Abstract
The field of vascular contributions to cognitive impairment and dementia (VCID) is evolving rapidly. Research in VCID encompasses topics aiming to understand, prevent, and treat the detrimental effects of vascular disease burden in the human brain. In this perspective piece, early career researchers (ECRs) in the field provide an overview of VCID, discuss past and present efforts, and highlight priorities for future research. We emphasize the following critical points as the field progresses: (a) consolidate existing neuroimaging and fluid biomarkers, and establish their utility for pharmacological and non‐pharmacological interventions; (b) develop new biomarkers, and new non‐clinical models that better recapitulate vascular pathologies; (c) amplify access to emerging biomarker and imaging techniques; (d) validate findings from previous investigations in diverse populations, including those at higher risk of cognitive impairment (e.g., Black, Hispanic, and Indigenous populations); and (e) conduct randomized controlled trials within diverse populations with well‐characterized vascular pathologies emphasizing clinically meaningful outcomes.
Keywords: early career researcher, vascular cognitive impairment, vascular dementia, VCID
1. INTRODUCTION
The study of vascular contributions to cognitive impairment and dementia (VCID) encompasses a broad range of research areas that aim to understand, prevent, and treat the detrimental effects of vascular disease burden on human brain structure, cognition, and overall function. 1 , 2 Neuropathological studies continue to demonstrate that dementia is often the result of multiple etiologies, with mixed vascular, amyloid beta (Aβ), and tau pathology observed in more than two‐thirds of cases. 3 , 4 By contrast, pure forms of vascular dementia are rare, accounting for only ≈10% of dementia cases. 5 Therefore, research on VCID is particularly challenging and requires a comprehensive understanding of the underlying pathophysiology. 6
With the goal of discussing key priorities for future research on VCID, the International Society to Advance Alzheimer's Research and Treatment (ISTAART) Vascular Cognitive Disorders Professional Interest Area (PIA) held an online discussion panel of early career researchers (ECRs) in October 2020, with 177 attendees from 19 countries. Panelist expertise ranged from basic and translational science to epidemiology, prevention, and clinical care. In this perspective piece, we review the following key concepts discussed: (1) the clinical definition of VCID, (2) past and present efforts toward prevention and treatment, and (3) perspectives and priorities for future research.
2. A BRIEF OVERVIEW OF VCID
In this section, we discuss the key elements of VCID, including terminology, diagnostic criteria, underlying neuropathology, and potential for therapeutic and/or preventive strategies. A conceptual model of these elements is presented in Figure 1.
FIGURE 1.
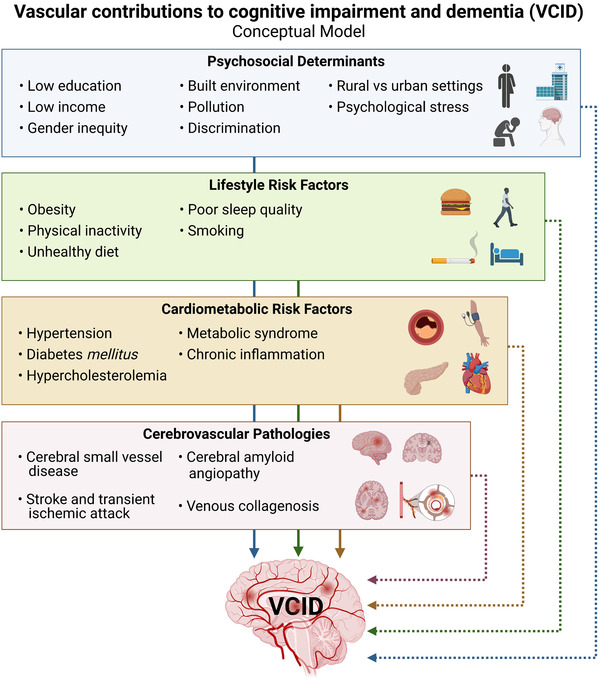
A conceptual model for vascular contributions to cognitive impairment and dementia (VCID). Note: This conceptual model highlights direct and indirect mechanisms in the causative chain of events yielding brain injury, ultimately leading to vascular cognitive impairment and dementia
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using conventional (e.g., PubMed) sources. An overview of vascular contributions to cognitive impairment and dementia (VCID) indicated the field is evolving rapidly; however, there are important aspects yet to be addressed.
Interpretation: From an early career researcher perspective, with a varied range of expertise, we discussed current limitations in VCID research in basic and translational models, as well as clinical research, including pharmacological and non‐pharmacological preventive and therapeutic interventions.
Future Directions: More research is needed to consolidate existing biomarkers as they apply to prevention and treatment. As well, it is critical to develop, refine, and increase access to new disease‐specific biomarkers and non‐clinical models that better recapitulate vascular pathologies. Also, it is imperative to conduct clinical research in diverse, high‐risk populations (e.g., Black, Hispanic, and Indigenous), and design randomized controlled trials emphasizing clinically meaningful outcomes.
HIGHLIGHTS
Vascular contributions to cognitive impairment and dementia were reviewed
Current limitations in basic, translational, and clinical research were discussed
Priorities for future research were provided from an early career researcher viewpoint
2.1. Terminology and clinical definition
Historically, research and clinical diagnoses of vascular cognitive impairment have focused on vascular dementia (VaD), large vessel disease, and stroke. Further characterization led to the recognition of other significant contributors to vascular mild cognitive impairment (MCI) and dementia. 1 , 2 , 7 These include cerebral small vessel disease (cSVD), systemic vascular disease, and cerebrovascular pathologies such as cerebral amyloid angiopathy (CAA) concomitant with Alzheimer's disease (AD) and/or Lewy body pathologies. 1 , 2 , 7 More recently, the term VCID has been used as it better captures the spectrum of associated pathologies. 2 , 8 However, use of the term VCIDis not yet widespread among the more than 21,000 publications related to vascular disease and cognitive impairment (see Figure 2 for publications through 2020).
FIGURE 2.
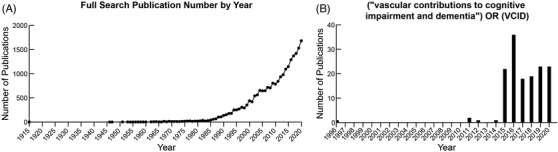
Vascular contributions to cognitive impairment and dementia (VCID) publication trends over time based on a July 13, 2021, PubMed search through December 31, 2020. Note: (A) Number of publications per year based on a full search using the following terms: ((“vascular contributions to cognitive impairment and dementia”) OR ((vascular) AND ((cognitive impairment) OR (dementia)))) OR ((vascular) AND (mild cognitive impairment)). (B) Number of publications per year based on searching specifically for “(“vascular contributions to cognitive impairment and dementia”) OR (VCID)”
Various diagnostic criteria have been proposed for independent categories that fall under the VCID umbrella, but integrated, overarching criteria covering all VCID have been difficult to develop and apply (historical evolution of various diagnostic criteria are reviewed in Dichgans et al. 6 and Gorelick et al. 8 ). Two key features are required for VCID diagnosis: (1) the presence of cerebrovascular disease or cerebral hypoperfusion, and (2) impairment on neuropsychological assessment in at least one cognitive domain (based on the American Heart Association/American Stroke Association Scientific Statement 8 and the Vascular Impairment of Cognition Classification Consensus Study 7 ). A causal link between these two criteria is used to distinguish between “probable” VCID where a causal link can be established, or “possible” VCID where a causal link cannot be established with certainty. 6 , 7 , 8
2.2. Neuropathology and neuroimaging
The most prevalent pathology underlying VCID is cSVD, which itself comprises several pathologies that affect the brain's small arteries, arterioles, veins, venules, and capillaries, the integrity of which are crucial to maintain adequate cerebral blood flow (CBF). Other VCID‐related vascular pathologies include the venous deposition of collagen and subsequent vessel wall thickening (venous collagenosis), lipohyalinosis, and CAA. 9 , 10 The consequences of cSVD are heterogenous in their manifestations; parenchymal lesions associated with cSVD vasculopathy include small focal infarcts (lacunes and microinfarcts), diffuse white matter (WM) lesions, microbleeds (also known as microhemorrhages), intracerebral hemorrhage, and subarachnoid hemorrhage. 11
Neuroimaging is heavily relied upon to assess the extent, location, and type of vascular lesion present, and to allow differential diagnosis. Individuals with VCID typically present evidence of prior strokes and diffuse WM lesions, the variability in size and distribution of which may reflect differences in etiology and pathological severity. T1‐weighted magnetic resonance imaging (MRI) is used to visualize atrophy, whereas T2‐weighted MRI aids in the visualization of lacunar infarcts and WM hyperintensities (WMH). WMH are diffuse areas of hyperintense signal (also seen on fluid‐attenuated inversion recovery [FLAIR] sequences) that occur in WM regions undergoing demyelination and subsequent axonal degeneration. 12 , 13 Although not required for the diagnosis, WMH are often interpreted clinically as a surrogate for cSVD contributing to VCID pathophysiology (reviewed in Alber et al. 10 ). T2*‐weighted MRI and susceptibility‐weighted imaging (SWI) are used to identify hemosiderin deposits indicative of microbleeds and other forms of hemorrhage. Therefore, the different neuroimaging techniques are useful because they can discriminate the different pathophysiological mechanisms underlying the VCID syndrome in individual persons.
2.3. Epidemiology and risk factors
Estimates for prevalence and incidence of VCID are incomplete and have been variable depending on what is included in the definition. Factors contributing to this include historical diagnostic fragmentation, whether milder forms of cognitive impairment are included in addition to dementia, uncertain diagnostic categorization due to mixed cerebrovascular and neurodegenerative pathologies, whether neuroimaging characterization is available, and the recent introduction of the term VCID. 2 , 8 , 11 Occurrence of VCID varies by sex, age, race/ethnicity, individual vascular and cardiometabolic risk factors, and comorbid conditions. 14
Inherited and modifiable factors traditionally associated with neurotoxicity, microglial activation, compromised neural repair mechanisms, thromboembolic phenomena, and blood‐brain barrier (BBB) dysfunction all increase dementia risk. 15 Cross‐sectional studies have shown that the apolipoprotein E (APOE) ε4 allele, midlife obesity, a family history of cardiovascular disease, and the number of cerebrovascular risk factors present are strongly associated with earlier dementia onset in selected populations. 16 Risk of post‐stroke dementia is also high, especially when individuals present with additional vascular and cardiometabolic risk factors. 17 In both familial and sporadic forms of AD, prior history of stroke has also been associated with increased dementia risk. 18 Prospective studies have shown that later onset of AD and lifetime alcohol use are associated with faster cognitive and functional decline, 19 and sex differences are also observed. 20
2.4. Genetics and RNA‐seq
Genome‐wide association studies (GWAS) of VCID constitute a growing area of research, with new genetic underpinnings being linked to stroke 21 , 22 and cSVD. 23 Furthermore, many of the genes identified in GWAS for AD have been linked to vascular dysfunction (e.g., APOE, PICALM, CLU, PSEN1, PSEN2, APP, MEOX2, and COL4A1). 24 , 25 , 26 Monogenic forms of cSVD (leading to cognitive impairment in some individuals), such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), are relatively rare. 27 Major efforts are under way to discover and understand genetic contributions to AD and VCID; nominated targets and other genes can be explored through the Agora database (https://agora.ampadportal.org/genes).
Genomic effects of cognitive reserve, cerebral perfusion, and hormonal changes interact to influence neurodegeneration in late life 19 , 20 (see the Box for specific effects of APOE). Nevertheless, validation in larger and diverse populations is needed. Future long‐term prospective studies that use GWAS data are needed to assess the risk of cognitive and functional decline in VCID in all populations.
3. PAST AND PRESENT STUDIES OF PREVENTION AND TREATMENT
3.1. Non‐clinical studies in animal models
Considering VCID is inherently heterogenous, animal models can aid in determining which treatments prove efficacious for specific VCID‐associated pathologies. Drugs targeting vascular and metabolic factors, such as statins, 28 anti‐platelet medications, 29 and anti‐hypertensives 30 have proven efficacious in models of chronic hypertension and chronic cerebral hypoperfusion (CCH), improving CBF and cognition, reducing inflammation, and protecting against neuronal damage (reviewed in Yang et al. 31 ). Anti‐inflammatory drugs, such as minocycline, which has been shown in multiple models of CCH to attenuate microglial activation, improve memory function, enhance CBF, and preserve WM integrity. 32 , 33 , 34 , 35 , 36 Immunosuppressants, including cyclosporin A 37 and free radical scavengers, 38 have also shown promise in CCH models. Medications that augment acetylcholine signaling have proven effective across models. 39 , 40
Estrogen and other sex hormones have demonstrated neuroprotective properties and play a role in vascular function and pathology (reviewed in Abi‐Ghanem et al. 41 and Robison et al. 42 ). While hormone therapy has been studied extensively in stroke models (reviewed in Robison et al. 43 ), few studies have investigated hormonal effects in other VCID‐relevant animal models. Estradiol is protective in models of CCH; however, these studies included only males. 44 , 45 Rodent studies treating females with estrogen are necessary, and should include models of menopause (e.g., ovariectomy, 4‐vinylcyclohexene) to provide evidence on the safety and efficacy of hormone replacement therapy for preventing and/or treating VCID in all populations, including post‐menopausal women. 46
The heterogenous nature of VCID requires that common comorbidities and clinically relevant risk factors such as sex, biological and endocrine aging, and vascular and metabolic risk factors, be commonly integrated in translational studies. Moreover, our ability to translate non‐clinical data to the clinic can likely be improved by conducting studies in non‐human primates or larger domesticated species, which share key attributes with humans, including lifespan, CBF, vascular architecture, immune function, and relative abundance of WM. 47 , 48 , 49 Finally, candidate therapies should be tested in animal models of multi‐etiology dementias, particularly models with combined vascular and AD pathology. 50
3.2. Pharmacological prevention and treatment strategies in humans
There are no approved drugs specifically for VCID. In some countries current therapeutic management includes off‐label use of cholinesterase inhibitors (particularly for those with multiple cortical infarcts and hippocampal atrophy) and of memantine (mainly for those with subcortical cSVD). 51 Additionally, management of cerebrovascular risk factors continues to be part of the patient care strategy, 2 though underlying neurobiological mechanisms are not yet fully understood. Furthermore, midlife arterial hypertension causes memory decline, vascular cognitive impairment, 52 and earlier onset of sporadic AD, particularly in combination with other cerebrovascular risk factors. 16 Blood pressure lowering has been shown to reduce risk of cognitive impairment in Systolic Blood Pressure Intervention Trial–Memory and Cognition in Decreased Hypertension (SPRINT‐MIND 53 , 54 and other cohorts, 55 , 56 with further evidence showing that intensive blood pressure management also reduces accrual of WMH compared to standard blood pressure management.
There are conflicting results regarding the effects of distinct anti‐hypertensive classes over incidence or course of cognitive decline. 55 , 56 , 57 Molecular mechanisms have been studied more often concerning the effects of angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers over cognitive decline. 58 , 59 A recent study of 193 patients with AD found evidence that angiotensin‐converting enzyme inhibitors slowed cognitive (but not functional) decline in 1 year by way of central or peripheral mechanisms that do not depend upon their anti‐hypertensive properties, particularly for APOE ε4 non‐carriers who also carried specific ACE genotypes of rs1800764 or rs4291. 58 Additionally, another study of 1689 patients with AD who used angiotensin II type 1 receptor blockers (n = 578) or angiotensin‐converting enzyme inhibitors (n = 1111) found that, among APOE ε4 non‐carriers, use of angiotensin II type 1 receptor blockers was associated with greater preservation of memory and attention, effects that were particularly notable compared to angiotensin‐converting enzyme inhibitors with lower brain penetration. 59 These findings support the importance of pharmacogenetic studies in VCID.
Thiazolidinediones such as pioglitazone and rosiglitazone are agonists of the nuclear peroxisome proliferator‐activated receptor γ (PPAR γ). They improve insulin sensitivity, and in animal models demonstrated enhanced Aβ clearance and reduced β‐secretase activity. 60 One small study demonstrated that pioglitazone conferred cognitive and functional benefits to patients with mild AD and diabetes mellitus, 61 while a phase III trial with APOE ε4 carrier stratification showed no benefits of rosiglitazone. 62
3.3. Non‐pharmacological treatment and prevention strategies in humans
In general, few studies have focused specifically on benefits of lifestyle modification for individuals with VCID or on VCID‐related outcomes. Evidence from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study, a randomized controlled trial (RCT) testing a multidomain intervention, suggests that administering diet, exercise, cognitive training, and vascular risk monitoring could maintain or improve cognitive function in older adults thought to be at increased risk of cognitive decline or dementia. 63 While promising, replication of these results is necessary in larger and more diverse populations; this is currently being undertaken by the World‐Wide FINGERS network (www.alz.org/wwfingers), which comprises >30 interventional studies around the world. 64 , 65 Among these studies, the US POINTER Study (NCT03688126) has an entire ancillary study dedicated to neurovascular function.
Differences in educational attainment are consistently associated with variations in cognitive and brain reserve. For instance, it is known that age‐related reductions in hippocampal volume are less pronounced among more highly educated individuals. 66 A few studies have shown significant effects of cognitive reserve over the expression of VCID: education and managerial or professional occupations buffer individuals against cognitive impairment caused by stroke and promote rapid cognitive recovery early after stroke; 67 higher education preserves cognitive function in individuals with similar degrees of subcortical hyperintensity burden; 68 and education impacts processing speed in patients with CADASIL who have mild and moderate (but not severe) degrees of neuroimaging‐confirmed brain pathology, reflecting faster cognitive decline once cognitive reserve is depleted. 69 However, one meta‐analysis showed that formal education had a small to medium effect on vascular cognitive impairment after stroke in young patients, while the effect of education on post‐stroke executive dysfunction was mediated by age, and below‐average performance in the attention domain was more frequent for patients with lower levels of education. 70 Future prospective studies are expected to address whether strategies to enhance cognitive reserve can help patients cope with more extensive vascular neurodegenerative mechanisms.
Several studies have focused specifically on exercise as a single intervention. One RCT reported that aerobic exercise improves global cognition in older adults with mild subcortical ischemic vascular cognitive impairment. 71 Results relating to neuroimaging outcomes are mixed. The results of a small ultra–high‐field MRI substudy of a 24‐month physical activity intervention RCT suggested that physical activity may be capable of beneficially altering small vessel morphology, 72 while another suggested resistance training may also be protective by demonstrating reduced WMH volume progression in community‐dwelling women. 73 Conversely, a 24‐month physical activity trial in individuals with high cardiovascular disease risk burden failed to demonstrate significant reductions in WMH progression or hippocampal atrophy, 74 coinciding with evidence from the FINGER trial which also showed no effects on WMH or other brain structural outcomes. 75 Another ongoing RCT 76 exploring the effects of resistance exercise on cognition and WMH progression in older adults with cSVD will provide further clarification. Additional exercise trials such as Exercise in Adults with Mild Memory Problems (EXERT; NCT02814526) and Investigating Gains in Neurocognition in an Intervention Trial of Exercise (IGNITE), 77 although not specifically targeting VCID, will include relevant neuroimaging data and blood‐based biomarkers that will aid in further understanding the impact of exercise on outcomes of interest.
4. PERSPECTIVES AND PRIORITIES FOR FUTURE RESEARCH
4.1. Inclusion, diversity, and justice in dementia research
To prevent and treat VCID in all groups of people, research must include diverse study participants; evaluate diverse disease determinants, including social and policy determinants; and carry out rigorous scientific study. As researchers in health equity, social determinants of health, aging, and AD and related dementias have recently pointed out, 78 , 79 inequitable representation in research is a barrier to both scientific accuracy and the human subjects research ethics principle of justice laid out in the Belmont report. 80 Much of the world's population with dementia (≈60%) live in low‐ and middle‐income countries (LMICs), 81 yet most VCID studies have been carried out in high‐income countries. Similarly, little is known about VCID in Indigenous and minoritized populations around the world, populations living in rural areas, by race/ethnicity, and by gender/sex. Furthermore, both neuroimaging and aging studies are known to suffer from selection and survival biases which limit the scope and accuracy of our VCID knowledge. 82
Understanding VCID in all populations will have significant impact considering that some bear a greater burden of or are at greater risk of cognitive impairment (e.g., Black/African Americans, American Indian/Alaska Natives, Latinx compared to Asian Americans or non‐Hispanic White Americans 83 , 84 ), some have more vascular risk factors and comorbidities (e.g., Latinx, Black/African Americans, American Indians 84 , 85 , 86 ), some have unique exposures or differing social and structural circumstances that increase risk or impact care (e.g., sex‐specific exposures such as preeclampsia and menopause; 87 , 88 discrimination and racism; 84 social stigma, and fewer potential caregivers among sexual and gender minorities 89 ). Studying diverse groups can also give unique insights into protective factors.
Finding the best interventions for all people with VCID can therefore be enhanced by jointly applying the science of inclusion and population neuroscience. The science of inclusion (which has also been called the science of recruitment and retention) develops systematic approaches to achieve equitable representation in research. 79 Population neuroscience, which integrates epidemiology and neuroscience methods, 79 , 90 provides a framework to further address existing research limitations in that it offers ways to harness population heterogeneity, incorporate neuroimaging and molecular markers, pool and coordinate data across studies and countries, and carefully and quantitatively address internal and external validity. 91 For example, this approach has found that mid‐ and late‐life vascular risk factors increase risk of poor late‐life brain health 17 , 92 and that simple physical activity, such as walking, is a protective factor. 93 A population neuroscience framework has been applied to dementia and cSVD, and can be applied to VCID more broadly. 91 , 94 Together, the science of inclusion and population neuroscience can improve the diversity of study samples and the quality of the conclusions drawn from VCID research.
Recommendations for future research on this topic:
Apply systematic approaches learned from the science of inclusion to carry out studies of VCID in diverse study samples and locations and to achieve equitable representation in research.
Apply epidemiologic methods to neuroscience research under a population neuroscience framework to enhance rigor of VCID study designs and analyses.
4.2. Exploring the genetic signature of VCID and improving non‐clinical models
As bioinformatics and sequencing technologies have advanced, studies have aimed to measure genetic changes at the cellular level by performing single cell (sc‐) or single nuclei (sn‐) RNA sequencing. However, the dense basement membrane enveloping blood vessels makes it challenging to isolate single brain vascular cells (e.g., myocytes, pericytes, endothelial cells) or their nuclei. In a recent study that sequenced >75,000 brain cells from control or AD patients, only 0.2% were pericytes and 0.2% were endothelial cells, both of which were excluded from the differential analyses due to their limited abundance. 95 A new method has been developed to successfully isolate nuclei from human brain vascular cells from control and AD hippocampus and cortex. 96 The study identified that, unlike the mouse brain, the human brain has two types of pericytes defined as matrix‐ or transporter‐type pericytes. 96 The authors determined that 30 of the top 45 AD GWAS genes are expressed in the human brain vasculature. Future research will be needed to elucidate the location and functions of matrix‐type pericytes that are reduced in AD and to further characterize vascular genetic changes throughout the brain in VCID. 96
BOX. Interactions of APOE ε4 carrier status with neurological features in specific populations with VCID
Variable age at dementia onset in selected populations 16 , 111
Cognitive activity and vascular health may reduce the risk of dementia also in APOE ε4 carriers 17 , 126
Education and lifetime sanitary conditions have protective effects against risk of AD particularly for APOE ε4 carriers 19
Modulation of frequency of most behavioral symptoms particularly in AD 111 , 112
Higher predisposition of APOE ε4 carriers to BBB dysfunction and subsequent cognitive decline 110
Predisposition to amyloid‐related imaging abnormalities (ARIA) 122
Rises in blood pressure may compensate for endothelial dysfunction and improve cerebral perfusion rates in APOE ε4 carriers with AD 57
APOE ε4 carriers with AD exhibit decreased participation in physical activities 109
Higher body mass index seems to be protective in late life across APOE haplotypes 20
Longitudinal benefits of a worsening lipid profile to APOE ε4 non‐carriers with AD as a result of enhanced lipid availability for protection of neuronal membranes 127
Modulation of effects of cerebrovascular metabolism modulators 58 , 59 , 128
Pleiotropic effects and interactions with other genes, thus affecting clinical response to angiotensin‐converting enzyme inhibitors 58 and statins 128
Experimental models (e.g., hypoperfusion, pericyte‐deficient, APOE ε4, Aβ‐ or tau‐overexpressing, and aged) emulate various aspects of VCID, including reduced CBF, BBB leakage, pericyte dysfunction, WM damage, fibrin deposits, BBB transporter expression changes, and cognitive impairment. 97 The Model Organism Development & Evaluation for Late‐Onset Alzheimer's Disease (MODEL AD, https://www.model‐ad.org/) and the UK Dementia Research Institute (https://ukdri.ac.uk/) are working to develop next‐generation animal models to recapitulate pathophysiological features of AD including vascular dysfunction. The aforementioned study using snRNA sequencing of vascular cells from control and AD human brains may inform the development of new transgenic and translational models, while the incorporation of relevant cardiovascular risk factors into existing animal models would enhance their translational value. 1 , 96 Human‐induced pluripotent stem cell models of the BBB and neurovascular unit are also promising new translational approaches, assuming they can re‐create physiological conditions (e.g., capillary diameter, actual CBF, glycocalyx changes, flexible basement membrane matrix, and proper incorporation of all neurovascular unit cell types).
Pericytes are contractile cells capable of reducing capillary red blood cell flow. 98 Pericyte‐deficient mice have disrupted neurovascular coupling resulting in reduced oxygen supply to the brain, metabolic stress, neurodegeneration, 99 and WM degeneration. 100 One recent study implicated stalled capillaries (blocked by neutrophils) contributing to CBF reduction and likely short‐term memory deficits. 101 Understanding the interplay between classic AD pathology (e.g., Aβ and tau) and CBF through capillaries is both timely and important. 52 , 102 , 103 A recent study showed that Aβ oligomers induce pericyte contraction and capillary constriction, which likely contribute to CBF reductions, vascular inflammation, and cognitive impairment. 104
Recommendations for future research on this topic:
Elucidate the location and functions of matrix‐type pericytes that are reduced in AD.
Further characterize vascular genetic changes throughout the brain in VCID.
Develop and rigorously validate new models that fully mirror the pathophysiological range of VCID.
Explore new VCID models to determine whether pericyte dysfunction and loss, oxidative stress, BBB breakdown, or increased chronic vascular inflammation could lead to stalled capillaries using state‐of‐the‐art methodologies such as intravital multiphoton microscopy (Figure 3D and E).
FIGURE 3.
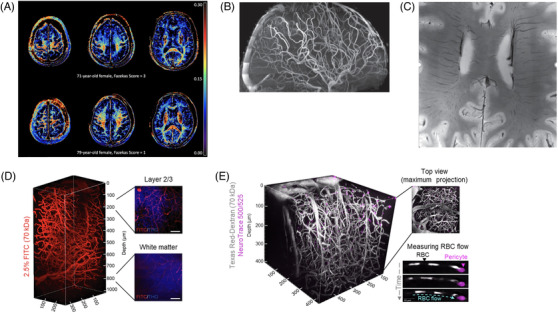
Emerging neuroimaging techniques in human and animal models. Note: (A) Myelin water fraction maps used to image in vivo myelin content in the human brain. Warmer regions indicate greater degree of myelination. In comparison are maps from two individuals with varying degrees of white matter lesion burden, courtesy of Dr. Teresa Liu‐Ambrose (The University of British Columbia). B, Time‐of‐flight angiography used to visualize small arteries that appear as thin thread‐like areas of flow‐related contrast in the human brain. C, Susceptibility‐weighted image used to visualize small veins in the human brain; (B) and (C) were adapted from Jorgensen et al. 94 and replicated with permission. Images were acquired without the use of any contrast agents at 7T using the Tic Tac Toe Radiofrequency Coil System (http://rf‐research‐facility.engineering.pitt.edu/). Images were provided by Dr. Tamer Ibrahim (University of Pittsburgh). D, 3D reconstruction of a wild‐type mouse using three‐photon microscopy, the blood vessels are labeled with a 2.5% fluorescein isothiocyanate (FITC)‐dextran (red). Selected z‐stacks labeled with 2.5% FITC‐dextran (blood vessels; red) and third‐harmonic generation (THG) (myelinated axons; blue) labeled at 200 μm (upper panel) and the white matter at 800 μm (lower panel). Scale bar, 50 μm. E, Vascular and pericyte architecture can be visualized in vivo in the mouse brain through a cranial window by multiphoton microscopy using Texas Red‐Dextran, 70 kDa (white) and NeuroTrace 500/525 (fuchsia), respectively. Dextran is not taken up by red blood cells (RBCs) allowing visualization and quantification of RBC flow when imaging speeds are 100 fps or greater
4.3. Efforts in biomarker identification and clinical diagnoses
Our ability to effectively identify and intervene for high‐risk individuals hinges on validated biomarkers. Multiple fluid and neuroimaging markers are used in VCID research, but the development of standardized pre‐analytic and analytic processes, harmonization of measures across multi‐center studies, proof of measurement reliability, and biological validation against clinically relevant outcomes has been difficult. The MarkVCID consortium is working to address this barrier and is presently testing 11 candidate fluid and neuroimaging biomarkers (see Table 1). We refer readers to the MarkVCID study design papers 105 , 106 and biomarker protocols (https://markvcid.partners.org/consortium‐protocols‐resources) for a full description. Another multinational effort to harmonize MRI measures of cSVD is the Harmonizing Brain Imaging Methods for Vascular Contributions to Neurodegeneration (HARNESS) initiative; 107 imaging protocols may be found at their website (https://harness‐neuroimaging.org/).
TABLE 1.
MarkVCID candidate neuroimaging and fluid biomarkers for vascular cognitive impairment and dementia
| Candidate biomarkers | |
|---|---|
| Neuroimaging |
A risk score for arteriolosclerosis based on multimodal MRI and demographic characteristics (ARTS) Cerebrovascular reactivity White matter hyperintensities volume White matter hyperintensities progression/regression Peak skeletonized mean diffusivity Mean white matter free water fraction Optical coherence tomography angiography retinal vessel skeleton density |
| Fluid |
Plasma endothelial signaling—VEGF‐D, PlGF, and bFGF Plasma exosome endothelial inflammation—C3b and Bb (activated complement factors) Plasma NfL Cerebrospinal fluid PlGF |
Abbreviations: bFGF, basic fibroblast growth factor; MRI, magnetic resonance imaging; NfL, neurofilament light; PIGF, placental growth factor; VEGF‐D, vascular endothelial growth factor.
Furthermore, VCID‐related biomarkers of interest to us as ECRs in this field include: cerebrospinal fluid soluble platelet‐derived growth factor receptor‐β (sPDGFRβ) as a marker of brain capillary and BBB damage, 108 ultra–high‐field (7T) MRI susceptibility‐weighted imaging of small veins and time‐of‐flight imaging of small arteries, cerebrospinal fluid flow imaging, and myelin water fraction via myelin water imaging (several of these techniques are shown in Figure 3). Overall, most existing neuroimaging measures of cSVD (reviewed in Wardlaw et al. 13 ) measure tissue damage thought to be due to cSVD but are unable to directly measure damage to the vessels themselves. Genetic factors may also be important prognostic biomarkers for VCID. For example, although this may vary by race/ethnicity, APOE ε4 increases risk of dementia both additively and synergistically with other vascular and cardiometabolic risk factors and may modify relationships of other biomarkers (such as sPDGFRβ) with cognitive decline. 17 , 19 , 20 , 109 , 110 Additional genetic variants involved in cerebrovascular metabolism may be better candidate markers of specific neuropsychiatric features rather than clinical diagnosis. 94 , 111 , 112
In addition to neuroimaging and fluid‐based biomarkers, neurocognitive testing is critical to VCID research as clinical diagnosis relies heavily on it. However, cross‐study collaborations and comparisons have been hampered by differing neurocognitive batteries. Efforts to harmonize neurocognitive assessments are crucial for progress in the field. 113
Recommendations for future research on this topic:
Further explore existing biomarkers and generate novel biomarkers of VCID.
Apply novel structural and functional neuroimaging techniques and fluid biomarkers to measure more direct vessel damage.
Effectively integrate neuroimaging and fluid biomarkers for diagnostic confirmation at enrollment and differential diagnoses with other dementia syndromes.
Develop and harmonize neurocognitive assessments to better address VCID.
4.4. Critical aspects of future randomized controlled trials
Understanding disease etiology will allow for identification of ideal targets for RCTs in the context of primary and secondary prevention. 6 Using neuroimaging techniques as a diagnostic marker to confirm presence of cerebrovascular disease at enrollment is critical. As well, neuroimaging to assess the efficacy of various interventions for VCID is appealing, especially when techniques continue to be optimized (see Figure 3). Nonetheless, it is crucial to identify markers that are consistently sensitive to intervention effects, because evidence indicates that commonly used markers, such as WMH progression, may respond differently to pharmacological 114 and non‐pharmacological interventions, 73 , 75 and may not directly correlate with changes in cognition. 115 Thus, understanding the sensitivity of other VCID neuroimaging markers, such as lacunar infarcts, microbleeds, enlargement of perivascular spaces, loss of microstructural tissue integrity, and secondary neurodegeneration will aid in designing future clinical trials. 115 Moreover, use of novel neuroimaging techniques that correlate well with clinical outcomes, such as diffusion tensor imaging and myelin water imaging, 116 will increase the clinical utility. Addressing these issues will allow for a mechanistic understanding of how, and to what degree, preventive or therapeutic interventions lead to clinical improvement. 117 In addition, it is essential that clinical trials include genetic association data (particularly regarding APOE ε4 carrier status) and analyses by sex/gender, and race/ethnicity. 20
Further, with the increasing emphasis on lifestyle RCTs, having a clear and efficient pipeline to move promising interventions from pilot studies to well‐powered RCTs could accelerate progress in the field. This is needed to overcome several limitations from previous investigations. For instance, many studies evaluate the effects of lifestyle interventions on dementia, without specifying subtype. Others include small sample sizes, short follow‐ups, and modest effect sizes on primary outcome measures; therefore, replication of their findings in larger samples is critical. Moreover, few studies consider gender/sex as important variables despite evidence suggesting the efficacy of these interventions may vary based on these factors. 118 Multicomponent intervention, such as that implemented in the FINGER study, may be more successful in mitigating disease burden than either intervention alone (e.g., exercise, cognitive training, diet, vascular risk monitoring); 51 however, it remains to be determined whether multicomponent interventions can be feasibly implemented in real‐world settings. Notably, interventions targeting factors beyond exercise, cognitive training, and diet are warranted. For instance, improving sleep quality is feasible and could be beneficial 119 given the potential impact of poor sleep on cerebrovascular health. 120
Last, in July 2021, the FDA gave accelerated approval for aducanumab as the first amyloid‐reducing drug for AD. 121 While the efficacy of aducanumab and other anti‐amyloid monoclonal antibodies is beyond the scope of this paper, its approval has widespread implications. Aside from potential benefits of anti‐amyloid immunotherapy, one of the noted side effects in some individuals is the subsequent occurrence of amyloid‐related imaging abnormalities (ARIA) in the form of vasogenic edema or hemorrhage. 122 , 123 , 124 ARIA has long been identified as an adverse event in AD trials of anti‐amyloid candidate drugs and could be problematic for patients with cSVD and CAA. 122 Except for APOE ε4 carrier status and history of CAA, little is known about what predisposes individuals to ARIA. 122 Thus, the use of anti‐amyloid drugs in individuals with cardiometabolic risk factors and comorbid vascular conditions will need to be monitored in future trials, considering these individuals are already at higher risk of cSVD. 125 Especially for CAA, immune response in ARIA appears to be targeted against vascular amyloid, indicating that study of vascular–amyloid–immune interactions may be critical for translating these therapies safely.
Recommendations for future research on this topic:
Validate and incorporate neuroimaging and fluid‐based biomarker outcomes to enable clearer understanding of effects of interventions in individuals along the VCID spectrum.
Establish minimal clinically important difference (i.e., the smallest change in outcome that a patient deems important) of primary outcomes in VCID subclasses.
Explore feasibility of multidomain lifestyle interventions in real‐world settings and assess long‐term benefits of such interventions.
Test the safety and efficacy of new therapies in diverse populations with measures that are inclusive of cardiometabolic risk factors and comorbid vascular conditions. 125
5. CONCLUSIONS
In this perspective piece, we provide an overview of VCID, discuss current limitations in the field, and highlight important areas of future research. Our recommendations should be interpreted as relevant research opportunities to advance the field in directions that span basic and clinical science. We acknowledge the need for support to ensure these recommendations can be realized. To that end, we call upon funders to support professional organizations and research institutions to train ECRs to do this work.
CONFLICTS OF INTEREST
Nárlon C. Boa Sorte Silva, Oliver Bracko, Amy R. Nelson, Fabricio Ferreira de Oliveira, Lisa S. Robison, C. Elizabeth Shaaban have nothing to disclose. Atticus H. Hainsworth has received honoraria from Eli‐Lilly and from NIA, he is a member of the Vascular Cognitive Disorders PIA within ISTAART and he leads the UK Medical Research Council Dementias Platform UK (DPUK) Vascular Experimental Medicine group. Brittani R. Price is a full‐time employee of Life Molecular Imaging, GmbH.
AUTHOR CONTRIBUTIONS
Original concept: Nárlon C. Boa Sorte Silva, Brittani R. Price, Atticus H. Hainsworth; first draft: Nárlon C. Boa Sorte Silva; writing of the manuscript: Nárlon C. Boa Sorte Silva, Oliver Bracko, Amy R. Nelson, Fabricio Ferreira de Oliveira, Lisa S. Robison, C. Elizabeth Shaaban, and Brittani R. Price; editing and formatting: Nárlon C. Boa Sorte Silva and Brittani R. Price. Revision for critical content: all authors.
ACKNOWLEDGMENTS
The authors would like to deeply thank Dr. Heather Snyder, PhD, from the Alzheimer's Association for her critical and insightful review of this article. NCBSS is a post‐doctoral fellow jointly funded by the Michael Smith Health Research BC, the Pacific Alzheimer Research Foundation, and the Canadian Institutes of Health Research. OB was funded by the DFG German Research Foundation. The research of ARN was supported by the National Institute on Aging of the National Institutes of Health (R00AG058780) and AlzOut (https://alzout.org/). FFO is supported by FAPESP–The State of São Paulo Research Foundation (grant #2015/10109‐5). CES was funded by T32AG055381 from the National Institute on Aging of the US National Institutes of Health. This article was facilitated by the Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART) through the Vascular Cognitive Disorders Professional Interest Area (PIA). The views and opinions expressed in this article represent those of the authors and do not necessarily reflect those of the PIA membership, ISTAART, the Alzheimer's Association, the National Institutes of Health, or Life Molecular Imaging.
Silva NCBS, Bracko O, Nelson AR, et al. Vascular cognitive impairment and dementia: An early career researcher perspective. Alzheimer's Dement. 2022;14:e12310. 10.1002/dad2.12310
Nárlon C. Boa Sorte Silva and Brittani R. Price organized, led the submission, and contributed equally to this manuscript.
Oliver Bracko, Amy R. Nelson, Fabricio Ferreira de Oliveira, Lisa S. Robison, and C. Elizabeth Shaaban contributed equally to this manuscript.
REFERENCES
- 1. Zlokovic B V., Gottesman RF, Bernstein KE, et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimer's Dement. 2020;16:1714‐1733. [DOI] [PubMed] [Google Scholar]
- 2. Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimer's Dement. 2015;11:710‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimer's Dement. 2018;14:148‐156. [DOI] [PubMed] [Google Scholar]
- 4. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community‐dwelling older persons. Neurology 2007;69:2197‐2204. [DOI] [PubMed] [Google Scholar]
- 5. Smith E, Wright C. Etiology, clinical manifestations, and diagnosis of vascular dementia—UpToDate. UpToDate. 2020:1‐31. [Google Scholar]
- 6. Dichgans M, Leys D. Vascular cognitive impairment. Circ Res 2017;120:573‐591. [DOI] [PubMed] [Google Scholar]
- 7. Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimer's Dement 2018;14:280‐292. [DOI] [PubMed] [Google Scholar]
- 8. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689‐701. [DOI] [PubMed] [Google Scholar]
- 10. Alber J, Alladi S, Bae H, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimer's Dement Transl Res Clin Interv. 2019;5:107‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, et al. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): a Framework for Advancing Research Priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36:281‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carnevale L, Lembo G. Innovative MRI techniques in neuroimaging approaches for cerebrovascular diseases and vascular cognitive impairment. Int J Mol Sci 2019;20:2656. 10.3390/ijms20112656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iadecola C, Duering M, Hachinski V, et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol 2019;73:3326‐3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hainsworth AH, Minett T, Andoh J, et al. Neuropathology of white matter lesions, blood‐brain barrier dysfunction, and dementia. Stroke 2017;48:2799‐2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Oliveira FF, Bertolucci PHF, Chen ES, Smith MC. Risk factors for age at onset of dementia due to Alzheimer's disease in a sample of patients with low mean schooling from São Paulo, Brazil. Int J Geriatr Psychiatry. 2014;29:1033‐1039. [DOI] [PubMed] [Google Scholar]
- 17. Shaaban CE, Jia Y, Chang CCH, Ganguli M. Independent and joint effects of vascular and cardiometabolic risk factor pairs for risk of all‐cause dementia: A prospective population‐based study. Int Psychogeriatrics. 2019;31. 10.1017/S1041610219001066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tosto G, Bird TD, Bennett DA, et al. The role of cardiovascular risk factors and stroke in familial Alzheimer disease. JAMA Neurol. 2016;73:1231‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Oliveira FF, De Almeida SS, Chen ES, Smith MC, Naffah‐Mazzacoratti MG, Bertolucci PHF. Lifetime risk factors for functional and cognitive outcomes in patients with Alzheimer's disease. J Alzheimer's Dis. 2018;65:1283‐1299. [DOI] [PubMed] [Google Scholar]
- 20. Oliveira FF, Chen ES, Smith MC, Bertolucci PH. Predictors of cognitive and functional decline in patients with Alzheimer disease dementia from Brazil. Alzheimer Dis Assoc Disord. 2016;30:243‐250. [DOI] [PubMed] [Google Scholar]
- 21. Traylor M, Malik R, Nalls MA, et al. Genetic variation at 16q24.2 is associated with small vessel stroke. Ann Neurol. 2017;81:383‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Persyn E, Hanscombe KB, Howson JMM, Lewis CM, Traylor M, Markus HS. Genome‐wide association study of MRI markers of cerebral small vessel disease in 42,310 participants. Nat Commun 2020;11:2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris SE, Malik R, Marioni R, et al. Polygenic risk of ischemic stroke is associated with cognitive ability. Neurology 2016;86:611‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic B V. Blood‐brain barrier: from physiology to disease and back. Physiol Rev 2019;99:21‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rannikmäe K, Davies G, Thomson PA, et al. Common variation in COL4A1/COL4A2 is associated with sporadic cerebral small vessel disease. Neurology 2015;84:918‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malik R, Rannikmäe K, Traylor M, et al. Genome‐wide meta‐analysis identifies 3 novel loci associated with stroke. Ann Neurol 2018;84:934‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ikram MA, Bersano A, Manso‐Calderón R, et al. Genetics of vascular dementia—review from the ICVD working group. BMC Med. 2017;15:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trigiani LJ, Royea J, Tong XK, Hamel E. Comparative benefits of simvastatin and exercise in a mouse model of vascular cognitive impairment and dementia. FASEB J. 2019;33:13280‐10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi B‐R, Kim D‐H, Bin BD, et al. Characterization of white matter injury in a rat model of chronic cerebral hypoperfusion. Stroke. 2016;47:542‐547. [DOI] [PubMed] [Google Scholar]
- 30. Amenta F. Vascular and neuronal hypertensive brain damage: protective effect of treatment with nicardipine. J Hypertens Suppl. 1996;14:S39‐S45. 10.1097/00004872-199610003-00006 [DOI] [PubMed] [Google Scholar]
- 31. Yang Y, Kimura‐Ohba S, Thompson J, Rosenberg GA. Rodent models of vascular cognitive impairment. Transl Stroke Res. 2016;7:407‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jalal FY, Yang Y, Thompson JF, Roitbak T, Rosenberg GA. Hypoxia‐induced neuroinflammatory white‐matter injury reduced by minocycline in SHR/SP. J Cereb Blood Flow Metab 2015;35:1145‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cho KO, HO La, Cho YJ, Sung KW, Kim SY. Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J Neurosci Res 2006;83:285‐291. [DOI] [PubMed] [Google Scholar]
- 34. Ma J, Zhang J, Hou WW, et al. Early treatment of minocycline alleviates white matter and cognitive impairments after chronic cerebral hypoperfusion. Sci Rep 2015;5:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Du B, Li H, Zheng H, et al. Minocycline ameliorates depressive‐like behavior and demyelination induced by transient global cerebral ischemia by inhibiting microglial activation. Front Pharmacol. 2019;0:1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan R, Xu F, Lou Previti M, et al. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057‐3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Protective effect of cyclosporin a on white matter changes in the rat brain after chronic cerebral hypoperfusion. Stroke. 1995;26:1415‐1422. [DOI] [PubMed] [Google Scholar]
- 38. Kim JS, Yun I, Bin Choi Y, Lee KS, Kim YI. Ramipril protects from free radical induced white matter damage in chronic hypoperfusion in the rat. J Clin Neurosci. 2008;15:174‐178. [DOI] [PubMed] [Google Scholar]
- 39. Kwon KJ, Kim MK, Lee EJ, et al. Effects of donepezil, an acetylcholinesterase inhibitor, on neurogenesis in a rat model of vascular dementia. J Neurol Sci. 2014;347:66‐77. [DOI] [PubMed] [Google Scholar]
- 40. Tayebati SK, Di Tullio MA, Tomassoni D, Amenta F. Neuroprotective effect of treatment with galantamine and choline alphoscerate on brain microanatomy in spontaneously hypertensive rats. J Neurol Sci. 2009;283:187‐194. [DOI] [PubMed] [Google Scholar]
- 41. Abi‐Ghanem C, Robison LS, Zuloaga KL. Androgens’ effects on cerebrovascular function in health and disease. Biol Sex Differ. 2020;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robison LS, Gannon OJ, Salinero AE, Zuloaga KL. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019;1710:43‐60. [DOI] [PubMed] [Google Scholar]
- 43. Robison LS, Popescu DL, Anderson ME, et al. Long‐term voluntary wheel running does not alter vascular amyloid burden but reduces neuroinflammation in the Tg‐SwDI mouse model of cerebral amyloid angiopathy. J Neuroinflammation 2019;16:144. 10.1186/s12974-019-1534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dominguez R, Zitting M, Liu Q, et al. Estradiol protects white matter of male C57BL6J mice against experimental chronic cerebral hypoperfusion. J Stroke Cerebrovasc Dis. 2018;27:1743‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu Y, Zhang Q, Zhang W, et al. Protective effect of 17β‐estradiol upon hippocampal spine density and cognitive function in an animal model of vascular dementia. Sci Rep 2017;7:42660. 10.1038/srep42660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mikkola TS, Savolainen‐Peltonen H, Tuomikoski P, et al. Lower death risk for vascular dementia than for Alzheimer's disease with postmenopausal hormone therapy users. J Clin Endocrinol Metab. 2017;102:870‐877. [DOI] [PubMed] [Google Scholar]
- 47. Madigan JB, Wilcock DM, Hainsworth AH. Vascular contributions to cognitive impairment and dementia: topical review of animal models. Stroke. 2016;47:1953‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Safaiyan S, Besson‐Girard S, Kaya T, et al. White matter aging drives microglial diversity. Neuron. 2021;109:1100‐1117.e10. [DOI] [PubMed] [Google Scholar]
- 49. Geirsdottir L, David E, Keren‐Shaul H, et al. Cross‐species single‐cell analysis reveals divergence of the primate microglia program. Cell. 2019;179:1609‐1622.e16. [DOI] [PubMed] [Google Scholar]
- 50. Weekman EM, Sudduth TL, Caverly CN, et al. Reduced efficacy of anti‐Aβ immunotherapy in a mouse model of amyloid deposition and vascular cognitive impairment comorbidity. J Neurosci. 2016;36:9896‐9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Prim. 2018;4:1‐16. [DOI] [PubMed] [Google Scholar]
- 52. Nelson AR, Sweeney MD, Sagare AP, Zlokovic B V. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer's disease. Biochim Biophys Acta ‐ Mol Basis Dis. 2016;1862:887‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA ‐ J Am Med Assoc. 2019;321:553‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nasrallah IM, Gaussoin SA, Pomponio R, et al. Association of intensive vs standard blood pressure control with magnetic resonance imaging biomarkers of Alzheimer disease: secondary analysis of the SPRINT MIND randomized trial. JAMA Neurol. 2021;78:E1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ding J, Davis‐Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta‐analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peters R, Yasar S, Anderson CS, et al. An investigation of antihypertensive class, dementia, and cognitive decline: A meta‐analysis. Neurology. 2020;94:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. De Oliveira FF, Chen ES, Smith MC, Bertolucci PHF. Associations of blood pressure with functional and cognitive changes in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2016;41:314‐323. [DOI] [PubMed] [Google Scholar]
- 58. De Oliveira FF, Chen ES, Smith MC, Bertolucci PHF. Pharmacogenetics of angiotensin‐converting enzyme inhibitors in patients with Alzheimer's disease dementia. Curr Alzheimer Res. 2017;15:386‐398. [DOI] [PubMed] [Google Scholar]
- 59. Ouk M, Wu CY, Rabin JS, et al. The use of angiotensin‐converting enzyme inhibitors vs. angiotensin receptor blockers and cognitive decline in Alzheimer's disease: the importance of blood‐brain barrier penetration and APOE ε4 carrier status. Alzheimer's Res Ther. 2021;13:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kehoe PG. The coming of age of the angiotensin hypothesis in Alzheimer's disease: progress toward disease prevention and treatment? J Alzheimer's Dis. 2018;62:1443‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR‐γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32:1626‐1633. [DOI] [PubMed] [Google Scholar]
- 62. Gold M, Alderton C, Zvartau‐Hind M, et al. Rosiglitazone monotherapy in mild‐to‐moderate Alzheimer's disease: results from a randomized, double‐blind, placebo‐controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255‐2263. [DOI] [PubMed] [Google Scholar]
- 64. Kivipelto M, Mangialsche F, Ngandu T, Solomon A, Tuomilehto J, Soininen H. From the finnish geriatric intervention study to prevent cognitive impairment and disability to the global dementia prevention initiative: applicability of multidomain interventions. Alzheimer's Dement. 2017;13:1221. [Google Scholar]
- 65. Baker L, Espeland M, Kivipelto M, et al. U.S. pointer: study design and trial kickoff. J Prev Alzheimer's Dis. 2018;5:S34‐S35. [Google Scholar]
- 66. Noble K, Grieve S, Korgaonkar M, et al. Hippocampal volume varies with educational attainment across the life‐span. Front Hum Neurosci. 2012;6:307. 10.3389/fnhum.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shin M, Sohn MK, Lee J, et al. Effect of cognitive reserve on risk of cognitive impairment and recovery after stroke: the KOSCO study. Stroke. 2020;51:99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lane EM, Paul RH, Moser DJ, Fletcher TD, Cohen RA. Influence of education on subcortical hyperintensities and global cognitive status in vascular dementia. J Int Neuropsychol Soc. 2011;17:531‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zieren N, Duering M, Peters N, et al. Education modifies the relation of vascular pathology to cognitive function: cognitive reserve in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiol Aging. 2013;34:400‐407. [DOI] [PubMed] [Google Scholar]
- 70. Kessels RPC, Eikelboom WS, Schaapsmeerders P, et al. Effect of formal education on vascular cognitive impairment after stroke: a meta‐analysis and study in young‐stroke patients. J Int Neuropsychol Soc. 2017;23:223‐238. [DOI] [PubMed] [Google Scholar]
- 71. Liu‐Ambrose T, Best JR, Davis JC, et al. Aerobic exercise and vascular cognitive impairment. Neurology. 2016;87:2082‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shaaban CE, Aizenstein HJ, Jorgensen DR, et al. Physical activity and cerebral small vein integrity in older adults. Med Sci Sports Exerc. 2019;51:1684‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bolandzadeh N, Tam R, Handy TC, et al. Resistance training and white matter lesion progression in older women: Exploratory analysis of a 12‐month randomized controlled trial. J Am Geriatr Soc. 2015;63:2052‐2060. [DOI] [PubMed] [Google Scholar]
- 74. Venkatraman VK, Sanderson A, Cox KL, et al. Effect of a 24‐month physical activity program on brain changes in older adults at risk of Alzheimer's disease: the AIBL active trial. Neurobiol Aging. 2020;89:132‐141. [DOI] [PubMed] [Google Scholar]
- 75. Stephen R, Liu Y, Ngandu T, et al. Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimer's Res Ther 2019;11:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu‐Ambrose T, Dao E, Crockett RA, et al. Reshaping the path of vascular cognitive impairment with resistance training: a study protocol for a randomized controlled trial. Trials 2021;22:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Erickson KI, Grove GA, Burns JM, et al. Investigating Gains in Neurocognition in an Intervention Trial of Exercise (IGNITE): Protocol. Contemp Clin Trials 2019;85:105832. 10.1016/j.cct.2019.105832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gilmore‐Bykovskyi A, Jackson JD, Wilkins CH. The urgency of justice in research: Beyond COVID‐19. Trends Mol Med 2021;27:97‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gilmore‐Bykovskyi A, Croff R, Glover CM, et al. Traversing the aging research and health equity divide: toward intersectional frameworks of research justice and participation. Gerontologist 2021. 10.1093/geront/gnab107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. Washington, DC: The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. 1979. [PubMed] [Google Scholar]
- 81. Gauthier S, Rosa‐Neto P, Morais J, Webster C. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London, England: Alzheimer's Disease International. 2021. [Google Scholar]
- 82. Ganguli M, Lee CW, Hughes T, et al. Who wants a free brain scan? Assessing and correcting for recruitment biases in a population‐based sMRI pilot study. Brain Imaging Behav 2015;9:204‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer's Dement. 2016;12:216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. 2021 Alzheimer's disease facts and figures. Alzheimer's Dement. 2021;17:327‐406. [DOI] [PubMed] [Google Scholar]
- 85. Carty CL, Noonan C, Muller C, et al. Risk factors for alzheimer's disease and related dementia diagnoses in American Indians. Ethn Dis 2020;30:671‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kirkpatrick AC, Stoner JA, Donna‐Ferreira F, et al. High rates of undiagnosed vascular cognitive impairment among American Indian veterans. GeroScience 2019;41:69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shaaban CE, Rosano C, Cohen AD, et al. Cognition and cerebrovascular reactivity in midlife women with history of preeclampsia and placental evidence of maternal vascular malperfusion. Front Aging Neurosci 2021;13. 10.3389/fnagi.2021.637574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gannon OJ, Robison LS, Custozzo AJ, Zuloaga KL. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem Int 2019;127:38‐55. [DOI] [PubMed] [Google Scholar]
- 89. Alzheimer's Association . Issues Brief: LGBT and Dementia. Chicago: Alzheimer's Association. 2021.
- 90. Indorewalla KK, O'Connor MK, Budson AE, Guess C, Jackson J. Modifiable barriers for recruitment and retention of older adults participants from underrepresented minorities in alzheimer's disease research. J Alzheimer's Dis. 2021;80:927‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ganguli M, Albanese E, Seshadri S, et al. Population neuroscience: dementia epidemiology serving precision medicine and population health. Alzheimer Dis Assoc Disord. 2018;32:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25‐year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74:1246‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Erickson KI, Raji CA, Lopez OL, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75:1415‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jorgensen DR, Shaaban CE, Wiley CA, Gianaros PJ, Mettenburg J, Rosano C. A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am J Physiol ‐ Hear Circ Physiol. 2018;314:H1117‐H1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mathys H, Davila‐Velderrain J, Peng Z, et al. Single‐cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570:332‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yang AC, Vest RT, Kern F, et al. A human brain vascular atlas reveals diverse mediators of Alzheimer's risk. Nature. 2022;603:885‐892. 10.1038/s41586-021-04369-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Montagne A, Zhao Z, Zlokovic B V. Alzheimer's disease: a matter of blood‐brain barrier dysfunction? J Exp Med. 2017;214:3151‐3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nelson AR, Sagare MA, Wang Y, Kisler K, Zhao Z, Zlokovic B V. Channelrhodopsin excitation contracts brain pericytes and reduces blood flow in the aging mouse brain in vivo. Front Aging Neurosci. 2020;0:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kisler K, Nelson AR, Rege S V., et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20:406‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Montagne A, Nikolakopoulou AM, Zhao Z, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24:326‐337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101. Cruz Hernández JC, Bracko O, Kersbergen CJ et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer's disease mouse models. Nat Neurosci. 2019;22:413‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bracko O, Cruz Hernández JC, Park L, Nishimura N, Schaffer CB. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer's disease. J Cereb Blood Flow Metab. 2021;41:1501‐1516. 10.1177/0271678X20982383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kisler K, Nelson AR, Montagne A, Zlokovic B V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nortley R, Korte N, Izquierdo P, et al. Amyloid b oligomers constrict human capillaries in Alzheimer's disease via signaling to pericytes. Science (80‐). 2019;365:6450. 10.1126/science.aav9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wilcock D, Jicha G, Blacker D, et al. MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols. Alzheimer's Dement. 2021;17:704‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lu H, Kashani AH, Arfanakis K, et al. MarkVCID cerebral small vessel consortium: II. Neuroimaging protocols. Alzheimer's Dement. 2021;17:716‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Smith EE, Biessels GJ, De Guio F, et al. Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimer's dement diagnosis, Assess Dis Monit. 2019;11:191‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nation DA, Sweeney MD, Montagne A et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. De Oliveira FF, Pivi GAK, Chen ES, Smith MC, Bertolucci PHF. Risk factors for cognitive and functional change in one year in patients with Alzheimer's disease dementia from São Paulo, Brazil. J Neurol Sci. 2015;359:127‐132. [DOI] [PubMed] [Google Scholar]
- 110. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Oliveira FF, Chen ES, Smith MC, Bertolucci PH. Associations of cerebrovascular metabolism genotypes with neuropsychiatric symptoms and age at onset of alzheimer's disease dementia. Rev Bras Psiquiatr. 2017;39:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. De Oliveira FF, De Almeida SS, Smith MC, Bertolucci PHF. Behavioural effects of the ACE insertion/deletion polymorphism in Alzheimer's disease depend upon stratification according to APOE‐ϵ4 carrier status. Cogn Neuropsychiatry. 2021;26:293‐305. [DOI] [PubMed] [Google Scholar]
- 113. Boccardi M, Monsch AU, Ferrari C, Altomare D, Berres M, Bos I, et al. Harmonizing neuropsychological assessment for mild neurocognitive disorders in Europe. Alzheimer's Dement, 2021;18:29‐42. 10.1002/alz.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nasrallah IM, Pajewski NM, Auchus AP, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA ‐ J Am Med Assoc. 2019;322:524‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684‐696. [DOI] [PubMed] [Google Scholar]
- 116. Bouhrara M, Reiter DA, Bergeron CM, et al. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimer's Dement. 2018;14:998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ackley SF, Zimmerman SC, Brenowitz WD, et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: Instrumental variable meta‐analysis. BMJ. 2021;372:n156. 10.1136/bmj.n156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Barha CK, Hsiung GYR, Best JR, et al. Sex difference in aerobic exercise efficacy to improve cognition in older adults with vascular cognitive impairment: secondary analysis of a randomized controlled trial. J Alzheimer's Dis. 2017;60:1397‐1410. [DOI] [PubMed] [Google Scholar]
- 119. Falck RS, Davis JC, Best JR, et al. Effect of a multimodal lifestyle intervention on sleep and cognitive function in older adults with probable mild cognitive impairment and poor sleep: a randomized clinical trial. J Alzheimer's Dis. 2020;76:179‐193. [DOI] [PubMed] [Google Scholar]
- 120. Lim ASP, Yu L, Schneider JA, Bennett DA, Buchman AS. Sleep fragmentation, cerebral arteriolosclerosis, and brain infarct pathology in community‐dwelling older people. Stroke. 2016;47:516‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. FDA Grants Accelerated Approval for Alzheimer's Drug | FDA 2021. Accessed July 25, 2021. https://www.fda.gov/news‐events/press‐announcements/fda‐grants‐accelerated‐approval‐alzheimers‐drug
- 122. Sperling RA, Jack CR, Black SE, et al. Amyloid‐related imaging abnormalities in amyloid‐modifying therapeutic trials: Recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimer's Dement. 2011;7:367‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ferrero J, Williams L, Stella H, et al. First‐in‐human, double‐blind, placebo‐controlled, single‐dose escalation study of aducanumab (BIIB037) in mild‐to‐moderate Alzheimer's disease. Alzheimer's Dement Transl Res Clin Interv. 2016;2:169‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384:1691‐704. [DOI] [PubMed] [Google Scholar]
- 125. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ferrari C, Xu WL, Wang HX, et al. How can elderly apolipoprotein E ε4 carriers remain free from dementia? Neurobiol Aging. 2013;34:13‐21. [DOI] [PubMed] [Google Scholar]
- 127. De Oliveira FF, Chen ES, Smith MC, Bertolucci PHF. Longitudinal lipid profile variations and clinical change in Alzheimer's disease dementia. Neurosci Lett. 2017;646:36‐42. [DOI] [PubMed] [Google Scholar]
- 128. De Oliveira FF, Chen ES, Smith MC, Bertolucci PHF. Selected LDLR and APOE polymorphisms affect cognitive and functional response to lipophilic statins in Alzheimer's disease. J Mol Neurosci. 2020;70:1574‐1588. [DOI] [PubMed] [Google Scholar]


