Summary
Retinal ganglion cell (RGC) transplantation has the potential to restore vision in optic neuropathy, but donor neuron survival and retinal integration remain challenging. Here, we present a protocol for ex vivo human RGC transplantation on flatmounted murine organotypic retinal explants, providing a robust platform for studying donor RGC survival, dendritic stratification, topographic distribution, donor-host interactions, and pro-engraftment strategies. The protocol includes microscopy-based analyses to evaluate donor cell engraftment and can be adapted to various donor cell types or culture systems.
For complete details on the use and execution of this protocol, please refer to Zhang et al. (2021a, 2021b).
Subject areas: Cell Biology, Cell culture, Microscopy, Neuroscience, Stem Cells, Tissue Engineering
Graphical abstract

Highlights
-
•
Protocol for murine organotypic retinal explant culture with RGC transplantation
-
•
Proteolytic digestion of the internal limiting membrane enhances engraftment
-
•
Microscopy-based analyses quantify donor RGC survival and topology
-
•
Three-dimensional microscopy reconstructions localize donor neurite engraftment
Retinal ganglion cell (RGC) transplantation has the potential to restore vision in optic neuropathy, but donor neuron survival and retinal integration remain challenging. Here, we present a protocol for ex vivo human RGC transplantation on flatmounted murine organotypic retinal explants, providing a robust platform for studying donor RGC survival, dendritic stratification, topographic distribution, donor-host interactions, and pro-engraftment strategies. The protocol includes microscopy-based analyses to evaluate donor cell engraftment and can be adapted to various donor cell types or culture systems.
Before you begin
The protocol below describes specific steps for culturing human pluripotent stem cell-derived retinal ganglion cells (RGCs) on mouse organotypic retinal explants to model xenographic intravitreal neuronal transplantation, followed by the quantitative characterization of donor neuronal behavior in situ. There are numerous methods for stimulating differentiation of stem cells into RGCs (Gill et al., 2016; Sluch et al., 2017; Teotia et al., 2017; Langer et al., 2018) and this protocol is amenable to cellular transplantation from various sources including freshly isolated primary RGCs (Venugopalan et al., 2016; Wu et al., 2018). Therefore, specific steps for donor neuron derivation are not specified but rather left to the end user. In addition, neurons can be co-cultured on retinal explants derived from other species including rat, pig, cow, and post-mortem human tissue. The reader is referred to complimentary sources for derivation of retinal explant cultures from those species (Bull et al., 2011; Wang et al., 2011; Januschowski et al., 2012; Peynshaert et al., 2017). We have documented that proteolytic digestion of the internal limiting membrane (ILM) with the enzyme Pronase facilitates the structural engraftment of donor RGCs into the host retina (Zhang et al., 2021a, 2021b), and so steps to achieve ILM disruption are included here. For complete details on the generation and use of this protocol, please refer to (Zhang et al., 2021a, 2021b).
Institutional permission and oversight information
Use of mice and all animal procedures performed were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University School of Medicine. The experimental use of stem cells was approved by the Institutional Stem Cell Research Oversight Committee of Johns Hopkins University School of Medicine.
Days before dissection
Timing: 0.5 days
-
1.Precoat a 96-well plate with poly-L-ornithine (PLO) and laminin for eventual plating of RGCs. A sample of RGCs used for explant transplantation will be cultured in parallel on PLO-laminin coated plates to evaluate batch-specific donor neuron viability independent of recipient retinal explant cultures.
-
a.Dilute PLO with water to yield a working concentration of 20 μg/mL for polystyrene plates or 50 μg/mL for glass plates.
-
b.Dilute laminin to a working concentration of 5 μg/mL.
-
c.Add 100 μL of PLO solution to cover the surface of each well. Place the plate in a 37°C incubator for 1 h.
-
d.Aspirate the PLO solution and rinse the wells once briefly with 200 μL of sterile water.
-
e.Aspirate the water from each well and add 100 μL of laminin solution to each well. Keep the plate at 4°C overnight (12 h).
-
f.Coated plates can be stored in the laminin solution at 4°C for 3 weeks. Prior to use, bring the plate to room temperature (20°C) and remove the laminin solution. Aspirate the laminin solution and rinse the wells with sterile PBS immediately before plating the RGCs.
-
a.
-
2.
Prepare Retinal Explant Culture Media. See the list of supplemental ingredients in the materials and equipment section.
Preparing dissection hood and euthanasia area
Timing: 1 h
-
3.Thoroughly sanitize the tissue culture hood and dissection microscope with 70% ethanol. Place sterile dissection instruments inside the hood. The list of tools required includes:
-
a.Dumont #5 forceps (x2).
-
b.Vannas scissors.
-
c.Bent metal vascular probe.
-
d.30-gauge needle.
-
e.1,000 μL and 200 μL pipettes with sterile barrier tips.
-
f.Straight razor blade.
-
g.Whatman filter paper.
-
h.Organotypic culture inserts.
-
a.
-
4.
In the euthanasia area, prepare 15 mL conical tubes filled with sterile ice-cold DPBS. Label each 15 mL conical tube for one dissected eye.
Preparing culture media and enzymes
Timing: 1 h
-
5.On the day of dissection, add 1.5 mL of culture media to each well of a 6-well plate. Each well will hold one culture insert and one organotypic retinal explant. Place the plates in the 37°C incubator with 5% CO2 to equilibrate the media to physiological pH.
-
a.Stock media is stable at 4°C in the dark for at least 1 month.
-
a.
CRITICAL: Add sterile water to the plate in the space outside of the wells to provide localized humidity and prevent excessive evaporation of culture media.
-
6.Prepare Pronase enzyme prior to dissection. Due to the low final concentration and the small volume of the enzyme solution, perform serial dilution by first making a 60 U/mL solution before diluting to the final 0.6 U/mL solution.
 CRITICAL: Check the original manufacturer’s website for lot-specific units of enzymatic activity per milligram (U/mg). This is used to calculate the mass of enzyme needed for the desired final concentration of 0.6 U/mL.
CRITICAL: Check the original manufacturer’s website for lot-specific units of enzymatic activity per milligram (U/mg). This is used to calculate the mass of enzyme needed for the desired final concentration of 0.6 U/mL.-
a.Using an analytical balance, weigh out 9.23 mg of the Pronase powder (assuming an enzymatic activity of 6.5 U/mg based on Lot# SLCB5965). Use a small piece of sterile aluminum foil as the weighing boat to reduce static. Add the Pronase powder to a 1.5 mL tube, then resuspend the enzyme in 1 mL of sterile BSS. This results in a 60 U/mL solution of Pronase, which can then be diluted to 0.6 U/mL with BSS.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-RFP antibody (1:300) | Rockland Immunochemicals | Cat#600-401-379 |
| Mouse monoclonal anti-human nuclei antibody (1:300) | Millipore | Cat#MAB1281 |
| Goat anti-mouse IgG (H+L), SuperclonalTM recombinant secondary antibody, Alex Fluor 488 (1:1000) | Thermo Fisher Scientific | Cat#A28175 |
| Goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 568 (1:1000) | Thermo Fisher Scientific | Cat#A11036 |
| Chemicals, peptides, and recombinant proteins | ||
| B-27 Plus Supplement | Thermo Fisher Scientific | Cat#A3582801 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat#A9418 |
| Balanced Salt Solution (BSS) | Gibco | Cat#14287080 |
| Calcein, AM, cell-permeant dye | Thermo Fisher Scientific | Cat#C1430 |
| DAPI | Thermo Fisher Scientific | Cat#D1306 |
| Dulbecco’s phosphate buffered saline (DPBS) | Thermo Fisher Scientific | Cat#14040133 |
| Fluorescent Mounting Medium | Dako | Cat#S3023 |
| GlutaMAX Supplement | Thermo Fisher Scientific | Cat#35050061 |
| Laminin | Sigma-Aldrich | Cat#L2020 |
| N-2 Supplement | Thermo Fisher Scientific | Cat#17502001 |
| Neurobasal-A Medium | Thermo Fisher Scientific | Cat#10888022 |
| Normal Goat Serum | Thermo Fisher Scientific | Cat#50197Z |
| Ovomucoid | Sigma-Aldrich | Cat#T9253 |
| Penicillin-Streptomycin | Thermo Fisher Scientific | Cat#15070063 |
| Poly-L-ornithine solution | Sigma-Aldrich | Cat#P4957 |
| Protease from Streptomyces griseus (Pronase) | Sigma-Aldrich | Cat#P8811 |
| Triton X-100 | Sigma-Aldrich | Cat#9036-19-5 |
| Experimental models: Cell lines | ||
| Human: H7 ES cell derived RGCs | Laboratory of Don Zack (Johns Hopkins University) | (Sluch et al., 2017) |
| Experimental models: Organisms/strains | ||
| 6- to 12-week-old male and female mice (C57BL/6J) | The Jackson Laboratory | RRID: IMSR_JAX:000664 |
| Software and algorithms | ||
| Fiji/ImageJ | National Institutes of Health (NIH) | https://imagej.nih.gov/ij/ |
| IMARIS | Bitplane | https://www.bitplane.com/imaris |
| Photoshop | Adobe | N/A |
| R | R Foundation for Statistical Computing | https://www.R-project.org/ |
| R package: sjedrp | Stephen Eglen, Ph.D. | https://github.com/sje30/sjedrp |
| R package: spatstat | (Baddeley et al., 2015) | https://spatstat.org/ |
| Other | ||
| 30G needle | BD | Cat#304000 |
| 1,000 μL, 200 μL, 10 μL pipettes with sterile barrier tips | N/A | N/A |
| Angled Vessel Probe | Fine Science Tools | Cat#10140-03 |
| Cover glasses | Thermo Fisher Scientific | Cat#12-541B |
| Crescent Knife | MicroSurgical Technology | Cat#AB-ACU26KL |
| Dissecting Microscope | Leica Microsystems | Cat#SP-S9D-TS |
| Dumont Forceps #5 | Fine Science Tools | Cat#11251-10 |
| Dumont #7 Curved Forceps | Fine Science Tools | Cat#11271-30 |
| EVOS FL Auto Imaging System | Thermo Fisher Scientific | Cat#AMC1000 |
| LSM 880 Confocal Microscope | Zeiss | N/A |
| Microscope Slides | Fisher Scientific | Cat#12-550-003 |
| Millex-GV Syringe Filter, 0.22 μm | Millipore | Cat#SLGV033RS |
| Organotypic culture inserts | Millipore | Cat#PICM0RG50 |
| Straight razor blade | N/A | N/A |
| Tissue Culture Hood | N/A | N/A |
| Tissue Culture Incubator | N/A | N/A |
| Vannas scissors | Fine Science Tools | Cat#15000-08 |
| Whatman filter paper | Whatman | Cat#1001090 |
Materials and equipment
Prepare Retinal Explant Culture Media, Pronase Solution, and Proteolytic Inhibitor Solution. Sterilize the Retinal Explant Culture Media using a 0.22 μm filter and store at 4°C in the dark. Pronase Solution should be freshly prepared under aseptic conditions as to avoid filtering low volumes. Proteolytic Inhibitor Solution should be freshly prepared and sterilized using a 0.22 μm filter; it can be stored at room temperature (20°C) for a few hours.
Retinal Explant Culture Media
| Reagent | Stock concentration | Final concentration | Amount |
|---|---|---|---|
| Neurobasal-A Medium | N/A | N/A | 47.5 mL |
| B27 Plus Supplement | 50× | 1× | 1 mL |
| N-2 Supplement | 100× | 1× | 500 μL |
| Penicillin-Streptomycin | 5,000 U/mL penicillin 5,000 μg/mL streptomycin |
50 U/mL penicillin 50 μg/mL streptomycin |
500 μL |
| GlutaMAX Supplement | 100× | 1× | 500 μL |
| Total | N/A | N/A | 50 mL |
Sterilize using a 0.22 μm filter, store at 4°C, do not store for more than 1 month.
Pronase Solution
| Reagent | Stock concentration | Final concentration | Amount |
|---|---|---|---|
| Protease from Streptomyces griseus | ∼3.5 U/mg | 0.6 U/mL | lot dependent |
| Balanced Salt Solution | N/A | N/A | lot dependent |
| Total | N/A | N/A | lot dependent |
Create a 0.6 U/mL Pronase Solution by dissolving the lyophilized powder in sterile BSS. Check the exact stock enzyme concentration by referencing the Lot Number on the manufacturer website. We recommend preparing an initial 100× concentrate in 1 mL of BSS (i.e., 60 U/mL in 1 mL BSS), and dilute the stock solution to the final concentration of 0.6 U/mL. Final solution can be kept at room temperature (20°C). Prepare enzyme solution fresh for each experiment and discard the unused enzyme solution.
CRITICAL: Practice sterile technique while preparing the enzyme solution.
Proteolytic Inhibitor Solution
| Reagent | Stock concentration | Final concentration | Amount |
|---|---|---|---|
| Bovine Serum Albumin | N/A | 0.15 mM | 150 mg |
| Ovomucoid | N/A | 0.357 mM | 150 mg |
| Balanced Salt Solution | N/A | N/A | 15 mL |
| Total | N/A | N/A | 15 mL |
Sterilize using a 0.22 μm filter and store at room temperature (20°C) prior to use. Prepare the inhibitor solution fresh for each experiment and discard the unused solution. Prepare enzyme inhibitor solution fresh for each experiment and discard the unused enzyme solution.
Step-by-step method details
Animal dissection and retinal explant preparation
Timing: 10–15 min for each pair of eyes; 2–3 h for dissecting a cohort of approximately 15–30 eyes
This section describes the detailed procedure of dissecting the retina and flatmounting the explant in culture. The techniques outlined can be adapted to retinal tissue derived from other species.
-
1.Euthanize 6- to 12-week-old mice using either deep anesthesia with intraperitoneal ketamine/xylazine or CO2 asphyxiation followed by cervical dislocation, in accordance with AVMA Guidelines for Euthanasia of Animals.Note: Consistency in animal age ensures homogeneity in retinal tissue architecture and transplantation results.
-
a.Enucleate eyes by clasping behind the globe using Dumont #7 curved forceps and applying brisk outward force.
-
b.Immediately place eyes in ice cold DPBS. Transport enucleated eyes to the dissection hood for explantation.
 CRITICAL: Euthanize one animal at a time. A skilled dissectionist can flatmount retinas from one pair of eyes in approximately 6–10 min. During retinal dissection, an assistant can expedite the process by euthanizing and enucleating the next animal.
CRITICAL: Euthanize one animal at a time. A skilled dissectionist can flatmount retinas from one pair of eyes in approximately 6–10 min. During retinal dissection, an assistant can expedite the process by euthanizing and enucleating the next animal.
-
a.
-
2.
Explant the retina from the globe. Begin by using a 30-gauge needle to puncture the sclera at the limbus and then use Vannas scissors to incise the sclera just posterior to the limbus circumferentially (Figures 1A–1D; Methods video S1).
-
3.Separate the anterior and posterior segments of the globe (Figures 1E and 1F).
Note: This will separate the neural retina from the retinal pigmented epithelium (Methods video S2).
Note: Small amount of RPE remain attached to the retina will not affect the explant culture or subsequent imaging.
CRITICAL: To avoid explant culture contamination, perform dissection in a sterilized hood. Additionally, sterilize all dissection tools prior to use and between animals.
CRITICAL: Avoid iatrogenic damage to the retinal tissue during dissection, as it induces surface disruption and glial reactivity. Do not touch the retinal tissue with dissecting instruments. Instead, peel the non-retinal tissue away from the neural retina to achieve retinal isolation.
-
4.
Prior to separating the retina from the eye cup, create 4 radial relaxing incisions in the retina from the periphery halfway to the optic nerve with Vannas scissors (Figures 1N–1P), which are necessary to mount the semispherical retina onto a flat membrane. Then, detach the retina by cutting the optic nerve posterior to the retina using Vannas scissors (Figure 1Q) (Methods video S3).
-
5.Carefully transfer the free-floating retinas in PBS onto organotypic culture inserts using a sterile P1000 pipette with tip widened by cutting with a straight razor (Figures 1R and 1S).
-
a.Orient the retina inside a culture insert with the RGC layer (RGCL) facing up (photoreceptors against the membrane). The retina naturally curls with the photoreceptor side outward, and the retinal vessels are visible on the RGCL side.
-
b.Using a P200 pipette, slowly aspirate excess PBS until the retina is flatmounted on the dry filter (Figures 1T and 1U).
-
c.Use surface tension between the pipette tip or vascular probe and the retinal tissue to draw the edges of retinal explant out such that they lie flat, rather than folded (Figures 1V and 1W). Avoid touching the retinal tissue directly with the pipette tip or vascular probe (Methods videos S4 and S5).
-
a.
CRITICAL: The natural curvature of the retinal tissue creates a tendency for the tissue to fold inward. The inner retinal surface (RGCL/vitreous side) must face up when flattened (Figure 1X).
CRITICAL: Cell and tissue culture inserts are available with several options for membrane composition. We have found that hydrophilic PTFE membrane with 0.4 μm pore size (PICM0RG50) works well for culturing organotypic retinal explants.
-
6.
Apply Whatman paper to the undersurface of the organotypic membrane to remove residual fluid and create suction to adhere the retinal tissue to the membrane.
-
7.Place the culture insert with the flatmounted retina in the 6-well plate.Note: Avoid introduction of air bubbles underneath the filter during this step.
-
a.The culture media underlying the filter feeds the organotypic retinal explants, which remain under a thin air-fluid interface.
-
b.Place the culture plate back in the incubator.
-
a.
Figure 1.
Retinal dissection from an enucleated mouse eye
(A) An enucleated mouse eye submerged in PBS.
(B) Grasp the sclera or a stump of residual extraocular muscle with Dumont Forceps #5 and secure the eye in position. Create a puncture in the sclera across the globe using a 30-gauge needle.
(C) The dotted circle indicates the scleral puncture.
(D) Grasp the anterior sclera at the edge of the puncture with the Dumont Forceps #5 and create a circumferential incision parallel to and just posterior to the limbus with Vannas scissors.
(E) Remove the cornea from the posterior eye cup by holding the eye at the limbus with forceps.
(F) Use a second pair of forceps to hold the posterior cup at the optic nerve to create traction.
(G) While securing the optic nerve with forceps, pull away the crystalline lens with a second pair of forceps.
(H) An inside view of an isolated posterior cup shows the neuroretina surrounded by retinal pigmented epithelium and sclera.
(I) Use a pair of forceps to grab the scleral edge and begin folding back the sclera from the retina.
(J) Continue to use both pairs of forceps to peel back the sclera from the retina and work circumferentially around the limbal edge.
(K) Evert the sclera to prolapse the retina.
(L) Continue to gently separate the retina from the sclera.
(M) The scleral cup is now completely everted and only attached to the retina at the optic nerve head.
(N) Secure the sclera with forceps and create four radial incisions within the retina.
(O) Even with relaxing cuts, the retina still retains a folded shape at this stage.
(P) Make the final radial incision in the retina. Relaxing incisions are 90 degrees apart.
(Q) Detach the retina from the sclera by axotomizing RGCs at the optic nerve head with the Vannas scissors.
(R) Aspirate the free-floating retina using a P1000 pipette with a widened tip.
(S) A view showing the retina inside the P1000 pipette tip.
(T) Transfer the retina onto the center of a culture insert and position the retina with the inner retina facing up.
(U) Aspirate the excess PBS around the retina. The retina edges will start unfolding and flattening.
(V) An alternative method of unfolding the edge is by using a bent vascular probe to engage the overlying fluid and use surface tension to draw the folded retina peripherally.
(W) Use the surface tension between the vascular probe and the folded surface to flatten out the retina.
(X) The flatmounted retina on the culture membrane. Scale bar, 2 mm.
Pronase treatment and enzyme inhibition
Timing: 2 h
This section describes the preparation of the Pronase enzyme to be used on flatmounted retinal explant. The enzyme should be prepared prior to dissection; it is stable for several hours at room temperature (20°C).
-
8.Work with one 6-well plate at a time in the dissection hood while the rest of the plates remain in the incubator.
-
a.Using a P10 pipette, dispense 5 μL of 0.6 U/mL Pronase on to the surface of each retina.
-
b.Directly visualize the enzyme application onto the retinal surface using a dissecting microscope as a centrally-placed droplet will remain confined to the retina due to the surface tension of the air-fluid interface (Methods video S6).
-
c.Start a timer for 60 min once the first retina receives the enzyme.
-
d.Return the completed 6-well plate back into the incubator before taking out the next plate for enzyme treatment.
-
a.
CRITICAL: To dry the external surface of the pipette tip prior to each application, gently wipe the tip with Kimwipe in a single forward motion. Failure to do so will result in the droplet adhering to the side of the pipette tip instead of dispensing onto the retinal surface. While contamination from this step is rare, use a new box of Kimwipe for each experiment to limit contamination. Avoid touching the surface of the Kimwipe that will contact the pipette tip.
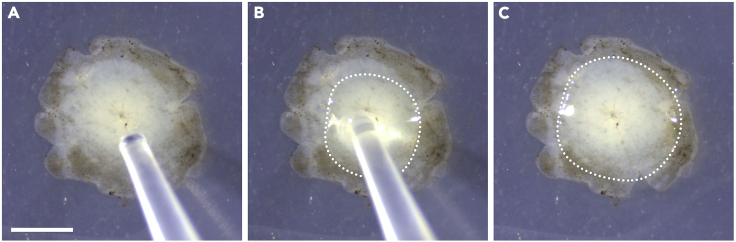
CRITICAL: The pipette should be angled as vertically as possible (Figures 2A and 2B), with pipette tip aimed at the optic nerve head to ensure equal distribution of the droplet on the retinal surface (Figure 2C) (Methods video S6).
Figure 2.
Pipetting technique
(A) Position the pipette tip over the optic nerve head.
(B) Pipette a droplet directly over the retina; the dotted line demarcates the edge of the droplet.
(C) The droplet distributes evenly around the central retina. Scale bar, 2 mm.
-
9.Prepare the Inhibitor Solution during enzymatic ILM digestion. The complete list of ingredients is in the materials and equipment section.Note: Since the Inhibitor Solution contains bovine serum albumin (BSA), it should be made fresh, immediately prior to use.
-
a.Filter the final Inhibitor Solution with a 0.22 μm syringe filter.
-
b.Keep the solution at room temperature (20°C) until use.
-
a.
-
10.After 1 h of enzyme treatment, add 300 μL of the Inhibitor Solution to the surface of each retina.Note: This volume of fluid will not remain confined to the retinal surface and will spill over onto the filter.
-
a.Space out the timing of Inhibitor Solution treatment to ensure that each individual retina has completed the 1-h enzymatic digestion.
-
b.After Inhibitor Solution has been added, place the plates back in the incubator for 15 min.
 CRITICAL: Reduce surface tension on the retina by ensuring the Inhibitor Solution completely submerges the retinal tissue. Failure to do so may result in partial or complete detachment of the retina from the membrane.
CRITICAL: Reduce surface tension on the retina by ensuring the Inhibitor Solution completely submerges the retinal tissue. Failure to do so may result in partial or complete detachment of the retina from the membrane.
-
a.
-
11.Fill the culture inserts with BSS (2 mL) to dilute the enzyme and inhibitors.
-
a.Add BSS to all the culture inserts before moving to the next step.
-
b.Dry the inside and the bottom of the culture membrane by removing the BSS and blotting the underside with Whatman paper.
-
c.After properly drying, the culture insert membrane becomes firm and taut, and can be placed in a fresh set of 6-well plates with 1.5 mL of culture media in each well.
-
d.Place the plates with the culture inserts back in the 37°C incubator until the next day.
-
a.
Note: As with using Kimwipe, Whatman paper does not require sterilization prior to use. However, practice proper antiseptic technique when handling fresh Whatman paper.
Retinal ganglion cell transplantation and co-culture
Timing: 2 h for RGC transplantation; 1 week for the culture
This section describes the technique of transplanting retinal ganglion cells on organotypic retinal explants, as well as the proper maintenance of the coculture.
-
12.
On the morning after explantation, replace 750 μL of the media in each well (underneath the membrane) with fresh media.
Note: This should be done several hours before RGC transplantation to allow the culture media to equilibrate inside the incubator.
-
13.Dilute donor RGCs to the desired concentration in Retinal Explant Culture Media.
-
a.Pipette 3 μL of the cell suspension onto each recipient retina using the technique detailed above in step-by-step method details step 8. We have found that 2 × 104 RGCs per retina works well for downstream quantitative applications.
-
b.Place the culture plates back in the incubator.Note: Optimize the total number of donor cells transplanted based on individual donor source and cell survival. Limit the transplant volume to 3 μL, as larger volumes may result in the cell suspension rolling off the retinal tissue, giving inaccurate survival counts and enabling RGCs to access the subretinal space.Note: When deriving donor RGCs through differentiation of pluripotent cells, we recommend using the lowest passage numbers possible. We typically differentiate RGCs from stem cells that have been passaged fewer than 10–15 times.
-
c.Exchange retinal explant culture media every other day after RGC transplantation. Media is exchanged by replacing half of the volume (750 μL) in each well with fresh culture media.
-
a.
-
14.Using the cells from the same preparation as step-by-step method details step 13, separately culture 8 × 103 RGCs in 150 μL of Retinal Explant Culture Media per well in the precoated 96-well plate from the “before you begin” section. Place the culture plate back in the incubator.
-
a.Allow the cells to settle onto the plate overnight (12 h).
-
b.On the following day, obtain microscopy images of the cell culture focusing on endogenous tdTomato expression using an epifluorescent microscope. Alternatively, cells can be quantified using a vital dye such as Calcein. Count the number of cells in each well to calculate survival rate.
-
c.Image the cell culture daily to examine RGC viability longitudinally.
-
a.
Retinal tissue immunofluorescent staining
Timing: 4–7 days for immunohistochemistry
This section describes the process of tissue fixation and immunohistochemistry of the cultured explants.
-
15.
Fix the retinal explants directly in the culture inserts by transferring inserts to new 6-well plates containing 3 mL per well of 4% cold PFA and adding 5 mL of 4% cold PFA directly into the culture insert.
Note: Add PFA to the culture insert slowly to avoid detaching the retinal explants. Maintain at 4°C for 1 h.
-
16.
Wash the explants by removing the PFA and replacing with 5 mL of PBS per well, and then remove the PBS. Fixed retinal explants can be stored in PBS at 4°C for 1–2 weeks prior to downstream histologic applications.
-
17.
Remove PBS from the culture inserts and cut the explants attached to the underlying membrane out of the culture insert by creating a circumferential incision in the membrane around the retinal tissue using a crescent blade.
Note: This step is best performed under a dissecting microscope. Take care to leave a margin on the membrane such that the membrane containing the explant can be picked up with forceps and transferred without touching the retina.
-
18.For flatmount immunofluorescence and microscopy, transfer the retinas attached to the culture membrane into a prelabeled plate and submerge in PBS.
-
a.Immunofluorescent staining can be performed in 48-well plate, which minimizes the amount of antibody required.
-
b.Alternatively, retinal explants at this step can be transferred to OCT and frozen for cryosectioning per standard protocols.
-
a.
-
19.To label donor RGCs, perform wholemount immunofluorescent staining for RFP.
-
a.Wash the retinas with PBS for 10 min while rocking at 80 revolutions per minute.
-
b.Block and permeabilize the tissues with 500 μL of blocking buffer (0.3% Triton X-100 and 10% normal goat serum in PBS) for 1 h at room temperature (20°C) while rocking.
-
c.Remove blocking buffer and immerse the retinas in 500 μL of primary antibodies diluted in blocking buffer for 2–5 days at 4°C while rocking. The length of primary antibody incubation should be titrated for specific antibodies.
-
d.Wash the retinas in PBS for 15 min while rocking; repeat 3 times.
-
e.Incubate the retinas in 500 μL of secondary antibodies in diluted blocking buffer overnight (12 h) at 4°C while rocking.
-
f.Wash the retinas in PBS for 15 min while rocking; repeat 3 times.
-
g.Counterstain the nuclei with DAPI in PBS for 15 min.
-
h.Wash the retinas with PBS for 15 min.
-
i.Mount the retinas on the membranes onto glass slides.
-
j.Add mounting medium (Dako, Denmark) and coverslip over the retinas. Keep the slides in the dark to dry until imaging. When dry, store in the dark at 4°C prior to imaging.
-
a.
-
20.
Perform low magnification wholemount confocal tiled imaging on each retina. See detailed procedure on confocal microscopy.
-
21.
Perform high resolution stacked confocal images for three-dimensional volumetric analysis. See detailed procedure on confocal microscopy.
-
22.
Perform computational volumetric and topographic analyses on donor cell survival and integration. See detailed procedure the quantification and statistical analysis section.
Confocal microscopy
Timing: 1–3 days
This section describes the methods for capturing images on a Zeiss 880 confocal microscope. Other microscope models may have different settings.
-
23.Wholemount tiled confocal imaging.Note: The superficial surface of the mounted retinal tissue is not necessarily parallel to the horizontal plane and tends to be slanted and irregular. Thus, capturing a wholemount retinal surface requires imaging tiled z-stacks.
-
a.Use low magnification objectives (10× or 20×) to survey and scan across the retinal surface.
-
b.Manually focus the objective and identify the most superficial area on the entire retina. Set this vertical position as the top of the z-stack.
-
c.Manually focus the objective and identify the deepest region of interest on the entire retina. Set this vertical position as the bottom of the z-stack.
-
d.Set the laser power and gain for optimal exposure. We recommend keeping the acquisition settings for master gain and digital gain constant at 650 and 1, respectively, while adjusting the laser power. Using the staining protocol above, a laser power between 1-3 would yield the optimal exposure.
-
e.Center the microscope view at the optic nerve head and acquire tiled z stacks.Note: The tile dimensions sufficient to capture the entire retina are typically 6 × 6 tiles for the 10× objective at 1.0× digital magnification and 11 × 11 tiles for the 20× objective at 1.0× digital magnification. A 512 × 512 pixel dimension for each tile provides sufficient resolution to quantify donor RGC cell bodies and visualize neurites. The z-stack interval can be set to 2.5–3.5 μm, since lower magnification objectives have larger depth of field and depth resolution is not a focus of this imaging modality.
-
a.
-
24.Detailed analysis of individual neurons demonstrating neurite integration with high-resolution and high-magnification confocal imaging (Zhang et al., 2021b).
-
a.Identify a region or a cell of interest with a 40× or 63× objective.
-
b.Set the top and bottom slices of the z-stack to capture the entire donor RGC from the soma to the deepest neurite branch. The bottom slice should extend through the outer nuclear layer to capture the entire host neural retina for characterization of laminar stratification.
-
c.Acquire fluorescent channels of interest. Our examples include acquisition of at least the tdTomato and DAPI signals.
-
a.
Note: Tiling may be required to capture the entire neurite extensions.
Note: Acquire high image resolution images to enhance downstream 3D rendering and analyses. Optimize image quality by increasing line averages and decreasing scanning speed. Pixel dimensions of 2048 × 2048 are preferred.
Expected outcomes
The main outcomes of interest for ex vivo RGC transplantation studies using this protocol include donor cell survival and localization within the host retinal tissue. This protocol can be used to visualize donor soma and neurites, and the data gathered allow for the derivation of numerous quantitative metrics, which include but are not limited to: donor cell survival (number and rate), topographical distribution (nearest neighbor distance, density recovery profile, Ripley’s functions), donor cell localization in each retinal layer, donor neurite localization in each retinal layer, donor neurite morphology (dendritic field area, number of branches, Sholl analysis), and donor cell identity (immunohistochemistry for donor-specific cytoplasmic markers, synaptic markers, and neurite markers).
Quantification and statistical analysis
Endogenous retinal neurons form mosaic arrangements across the retina to ensure uniform sampling of the visual field. A variety of statistical measures can quantify the spatial patterns of such mosaics. These metrics include nearest neighbor distance (NND), density recovery profile (DRP), and Ripley’s L-function, and have been elegantly summarized in a recent review (Keeley et al., 2020).
The NND measures the distance between a reference neuron and its closest neighboring neuron. The frequency distribution constructed from all NND in a population informs the relative spacing among neurons. A narrower peak in the frequency distribution indicates higher uniformity. The spatial uniformity is quantitatively expressed by the regularity index (RI), which is the ratio of mean NND and the standard deviation of NND (Wässle and Riemann, 1978; Cook, 1996). Higher RI correlates with increased regularity and suggests non-random organization (Hutsler and Chalupa, 1994). The RI of randomly arranged points is approximately 1.9; retinal neurons within a sub-type typically have an RI between 3 and 8, indicating mosaic organization (Eglen, 2012).
The density recovery profile (DRP) is a spatial metric that describes densities over large distances and detects cellular clustering (Rodieck, 1991). The distances from a reference cell to every other cell in the sample is measured, and the process is repeated with each cell taken as the reference cell. The resulting measurements are combined and binned at specific intervals and graphed as a histogram. The characteristic DRP of a mosaic pattern shows low cell density at close by distances and a density plateau at a threshold distance (indicating spacing regularity). Low density around the reference cell indicates a zone of exclusion that is expected with regular mosaicism between neighboring cells of a similar subtype (Bleckert et al., 2014). In contrast, a clustered pattern exhibits high densities at nearby distances, followed by an exponential decrease in density with increasing eccentricity from the reference point.
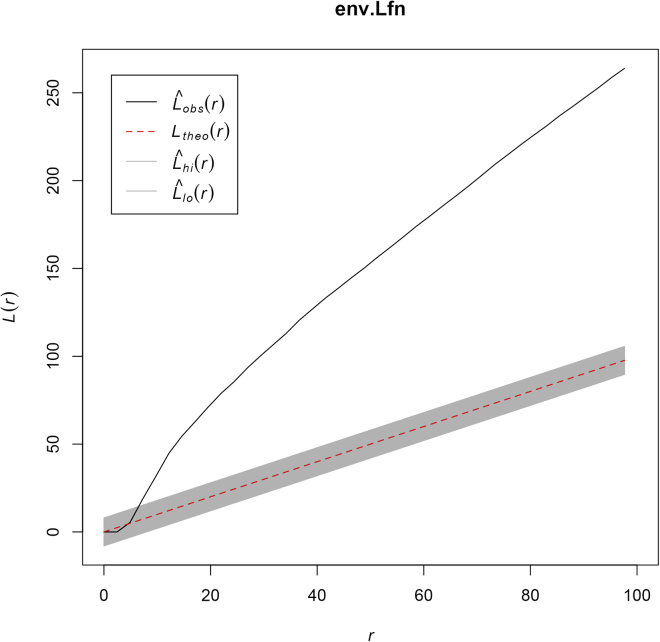
Ripley’s L-function determines whether a population distribution deviates from spatial homogeneity, and categorizes the pattern as dispersed, clustered, or random (Ripley, 1988). Complete spatial randomness is calculated using a random Poisson distribution (Besag, 1977). L-function values greater than the expected distribution under complete spatial randomness indicates clustering; L-function values lower than the expected distribution suggests dispersion.
Applying these objective spatial analytic methods to RGC transplantation studies informs donor RGC topographic distribution and facilitates comparative analyses across experimental studies. Building topographic maps of donor RGCs provides crucial information regarding transplantation efficiency and assessing progress in achieving retinotopic coverage on host tissues.
-
1.Quantify donor cell survival from the tiled wholemount images.Note: In the method described below, donor RGCs are discriminated based on overlapping tiled confocal images of both intracellular (tdTomato) and nuclear (human nuclei antigen/HuNu) immunofluorescent markers. In our human stem cell derived donor RGCs, tdTomato is expressed in the RGC-specific BRN3B locus. Its expression is specific to differentiated RGCs (Figure 3A). We additionally immunolabel for a human-specific nuclear marker (HuNu, Figure 3B), and overlap the two immunofluorescent signals to accurately account for clustered donor cells (Figures 3C and 3C′) and rule out potential material transfer as a theoretical confound which could lead to artifactual identification of host neurons as donor-derived (Pearson et al., 2016; Santos-Ferreira et al., 2016; Singh et al., 2016). In the example, the HuNu immunolabel is also necessary to exclude HuNu+RFP- cells that are human stem cell derived but undifferentiated.
-
a.Save each tiled z-stack acquired from step-by-step method details step 23 as an individual TIFF file.
-
b.Merge all the z-stacks into a single image using Photoshop or ImageJ.
-
c.Use the user-preferred manual, semi-automated, or automated method for quantifying donor cell survival and record in Table 1.
-
a.
-
2.Compute the nearest neighbor distance (NND) of donor cells (Figure 4).Note: Use ImageJ to scale the wholemount image in microns (Analyze -> Set Scale…). Failure to set scale will result in coordinates and distances reported in pixels rather than micrometers.
-
a.Generate spatial positions of donor cells across the wholemount tiled confocal image either manually with the “multi-point tool” or using semi-automatic particle detection methods in ImageJ (Figure 4A).
-
b.Save the detected points as an .ROI file (File -> Save As -> Selection…).
-
c.Measure the X,Y coordinates in ImageJ (Analyze -> Measure) (Figure 4B).
- d.
-
e.Copy the NND values into an Excel spreadsheet (Figure 4D).
-
f.Create a Pivot Table from these values. Summarize the data by “Count” and show the data as “% of Column Total” (Figure 4E).
-
g.Group the values in the newly created Pivot Table by setting the Starting point at “0” and bin size at “10” (Figure 4F).
- h.
-
a.
-
3.Density recovery profile (DRP).
-
a.Copy the X and Y coordinates from the Results window from step 2b into Excel and save as a .CSV file.
-
b.Use the following codes in R to analyze the DRP for the positional values in the .CSV file.> install.packages("sjedrp") # If not alreadyinstalled> require(sjedrp)> data = read.csv(“/users/destination/Results.csv”,sep=’,’, header=FALSE)> x = data[,1]> y = data[,2]> drp = autodrp(x, y, nbins=20, r=10, a=NULL)> plot(drp, scale=1e6)
-
i.Install the R package “sjedrp” and run the scripts.
-
ii.Retrieve the data file by replacing the file name and location with the user defined values.
-
iii.Define the x and y values by the coordinates in columns 1 and 2 of the .CSV file, respectively.
-
iv.Define the DRP parameters. “nbins” sets the number of bins analyzed; “r” sets the size of the bin in microns.
-
v.DRP histogram (Figure 5) plots the X-axis in microns and Y-axis in cells/mm2.
-
i.
-
a.
-
4.Ripley’s L function and Density plot (Figures 6 and 7).
-
a.Load the Spatstat package in R.
-
b.Use the following codes to analyze clustering functions for the .CSV file.> install.packages("spatstat") # If not alreadyinstalled> library(spatstat)> data = read.csv(“/users/destination/Results.csv”,sep=’,’, header=FALSE) > Lfn <- ppp(data[,1],data[,2],c(0,5000),c(0,5000))> env.Lfn <- envelope(Lfn, fun=Lest, nsim=99,rank=1, global=TRUE)> plot(env.Lfn, xlim=c(0,100))
-
c.Use the following codes to generate a density heatmap of the sample population from the .CSV file.> plot(Lfn,cex=0.5)> plot(density(Lfn,50))
-
a.
-
5.Quantify neurite integration using IMARIS (Figure 8).
-
a.Load high-magnification, high-resolution confocal z-stacks (step-by-step method details step 24) in IMARIS (Oxford Instruments, Zurich, Switzerland).
- b.
- c.
-
d.The DAPI channel marks three distinct nucleated retinal layers (RGCL, INL, ONL). The IPL is the non-nucleated layer between the RGCL and the INL. Segment each neurite at retinal layer junctions demarcated by the DAPI labeling and measure the total neurite length within each retinal layer.Note: This is best achieved by rotating the three-dimensional z-stack to an orthogonal view, such that the vertical profile is projected on the screen (Figure 9C).Note: While the goal of RGC replacement is to form mature dendrites restricted to the IPL, ectopic neurite growth into the INL or ONL is not uncommon in experimental settings. Hence, documentation of the relative neurite distribution within each retinal layer becomes important toward optimizing donor neurite lamination.
- e.
- f.
-
g.Tally the number of intralaminar neurite segments in each retinal layer and record the values in Table 2.
-
h.To derive additional measures from the entire neurite tracing, select “Filament” option under “Mouse Selects”, and choose the menu options “Number of Branch Points” or “Number of Terminal Points” in the “Statistics” tab. These metrics provide further quantitative description of the overall neurite complexity.
-
a.
-
6.Quantify neurite density and perform Sholl analysis using ImageJ (Figure 10).
-
a.Save the neurite tracing from step 5b as a flatmount projection in a .TIFF file. Open the .TIFF file with ImageJ. Ensure the image is properly scaled.
-
b.Use the default settings in the Sholl analysis plugin (Ferreira et al., 2014) to determine the number of neurite intersections at increasing eccentricities from the soma (Figure 10A).
- c.
-
a.
Note: The steps outlined above perform the Sholl analysis on a 2-dimensional projection of the neurite tracing. A 3-dimensional Sholl analysis may be performed in IMARIS as an alternative approach.
Figure 3.
Immunohistochemistry of wholemount tiled confocal images of the retina
(A) Immunolabeling for tdTomato.
(B) Immunolabeling for human nuclei (HuNu).
(C and C′) (C) Merged image to discriminate individual cells in high density regions (C′). Scale bars, 1 mm in (A–C), 50 μm in (C′).
Table 1.
Example of data on RGC survival and integration
| Explant ID | Treatment | Dissection notes | RGC survival | RGCs with dendrites in the neural retina |
|---|---|---|---|---|
| #001 | Pronase | Good dissection | 1965 | 15 |
Figure 4.
Derivation of NND from donor RGC topography
(A) Spatial position of donor RGCs marked on wholemount recipient retina.
(B) ImageJ readout of donor RGC coordinates.
(C) ImageJ readout of NND derived from donor RGC coordinates.
(D) Excel copy of the NND values.
(E) Excel Pivot Table “Field” settings.
(F) Excel Pivot Table “Grouping” settings.
(G) Excel Pivot Table showing NND values binned at 10 μm intervals and the frequency of each bin.
(H) Histogram displaying the grouped data from the Pivot Table.
Figure 5.
DRP derived from the sample data
The peak at near distances followed by an exponential decay in density indicates clustering within this sample population. X-axis shows distance in μm; Y-axis shows density in cells/mm2).
Figure 6.
L-function derived from the sample data
The L-function of the sample population is plotted as a black line, Lobs(r). The L-function of the expected distribution under complete spatial randomness, Ltheo(r), is indicated by the red dotted line with 95% confidence interval in gray. The observed L-function in this example lies above the expected values under complete spatial randomness and indicates clustering of donor RGCs. r (radius from cell, μm).
Figure 7.
Ripley’s L function
(A and B) Sample data illustrated by coordinates (A) as well as by local density (B), where yellow and purple correspond to high and low densities, respectively.
Figure 8.
IMARIS three-dimensional rendering of high-resolution confocal z stacks
(A and A′) Z plane projection of an integrated tdTomato+ donor RGC (A) and its neurite tracing (A′).
(B and B′) Profile view of the confocal z stacks showing an overlay of tdTomato (red), DAPI (blue), and neurite tracing (B). Neurite tracing overlaid with DAPI (B′) enables neurite length quantification within each retinal layer (RGCL, IPL, INL, ONL). Scale bars: 20 μm (A, A′, and B); 10 μm (B′).
Figure 9.
Donor RGC neurite tracing
(A) Traced neurite filaments with the overlaid DAPI labeling of host retinal cells.
(B) Select a single neurite segment (arrow).
(C) Profile view of a selected neurite segment and its measured length.
(D) Select a single point (arrow) on the neurite filament at the border of adjacent retinal layers. Delete the selected point to separate the neurite into components in each retinal layer.
(E) Select the neurite component within retinal layers and measure the segment length within a specific retinal layer.
Table 2.
Example of data from one integrated RGCs
| Dendritic area | Neurite density | Total neurite length | Neurite length in RGCL | Neurite length in IPL | Neurite length in INL | Neurite length in ONL | Total neurite segments | Neurite segments in RGCL | Neurite segments in IPL | Neurite segments in INL | Neurite segments in ONL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 894.3 μm2 | 82,809 μm/mm2 | 1,342 μm | 1,034 μm | 180 μm | 308 μm | 0 μm | 23 | 20 | 5 | 3 | 0 |
Figure 10.
Sholl analysis of donor RGC dendritic arbors
(A) Perform Sholl analysis using the default settings in the ImageJ Plugin.
(B) Numeric printout of the number of neurite intersections at increasing eccentricities from the cell body.
(C) A graphical demonstration of the Sholl analysis for the example neuron.
Limitations
The greatest obstacle associated with ex vivo RGC transplantation using organotypic retinal explant is maintaining tissue health and normal physiologic function throughout the culture period. Despite optimizing the culture conditions, organotypic retinal explant tissues undergo progressive neurodegeneration, and typically cannot be cultured for more than 1–2 weeks, though adding growth factors or neuroprotective agents may prolong survival. This significantly constrains the utility of assessing long-term (>2 weeks) donor engraftment and functional connectivity with the recipient. Therefore, while this protocol allows for the visualization of donor cellular dynamics in the short-term, the influences of ex vivo environmental factors compared to an in vivo counterpart may reveal differences in donor cell behavior.
Troubleshooting
Problem 1
Retinal tissue appears grossly unhealthy at early culture time points, and photoreceptor survival is especially poor (step-by-step method details steps 12 and 13).
Potential solution
Degenerative retinal phenotypes may be unexpectedly observed before or after enucleation due to previously unrecognized mutations in the Crb1 gene (the so called retinal degeneration (rd)-8 mutation) in some strains bred on a C57BL/6N background (Mattapallil et al., 2012). It is important to exclude such animals from the experiment. If degenerative phenotypes are observed in multiple littermates, breeders may carry recessive mutations and should be replaced by new breeders. In addition, handling the retinal tissue too roughly during dissection or taking too long with dissection can also undermine host tissue survival.
Problem 2
Organotypic retinal explants can sometimes detach from the culture insert at various stages of the culture (step-by-step method details steps 10–13).
Potential solution
The retinal explant may detach from the underlying culture insert as a result of insufficient membrane adherence. Blotting the undersurface of the membrane to create adhesion is critical prior to introduction fluid onto the retinal explant. Explant tissue detachment can also be exacerbated by enzymatic digestion. Verify the enzyme concentration and limit the digestion to 1 h. Pipetting Inhibitor Solution or BSS onto the explant surface can also cause tissue disruption, thus avoid vigorous pipetting when working with explant tissues. When the explant is partially detached, carefully flatten the lifted edge with a bent vascular probe and blot the membrane undersurface dry before proceeding onto the next step. If the entire explant tissue is detached, ensure the inner retina faces up before reattaching the tissue by blotting the membrane.
Problem 3
Organotypic retinal explants can become unhealthy or die during culturing (step-by-step method details steps 10–13).
Potential solution
Explants may exhibit poor survival during the culture period for several reasons, including non-physiologic pH of the culture media, irregular temperature regulation, and prolonged or traumatic enzymatic digestion. The Retinal Explant Media is buffered by bicarbonate and requires incubation in 5% CO2 to maintain physiologic pH. Accordingly, limit the amount of time explants are outside of the incubator to prevent media alkalinization and exchange media at least every other day to prevent acidification. Timing Pronase treatment to 1 h is critical, as prolonged enzymatic digestion can cause tissue damage.
Problem 4
Organotypic retinal explants can become contaminated during culturing (step-by-step method details steps 10–13).
Potential solution
Despite the fact that supplemental antibiotics are added to the culture media, maintaining sterility is critical, especially for primary tissue cultures. This includes sterilizing surgical instruments, dissection hood, dissection microscope, and filtering buffer solutions. Fungal contamination is the most common (and we typically do not include antimycotics in the culture media), and easily spreads among culture inserts and sometimes culture plates. Contamination compromises experimental data integrity. If found, abandon the plate immediately and restart the experiment, sterilize all equipment, and decontaminate the incubator.
Problem 5
Difficulty tracing neurites in IMARIS (step-by-step method details step 24; Analysis step 5).
Potential solution
The automatic filament detection in IMARIS relies on excellent image quality, which is dependent on optimized immunohistochemistry and fluorescent image acquisition. Avoid overexposure and reduce background noise when imaging the tissues. Adjustments to acquisition settings, such as increasing pixel resolution to 2048 × 2048, increasing averaging number to 2 or 4, and decreasing acquisition speed can improve the final image quality. However, be mindful of the balance between higher image quality and increased acquisition time and risk of photobleaching.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Thomas V Johnson (johnson@jhmi.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
Funding sources: National Eye Institute (K08-EY031901 [T.V.J.] and P30-EY001765 [Wilmer Eye Institute]), Research to Prevent Blindness (Career Development Award [T.V.J.] and unrestricted scientific support [Wilmer Eye Institute]), and The Alan & Shelley Holt Rising Professorship in Ophthalmology (T.V.J.).
Author contributions
Conceptualization, T.V.J.; methodology, K.Y.Z. and T.V.J.; software, K.Y.Z.; validation, K.Y.Z.; formal analysis, K.Y.Z. and T.V.J.; writing – original draft, K.Y.Z. and T.V.J.; writing – review & editing, K.Y.Z. and T.V.J.; funding acquisition, T.V.J.; resources, T.V.J.; supervision, T.V.J.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101328.
Contributor Information
Kevin Y. Zhang, Email: yzhan427@jhmi.edu.
Thomas V. Johnson, Email: johnson@jhmi.edu.
Data and code availability
The data used in this study are available from the lead author upon request. The codes used in analyses are available from cited sources.
References
- Baddeley A., Rubak E., Turner R. CRC Press; 2015. Spatial Point Patterns: Methodology and Applications with R.https://www.routledge.com/Spatial-Point-Patterns-Methodology-and-Applications-with-R/Baddeley-Rubak-Turner/p/book/9781482210200 [Google Scholar]
- Besag J. Contribution to the discussion on Dr Ripley’s paper. J. R. Stat. Soc. 1977;39:193–195. [Google Scholar]
- Bleckert A., Schwartz G.W., Turner M.H., Rieke F., Wong R.O. Visual space is represented by nonmatching topographies of distinct mouse retinal ganglion cell types. Curr. Biol. 2014;24:310–315. doi: 10.1016/j.cub.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull N.D., Johnson T.V., Welsapar G., DeKorver N.W., Tomarev S.I., Martin K.R. Use of an adult rat retinal explant model for screening of potential retinal ganglion cell neuroprotective therapies. Invest. Ophthalmol. Vis. Sci. 2011;52:3309–3320. doi: 10.1167/iovs.10-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.E. Spatial properties of retinal mosaics: an empirical evaluation of some existing measures. Vis. Neurosci. 1996;13:15–30. doi: 10.1017/S0952523800007094. [DOI] [PubMed] [Google Scholar]
- Eglen S.J. In: Computational Systems Neurobiology. Le Novère N., editor. Springer Netherlands; 2012. Cellular spacing: analysis and modelling of retinal mosaics; pp. 365–385. [Google Scholar]
- Ferreira T.A., Blackman A.V., Oyrer J., Jayabal S., Chung A.J., Watt A.J., Sjöström P.J., van Meyel D.J. Neuronal morphometry directly from bitmap images. Nat. Methods. 2014;11:982–984. doi: 10.1038/nmeth.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill K.P., Hung S.S., Sharov A., Lo C.Y., Needham K., Lidgerwood G.E., Jackson S., Crombie D.E., Nayagam B.A., Cook A.L., et al. Enriched retinal ganglion cells derived from human embryonic stem cells. Sci. Rep. 2016;6:30552. doi: 10.1038/srep30552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler J.J., Chalupa L.M. Neuropeptide Y immunoreactivity identifies a regularly arrayed group of amacrine cells within the cat retina. J. Comp. Neurol. 1994;346:481–489. doi: 10.1002/cne.903460402. [DOI] [PubMed] [Google Scholar]
- Januschowski K., Mueller S., Spitzer M.S., Schramm C., Doycheva D., Bartz-Schmidt K.U., Szurman P. Evaluating retinal toxicity of a new heavy intraocular dye, using a model of perfused and isolated retinal cultures of bovine and human origin. Graefes Arch. Clin. Exp. Ophthalmol. 2012;250:1013–1022. doi: 10.1007/s00417-012-1989-5. [DOI] [PubMed] [Google Scholar]
- Keeley P.W., Eglen S.J., Reese B.E. From random to regular: variation in the patterning of retinal mosaics. J. Comp. Neurol. 2020;528:2135–2160. doi: 10.1002/cne.24880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer K.B., Ohlemacher S.K., Phillips M.J., Fligor C.M., Jiang P., Gamm D.M., Meyer J.S. Retinal ganglion cell diversity and subtype specification from human pluripotent stem cells. Stem Cell Rep. 2018;10:1282–1293. doi: 10.1016/j.stemcr.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y. Nearest neighbor distances calculation with ImageJ. 2016. https://icme.hpc.msstate.edu/mediawiki/index.php/Nearest_Neighbor_Distances_Calculation_with_ImageJ.html
- Mattapallil M.J., Wawrousek E.F., Chan C.C., Zhao H., Roychoudhury J., Ferguson T.A., Caspi R.R. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest. Ophthalmol. Vis. Sci. 2012;53:2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.A., Gonzalez-Cordero A., West E.L., Ribeiro J.R., Aghaizu N., Goh D., Sampson R.D., Georgiadis A., Waldron P.V., Duran Y., et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 2016;7:13029–13115. doi: 10.1038/ncomms13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peynshaert K., Devoldere J., Forster V., Picaud S., Vanhove C., De Smedt S.C., Remaut K. Toward smart design of retinal drug carriers: a novel bovine retinal explant model to study the barrier role of the vitreoretinal interface. Drug Deliv. 2017;24:1384–1394. doi: 10.1080/10717544.2017.1375578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley B.D. Cambridge University Press; 1988. Statistical Inference for Spatial Processes. [Google Scholar]
- Rodieck R.W. The density recovery profile: a method for the analysis of points in the plane applicable to retinal studies. Vis. Neurosci. 1991;6:95–111. doi: 10.1017/S095252380001049X. [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira T., Llonch S., Borsch O., Postel K., Haas J., Ader M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat. Commun. 2016;7:13028–13037. doi: 10.1038/ncomms13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.S., Balmer J., Barnard A.R., Aslam S.A., Moralli D., Green C.M., Barnea-Cramer A., Duncan I., MacLaren R.E. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 2016;7:13537–13545. doi: 10.1038/ncomms13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluch V.M., Chamling X., Liu M.M., Berlinicke C.A., Cheng J., Mitchell K.L., Welsbie D.S., Zack D.J. Enhanced stem cell differentiation and immunopurification of genome engineered human retinal ganglion cells. Stem Cells Transl. Med. 2017;6:1972–1986. doi: 10.1002/sctm.17-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teotia P., Chopra D.A., Dravid S.M., Van Hook M.J., Qiu F., Morrison J., Rizzino A., Ahmad I. Generation of functional human retinal ganglion cells with target specificity from pluripotent stem cells by chemically defined recapitulation of developmental mechanism. Stem Cells. 2017;35:572–585. doi: 10.1002/stem.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan P., Wang Y., Nguyen T., Huang A., Muller K.J., Goldberg J.L. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 2016;7:10472. doi: 10.1038/ncomms10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Kolomeyer A.M., Zarbin M.A., Townes-Anderson E. Organotypic culture of full-thickness adult porcine retina. J. Vis. Exp. 2011:e2655. doi: 10.3791/2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H., Riemann H.J. The mosaic of nerve cells in the mammalian retina. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 1978;200:441–461. doi: 10.1098/rspb.1978.0026. [DOI] [PubMed] [Google Scholar]
- Wu S., Chang K.C., Nahmou M., Goldberg J.L. Induced pluripotent stem cells promote retinal ganglion cell survival after transplant. Invest. Ophthalmol. Vis. Sci. 2018;59:1571–1576. doi: 10.1167/iovs.17-23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.Y., Tuffy C., Mertz J.L., Quillen S., Wechsler L., Quigley H.A., Zack D.J., Johnson T.V. Role of the internal limiting membrane in structural engraftment and topographic spacing of transplanted human stem cell-derived retinal ganglion cells. Stem Cell Rep. 2021;16:149–167. doi: 10.1016/j.stemcr.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.Y., Aguzzi E.A., Johnson T.V. Retinal ganglion cell transplantation: approaches for overcoming challenges to functional integration. Cells. 2021;10:1426. doi: 10.3390/cells10061426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available from the lead author upon request. The codes used in analyses are available from cited sources.