Abstract
Candida vulturna is a newly emerging candida species belong to Candida haemulonii species complex of Metschnikowiaceae family. Numerous clinical samples have been reported to isolate C. vulturna since discovery. We report a case of catheter related blood stream infection in which C. vulturna was isolated from blood in patient after prolong antibiotic therapy for recurrent infection of retroperitoneal cyst. The blood isolate was identified to species level by molecular assay targeting D1/D2 regions of 26s rDNA gene. The patient improved with administration of intravenous micafungin despite lack of antifungal susceptibility breakpoints.
Keywords: Candida vulturna, Fungemia, Catheter-related blood stream infection, Candida haemulonii species complex, Prolonged antibiotic therapy, Candida spp.
1. Introduction
Candida vulturna is a newly emerging opportunistic fungal pathogen from Candida haemulonii species complex. Although regarded as a rare Candida species, it deserves attention as it is considered a multidrug-resistant yeast, especially to amphotericin B [1]. This species was first isolated from flowers and later it was described to cause invasive candidiasis in human [1,2]. The potential of this organism for human infection remains uncertain as no detailed clinical data have been reported. Here, we describe a case of C. vulturna fungemia in a hospitalized patient with an intractable infected retroperitoneal cyst and sepsis. To our knowledge, this is the first reported case of C. vulturna fungemia.
2. Case
A 83-year-old man underlying acquired cystic kidney disease, essential hypertension and dyslipidemia was admitted to University Malaya Medical Centre in Kuala Lumpur, Malaysia for intermittent fever and chills with rigors secondary to recurrent infection of a large multilobulate retroperitoneal cyst (12.4cm × 9.6cm). Upon admission (day 0), he was treated with empirical antibiotics after 2 sets of blood culture drawn from peripheral veins and peripherally inserted central catheter (PICC). C. vulturna was diagnosed based on positive blood cultures were drawn from peripheral veins and PICC on day 33 of hospitalization.
The patient had 6 months history of retroperitoneal cyst diagnosed incidentally during follow up computer tomography imaging for his renal cyst. Since the time of diagnosis, he had multiple re-admissions for recurrent sepsis secondary to infected retroperitoneal cyst.
He had one episode of bacteremia on his 1st hospitalization caused by methicillin resistant Staphylococcus aureus (MRSA) and was treated with intravenous vancomycin, dose depending on biweekly therapeutic drug measurement. As he was not fit for surgical intervention, the infected cyst was drained via pig tail and left in situ due to persistently enlarging cyst despite multiple intravenous antibiotic coverage and drainage. At the same time, in view of poor peripheral venous access for antibiotic therapy, PICC was inserted before this admission. Throughout all these hospitalizations, he responded well to the antibiotic therapy and remained hemodynamically stable with improving infective parameters.
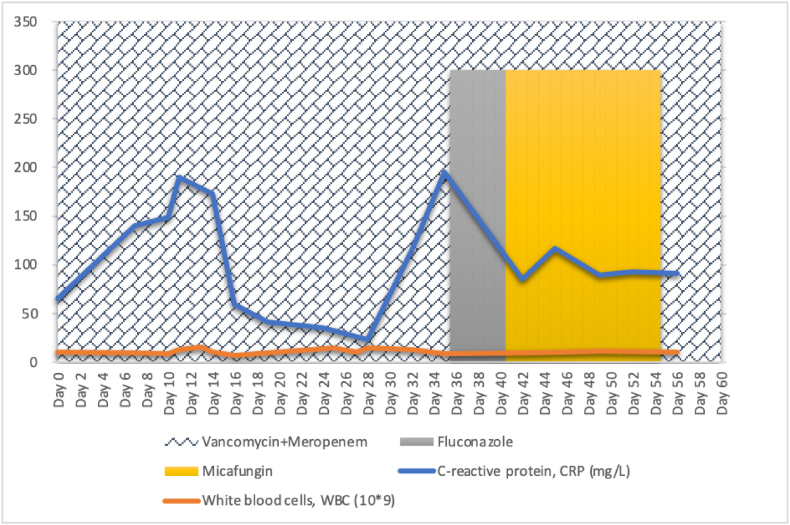
In this current admission which is his 3rd hospitalization, the culture from the infected retroperitoneal cyst grew extended spectrum beta-lactamase (ESBL) Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA) while his blood cultures (Day 0 and Day 3) from peripheral vein and PICC had no growth. He was treated with intravenous meropenem 500mg BD and vancomycin 650mg OD based on antibiotic susceptibility testing performed on pigtail drainage discharge from his previous admission which grew Enterococcus faecalis, extended spectrum beta-lactamase (ESBL) Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA). He became afebrile and hemodynamical stable thereafter with reducing trend of infective markers and scanty discharge volume from the pig tail drainage. However, on day 33, he began to developed intermittent fever (Fig. 1). Septic workout was performed from urine, pig tail drainage and both peripheral veins and PICC using BD BACTEC Aerobic, Anaerobic and MYCO Lytic Blood Culture System vials which were then processed in the automated BACTEC FX system.
Fig. 1.
Clinical course and treatment of the patient with C. vulturna fungemia. The highlighted areas of the chart are indicating periods of antimicrobial therapy along with the changes of infective parameters of the patient.
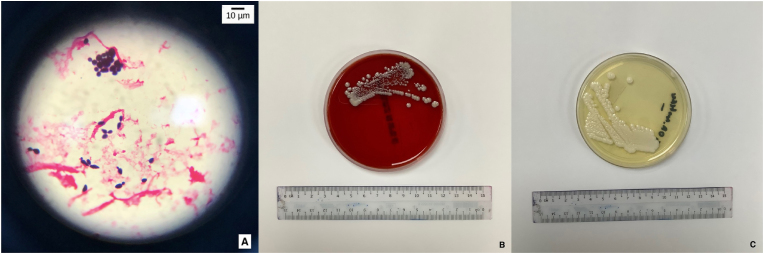
An alert notification was sent by the Epicenter system, signaling growth in both Aerobic and MYCO Lytic Blood Culture vials after 3 days of incubation (Day 36 of admission). Gram stain done from these vials showed large gram positive large budding yeast cells, oval in shape with no hyphae visualized. Intravenous fluconazole 800mg immediate dose (stat) followed by 400mg OD of antifungal therapy was initiated empirically thereafter. Both PICC and peripheral vein blood cultures yielded same yeast isolate, while urine and pig tail drainage cultures rendered no growth. The isolates grew on sheep blood agar and Sabouraud dextrose agar (SDA) as non-hemolytic, white, moist yeast-like colonies after 48hrs of incubation at 35′C in an aerobic environment, and yielded lavender moist colonies on BBL CHROMagar Candida (BD Diagnostics, Franklin Lakes, NJ, USA) (Fig. 2).
Fig. 2.
The above pictographs labelled (A) was taken from gram stain of blood culture vials and viewed at 100× magnification with oil immersion. As shown by the microscope indicator are the large oval, gram positive budding yeast-like cells. No hyphae are present. (B) & (C): Non-hemolytic, white, smooth and moist yeast-like colonies were seen after 48hrs of incubation at 35′C in aerobic environment on blood agar (BA) and sabouraud dextrose agar (SDA).
The isolate was identified as Candida auris (93% probability) by Vitek MS card (bioMérieux, Marcy l’Étoile, France) system whereas commercial matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS) system, the VITEK MS (bioMérieux) with Knowledge Base version 3.2 failed to generate a meaningful database match. Therefore, the isolates were analyzed by sequencing the D1/D2 regions of the 26s ribosomal DNA of their rRNA gene. The D1/D2 domains of the 26S rRNA gene were amplified using the universal primer pairs NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) [3]. The yeast was identified as C. vulturna (GenBank accession: OM189536) with 99.8% homology using BLAST (www.ncbi.nlm.nih.gov/blast).
Although there are currently no interpretative breakpoints available, invitro antifungal susceptibility testing (AST) performed using the broth microdilution M27-A3 method of Clinical and Laboratory Standards Institute (CLSI) via Vitek2 AST card (bioMérieux, Marcy l’Étoile, France) system produced following MICs: amphotericin B, 8mg/L; Flucytosine, <1mg/L; Caspofungin, 0.25mg/L; Fluconazole, 2mg/L; Voriconazole, <0.12mg/L; and Micafungin, 0.12mg/L. Intravenous fluconazole 400mg OD was substituted to intravenous micafungin 100mg OD after receiving complete report of isolate identification and antifungal sensitivity, 5 days (Day 41 of admission) after the initiation of antifungal therapy. The PICC was removed thereafter. An ophthalmological examination showed no evidence of endophthalmitis, and transthoracic echocardiography showed no evidence of endocarditis. The patient was on intravenous micafungin in addition to meropenem and vancomycin for 14 days. Two sets of blood cultures repeated on Day 36 and Day 39 were negative. He responded well to this regime and his symptoms relieved. Eventually, the patient was discharged well after the completion of antimicrobial therapy.
3. Discussion
Candida vulturna is an ascomycetous yeast from Candida haemulonii species complex of Metschnikowiaceae family, that is often resistant to antifungal drugs [4]. Its natural habitat has been presumed to be associated with plants after the discovery in year 2016 from Mindanao Island, the Philippine. Despite uncommonly found in healthy individuals, C. vulturna has been documented to cause human infection and isolated from human blood, gastric secretion and wounds, indicating possible exogenous origin [1,2]. Although, it has been documented to cause human disease, the pathogenesis and manifestation of infection by C. vulturna is largely unknown to this day as no detailed clinical data have been reported [1,2,5].
Prior or prolonged antibiotic therapy, use of an indwelling central venous catheter, parenteral nutrition and immunocompromised disease are among the many risk factors associated with candidemia [6]. C. haemulonii species complex are known for causing catheter related blood stream infection by colonizing and invading blood stream through indwelling intravascular catheter by forming biofilms on the surface of biomedical device. These biofilms confer protection against host immune responses and antimicrobials, which directly impact the effectiveness of treatment [7,8]. In this case report, C. vulturna fungemia was associated with central venous catheter, the prolonged usage of broad-spectrum antibiotic therapy prior the onset of fungemia and presumably age-related immune dysfunction status of our patient [9].
The epidemiology of Candida species associated with invasive candidemia is ever evolving. Identification of new Candida species can be challenging and often misidentified. C. haemulonii species complex are often misidentified as Candida auris, especially in laboratories that do not have access to high-end molecular assay or matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS) system with poorly updated databases [10,11]. C. vulturna isolated from our case cannot be correctly identified with commercial identification Vitek MS and MALDI-TOF-MS systems used in routine microbiology laboratories because of the absence of this organism from the databases. Therefore, molecular methods were required for accurate identification. Several researchers have reported that sequence analyses of D1/D2 regions were able to clearly differentiate between all the species in candida genus [12].
Antifungal resistance is a great concern in the management of patient with invasive candidiasis [13]. At the time of writing, there are no official guidelines for the performance, interpretation or quality control of in vitro susceptibility test for C. vulturna [14,15]. Unlike other species within C. haemulonii species complex, C. vulturna has shown to be sensitive to echinocandins and the azoles [1,2]. In case of human blood infection (candidemia), a good outcome was observed in our patient following selection of micafungin as an option of treatment.
Although rare, C. vulturna should not be ignored as a newly emerging infection. The study of pathogenicity and epidemiology of this organism would be beneficial for both clinicians and laboratory personnel in providing accurate diagnosis and effective treatment. Unusual Candida species may vary greatly in their susceptibility to current antibiotic agents. Therefore, studies to determine antifungal sensitivity breakpoints are of paramount importance as more cases are expected to be encountered.
Sources of funding
This study was funded by University Malaya Specialist Centre Care Fund.
Consent
Written informed consent was obtained from the patient or legal guardian(s) for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of competing interest
There are none.
Acknowledgements
The work was done in Medical Microbiology Laboratory of University Malaya Medical Centre.
References
- 1.Gade L., Muñoz J.F., Sheth M., Wagner D., Berkow E.L., Forsberg K., et al. Understanding the emergence of multidrug-resistant Candida: using whole-genome sequencing to describe the population structure of Candida haemulonii species complex. Front. Genet. 2020;11(June):1–15. doi: 10.3389/fgene.2020.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sipiczki M., Tap R.M. Candida vulturna pro tempore sp. nov., A dimorphic yeast species related to the Candida haemulonis species complex isolated from flowers and clinical sample. Int. J. Syst. Evol. Microbiol. 2016;66(10):4009–4015. doi: 10.1099/ijsem.0.001302. [DOI] [PubMed] [Google Scholar]
- 3.Kurtzman C.P., Robnett C.J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997;35(5):1216–1223. doi: 10.1128/jcm.35.5.1216-1223.1997. https://journals.asm.org/journal/jcm [Internet] [cited 2022 Jan 5] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson B.R., Chow N., Forsberg K., Litvintseva A.P., Lockhart S.R., Welsh R., et al. On the origins of a species: what might explain the rise of Candida auris? J. Fungi. 2019;5:58. doi: 10.3390/jof5030058. https://www.mdpi.com/2309-608X/5/3/58/htm [Internet]. 2019 Jul 6 [cited 2022 Jan 7];5(3):58. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro-Muñoz J.C., de Jong A.W., Gerrits van den Ende B., Haas P.J., Then E.R., Mohd Tap R., et al. The high-quality complete genome sequence of the opportunistic fungal pathogen Candida vulturna CBS 14366T. Mycopathologia. 2019 Dec 1;184(6):731–734. doi: 10.1007/s11046-019-00404-0. https://link.springer.com/article/10.1007/s11046-019-00404-0 [Internet] [cited 2022 Jan 4] Available from: [DOI] [PubMed] [Google Scholar]
- 6.Catheter-related fungemia caused by Candida intermedia | Elsevier Enhanced Reader [Internet] https://reader.elsevier.com/reader/sd/pii/S1201971209001519?token=6D8425C9178E926B72269265D6CEEF50ECB2051E9BB9A9EB04AF59AD46FEBA34B1358057E8E29CC14966CCD6ECC0EB69&originRegion=eu-west-1&originCreation=20220105114254 [cited 2022 Jan 5], Available from.
- 7.Ramos L.S., Mello T.P., Branquinha M.H., Santos A.L.S. Biofilm formed by candida haemulonii species complex: structural analysis and extracellular matrix composition. J. Fungi. 2020;6(2) doi: 10.3390/jof6020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghi E., Borgo F., Morace G. 2016. Fungal Biofilms: Update on Resistance; pp. 37–47.https://link.springer.com/chapter/10.1007/5584_2016_7 [cited 2022 Jan 7] Available from: [DOI] [PubMed] [Google Scholar]
- 9.Butcher S.K., Killampalli V., Lascelles D., Wang K., Alpar K.E., Lord J.M. Raised cortisol:DHEAS ratios in the elderly after injury: potential impact upon neutrophil function and immunity. Aging Cell. 2005 Dec;4(6):319–324. doi: 10.1111/j.1474-9726.2005.00178.x. https://pubmed.ncbi.nlm.nih.gov/16300484/ [Internet] [cited 2022 Jan 7] Available from: [DOI] [PubMed] [Google Scholar]
- 10.Hou X., Xiao M., Chen S.C.A., Wang H., Cheng J.W., Chen X.X., et al. Identification and antifungal susceptibility profiles of candida haemulonii species complex clinical isolates from a multicenter study in China. J. Clin. Microbiol. [Internet] 2016 Nov 1;54(11):2676. doi: 10.1128/JCM.01492-16. https://journals.asm.org/journal/jcm [cited 2022 Jan 7] 80. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araúz A.B., Caceres D.H., Santiago E., Armstrong P., Arosemena S., Ramos C., et al. Isolation of Candida auris from 9 patients in Central America: importance of accurate diagnosis and susceptibility testing. Mycoses. 2018 Jan 1;61(1):44–47. doi: 10.1111/myc.12709. https://onlinelibrary.wiley.com/doi/full/10.1111/myc.12709 [Internet] [cited 2022 Jan 7] Available from: [DOI] [PubMed] [Google Scholar]
- 12.Aydin M., Kustimur S., Kalkanci A., Duran T. Identification of medically important yeasts by sequence analysis of the internal transcribed spacer and D1/D2 region of the large ribosomal subunit. Rev. Iberoam Micol. 2019 Jul 1;36(3):129–138. doi: 10.1016/j.riam.2019.05.002. https://www.elsevier.es/es-revista-revista-iberoamericana-micologia-290-articulo-identification-medically-important-yeasts-by-S1130140619300543 [Internet] [cited 2022 Mar 30] Available from: [DOI] [PubMed] [Google Scholar]
- 13.Pfaller M.A., Woosley L.N., Messer S.A., Jones R.N., Castanheira M. Significance of molecular identification and antifungal susceptibility of clinically significant yeasts and moulds in a global antifungal surveillance programme. Mycopathologia. 2012 May 13;174(4):259–271. doi: 10.1007/s11046-012-9551-x. https://link.springer.com/article/10.1007/s11046-012-9551-x 1744 [Internet] [cited 2022 Jan 7] Available from: [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Tudela J.L., Arendrup M.C., Cuenca-Estrella M., Donnelly J.P., Lass-Flörl C. EUCAST breakpoints for antifungals [Internet] Drug News Perspect. 2010;23:93. doi: 10.1358/dnp.2010.23.2.1400855. https://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ [cited 2022 Jan 7] 7. Available from: [DOI] [PubMed] [Google Scholar]
- 15.CLSI Standards & guidelines: shop for CLSI Standards [Internet] https://clsi.org/standards/ [cited 2022 Jan 7], Available from: