Abstract
Aim:
Epicutaneous immunotherapy (EPIT) with peanut has been demonstrated to be safe but efficacy may be limited by allergen uptake through the skin barrier. To enhance allergen uptake into the skin, the authors used peanut-coated microneedles and compared them with EPIT in a peanut allergy mouse model.
Methods:
Sensitized mice were treated with peanut-coated microneedles or peanut-EPIT and then challenged with peanut to determine protection.
Results:
Treatment with peanut-coated microneedles was safe and showed enhanced desensitization to peanut compared with peanut-EPIT administered via a similar schedule. Protection was associated with reduced Th2 immune responses and mast cell accumulation in the intestine.
Conclusion:
Peanut-coated microneedles have the potential to present a safe method of improving allergen delivery for cutaneous immunotherapy.
Keywords: : allergen immunotherapy, coated microneedles, cutaneous immunotherapy, desensitization, epicutaneous immunotherapy, food allergy, peanut allergy, skin vaccination
Plain language summary
Epicutaneous immunotherapy (EPIT) with peanut has been demonstrated to be safe but efficacy has been varied. The tight barrier provided by the skin may limit the amount of allergen taken up through the skin and thus reduce efficacy. The authors evaluated a microneedle-based approach to improve the amount of allergen deposited into the skin to improve efficacy. Mice were made allergic to peanut and then treated with peanut-coated microneedles or peanut-EPIT. Mice were challenged with peanut to determine suppression of allergic reactivity. In mice, treatment with peanut-coated microneedles was safe and enhanced desensitization to peanut compared with peanut-EPIT administered via a similar schedule. Peanut-coated microneedles may present a novel method of improving allergen immunotherapy delivered through the skin.
Food allergy is a significant and increasing public health problem, with approximately 1 in 10 American adults and 1 in 13 children are allergic to at least one food [1,2]. Despite the high prevalence, few treatment options exist beyond strict allergen avoidance with epinephrine use upon accidental exposure to the allergen. In recent years, significant progress has been made toward the development of allergen-specific immunotherapies in which desensitization is achieved through repeated allergen exposure. Large clinical trials have been conducted for oral immunotherapy (OIT) and epicutaneous immunotherapy (EPIT), showing varying degrees of success [3]. Even the successful approaches typically require daily exposure to the food to maintain desensitization. Despite recent advances, OIT for peanut allergy is the only US FDA-approved therapy [4]. Thus, novel therapies are needed to improve outcomes and alleviate strict protocols for patients with food allergies.
The skin is an attractive target for allergen-specific immunotherapy. The skin is enriched with antigen-presenting cells (APCs) capable of activating signaling pathways to maintain a balance between immunogenicity against microorganisms and tolerance [5]. Delivery of antigen into the skin has been shown to activate APCs and induce immunoregulatory cells, resulting in attenuation of allergic reactivity [6,7]. Because the epidermis is non-vascularized, the risk of antigen entry into the bloodstream and adverse events is limited [8]. While EPIT has been demonstrated to be safe, efficacy has been limited [3]. Conversely, subcutaneous immunotherapy (SCIT) has been shown to be effective for suppressing allergic reactivity for aeroallergens [9]. However, an early trial of SCIT for peanut allergy had severe adverse events, so the investigation of SCIT for food allergy has been limited due to safety concerns [10]. Results from these two approaches to allergen immunotherapy demonstrate that the route of allergen exposure has implications for both safety and efficacy.
The most well-studied EPIT approach involved 12 months of daily treatment with the transdermal patch (Viaskin®) formulated with 250 μg of peanut protein [11]. Viaskin demonstrated moderate efficacy with a 50% and 35% response rate in phase IIb and III clinical trials, respectively [12,13]. There was no significant response in adults, and the phase III clinical trial did not meet the primary end point for increase in efficacy compared with placebo, as there was a 13.6% response rate in placebo controls. The reasons for these variations are not entirely clear, but the transdermal patch requires passive diffusion of peanut protein through the skin to interact with immune cells. Because the skin barrier varies greatly between patients and across areas of the skin on a single patient, differences in skin permeability may account for a proportion of the low response rates, especially in adults [14]. Therefore, improved dermal delivery of peanut protein may improve treatment efficacy.
One method to improve cutaneous immunotherapy is the use of delivery devices such as micron-sized needles (microneedles [MNs]) to administer controlled amounts of allergen directly into the skin [15]. MNs bypass the physiological barrier created by the outermost layer of the skin. Through this method of targeted allergen-specific immunotherapy within the skin, the amount of allergen delivered can be controlled and is not influenced by differences in skin permeability. MNs coated with ovalbumin or Der p 1 have shown efficacy in mouse models of allergic lung disease [16,17]. The authors have previously demonstrated that peanut-coated MNs (PN-MNs) can desensitize peanut allergic mice [18]; however, there are no direct comparisons reported between PN-MNs and epicutaneous peanut protein immunotherapy (PN-EPIT). In a randomized clinical trial, the safety of the epicutaneous route appeared to be favorable, but the efficacy was limited. Epicutaneous treatment appeared to have limited desensitization, as only about 35% of 4- to 11-year-old children met the clinical end point in the phase III pivotal trial, while there was a 13.6% response rate in placebo controls [13]. PN-MNs and PN-EPIT differ substantially in how they deliver the allergen into the skin. While PN-MNs provide quantifiable targeted allergen delivery into the skin within a few minutes, in contrast, PN-EPIT relies on passive diffusion of allergen into the skin with an unknown amount penetrating the skin after continuous PN-EPIT application for days in a mouse model. Therefore, a direct comparison between the two approaches can help provide insight into the role the skin barrier and allergen uptake might play in allergen immunotherapy via the skin. This information could help in the development of strategies to improve skin-based peanut allergen immunotherapy and, in particular, provide insight into the clinical potential of PN-MNs.
Accordingly, in this study the authors evaluated the relative efficacy of PN-MNs as compared with PN-EPIT. In their previous study, the authors had used Balb/c mice; however, to create a more stringent test of efficacy of peanut allergen immunotherapy, here the authors performed the comparison in C3H/HeJ mice, a strain known to possess increased reactivity to oral peanut challenge [19,20]. Furthermore, the suppression of allergen-specific reaction was studied following both oral and intradermal allergen challenges. Since mast cells are a key mediator of allergic response [21–23], the authors also directly compared the effect of PN-MNs with that of PN-EPIT on intestinal mast cells. The safety between PN-MNs and PN-EPIT was also compared. Overall, this is the first study directly comparing PN-EPIT with PN-MNs.
Materials & methods
MNs & coating formulation
MN arrays were made from 50 μm thick stainless steel (316 grade) sheets via a wet etch process as described previously [15]. The MN array measured 1 × 1 cm and contained 50 MNs, each 700 μm long. Upon receiving the fabricated MN arrays, individual MNs in an array were manually pushed to position them perpendicular to the base. The peanut protein coating formulation contained peanut extract (Moonlight Therapeutics, GA, USA), carboxymethyl cellulose (low viscosity, USP grade, CarboMer, CA, USA) (1%, w/v) as a viscosity enhancer, and POLOXAMER 188 (Spectrum Chemical, CA, USA) as a surfactant. The ovalbumin (OVA) coating formulation contained carboxymethyl cellulose (1%, w/v; low viscosity, USP grade, CarboMer) as a viscosity enhancer, POLOXAMER 188 (Spectrum Chemical) (0.5% w/v) and ovalbumin (Abnova, Taiwan; 0.8% w/v) in 20 mM 1x Tris buffer (1 M Tris [VWR, PA, USA] and sterile deionized [DI] water). Coated MNs were prepared using a micro-precision dip coating machine as described previously [24]. For characterization, MN arrays were washed in 0.3 ml DI water for 2 min. The protein concentration in the solution was assessed by microBCA test (Thermo Fisher Scientific, IL, USA). Coating amount was 11.3 +/- 0.9 (std, n = 4) micrograms of peanut protein or 9.4 +/- 0.8 (std, n = 3) micrograms ovalbumin per array. Each coated array was attached to the flat cap of 15 ml centrifuge tube (VWR) using double-sided tape (300LSE, 3M, MN, USA) with needle side up. The main body of the tube was used to apply the array to mouse skin by hand.
Visualization of coatings on MNs & determination of delivery efficiency
To visualize peanut protein coatings on MNs and determine delivery efficiency, peanut proteins were fluorescently labeled with N-Hydroxysuccinamide activated-fluorescein reagent (Thermo Fisher Scientific; Pierce Biotechnology, MA, USA), dialyzed to remove unconjugated dye molecules and coated on MNs. Coated MNs were imaged using a scanning electron microscope to visualize coatings. Three MN patches coated with fluorescently labeled peanut protein were each inserted into the skin of a mouse (Balb/c mice, Charles River, Laboratories, Inc., MA, USA) and manually held in place for 5 min. Subsequently, as described previously [18], the amount of peanut protein delivered into the skin, left on the needle and left on the skin surface were quantified with fluorescence spectroscopy (Cary Eclipse, Agilent Technologies, CA, USA), at settings of an excitation of 480 nm and emission of 530 nm wavelength.
Mice & allergic sensitization
Peanut extract was prepared from peanut flour (Byrd Mill, 12% fat, light roast) as previously described [25]. Briefly, peanut flour was solubilized in phosphate-buffered saline (PBS) with 1 mol/l NaCl and centrifuged to remove particulates. Supernatant was sterilized by sequential filtration through 0.4 μm and 0.2 μm filters and concentrated using 10,000 MWCO Centricon filters (Millipore, MA, USA). Protein content was determined by bicinchoninic acid (BCA) assay. C3H/HeJ mice (females 4–5-weeks old), a strain commonly used in peanut allergy models due to increased reactivity to oral challenge [19,20], were purchased from Jackson Laboratory. All animal procedures were approved by the University of Michigan Institutional Animal Care and Use Committee. The peanut allergy model utilized here has been optimized from previous reports [19,21,25,26] to generate IgE-mediated reactions to oral challenge with peanut without the need for systemic (intraperitoneal/intravenous) challenge for which anaphylaxis is largely IgG-mediated [27]. Allergic sensitization to peanut was induced by intragastric administration of 2 mg peanut extract and 10 μg cholera toxin (CTx, List Biological Labs, CA, USA) administered weekly for 6 weeks [26].
Immunotherapy
Mice were anesthetized with isoflurane using the IMPAC 6 system. Hair was removed from an approximately 2 × 3 cm region of the back 1 day prior to MN application. Hair was shaved with an electric hair-trimmer followed by a 30 sec application of hair removal cream. Coated MNs were inserted into the hairless skin and manually held in place for 5 min to allow coated protein to dissolve inside the skin. For PN-EPIT or sham PBS treatment, a peanut extract solution (100 μg in 50 μl) or PBS was deposited on a patch of sterile gauze (1 cm × 1 cm) that was maintained on depilated skin with bio-occlusive dressing (Tegaderm, 3M™) for 24 h. Immunotherapy was performed weekly for 5 weeks.
Assessment of hypersensitivity reactions to oral peanut challenge
Mice were challenged orally 7 alternating days during the final 2 weeks of the studies. These repeated oral challenges in a mouse model are known to increase the severity of allergic reactions, and this has been suggested to occur because mice have low basal levels of goblet cells and mast cells in the intestine that increase over the course of the challenge, reaching a threshold for significant reactivity after more than four oral challenges [21–23,26,28]. For each challenge, mice were fasted for 5–6 h before oral gavage with 20 mg peanut extract. Mice were monitored for reactivity for 1 h after the final oral challenge using the following scoring system [19,26]: 0, no symptoms; 1, itching around the eyes/snout; 2, puffiness around the eyes/snout, diarrhea, piloerection and/or decreased activity; 3, labored respiration, stridor and/or cyanosis around the mouth; 4, convulsion, no activity after prodding and/or moribund; 5, death. Rectal temperature was monitored every 15 min for 60 min and the maximum change in temperature recorded. Mice were bled 60 min following challenge, and serum concentrations of mouse mast cell protease-1 (MCPT-1) were determined by ELISA (Invitrogen, MA, USA).
Acute allergic skin response
The acute allergic skin response was determined after intradermal injection of 10 μg peanut extract in the ear pinnae of mice anesthetized with isoflurane. Ear thickness was measured in duplicate at 1 h using a digital micrometer. Reactogenicity at the local site of the immunotherapy was also determined; 20 μg peanut extract in 20 μl was injected intradermally into the skin on the back of isoflurane-anesthetized mice. The injection site was monitored for swelling at 20 min.
Mast cell quantification
1 day after the final oral challenge, the jejunum was fixed in 10% formalin, embedded in paraffin and cut into 5 μm sections. Sections were stained for chloroacetate esterase (CAE) activity as previously described [21,29,30]. Quantification of mast cells was performed by counting the number of CAE-positive cells from 25 random fields of view at 40× magnification. Additional sections were stained with hematoxylin and eosin (H&E) and eosinophils were morphometrically identified from 25 random fields of view at 400× magnification and reported as the average cell number per high powered field (HPF).
Analysis of cytokine expression
Spleens and mesenteric lymph nodes (mLNs) were dissected and manually disrupted to generate single cell suspensions. Red blood cells were depleted from splenocytes with ACK lysing buffer (Lonza, Basel, Switzerland). Lymphocytes were plated at 8 × 105 cells per well in 96-well plates. Cells were cultured ex vivo ± peanut extract (5 μg/ml). After 72 h, cytokine secretion was measured in supernatants using a Luminex Multiplex detection system (Millipore). Data were determined as [peanut stimulated] – [unstimulated] = Total (pg/ml) for each cytokine (mean of duplicate determinations).
Measurement of serum antibodies
Serum samples were obtained by cardiac puncture 1 day after the final oral peanut challenge. Peanut-specific IgE, IgG1, IgG2a and IgG2b antibodies were determined in serially diluted serum using peanut-coated 96-well plates and alkaline phosphatase-conjugated detection antibodies as described previously [26]. For quantification of peanut-specific IgE in IgG-depleted serum, serum samples were pooled from groups of two mice prior to IgG depletion in order to have sufficient sample volume. Serum samples were run over Nab Protein G spin columns (Thermo Scientific) to bind and remove IgG. >95% depletion of IgG was confirmed in each sample prior to determining peanut-specific IgE as described above.
Statistics
Statistical comparisons were assessed by the nonparametric Mann–Whitney test using GraphPad Prism version 8. p < 0.05 was considered significant.
Results
MNs are uniformly coated with peanut proteins & deliver allergen into the skin
The authors first assessed the coating of peanut proteins onto MNs and their ability to deliver the peanut protein into the skin. Figure 1A provides an overview of the approach of using allergen coated-MNs to deliver peanut proteins into the skin. Figure 1B shows a portion of the MN patch before insertion. Figure 1C is an image of the MNs after allergen coating. It can be noted that the coatings are localized only on the MN shafts, and the base of the patch is not contaminated with coatings. This allows for control and consistency over the dose being delivered into the skin. Indeed, delivery efficiency analysis in mouse skin showed that 65.1% of the coated mass was delivered into the skin, 28.1% remained on the MNs and 6.8% remained on the skin surface.
Figure 1. . Microneedles coated with peanut allergen and their delivery efficiency.
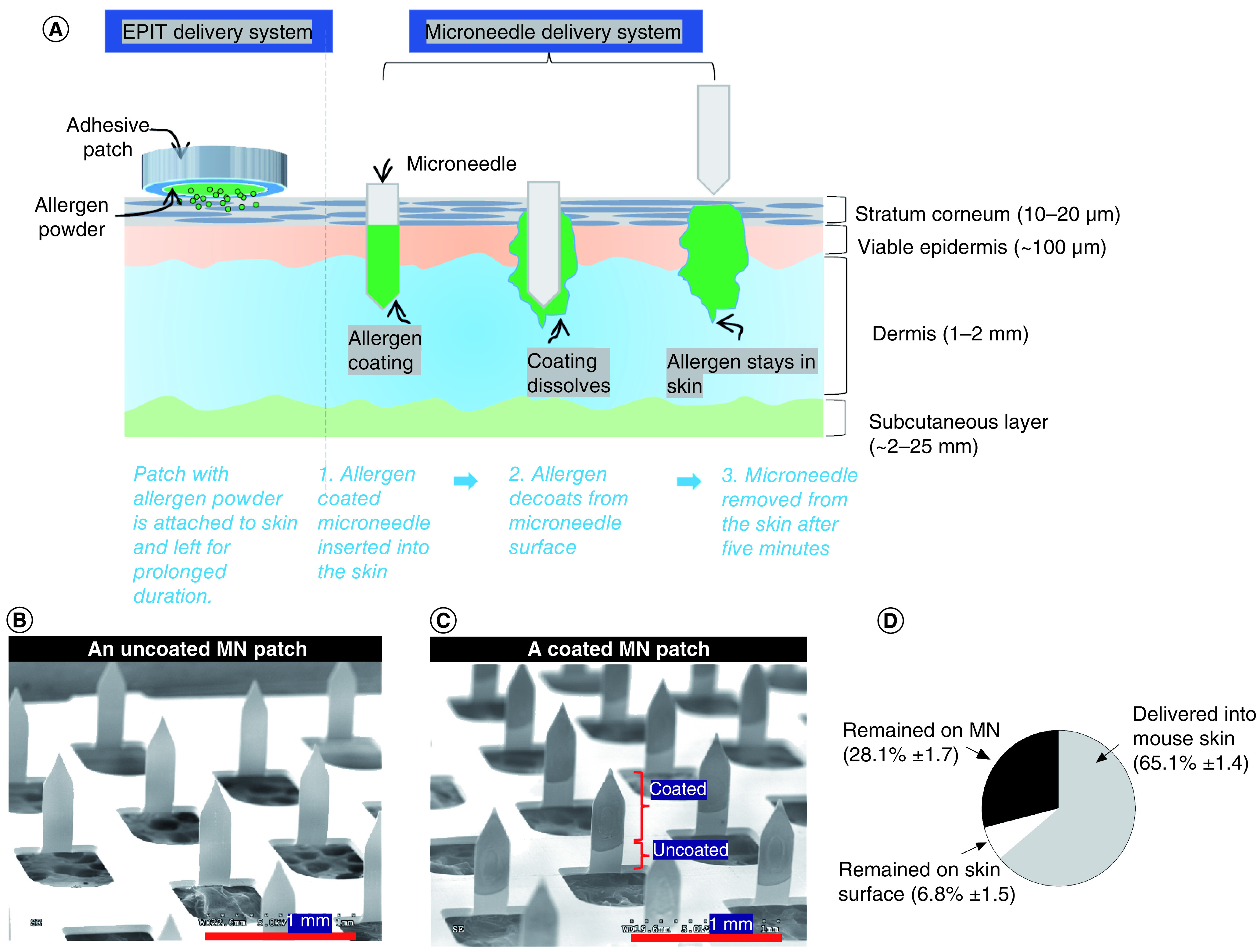
(A) Schematic representation of MN-mediated allergen delivery into skin layers (for reference, layer thicknesses represent human skin). Scanning electron microscopy images of (B) an uncoated patch and (C) a peanut protein-coated MN patch. (D) Delivery efficiency of peanut protein conjugated with fluorescein isothiocyanate into mouse skin.
MN: Microneedle.
Investigation of allergic reactivity following PN-MN treatment of peanut-sensitized mice
This MN technology delivers low μg quantities of allergen into the skin, penetrating the stratum corneum and epidermis (Figure 1). The low quantity of allergen should be shielded from interaction with effector cells. However, the potential exists for allergic reactions in sensitized animals. Therefore, the authors first confirmed that application of PN-MNs did not trigger reactivity in peanut-sensitized mice. Mice were sensitized to peanut through oral gavage with peanut extract and cholera toxin weekly for 6 weeks. Two weeks following the last sensitization, the mice were treated with PN-MNs, OVA-coated MNs (OVA-MNs) or epicutaneous application of peanut or PBS and monitored for symptoms of allergic reactions following application. Because application was performed under anesthesia, all mice experienced some degree of temperature loss following the procedure, which returned to baseline within 60 min; however, all mice, including those treated with PBS only, experienced the same drop in body temperature (Supplemental Figure 1A). These data suggest that all changes in body temperature were due to the expected effects of anesthesia. No mice displayed symptoms or mast cell degranulation (MCPT-1 release) that would indicate allergic reactions (Supplemental Figure 1B & C). The application sites also demonstrated no signs of inflammation. These data demonstrate that, like PN-EPIT, PN-MNs do not induce adverse events in sensitized mice.
Effects of immunotherapy in peanut-sensitized mice
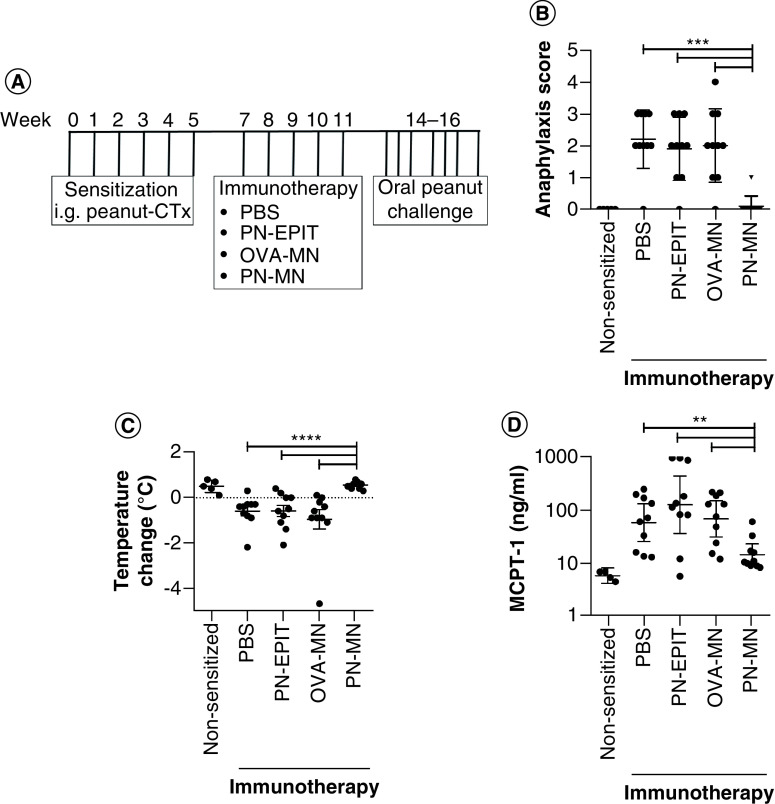
In order to compare PN-MN treatment with PN-EPIT, the authors tested a time course of 5, 8 and 12 weeks of PN-EPIT treatment for the ability to suppress reactivity to oral peanut challenge. Mice were orally sensitized to peanut, followed by weekly epicutaneous applications of peanut extract or PBS (sham) on shaved, intact skin. Significant suppression of reactivity was observed following 8 or 12 weeks of PN-EPIT; however, significant effects were not observed following 5 weeks of PN-EPIT (Supplementary Figure 2). In contrast, in their previous study the authors had observed that PN-MNs can provide protection against oral peanut challenge in peanut-sensitized mice after just 3 weeks of treatment (once-a-week treatment). Therefore, the authors decided to directly compare the shorter treatment times (5 weeks), since this schedule was not sufficient to produce effects with PN-EPIT alone, providing a therapeutic window to see improvement with PN-MNs. Mice were treated as described in Figure 2A and challenged orally with peanut to determine protection against IgE-mediated food allergic reactions [21,22,28,31]. All sensitized control mice displayed symptoms of allergic reactions such as swelling of the eyes/snout, wheezing and body temperature loss. Mice that were treated with PN-MNs were significantly protected from reactivity, with 90% of the mice displaying no symptoms (Figure 2B & C). MCPT-1 release into the serum was quantified as a marker of mast cell degranulation. Similar to the other outcomes of reactogenicity, PN-MN-treated mice had significant reductions in MCPT-1. This suppression of reactivity was allergen-specific, as treatment with MNs coated with an irrelevant antigen (OVA-MNs) did not produce any effects. Five PN-EPIT applications were not sufficient to desensitize the mice. PN-EPIT-treated mice displayed reductions in body temperature and increased clinical symptoms and MCPT-1 release similar to those of sensitized control mice.
Figure 2. . Effects of peanut-coated microneedles on allergic reactivity to oral peanut challenge.
(A) Experimental schedule. Mice were sensitized orally with peanut and cholera toxin weekly for 6 weeks. 2 weeks later, sensitized mice received 5 weekly treatments with PBS (sensitized control), peanut (PN-EPIT), OVA-MNs or PN-MNs. Mice were challenged orally with peanut. (B) Symptoms of anaphylaxis and (C) temperature change were monitored for 60 min after the final challenge. (D) Levels of mast cell protease-1 in the serum 60 min after the final challenge were determined by ELISA. n = 10 mice/group. Data are presented as mean ± standard error of the mean.
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
MN: Microneedle; OVA: Ovalbumin; OVA-MNs: OVA-coated MNs; PBS: Phosphate-buffered saline; PN-EPIT: Epicutaneous peanut protein immunotherapy; PN-MNs: Peanut-coated MNs.
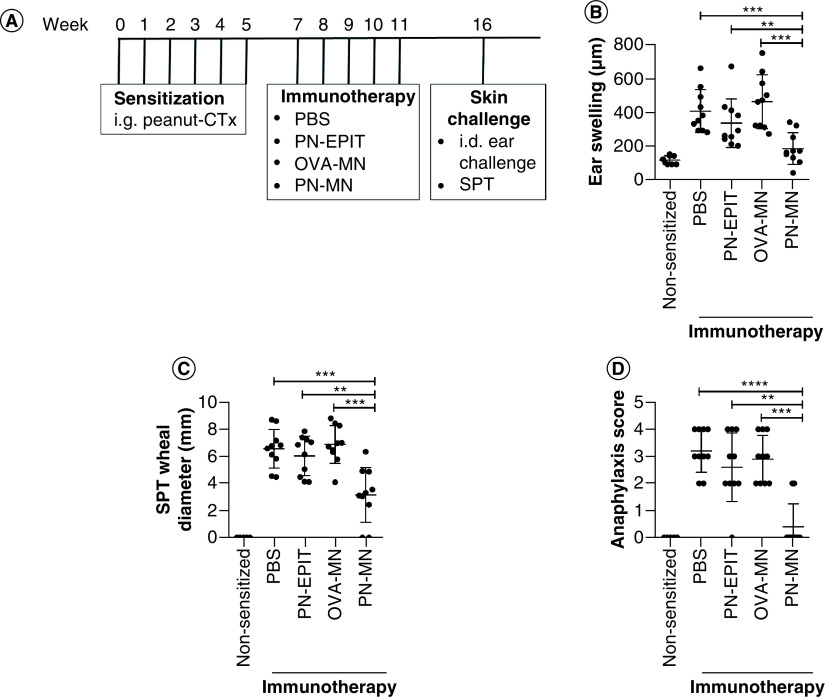
Mice were also challenged intradermally into the ear as another method of observing IgE-mediated reactivity. Edema in the ear at 1 h after intradermal injection has been demonstrated to be IgE-mediated, as local cutaneous anaphylaxis is mediated by the cross-linking of IgE on the surface of mast cells and basophils present in the skin [32,33]. Similar to the results obtained with oral challenge, intradermal injection of peanut caused significant swelling of the ears in sensitized mice, and this was largely prevented in PN-MN-treated mice (Figure 3B). The authors also determined the effect of reactogenicity at the local site of the immunotherapy. Peanut was injected into the skin on the back at the same location where MNs had been applied. Peanut-sensitized mice developed significant swelling at the injection site, and this was significantly reduced in PN-MN-treated mice (Figure 3B). Mice were also monitored for allergic reactivity following intradermal challenge, as other studies have reported systemic reactivity following challenge via this route [34–36]. This injection caused severe anaphylaxis in sensitized mice, but reactivity was significantly reduced in mice that had been treated with PN-MNs, with 80% of the mice having no reaction to the allergen injection (Figure 3D). Overall, these data demonstrate that PN-MNs suppress the reactogenicity of peanut-sensitized mice, resulting in protection from IgE-mediated reactions from allergen delivered via multiple routes. In contrast, PN-EPIT with an identical frequency of treatment did not produce significant protection.
Figure 3. . Effects of peanut-coated microneedles on acute allergic skin response.
(A) Experimental schedule. Mice were sensitized orally with peanut and cholera toxin weekly for 6 weeks. 2 weeks later, sensitized mice received 5 weekly treatments with PBS (sensitized control), peanut (PN-EPIT), OVA-MNs or PN-MNs. (B) Mice were challenged intradermally into the ear with peanut, and ear swelling was measured 1 h after challenge. (C & D) Mice were challenged intradermally into the skin on the back and (C) the wheal diameter was measured 20 min after injection. (D) Symptoms of anaphylaxis were monitored for 60 min after challenge. n = 10 mice/group. Data are presented as mean ± standard error of the mean.
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
MN: Microneedle; OVA: Ovalbumin; OVA-MNs: OVA-coated MNs; PBS: Phosphate-buffered saline; PN-EPIT: Epicutaneous peanut protein immunotherapy; PN-MNs: Peanut-coated MNs.
Cutaneous delivery of peanut with MNs results in modulation of peanut-specific humoral & cellular immune responses
The mechanisms of desensitization to food allergens are not completely understood; however, correlates of protection include suppression of Th2 cytokines and induction of Th1 and regulatory responses [37]. To determine the effect of PN-MN treatment on T cell polarization, peanut-specific cytokine secretion was determined in splenocytes and gut-draining mLNs. PN-MN treatment significantly suppressed Th2 cytokines (IL-4 and IL-5) in lymphocytes from the mLN and increased IL-10 and IFN-γ (Figure 4) compared with control sensitized mice. IL-21, a cytokine that promotes allergic disease, was also significantly decreased in the mLN of PN-MN-treated mice. Similar results were obtained from splenocytes, with significant decreases in IL-4, IL-5 and IL-21 and increased IFN-γ. IL-10 secretion was not increased in the spleen, suggesting that induction of IL-10 may be restricted to the local site of allergen challenge, while modulation of the Th1/Th2 bias is more systemic. Cytokine secretion was largely unchanged in mice that were treated with OVA-MNs or PN-EPIT. The notable exception was a reduction in IL-4 following PN-EPIT. Taken together, these data demonstrate that PN-MN treatment modulates the peanut-specific cellular immune response, resulting in suppression of Th2 immunity and induction of IFN-γ and local IL-10.
Figure 4. . Modulation of the peanut-specific cellular immune response by peanut-coated microneedles.
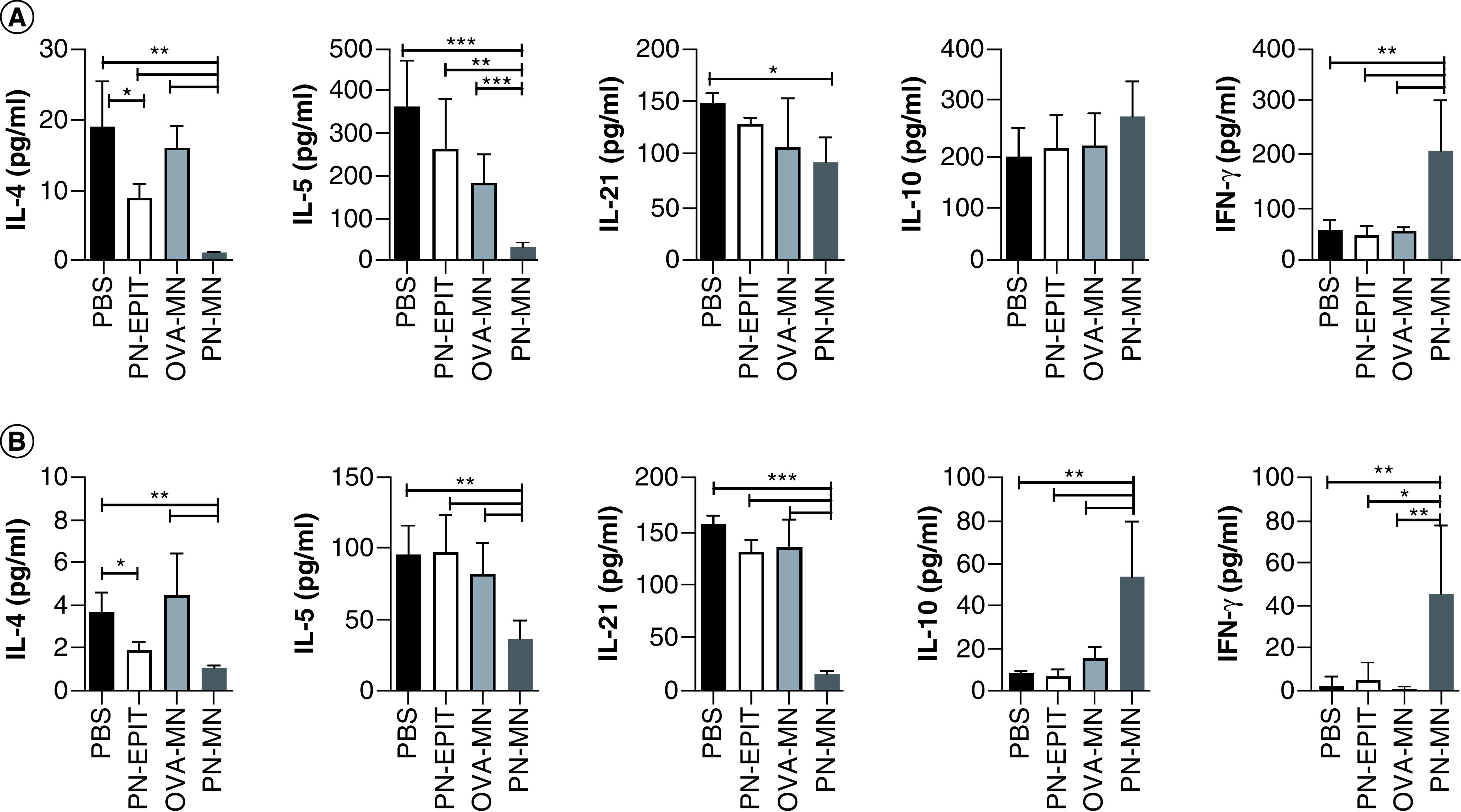
Cellular recall immune responses to peanut protein were measured in lymphocytes harvested from the (A) spleen and (B) mesenteric lymph node, following the experimental design shown in Figure 1. Lymphocytes were harvested and restimulated ex vivo with peanut for 72 h. Cytokine secretion was measured in cell culture supernatants using a Luminex multiplex assay. Cytokine production has been normalized to matched control unstimulated lymphocyte cultures from each individual animal and tissue. n = 10 mice/group. Data are presented as mean ± standard error of the mean.
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
MN: Microneedle; OVA: Ovalbumin; OVA-MNs: OVA-coated MNs; PBS: Phosphate-buffered saline; PN-EPIT: Epicutaneous peanut protein immunotherapy; PN-MNs: Peanut-coated MNs.
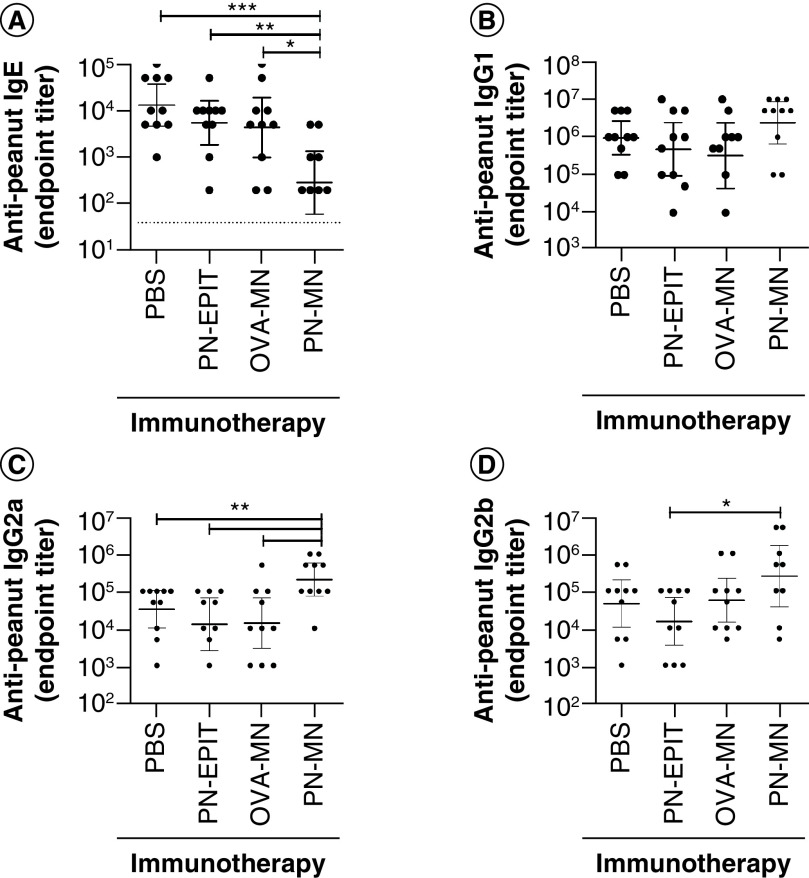
Allergen-specific immunotherapies often change the humoral immune response, resulting in modulation of antibody subclasses. PN-MN treatment did not alter peanut-specific IgG1 antibodies; however, IgG2a and IgG2b were significantly increased compared with sensitized control mice (Figure 5). The most significant change in antibodies was a 2-log reduction in peanut-specific IgE in PN-MN-treated mice (Figure 5A). Because peanut-specific IgG titers are >2 logs higher than IgE, the authors confirmed that the lower IgE titers were not due to technical limitations caused by competition of IgG for binding to the peanut antigens in the ELISA assay [38]. Peanut-specific IgE was quantified from serum that had been depleted of >95% of IgG to minimize competition for IgE binding to peanut proteins. While IgG removal resulted in an approximately two- to three-fold increase in the measured levels of peanut-specific IgE, this was true for all treatment groups, and the significant reduction in IgE in the mice that received PN-MNs was confirmed (Supplementary Figure 3). As IgE bound to FcεR1 on effector cells is responsible for the initiation of allergic reactions, this strong reduction of serum IgE may demonstrate a unique aspect of this approach for suppressing peanut allergy.
Figure 5. . Characterization of the peanut-specific antibody repertoire following treatment with peanut-coated microneedles.
Peanut-specific (A) IgE, (B) IgG1, (C) IgG2a and (D) IgG2b antibody titers were determined in the serum collected 1 day after the final challenge. n = 10 mice/group. Data are presented as mean ± standard error of the mean.
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
MN: Microneedle; OVA: Ovalbumin; OVA-MNs: OVA-coated MNs; PBS: Phosphate-buffered saline; PN-EPIT: Epicutaneous peanut protein immunotherapy; PN-MNs: Peanut-coated MNs.
Protection conferred by PN-MNs is associated with decreased tissue mast cell accumulation
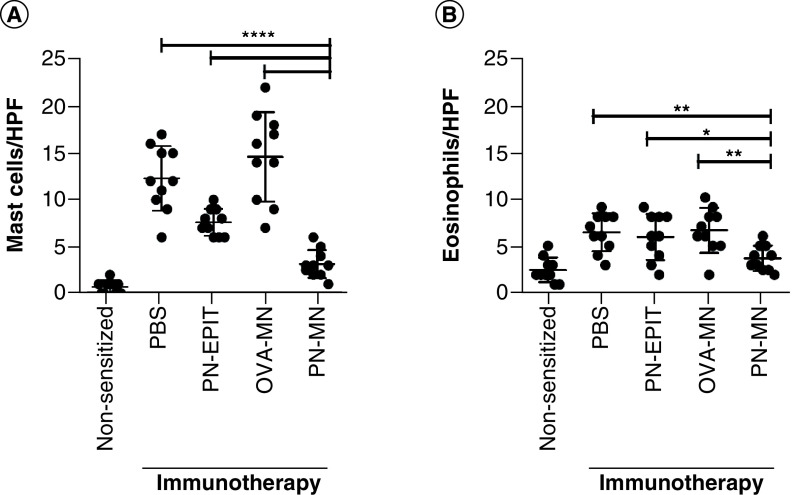
Mast cells are one of the primary effector cells that accumulate in the intestine following oral allergen exposure and are a key effector cell driving reactivity in this mouse model [21,39]. Peanut-sensitized mice had significant mast cell infiltration in the intestine compared with naive mice (Figure 6A). No significant differences were observed following treatment with OVA-MNs. There was also a significant decrease in mast cells in the intestine of mice treated with PN-EPIT compared with control mice; however, PN-MN treatment resulted in an even greater reduction. There was a modest, approximately twofold increase in eosinophils in the intestine of sensitized mice after challenge, and this was significantly suppressed only in mice treated with PN-MNs (Figure 6B). Overall, these data demonstrate increased accumulation of effector cells into the small intestine of sensitized mice that is significantly blocked in mice that received the PN-MNs.
Figure 6. . Effects of peanut-coated microneedles on effector cell infiltration in the intestine.
Following oral challenge with peanut, small intestines were stained by (A) chloroacetate esterase staining to visualize mast cells and (B) hematoxylin and eosin to visualize eosinophils. (A) Mast cells were enumerated by counting the number of cells per field of vision under 40× magnification. (B) Eosinophils were enumerated by counting the number of cells per field of vision under 1000× magnification. 25 random fields of vision were counted for each sample and reported as the average per high powered field. n = 10 mice/group. Data are presented as mean ± standard error of the mean.
*p < 0.05.
MN: Microneedle; OVA: Ovalbumin; OVA-MNs: OVA-coated MNs; PBS: Phosphate-buffered saline; PN-EPIT: Epicutaneous peanut protein immunotherapy; PN-MNs: Peanut-coated MNs.
Discussion
The skin is a critical component of the immune system, involved in both host defense and tolerance, and is densely populated with immune cells that are important for the initiation of immune responses [5]. Delivery of antigen via intact skin has been shown to induce immunoregulatory events, including Tregs, in mice and to provide some degree of desensitization in both mice and humans [6,7,40,41]. EPIT also may improve the induction of Tregs compared with other routes of delivery for allergen-specific immunotherapy ; however, these results are based on mouse experiments [42]. These attributes make cutaneous allergen delivery a prime target for the development of immunotherapies for food allergies.
EPIT has been under development over the past two decades, with some demonstrated efficacy in clinical trials [43]. The Viaskin peanut patch is the most well-studied EPIT. When placed on the skin, the Viaskin patch provides an occlusive chamber in which peanut protein on the patch combines with moisture from the skin to facilitate the uptake of allergen. However, this process requires uptake of peanut protein through the skin, which is largely inefficient because of the tight barrier provided by the stratum corneum [44,45]. Protein uptake therefore may be a limiting factor for the success of EPIT, as studies have suggested that the amount of protein delivered is critical for efficacy [12].
The stratum corneum is the outermost layer of the skin, which provides a physiological barrier that impedes passive diffusion of antigen into and through non-damaged skin [46], and permeability varies with age and location of the skin [14,47,48]. The most notable difference is between infant and adult skin, with adult skin providing a stronger barrier [49]. Because Viaskin peanut was demonstrated to have increased efficacy in younger patients, differences in barrier may be responsible for the variable response rate to EPIT [12,13]. This suggests that immunotherapy delivered via the skin may be improved by increasing allergen uptake in a controlled manner to ensure consistency.
Multiple approaches are under development to improve cutaneous delivery of antigen. Lasers have been used to introduce micropores into the stratum corneum, which improves antigen uptake and trafficking of dendritic cells (DCs) loaded with antigen to the draining lymph nodes [50–52]. Thus, increasing allergen delivery in the skin may improve cutaneous immunotherapy. Another approach to improving allergen delivery into the skin is to use MN arrays, which also create micropores to deliver antigen [46,53,54]. Multiple types of MN arrays, including dissolvable polymer needles and those coated with antigen, have been developed for antigen delivery into the skin [15,18,24,55–57]. MNs are minimally invasive and well tolerated by patients, who reported pain-free application [58–60]. Because this process directly delivers antigen into the skin, there is no variability due to strength of the barrier or requirement for passive diffusion of the antigen. This allows for increased antigen uptake with controlled doses of allergen. The prevalence of immune cells in the skin also yields immune responses from MN delivery of antigen that are similar to intramuscular antigen injection but require 100-fold less antigen [61,62].
In the mouse experiments described here, no erythema, inflammation, reactivity or mast cell degranulation was observed following MN insertion. These data demonstrate that this targeted immunotherapy approach to delivering peanut allergen into the skin with MNs is safe and well tolerated in peanut-sensitized mice. Clinical trials will be critical for demonstrating if similar safety profiles occur in humans.
In contrast to OIT and EPIT, which require daily allergen exposure to induce clinical benefit, in the present study, mice were treated with PN-MNs once weekly for 5 weeks. Significant efficacy was achieved for PN-MNs, as demonstrated by protection from clinical symptoms of anaphylaxis, temperature loss and mast cell degranulation. In contrast, the same number (five) of applications of PN-EPIT did not provide significant suppression of allergic reactivity. However, there was a trend toward reduced mast cell accumulation in the intestine following PN-EPIT, and IL-4 production was significantly reduced. While 5 weeks of PN-EPIT was suboptimal, increasing the duration of treatment to 8–12 weeks significantly improved efficacy (Supplemental Figure 2), similar to what has been reported [44,45]. This highlights the advantage of using MNs to deliver peanut protein. The increase in therapeutic efficacy with nearly tenfold reduced allergen (11 μg applied via MNs vs 100 μg applied epicutaneously) is likely due to increased efficiency in allergen delivery with the MNs compared with EPIT. It is unknown how much allergen is actually delivered into the skin with EPIT. The ability to reduce the amount of allergen as well as the frequency and duration of treatment may allow for reduced burden on patients. This may present an additional improvement upon allergen immunotherapies that require daily allergen exposure. It should be noted that the MN array used in this study measured 1 cm × 1 cm and contained 50 individual MNs. This MN array design is sufficient to deliver tens to hundreds of micrograms of material and can be used in human patients without increasing the MN array size [63].
Protection from allergic reactivity conferred by the peanut-coated MNs was associated with a strong suppression of peanut-specific Th2 immune responses and IgE. These Th2 immune responses are critical for the maintenance of effector cells, such as mast cells, in the tissue. Indeed, tissue mast cell accumulation was also inhibited in the mice that were treated with the peanut-coated MNs. The concomitant induction of Th1 immune responses suggests that peanut MNs may not be simply desensitizing, as desensitization is mostly associated with an overall suppression of the allergen-specific immune response.
The duration of sustained unresponsiveness observed here may also suggest more durable immune modulation compared with traditional desensitization. Both clinical protection outcomes and modulation of the peanut-specific cytokine response were sustained over more than 5 weeks. This duration of sustained unresponsiveness also suggests a more rapid induction of durable immune modulation than is typical for EPIT, which may require years on therapy to achieve durable protection [64]. Additionally, the length of time between immunotherapy and challenge may be responsible for the lack of efficacy of PN-EPIT, as prior studies of EPIT have not assessed sustained unresponsiveness in mice [44,45]. Future studies will more fully investigate the total duration of protection achieved following immunotherapy with PN-MNs. However, PN-MNs present an effective strategy to enhance peanut-specific immunotherapy via the skin to confer protection against reactivity to peanut in mice. Future clinical studies will be important to determine the efficacy of this approach in humans.
Conclusion
Allergen-coated MNs may present a novel mechanism to improve cutaneous immunotherapy for the treatment of food allergy. PN-MNs are safe and well tolerated in peanut-sensitized mice and provide efficacy following only five weekly applications, which was not a sufficient duration of therapy to produce protective effects for PN-EPIT. Protection and immune modulation were maintained for at least 5 weeks following the final MN application. Taken together, the data presented here demonstrate a potential advantage of delivering peanut into the skin with MNs to improve immune modulation and efficacy compared with PN-EPIT. Thus, MNs may be a useful strategy to develop next-generation therapeutics for allergen-specific immunotherapy through the skin.
Summary points.
Despite the high prevalence of food allergies in the developed world, treatment options are limited.
Allergen-specific immunotherapies are the most well-studied therapeutics for food allergies.
Immunotherapy through the skin has shown promise for treating food allergies; however, the efficacy of epicutaneous immunotherapy has been varied, with the phase III clinical trial of epicutaneous peanut protein immunotherapy not reaching its primary clinical end point.
Because the skin provides a tight barrier, methods to improve allergen uptake into the skin may improve efficacy.
The ability of peanut-coated microneedles (PN-MNs) to enhance desensitization to peanut was tested in a mouse model of IgE-mediated peanut allergy.
PN-MN application to peanut-sensitized mice did not cause allergic reactivity.
Five weekly applications of PN-MNs significantly suppressed allergic reactivity. Comparatively, five weekly applications of epicutaneous peanut protein immunotherapy did not significantly suppress disease.
Suppression of allergic reactivity by PN-MNs was associated with decreased Th2-polarized immune responses and increased Th1 and IL-10.
PN-MN treatment significantly reduced peanut-specific IgE in the serum as well as mast cell infiltration in the small intestine.
PN-MNs induced significantly enhanced efficacy compared with epicutaneous peanut protein immunotherapy, allowing for immune modulation and suppression of reactivity with only five treatments.
Based on these data from a mouse model, PN-MNs have the potential to improve food allergen immunotherapy through the skin. Future studies, including clinical studies, are warranted.
Supplementary Material
Acknowledgments
The authors acknowledge the University of Michigan Immunology Core and J Whitfield for assistance with cytokine measurements. The authors thank S Boese for his help in taking the scanning electron microscopy pictures.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/imt-2021-0206
Author contributions
SR Patel, V Zarnitsyn, HS Gill, JR Baker and JJ O'Konek designed the studies. JJ Landers, KW Janczak, AK Shakya, V Zarnitsyn and JJ O'Konek performed experiments and analyzed data. JJ Landers, V Zarnitsyn, SR Patel, JR Baker, HS Gill and JJ O'Konek prepared the manuscript. All authors read and approved the final version of the manuscript.
Financial & competing interests disclosure
Research reported in this publication was supported in part by the Michigan Food Allergy Research Accelerator (M-FARA) (JR Baker and JJ O'Konek) and the Vondell Family Research Fund (JR Baker and JJ O'Konek). Research was also supported the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R42AI143011 (SR Patel) and R01AI135197 (HS Gill) and the Whitacre Endowed Chair in Science and Engineering (HS Gill). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
HS Gill and AK Shakya are co-inventors on a patent related to coated microneedles for allergen immunotherapy. Moonlight Therapeutics is pursuing this technology for developing microneedles for peanut and other food allergy immunotherapies. HS Gill and AK Shakya have equity in Moonlight Therapeutics. SR Patel and V Zarnitsyn are employees of Moonlight Therapeutics and have equity in the company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional approval for all animal experimental investigations. All animal procedures were approved by the University of Michigan Institutional Animal Care and Use Committee.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gupta RS, Warren CM, Smith BM et al. Prevalence and severity of food allergies among US adults. JAMA Netw. Open 2(1), e185630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta RS, Warren CM, Smith BM et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics 142(6), e20183835 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim EH, Burks AW. Food allergy immunotherapy: oral immunotherapy and epicutaneous immunotherapy. Allergy 75(6), 1337–1346 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Palisade Group of Clinical Investigators, Vickery BP, Vereda A et al. AR101 oral immunotherapy for peanut allergy. N. Engl. J. Med. 379(21), 1991–2001 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 14(6), 417–428 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Spina L, Weisskopf M, Von Moos S, Graf N, Kundig TM, Senti G. Comparison of microneedles and adhesive-tape stripping in skin preparation for epicutaneous allergen delivery. Int. Arch. Allergy Immunol. 167(2), 103–109 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Dioszeghy V, Mondoulet L, Dhelft V et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J. Immunol. 186(10), 5629–5637 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Guttman-Yassky E, Zhou L, Krueger JG. The skin as an immune organ: tolerance versus effector responses and applications to food allergy and hypersensitivity reactions. J. Allergy Clin. Immunol. 144(2), 362–374 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Akdis CA, Akdis M. Advances in allergen immunotherapy: aiming for complete tolerance to allergens. Sci. Transl. Med. 7(280), 280ps286 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J. Allergy Clin. Immunol. 99(6 Pt 1), 744–751 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Mondoulet L, Dioszeghy V, Thebault C, Benhamou PH, Dupont C. Epicutaneous immunotherapy for food allergy as a novel pathway for oral tolerance induction. Immunotherapy 7(12), 1293–1305 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Sampson HA, Shreffler WG, Yang WH et al. Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: a randomized clinical trial. JAMA 318(18), 1798–1809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischer DM, Greenhawt M, Sussman G et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: the PEPITES randomized clinical trial. JAMA 321(10), 946–955 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This phase III clinical study of epicutaneous immunotherapy with peanut did not meet the primary end point for efficacy.
- 14.Mack MC, Chu MR, Tierney NK et al. Water-holding and transport properties of skin stratum corneum of infants and toddlers are different from those of adults: studies in three geographical regions and four ethnic groups. Pediatr. Dermatol. 33(3), 275–282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J. Control Rel. 117(2), 227–237 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakya AK, Lee CH, Gill HS. Cutaneous vaccination with coated microneedles prevents development of airway allergy. J. Control Rel. 265, 75–82 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakya AK, Lee CH, Gill HS. Coated microneedle-based cutaneous immunotherapy prevents Der p 1-induced airway allergy in mice. J. Allergy Clin. Immunol. 142(6), 2007–2011.e2003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is the first study evaluating allergen-coated microneedles for prevention of airway allergy development in a mouse model.
- 18.Shakya AK, Ingrole RSJ, Joshi G et al. Microneedles coated with peanut allergen enable desensitization of peanut sensitized mice. J. Control Rel. 314, 38–47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is the first study using peanut allergen-coated microneedles demonstrating the desensitization potential of microneedles in a mouse model of peanut allergy.
- 19.Li XM, Serebrisky D, Lee SY et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J. Allergy Clin. Immunol. 106(1 Pt 1), 150–158 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Srivastava KD, Siefert A, Fahmy TM, Caplan MJ, Li XM, Sampson HA. Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J. Allergy Clin. Immunol. 138(2), 536–543 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Ahrens R, Osterfeld H, Wu D et al. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. Am. J. Pathol. 180(4), 1535–1546 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper characterizes a mouse model of IgE-dependent food allergy to oral antigen exposure.
- 22.Chen CY, Lee JB, Liu B et al. Induction of interleukin-9-producing mucosal mast cells promotes susceptibility to IgE-mediated experimental food allergy. Immunity 43(4), 788–802 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes EE, Groschwitz K, Abonia JP et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med. 205(4), 897–913 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shakya AK, Gill HS. A comparative study of microneedle-based cutaneous immunization with other conventional routes to assess feasibility of microneedles for allergy immunotherapy. Vaccine 33(33), 4060–4064 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Orgel K, Kulis M. A mouse model of peanut allergy induced by sensitization through the gastrointestinal tract. Methods Mol. Biol. 1799, 39–47 (2018). [DOI] [PubMed] [Google Scholar]
- 26.O'Konek JJ, Landers JJ, Janczak KW et al. Nanoemulsion adjuvant-driven redirection of TH2 immunity inhibits allergic reactions in murine models of peanut allergy. J. Allergy Clin. Immunol. 141(6), 2121–2131 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Finkelman FD. Anaphylaxis: lessons from mouse models. J. Allergy Clin. Immunol. 120(3), 506–515 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Noah TK, Knoop KA, Mcdonald KG et al. IL-13-induced intestinal secretory epithelial cell antigen passages are required for IgE-mediated food-induced anaphylaxis. J. Allergy Clin. Immunol. 144(4), 1058–1073.e1053 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J. Cell Biol. 135(1), 279–290 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Konek JJ, Landers JJ, Janczak KW et al. Intranasal nanoemulsion vaccine confers long-lasting immunomodulation and sustained unresponsiveness in a murine model of milk allergy. Allergy 75(4), 872–881 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Lee JB, Chen CY, Liu B et al. IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J. Allergy Clin. Immunol. 137(4), 1216–1225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katayama I, Tanei R, Yokozeki H, Nishioka K, Dohi Y. Induction of eczematous skin reaction in experimentally induced hyperplastic skin of Balb/C mice by monoclonal anti-DNP IgE antibody: possible implications for skin lesion formation in atopic dermatitis. Int. Arch. Allergy Appl. Immunol. 93(2–3), 148–154 (1990). [DOI] [PubMed] [Google Scholar]
- 33.Evans H, Killoran KE, Mitre E. Measuring local anaphylaxis in mice. J. Vis. Exp. (92), e52005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vonk MM, Wagenaar L, Pieters RHH et al. The efficacy of oral and subcutaneous antigen-specific immunotherapy in murine cow's milk- and peanut allergy models. Clin. Transl. Allergy 7, 35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schouten B, Van Esch BC, Hofman GA, Van Den Elsen LW, Willemsen LE, Garssen J. Acute allergic skin reactions and intestinal contractility changes in mice orally sensitized against casein or whey. Int. Arch. Allergy Immunol. 147(2), 125–134 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Hogenkamp A, Knippels LM, Garssen J, Van Esch BC. Supplementation of mice with specific nondigestible oligosaccharides during pregnancy or lactation leads to diminished sensitization and allergy in the female offspring. J. Nutr. 145(5), 996–1002 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Hardy LC, Smeekens JM, Kulis MD. Biomarkers in food allergy immunotherapy. Curr. Allergy Asthma Rep. 19(12), 61 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Lehrer SB, Reish R, Fernandes J, Gaudry P, Dai G, Reese G. Enhancement of murine IgE antibody detection by IgG removal. J. Immunol. Methods 284(1–2), 1–6 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Arias K, Alvarez D et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J. Immunol. 179(10), 6696–6703 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Jones SM, Agbotounou WK, Fleischer DM et al. Safety of epicutaneous immunotherapy for the treatment of peanut allergy: a phase 1 study using the Viaskin patch. J. Allergy Clin. Immunol. 137(4), 1258–1261.e1210 (2016). [DOI] [PubMed] [Google Scholar]; •• This study demonstrated the safety of peanut allergen immunotherapy via the skin route.
- 41.Yasuda T, Ura T, Taniguchi M, Yoshida H. Intradermal delivery of antigens enhances specific IgG and diminishes IgE production: potential use for vaccination and allergy immunotherapy. PLoS One 11(12), e0167952 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dioszeghy V, Mondoulet L, Puteaux E et al. Differences in phenotype, homing properties and suppressive activities of regulatory T cells induced by epicutaneous, oral or sublingual immunotherapy in mice sensitized to peanut. Cell. Mol. Immunol. 14(9), 770–782 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senti G, Von Moos S, Tay F, Graf N, Johansen P, Kundig TM. Determinants of efficacy and safety in epicutaneous allergen immunotherapy: summary of three clinical trials. Allergy 70(6), 707–710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Dupont C, Benhamou PH. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin. Exp. Allergy 40(4), 659–667 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Mondoulet L, Dioszeghy V, Vanoirbeek JA, Nemery B, Dupont C, Benhamou PH. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int. Arch. Allergy Immunol. 154(4), 299–309 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Lv Y, Qi J, Zhu Q, Lu Y, Wu W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov. Today 23(1), 181–186 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Kong F, Galzote C, Duan Y. Change in skin properties over the first 10 years of life: a cross-sectional study. Arch. Dermatol. Res. 309(8), 653–658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walters RM, Khanna P, Chu M, Mack MC. Developmental changes in skin barrier and structure during the first 5 years of life. Skin Pharmacol. Physiol. 29(3), 111–118 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Stamatas GN, Nikolovski J, Luedtke MA, Kollias N, Wiegand BC. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr. Dermatol. 27(2), 125–131 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Kumar MNK, Zhou C, Wu MX. Laser-facilitated epicutaneous immunotherapy to IgE-mediated allergy. J. Control Rel. 235, 82–90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herve PL, Dhelft V, Plaquet C et al. Epidermal micro-perforation potentiates the efficacy of epicutaneous vaccination. J. Control Rel. 298, 12–26 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Machado Y, Duinkerken S, Hoepflinger V et al. Synergistic effects of dendritic cell targeting and laser-microporation on enhancing epicutaneous skin vaccination efficacy. J. Control Rel. 266, 87–99 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Ingrole RSJ, Azizoglu E, Dul M, Birchall JC, Gill HS, Prausnitz MR. Trends of microneedle technology in the scientific literature, patents, clinical trials and Internet activity. Biomaterials 267, 120491 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin. Hum. Vaccin. Immunother. 12(11), 2975–2983 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernando GJ, Chen X, Primiero CA et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J. Control Rel. 159(2), 215–221 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Pearson FE, Mcneilly CL, Crichton ML et al. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS One 8(7), e67888 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu Y, Kiran Kumar MN, Wu MX. Delivery of allergen powder for safe and effective epicutaneous immunotherapy. J. Allergy Clin. Immunol. 145(2), 597–609 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arya J, Henry S, Kalluri H, Mcallister DV, Pewin WP, Prausnitz MR. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials 128, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 24(7), 585–594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Study demonstrating the painless nature of microneedles arrays. One of the arrays in the pain study is similar to the one used in the current study with 50 microneedles.
- 60.Haq MI, Smith E, John DN et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed. Microdevices 11(1), 35–47 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Fernando GJ, Chen X, Prow TW et al. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS One 5(4), e10266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J. Infect. Dis. 201(2), 190–198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ingrole RSJ, Gill HS. Microneedle coating methods: a review with a perspective. J. Pharmacol. Exp. Ther. 370(3), 555–569 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown-Whitehorn TF, De Blay F, Spergel JM et al. Sustained unresponsiveness to peanut after long-term peanut epicutaneous immunotherapy. J. Allergy Clin. Immunol. Pract. 9(1), 524–526 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.