Abstract
We have recently reported an influenza virus neuraminidase inhibitor, RWJ-270201 (BCX-1812), a novel cyclopentane derivative discovered through structure-based drug design. In this paper, we compare the potency of three compounds, RWJ-270201, oseltamivir, and zanamivir, against neuraminidase enzymes from various subtypes of influenza. RWJ-270201 effectively inhibited all tested influenza A and influenza B neuraminidases in vitro, with 50% inhibitory concentrations of 0.09 to 1.4 nM for influenza A neuraminidases and 0.6 to 11 nM for influenza B neuraminidases. These values were comparable to or lower than those for oseltamivir carboxylate (GS4071) and zanamivir (GG167). RWJ-270201 demonstrated excellent selectivity (>10,000-fold) for influenza virus neuraminidase over mammalian, bacterial, or other viral neuraminidases. Oral administration of a dosage of 1 mg/kg of body weight/day of RWJ-270201 for 5 days (beginning 4 h preinfection) showed efficacy in the murine model of influenza virus infection as determined by lethality and weight loss protection. RWJ-270201 administered intranasally at 0.01 mg/kg/day in the murine influenza model demonstrated complete protection against lethality, whereas oseltamivir carboxylate and zanamivir at the same dose demonstrated only partial protection. In the delayed-treatment murine influenza model, oral administration of a 10-mg/kg/day dose of RWJ-270201 or oseltamivir (GS4104, a prodrug of GS4071) at 24 h postinfection showed significant protection against lethality (P < 0.001 versus control). However, when the treatment was delayed for 48 h, no significant protection was observed in either drug group. No drug-related toxicity was observed in mice receiving 100 mg/kg/day of RWJ-270201 for 5 days. These efficacy and safety profiles justify further consideration of RWJ-270201 for the treatment and prevention of human influenza.
Influenza is a respiratory infection associated with significant morbidity in the general population and mortality in elderly and high-risk patients. Influenza virus neuraminidase inhibitors, zanamivir and oseltamivir, have demonstrated efficacy in animal models of influenza virus infection (6, 10, 11, 15) and in studies in humans (2, 3, 4, 8) and were recently approved for treatment of influenza. Zanamivir is applied topically to the respiratory tract as an inhaled preparation because the drug is poorly absorbed orally. Oseltamivir is administered orally and may be associated with gastrointestinal tract-related adverse events (4). Also, a general concern in antiviral therapy is the occurrence of resistance. Currently, it is not known how important an issue this will be in the case of neuraminidase inhibitors. Combination therapy might be the future strategy for the treatment of influenza virus infection to potentiate the efficacy of the drugs and to minimize the risk of spreading resistant viruses. Hence, new antiviral agents that can be used to prevent and treat influenza virus infection are always desirable.
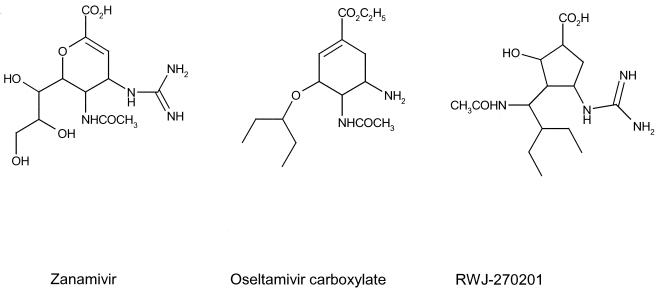
In an attempt to identify structurally novel and potent inhibitors of influenza virus neuraminidase, we have used structure-based drug design to synthesize a cyclopentane derivative, RWJ-270201 (1). RWJ-270201 has been shown to inhibit the growth of influenza virus in tissue culture (13) and demonstrated oral efficacy in a mouse influenza model (12). RWJ-270201 is currently undergoing clinical evaluation as an oral treatment for influenza in humans. The structures of the three compounds, RWJ-270201, zanamivir, and oseltamivir, included in this study are shown in Fig. 1. The present study was designed to compare the potency of these three compounds against neuraminidase enzymes from various subtypes of influenza (both A and B) virus. We investigated the specificity of RWJ-270201 as an influenza neuraminidase inhibitor by comparing its inhibition of neuraminidase from 23 different representative influenza strains, as well as against several noninfluenza neuraminidases. We present evidence that oral administration of RWJ-270201 in both prophylaxis and treatment models protects mice against the effects of influenza virus infection. A comparison of the efficacies of the three compounds by intranasal administration in the mouse influenza model is also presented.
FIG. 1.
Structures of compounds under investigation.
MATERIALS AND METHODS
Viruses.
The influenza viruses used in this study, together with their sources, are shown in Table 1.
TABLE 1.
Influenza viruses used in the study
| Virus | Type | Source |
|---|---|---|
| A/PR/8/34 | H1N1 | ATCC, USAa |
| A1/FM/1/47 | H1N1 | ATCC, USAa |
| A/NWS/33 | H1N1 | ATCC, USAa |
| A/Brazil/11/78 | H1N1 | Skehel, United Kingdomb |
| A/Taiwan/1/86 | H1N1 | Hayden, USAc |
| A2/Hong Kong/8/68 | H2N2 | ATCC, USAa |
| A/Singapore/1/57 | H2N2 | Memphis, Tenn., USAd |
| A/Tokyo/3/67 | H2N2 | Canberra, Australiae |
| A/Japan | H2N2 | ATCC, USAa |
| A/Aichi | H3N2 | ATCC, USAa |
| A/Belgium/2/81 | H3N2 | Skehel, United Kingdomb |
| A/Shangdong/09/93 | H3N2 | Logan, Utah, USAf |
| A/Virginia/88 | H3N2 | Hayden, USAc |
| A/Turkey/Mass/76A/Beijing/32/92 [R] | H6N2 | Valhalla, N.Y., USAg |
| B/Hong Kong/5/72 | B | ATCC, USAa |
| B/Hong Kong/5/72 | B | Hayden, USAc |
| B/Lee/40 | B | ATCC, USAa |
| B/Victoria/70 | B | Canberra, Australiae |
| B/Harbin/07/94 | B | Logan, Utah, USAf |
| B/Y177 | B | Pasteur Institute, Brussels, Belgiumh |
| B/Y253 | B | Pasteur Institute, Brussels, Belgiumh |
| B/Y263 | B | Pasteur Institute, Brussels, Belgiumh |
American Type Culture Collection, Manassas, Va.
Skehel, World Influenza Centre, Mill Hill, United Kingdom.
Frederic Hayden, University of Virginia, Charlottesville, Va.
Robert Webster, St. Jude Children's Hospital, Memphis, Tenn.
Graeme Laver, Australian National University, Canberra, Australia.
Robert Sidwell, Utah State University, Logan, Utah.
Bert Johannson, New York Medical College, Valhalla, N.Y.
Ferdinand Yane, Pasteur Institute, Brussels, Belgium.
Other neuraminidases.
Neuraminidases from Vibrio cholerae, Clostridium perfringens, and Newcastle disease virus were obtained from Sigma (St. Louis, Mo.). Parainfluenza virus was obtained from the American Type Culture Collection. Rat liver sialidase was partially purified from livers of Sprague-Dawley rats by using the method of Miyagi and Tsuiki (7).
Mice.
Specific-pathogen-free female BALB/c mice (10 to 19 g) were obtained from Charles River Laboratories (Raleigh, N.C.). They were quarantined 24 h prior to infection and were maintained on rodent diet from Harlan Teklad and tap water from the laboratory animal research center of BioCryst Pharmaceuticals, Inc.
Compounds and reagents.
RWJ-270201, oseltamivir, oseltamivir carboxylate, and zanamivir were synthesized by BioCryst Pharmaceuticals, Inc. (Birmingham, Ala.). Each compound was prepared in sterile 0.9% sodium chloride or 0.5% carboxymethyl cellulose for in vivo experiments. Ketamine (25 mg/ml; Merritt Veterinary Supplies, Birmingham, Ala.) and rompun (2.5 mg/ml; Phoenix Pharmaceuticals, Inc., St. Joseph, Mo.) were prepared in saline and administered intraperitoneally as anesthesia.
Neuraminidase assay.
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity (9). The substrate [2′-(4-methylumbelliferyl)-α-d-acetylneuraminic acid] is cleaved by neuraminidase to yield a fluorescent product that can be quantified. The assay mixture contained inhibitor at various concentrations and neuraminidase enzyme or virus suspension in 32.5 mM MES [2-(N-morpholino)-ethanesulfonic acid] buffer–4 mM calcium chloride (pH 6.5) and was incubated for 10 to 30 min. The reaction was started by the addition of the substrate. After incubation for 30 to 120 min (different times for different viruses), the reaction was terminated by adding 0.2 M glycine–NaOH (pH 10.2) or 0.034 M NaOH in water. Fluorescence was recorded (excitation at 360 nm and emission at 450 nm), and substrate blanks were subtracted from the sample readings. The 50% inhibitory concentration (IC50) was calculated by plotting percent inhibition of neuraminidase activity versus the inhibitor concentration. The results are reported as the average of two to seven experiments.
General procedure for in vivo antiviral experiments.
Mice (13 to 16 g) were anesthetized by intraperitoneal injection of ketamine-rompun solution and exposed to 100 μl of virus by intranasal instillation. The viral dose utilized was an approximately 90% lethal dose, which was equivalent to 102.3 of the 50% cell culture infectious dose in the case of H1N1 virus and 102.7 of the 50% cell culture infectious dose in the case of H6N2 virus. In the prophylaxis model, drug was administered 4 h before viral infection and 4 h after infection, and treatment continued twice a day (BID) for a total of 5 days; in the treatment model, drug was given at times indicated after the viral infection. Each infected group contained 7 to 10 mice, and normal controls contained five mice. All mice were observed daily for changes in weight and for any deaths. Parameters for evaluation of antiviral activity included weight loss, reduction in mortality, and/or increase in mean day to death determined through 21 days.
In vivo anti-influenza virus efficacy of RWJ-270201.
Mice were infected intranasally with an approximately 90% lethal dose of the A/NWS/33(H1N1) or A/Turkey/Mass/76 X A/Beijing/32/92(H6N2) influenza virus. Oral treatment with RWJ-270201 (prepared in 0.5% carboxymethyl cellulose) was begun 4 h before virus exposure (prophylaxis model) or at the indicated times (treatment model) and continued daily for 5 days. Infected and uninfected vehicle-treated control mice were included in the same treatment schedule.
For the intranasal studies, mice were infected with A/Turkey/Mass/76 X A/Beijing/32/92(H6N2). Intranasal treatment with doses of 0.1 and 0.01 mg/kg of body weight/day of RWJ-270201, oseltamivir carboxylate, or zanamivir (prepared in 0.9% sodium chloride) was begun 4 h before virus exposure and continued once daily for 5 days. Vehicle-treated uninfected and infected control mice were included. Parameters studied were reduction in mortality and/or increase in mean day to death.
Statistical analysis.
The data were analyzed by Sigma Plot (Windows version 4.01; SPSS, Chicago, Ill.) and Sigma Stat (Windows version 2.0; Jandel Corporation, San Rafael, Calif.). The t test was used to evaluate differences in mean day to death. Fischer least significant difference tests were used to evaluate differences in weight loss. Fisher exact tests were applied to survival differences.
RESULTS
In vitro neuraminidase inhibition.
The ability of RWJ-270201 to inhibit the neuraminidase activity of several influenza A and B strains was tested and compared to those of zanamivir and oseltamivir carboxylate. The IC50 of RWJ-270201 ranged from 0.09 to 1.4 nM for influenza A strains and from 0.60 to 11 nM for influenza B strains (Table 2). The IC90s for influenza A strains were six to nine times higher than the corresponding IC50s, but the IC90s for the B strains exceeded the IC50s by a factor of 19 to 24 (Table 2). The greatest overall IC90 of RWJ-270201 was 30 nM.
TABLE 2.
Comparison of IC50s and IC90s of RWJ-270201, oseltamivir carboxylate, and zanamivir against various influenza neuraminidases
| Virus | Type | IC50 (nM)
|
IC90 (nM)
|
||||
|---|---|---|---|---|---|---|---|
| RWJ-270201 | Oseltamivir carboxylate | Zanamivir | RWJ-270201 | Oseltamivir carboxylate | Zanamivir | ||
| A/Brazil/11/78 | H1N1 | 0.10a | 0.86a | 0.30a | 0.59a | 9.92a | 2.83a |
| A/FM/1/47 | H1N1 | 0.81b | 1.50b | 0.48b | NDd | ND | ND |
| A/NWS/33 (grown in MDCK cells) | H1N1 | 0.63b | 2.20b | 0.49b | ND | ND | ND |
| A/NWS/33 | H1N1 | 0.11a | 0.69a | 0.38a | 0.72a | 9.14a | 3.57a |
| A/PR/8/34 | H1N1 | 0.09b | 0.99b | 0.34b | 0.83a | 11.3a | 4.49a |
| A/Taiwan/1/86 | H1N1 | 0.37a | 2.24a | 0.80a | 2.30ac | 40.9a | 7.23ac |
| A2/Hong Kong/8/68 | H2N2 | 0.18a | 0.25a | 0.76a | 0.96a | 2.19a | 5.38a |
| A/Japan | H2N2 | 0.17a | 0.21ac | 0.99a | 0.96a | 2.27a | 9.77a |
| A/Singapore/1/57 | H2N2 | 0.20b | 0.01b | 1.78b | ND | ND | ND |
| A/Tokyo/3/67 | H2N2 | 1.39b | 1.45b | 1.38b | ND | ND | ND |
| A/Turkey/Mass/76 X A/Beijing/32/92 | H6N2 | 1.08b | 0.84b | 1.07b | ND | ND | ND |
| A/Aichi | H3N2 | 0.14a | 0.23ac | 0.68a | 0.84a | 2.04a | 5.02a |
| A/Belgium/2/81 | H3N2 | 0.29a | 0.24a | 2.32a | 1.37a | 2.00a | 22.5a |
| A/Shangdong/09/93 | H3N2 | 0.83b | 0.56b | 1.19b | ND | ND | ND |
| A/Virginia/88 | H3N2 | 0.17a | 0.21ac | 1.40a | 0.96a | 2.13a | 12.9a |
| B/Harbin/07/94 | B | 0.60b | 6.39b | 3.26b | ND | ND | ND |
| B/Hong Kong/5/72 (grown in MDCK cells) | B | 9.78a | 24.3a | 6.29a | ND | ND | ND |
| B/Hong Kong/5/72 | B | 1.53a | 7.70a | 3.31a | 29.7a | 14.0a | 58.9a |
| B/Lee/40 | B | 10.8b | 5.74b | 3.38b | ND | ND | ND |
| B/Victoria/70 | B | 5.38b | 5.00b | 17.0b | ND | ND | ND |
| B/Y177 | B | 0.82a | 8.90a | 1.61a | 19.7a | 15.4a | 18.7a |
| B/Y253 | B | 0.84a | 9.01a | 1.53a | 18.8a | 151.9a | 20.1a |
| BY263 | B | 0.99a | 10.4a | 1.63a | 21.0a | 162.8a | 21.7a |
Average of seven determinations; the highest value is less than three times the lowest value except where indicated.
Average of two determinations; the difference between the two values is less than threefold.
The highest value is less than five times the lowest value.
ND, not determined.
The IC50s obtained for zanamivir were somewhat higher than those for RWJ-270201. The IC50 range for zanamivir was 0.3 to 2.3 nM for influenza A strains and 1.6 to 17.0 nM for influenza B strains. The IC90s exceeded IC50s by a factor of 7 to 13 for influenza A strains and 12 to 18 for influenza B strains. (For zanamivir, the highest overall IC90 was 59 nM.) The IC90s for influenza A strains were 5 to 16 times higher than those obtained with RWJ-270201, but the IC90s for influenza B strains were one- to twofold higher.
The IC50 range of oseltamivir carboxylate was 0.01 to 2.2 nM for influenza A strains and 6.4 to 24.3 nM for influenza B strains. The oseltamivir carboxylate IC90s exceeded the IC50s by a factor of 8 to 18 for influenza A strains and 16 to 18 for influenza B strains. For oseltamivir carboxylate, the highest overall IC90 was 163 nM. The IC90s for influenza A strains were 2 to 18 times higher than those obtained with RWJ-270201, and the IC90s for influenza B strains were 5 to 8 times higher than those for RWJ-270201.
A comparison of mean IC90s of RWJ-270201, oseltamivir carboxylate, and zanamivir for the different virus types is presented in Table 3. RWJ-270201 is at least as effective as zanamivir in inhibiting A/H1N1 and B strain neuraminidases, but both drugs are more effective than oseltamivir carboxylate. RWJ-270201 is at least as effective as oseltamivir carboxylate against A/H3N2 and A/H2N2 strain neuraminidases, and both of these drugs are more effective than zanamivir.
TABLE 3.
Mean IC90s according to influenza virus type
| Virus type | No. of subtypes | Mean IC90 (nM) (range)
|
||
|---|---|---|---|---|
| RWJ-270201 | Oseltamivir carboxylate | Zanamivir | ||
| H1N1 | 4 | 1.11 (0.59–2.30) | 17.8 (9.14–40.9) | 4.53 (2.83–7.23) |
| H2N2 | 2 | 0.96 (0.96) | 2.23 (2.19–2.27) | 7.57 (5.38–9.77) |
| H3N2 | 3 | 1.06 (0.84–1.37) | 2.05 (2.00–2.13) | 13.5 (5.02–22.5) |
| B | 4 | 22.3 (18.8–29.7) | 152 (140–162.8) | 29.9 (18.7–58.9) |
The specificity of RWJ-270201 as an influenza virus neuraminidase inhibitor was investigated by determining its inhibitory activity against neuraminidases from a variety of sources in an in vitro enzymatic assay. The IC50 of RWJ-270201 against neuraminidases derived from other sources, including mammalian (rat liver), bacterial (V. cholerae and C. perfringens), and other viral (parainfluenza virus and Newcastle disease virus) neuraminidases was >300 μM, which is at least 4 orders of magnitude less potent than that for influenza virus neuraminidase. This indicates that RWJ-270201 is a highly selective inhibitor for influenza A and B neuraminidases.
Influenza A mouse model: treatment and prophylaxis.
In the mouse influenza model, viral infection leads to loss of body weight and high mortality, and this decrease in body weight correlates with pulmonary viral titer and pulmonary lesion score (5). Therefore, the efficacy of orally administered RWJ-270201 was evaluated on the basis of weight loss and survival rate measured for 21 days postinfection, for treated infected animals relative to untreated infected (control) animals.
In the prophylaxis treatment model, RWJ-270201 was administered orally 4 h before the infection. Complete protection was observed at a dose of 1 mg/kg/day BID in the H6N2 mouse influenza model, with all nine mice surviving (Table 4). By comparison, only one of the nine vehicle-treated control mice survived. Lowering the dosage to 0.1 mg/kg/day in the same model also prevented lethality in seven of nine mice. In the H1N1 mouse influenza model, four of the seven mice survived at a dose of 1 mg/kg/day BID, and none of the mice in the control group (treated with vehicle) survived. Complete protection against lethality was observed at the 10 mg/kg/day dose level (Table 4). No signs of drug-related toxicity were observed when RWJ-270201 was administered orally for 5 days at a dose of 100 mg/kg/day. RWJ-270201 showed a dose-response relationship when the weight loss of infected mice over time was monitored. In general, a lower dose resulted in greater weight loss than higher doses.
TABLE 4.
Effect of oral gavage treatment with RWJ-270201 on influenza virus infections in mice (prophylaxis model)
| Virus | Dosage (mg/kg/day BID) | No. of survivorsa | Mean day to deathab |
|---|---|---|---|
| A/Turkey/Mass/76A/ | 1 | 9/9∗∗ | |
| Beijing/32/92(H6N2) | 0.1 | 7/9∗ | 9.0 ± 1.0 |
| 0 | 1/9 | 8.8 ± 0.81 | |
| A/NWS/33 (H1N1) | 10 | 8/8∗∗ | |
| 1 | 4/7∗ | 10.7 ± 0.33∗∗ | |
| 0 | 0/10 | 7.6 ± 0.22 | |
| RWJ-270201 uninfected | 100 | 5/5 | |
| Saline uninfected | 5/5 |
∗, P < 0.015 versus vehicle; ∗∗, P < 0.001 versus vehicle.
Mean day to death of mice dying prior to day 22.
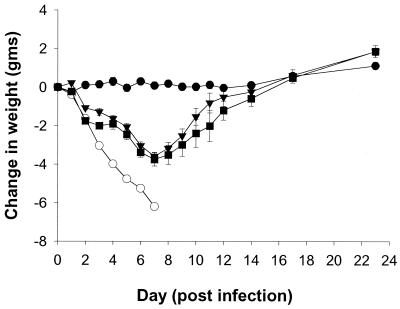
In the delayed-treatment model, oral administration of 10 mg/kg/day BID of RWJ-270201 or oseltamivir at 24 h postinfection gave essentially complete protection against lethality (Table 5). In the RWJ-270201 group 9 of 10 animals survived, and in the oseltamivir group 10 of 10 survived. However, when treatment was started 48 h postinfection, no significant protection against lethality was observed in any drug group. When the weight loss of the infected mice was monitored over time, the RWJ-270201- and oseltamivir-treated groups demonstrated comparable responses (Fig. 2).
TABLE 5.
Effect of delayed treatment with RWJ-270201 and oseltamivir on influenza A/Turkey/Mass/76 X A/Beijing/32/92(H6N2) virus infections in mice
| Compounda | Beginning of therapy (h) | No. of survivors/total no. treatedb | Mean day to deathc |
|---|---|---|---|
| RWJ-270201 | 24 | 9/10∗∗ | 12.0 ± 0.0 |
| RWJ-270201 | 48 | 0/10 | 8.5 ± 1.11 |
| Oseltamivir | 24 | 10/10∗∗ | |
| Oseltamivir | 48 | 1/10 | 8.4 ± 1.05 |
| Vehicle | 0/8 | 6.1 ± 0.35 | |
| Vehicle uninfected | 5/5 |
Dosage of each drug was 10 mg/kg/day BID.
∗∗, P < 0.001 versus vehicle.
Mean day to death of mice dying prior to day 23.
FIG. 2.
Effects of oral treatment of RWJ-270201 and oseltamivir, at 10 mg/kg/day given 24 h postinfection, on weight loss in influenza A (H6N2)-infected mice. The number of mice in each group was 10 except for the vehicle infected group (n = 8) and vehicle uninfected group (n = 5). ●, vehicle uninfected group; ○, vehicle infected group; ■, RWJ-270201 treatment group; ▾, oseltamivir treatment group.
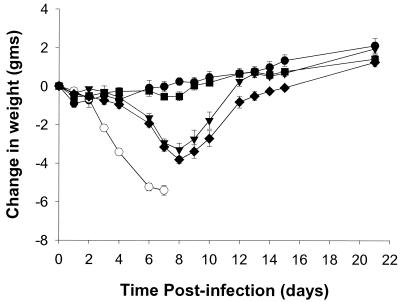
To compare the in vivo efficacy of RWJ-270201 to zanamivir, intranasal studies were performed, as zanamivir has poor oral bioavailability. The efficacy of intranasal administration of RWJ-270201 was also compared to oseltamivir carboxylate in the same mouse influenza model. Each drug was applied directly to the respiratory tract via intranasal treatment. All three drugs, RWJ-270201, zanamivir, and oseltamivir carboxylate, demonstrated complete protection against lethality at 0.1 mg/kg/day once a day (QD) (Table 6). At a lower dose of 0.01 mg/kg/day QD, RWJ-270201 was completely protective, whereas oseltamivir carboxylate was partially effective and zanamivir showed no significant protection against lethality. RWJ-270201 groups administered 0.1 mg/kg/day QD showed no significant weight loss compared to uninfected controls over time (Fig. 3). On the other hand, both the oseltamivir carboxylate and zanamivir groups given 0.1 mg/kg/day QD lost significant weight compared to the uninfected control group.
TABLE 6.
Effects of intranasal treatment with RWJ-270201, oseltamivir carboxylate, and zanamivir on influenza A (H6N2) virus infections in mice
| Compound | Dosage (mg/kg/day QD) | No. of survivors/total no. treatedb | Mean day to deathab |
|---|---|---|---|
| RWJ-270201 | 0.1 | 10/10∗∗ | |
| RWJ-270201 | 0.01 | 10/10∗∗ | |
| Oseltamivir carboxylate | 0.1 | 10/10∗∗ | |
| Oseltamivir carboxylate | 0.01 | 6/10∗∗∗ | 13.5 ± 1.85∗∗ |
| Zanamivir | 0.1 | 10/10∗∗ | |
| Zanamivir | 0.01 | 1/9 | 12.1 ± 0.97∗∗ |
| Vehicle | 0/10 | 7.2 ± 0.25 | |
| Vehicle uninfected | 5/5 |
Mean day to death of mice dying prior to day 21.
∗∗, P < 0.001 versus vehicle; ∗∗∗, P < 0.05 versus vehicle.
FIG. 3.
Effects of intranasal treatment of RWJ-270201, oseltamivir carboxylate, and zanamivir, at a dose of 0.1 mg/kg/day, on weight loss in influenza A (H6N2)-infected mice. The number of mice in each group was 10 except for the vehicle uninfected group (n = 5). ●, vehicle uninfected group; ○, vehicle infected group; ■, RWJ-270201 treatment group; ▾, oseltamivir carboxylate treatment group; ⧫, zanamivir treatment group.
DISCUSSION
Because of the importance of influenza neuraminidase in viral replication and pathogenesis, interest has focused on the development of selective inhibitors of this enzyme. Using structure-based drug design, we have previously synthesized a novel and potent inhibitor of influenza virus neuraminidase, RWJ-270201 (1). RWJ-270201 has five chiral centers, and initially it was synthesized as a mixture of isomers. Using crystallography, the isomer that bound to the active site was identified, and stereospecific synthesis was performed to obtain the right isomer. RWJ-270201 has multiple interactions with the active site residues of influenza neuraminidase and has been shown to inhibit viral growth in tissue culture (1, 12). This study compared the potency (IC50s and IC90s) of RWJ-270201, zanamivir, and oseltamivir carboxylate against neuraminidases from different virus subtypes. Overall, RWJ-270201 had either comparable or better potency compared to zanamivir and oseltamivir carboxylate on all the virus subtypes tested. RWJ-270201 was more effective in inhibiting A/H1N1 and B strain neuraminidases than oseltamivir carboxylate. This difference in potency can be explained by the subtle differences in the active site of the neuraminidase enzymes from different subtypes and by how these compounds bind into the active site. When oseltamivir carboxylate or RWJ-270201 binds to the active site of the neuraminidase, the 3-pentyl hydrophobic group occupies a region formed by a reorientation of the side chain of Glu 276. In the case of influenza B neuraminidase, it has been reported that the rearrangement of Glu 276 is energetically less favorable (1, 14). Although both oseltamivir carboxylate and RWJ-270201 have hydrophobic groups. RWJ-270201 demonstrates better potency on influenza B neuraminidase than oseltamivir carboxylate. A possible reason for this is that the guanidinium group of RWJ-270201 has an additional interaction with the active site amino acid residues that compensates the energy spent for the unfavorable reorientation of the Glu 276 side chain in the influenza B neuraminidase active site. Oseltamivir carboxylate has an amino group in this position. Zanamivir and RWJ-270201 have comparable potencies for influenza B neuraminidase. In the case of zanamivir, interactions of guanidinium and a glycerol group with the active site residues contribute to the overall potency of the compound. The glycerol group binds in the same pocket as the 3-pentyl group of RWJ-270201 and oseltamivir carboxylate, and it does not require reorientation of Glu 276 to create a hydrophobic pocket. The guanidinium groups of zanamivir and RWJ-270201 occupy the same pocket in the active site. In spite of this fact, RWJ-270201 is active against the zanamivir-resistant strain containing Glu 119 Gly, which forms part of the guanidinium binding pocket (L. V. Guboreva, D. Schallon, and F. G. Hayden, II Int. Symp. Influenza Other Resp. Viruses, abstr. p24, 1999). This is due to altered orientation of the guanidinium group of RWJ-270201, compared to that of zanamivir, in the active site binding pocket (1).
RWJ-270201 is a specific and potent inhibitor of influenza virus neuraminidase. No significant inhibition was observed against neuraminidases from other sources, including those of parainfluenza virus and Newcastle disease virus, which share homology with the influenza virus neuraminidase.
RWJ-270201 is active when administered orally in both prophylaxis and treatment mouse influenza virus infection models. At a dose of 1 mg/kg/day administered for 5 days beginning 4 h prior to infection, RWJ-270201 provided protection against lethality in two influenza A mouse models. Sidwell et al. reported similar results using different influenza virus strains (12). A good dose response was observed when weight loss in infected mice was monitored over time, and thus weight loss represents a rapid way to screen potential influenza virus neuraminidase inhibitors. It is interesting that, despite having similar potency against the neuraminidase activity of the two influenza A viruses (1.08 and 0.63 nM), RWJ-270201 was more effective in protecting mice against challenge with H6N2 virus (9 of 9 mice survived at 1 mg/kg/day) than against challenge with H1N1 virus (4 of 7 survived at 1 mg/kg/day). A similar observation has been made with oseltamivir (11). The reason for this remains unclear; it could be attributed to subtle differences in virus growth pattern or in the viral challenge. In other experiments, we were able to show that RWJ-270201 was also effective in the mouse influenza model when treatment was initiated as late as 24 h postinfection. Protection against lethality and weight loss was comparable to that with oseltamivir. However, when treatment was delayed for 48 h, administration of either RWJ-270201 or oseltamivir did not offer significant protection. It has previously been demonstrated that oseltamivir is highly effective when treatment is begun as late as 60 h postinfection (11). However, when viral challenge was increased 100-fold, the efficacy was affected markedly, with only 20% survival when therapy began at 48 h. Therefore, viral challenge dose and timing to begin treatment are extremely critical in these experiments, and how these factors correlate in the clinic remains to be seen.
Zanamivir has been approved by the Food and Drug Administration as a topical treatment (via inhaler) for influenza. In order to compare RWJ-270201 to zanamivir in vivo, intranasal treatment in a mouse influenza model was performed. These studies indicated that RWJ-270201 has superior activity when compared to zanamivir and oseltamivir carboxylate. The precise reason for this is unclear; it could possibly be due to slower dissociation of the compound from the enzyme or slower release of the drug from the lungs. RWJ-270201 was well tolerated by the mice given oral doses up to 100 mg/kg/day.
In summary, RWJ-270201 is a specific and potent inhibitor of the neuraminidase activity of both influenza A and B virus. Its in vitro activity is better than or comparable to oseltamivir carboxylate and zanamivir. Prophylactic and delayed oral administration of RWJ-270201 was effective in preventing lethality and weight loss in a mouse influenza model. In view of the in vivo and in vitro data, we conclude that RWJ-270201 is a novel, orally active agent that could be used in the prevention and treatment of human influenza virus infections.
ACKNOWLEDGMENTS
We thank Claude Bennett and Scott Rowland for many valuable discussions.
REFERENCES
- 1.Babu Y S, Chand P, Bantia S, Kotian P L, Dehghani A, El-Kattan Y, Lin T, Hutchinson T L, Elliot A J, Parker C D, Ananth S L, Horn L L, Laver G W, Montgomery J A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 2.Hayden F G, Atmar R L, Schilling M, Johnson C, Poretz D, Paar D, Huson L, Ward P, Mills R G. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–1343. doi: 10.1056/NEJM199910283411802. [DOI] [PubMed] [Google Scholar]
- 3.Hayden F G, Treanor J J, Betts R F, Lobo M, Esinhart J D, Hussey E K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996;275:295–299. [PubMed] [Google Scholar]
- 4.Hayden F G, Treanor J J, Fritz R S, Lobo M, Betts R F, Miller M, Kinnersley N, Mills R G, Ward P, Straus S E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 5.Johansson B E, Grajower B, Kilbourne E D. Infection-permissive immunization with influenza virus neuraminidase prevents weight loss in infected mice. Vaccine. 1993;11:1037–1039. doi: 10.1016/0264-410x(93)90130-p. [DOI] [PubMed] [Google Scholar]
- 6.Mendel D B, Tai C Y, Escarpe P A, Li W, Sidwell R W, Huffman J H, Sweet C, Jakeman K J, Merson J, Lacy S A, Lew W, Williams M A, Zhang L, Chen M S, Bischofberger N, Kim C U. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyagi T, Tsuiki S. Purification and characterization of cytosolic sialidase from rat liver. J Biol Chem. 1985;260:6710–6716. [PubMed] [Google Scholar]
- 8.Monto A S, Robinson D P, Herlocher M L, Hinson J M, Jr, Elliott M J, Crisp A. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999;282:31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Potier M, Mameli L, Belislem M, Dallaire L, Melanxon S B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 10.Ryan D M, Ticehurst J, Dempsey M H. GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is a potent inhibitor of influenza virus in ferrets. Antimicrob Agents Chemother. 1995;39:2583–2584. doi: 10.1128/aac.39.11.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidwell R W, Huffman J H, Barnard D L, Bailey K W, Wong M H, Morrison A, Syndergaard T, Kim C U. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antivir Res. 1998;37:107–120. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 12.Sidwell R W, Smee D F, Huffman J H, Barnard D L, Bailey K W, Morrey J D, Babu Y S. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RWJ-270201. Antimicrob Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smee D F, Huffman J H, Morrison A C, Barnard D L, Sidwell R W. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob Agents Chemother. 2001;45:743–748. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor N R, Cleasby A, Singh O, Skarzynski T, Wonacott A J, Smith P W, Sollis S L, Howes P D, Cherry P C, Bethell R, Colman P, Varghese J. Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. 2. Crystallographic and molecular modeling study of complexes of 4-amino-4H-pyran-6-carboxamides and sialidase from influenza virus types A and B. J Med Chem. 1998;41:798–807. doi: 10.1021/jm9703754. [DOI] [PubMed] [Google Scholar]
- 15.Von Itzstein M, Wu W Y, Kok G B, Pegg M S, Dyason J C, Jin B. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]