent solution of the Ag nanoparticles dispersed in 10 mL chloroform was added to 250 mL flask containing 50 mL of 0.06 M CTAB aqueous solution under vigorous mechanic stirring. After a moment, the chloroform was removed by heating the mixture at 70 °C, then centrifugated at 10 000 r.p.m. for 2 minutes in order to remove the large nanoparticles. The filtrate was mixed with 140 mL water and 40 mL ethanol, and then 2.5 mL of 0.2 M NaOH solution was added under stirring. Subsequently, 20 mL of 5% TEOS in ethanol was injected slowly. The product was precipitated by ethanol or acetone after four days, and isolated by centrifugating. Then the CTAB was removed by extraction using acetone at 70 °C. Finally, the sample was dried in vacuum at 50 °C for characterization.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors are grateful for National Natural Science Foundation of China (No. 21671117).
Electronic supplementary information (ESI) available: Experimental section, EDS of A-MSS, Tables S1 and S2 of different catalysts catalytic activity and recycle performance. See DOI: 10.1039/d1ra06853a
Notes and references
- Li J. Zhao T. Chen T. Liu Y. Ong C. N. Xie J. Nanoscale. 2015;7:7502. doi: 10.1039/C5NR00857C. [DOI] [PubMed] [Google Scholar]
- Gao J. Ma R. Feng L. Liu Y. Jackstell R. Jagadeesh R. V. Beller M. Angew. Chem. 2021;133:18739. doi: 10.1002/ange.202105492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. Zheng T. Zheng J. Yu C. Zhang N. Zhou Q. Zhang W. Chen B. CrystEngComm. 2018;20:113. doi: 10.1039/C7CE01847A. [DOI] [Google Scholar]
- Song J. Huang Z. Pan L. Li K. Zhang X. Wang L. Zou J. Appl. Catal., B. 2018;227:386. doi: 10.1016/j.apcatb.2018.01.052. [DOI] [Google Scholar]
- Alexander A.-M. Hargreaves J. S. Chem. Soc. Rev. 2010;39:4388. doi: 10.1039/B916787K. [DOI] [PubMed] [Google Scholar]
- Song J. Huang Z. Pan L. Li K. Zhang X. Wang L. Zou J. Appl. Catal., B. 2018;227:386. doi: 10.1016/j.apcatb.2018.01.052. [DOI] [Google Scholar]
- Serna P. Corma A. ACS Catal. 2015;5:7114. doi: 10.1021/acscatal.5b01846. [DOI] [Google Scholar]
- Jagadeesh R. V. Surkus A. E. Junge H. Pohl M. M. Radnik J. Rabeah J. Huan H. Schunemann V. Bruckner A. Beller M. Science. 2013;342:1073. doi: 10.1126/science.1242005. [DOI] [PubMed] [Google Scholar]
- Grirrane A. Corma A. Garcia H. Science. 2008;322:1661. doi: 10.1126/science.1166401. [DOI] [PubMed] [Google Scholar]
- Zhu L. Zheng T. Zheng J. Yu C. Zhang N. Liao Q. Shu Q. Chen B. H. CrystEngComm. 2017;19:3430. doi: 10.1039/C7CE00702G. [DOI] [Google Scholar]
- Concepcion P. Corma A. Silvestre-Albero J. Franco V. Chane-Ching J. Y. J. Am. Chem. Soc. 2004;126:5523. doi: 10.1021/ja031768x. [DOI] [PubMed] [Google Scholar]
- Sepulveda-Escribano A. Coloma F. Rodriguez-peinoso F. J. Catal. 1998;178:649. doi: 10.1006/jcat.1998.2199. [DOI] [Google Scholar]
- Amali A. J. Rana R. K. Chem. Commun. 2008;35:4165. doi: 10.1039/B807736C. [DOI] [PubMed] [Google Scholar]
- Visentin F. puxty G. Kut O. M. Hungerbuehler K. Ind. Eng. Chem. Res. 2006;45:4544. doi: 10.1021/ie0509591. [DOI] [Google Scholar]
- Corma A. Sema P. Conerpcion P. Calvino J. J. J. Am. Chem. Soc. 2008;130:8478. [Google Scholar]
- Liu L. Qiao B. Chen Z. Zhang J. Deng Y. Chem. Commun. 2009;6:653. doi: 10.1039/B816547E. [DOI] [PubMed] [Google Scholar]
- Corma A. Serna P. Science. 2006;313:332. doi: 10.1126/science.1128383. [DOI] [PubMed] [Google Scholar]
- Corma A. Serna P. Garcia H. J. Am. Chem. Soc. 2007;129:6358. doi: 10.1021/ja0704131. [DOI] [PubMed] [Google Scholar]
- Corma A. ConcepciÓn P. Serna P. Angew. Chem., Int. Ed. 2007;46:7266. doi: 10.1002/anie.200700823. [DOI] [PubMed] [Google Scholar]
- Boronat M. Concepción P. Corma A. González S. Illas F. Serna P. J. Am. Chem. Soc. 2007;129:16230. doi: 10.1021/ja076721g. [DOI] [PubMed] [Google Scholar]
- Lang L. Pan Z. Yan J. J. Alloys Compd. 2019;792:286. doi: 10.1016/j.jallcom.2019.03.323. [DOI] [Google Scholar]
- Shang L. Bian T. Zhang B. Zhang D. Wu L. Z. Tung C. H. Yin Y. Zhang T. Angew. Chem., Int. Ed. 2014;53:250. doi: 10.1002/anie.201306863. [DOI] [PubMed] [Google Scholar]
- Pashaei M. Mehdipour E. Appl. Organomet. Chem. 2018;32:e4226. doi: 10.1002/aoc.4226. [DOI] [Google Scholar]
- Abbas M. Toratia S. R. Kim C. Nanoscale. 2015;7:12192. doi: 10.1039/C5NR02680F. [DOI] [PubMed] [Google Scholar]
- Zheng J. Duan X. Lin H. Gu Z. Fang H. Li J. Yuan Y. Nanoscale. 2016;8:5959. doi: 10.1039/C5NR08651E. [DOI] [PubMed] [Google Scholar]
- Han M. Li X. Li B. Shi N. Chen K. Zhu J. Xu Z. J. Phys. Chem. C. 2008;112:17893. doi: 10.1021/jp806119w. [DOI] [Google Scholar]
- Li B. Li H. Xu Z. J. Phys. Chem. C. 2009;113:21526. doi: 10.1021/jp906821h. [DOI] [Google Scholar]
- Li B. Xu Z. J. Am. Chem. Soc. 2009;131:16380. doi: 10.1021/ja9061097. [DOI] [PubMed] [Google Scholar]
- Cai M. Li Y. Liu Q. Xue Z. Wang H. Fan Y. Zhu K. Ke Z. Su C. Li G. Adv. Sci. 2019;6:1802365. doi: 10.1002/advs.201802365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purbiaa R. Paria S. Nanoscale. 2015;7:19789. doi: 10.1039/C5NR04729C. [DOI] [PubMed] [Google Scholar]
- Arnal P. M. Comotti M. Schüth F. Angew. Chem., Int. Ed. 2006;45:8224. doi: 10.1002/anie.200603507. [DOI] [PubMed] [Google Scholar]
- Banerjee B. Singuru R. Kundu S. K. Dhanalaxmi K. Bai L. Zhao Y. Reddy B. M. Bhaumik A. Mondal J. Catal. Sci. Technol. 2016;6:5102. doi: 10.1039/C6CY00169F. [DOI] [Google Scholar]
- Lang L. Shi Y. Wang J. Wang F. Xia X. ACS Appl. Mater. Interfaces. 2015;7:9098. doi: 10.1021/acsami.5b00873. [DOI] [PubMed] [Google Scholar]
- Sun J. Y. Wang Z. K. Lim H. S. Ng S. C. Kuok M. H. Tran T. T. Lu X. ACS Nano. 2010;4:7692. doi: 10.1021/nn102581g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






