Summary
Background
Magnesium sulphate given to women prior to very preterm birth protects the perinatal brain, so fewer babies die or develop cerebral palsy. How magnesium sulphate exerts these beneficial effects remains uncertain. The MagNUM Study aimed to assess the effect of exposure to antenatal magnesium sulphate on MRI measures of brain white matter microstructure at term equivalent age.
Methods
Nested cohort study within the Magnesium sulphate at 30 to <34 weeks’ Gestational age Neuroprotection Trial (MAGENTA). Australian New Zealand Clinical Trials Registry ACTRN12611000491965. Mothers at risk of preterm birth at 30 to <34 weeks’ gestation were randomised to receive either 4 g of magnesium sulphate heptahydrate [8 mmol magnesium ions], or saline placebo, when preterm birth was planned or expected within 24 h. Participating babies underwent diffusion tensor MRI at term equivalent age. The main outcomes were fractional anisotropy across the white matter tract skeleton compared using Tract-based Spatial Statistics (TBSS), with adjustment for postmenstrual age at birth and at MRI, and MRI site. Researchers and families were blind to treatment group allocation during data collection and analyses.
Findings
Of the 109 babies the demographics of the 49 babies exposed to magnesium sulphate were similar to the 60 babies exposed to placebo. In babies whose mothers were allocated to magnesium sulphate, fractional anisotropy was lower within the corticospinal tracts and corona radiata, the superior and inferior longitudinal fasciculi, and the inferior fronto-occipital fasciculi compared to babies whose mothers were allocated placebo (P < 0·05).
Interpretation
In babies born preterm after 30 weeks’ gestation, antenatal magnesium sulphate exposure did not promote development of white matter microstructure in pathways affecting motor or cognitive function. This suggests that if the neuroprotective effect of magnesium sulphate treatment prior to preterm birth is confirmed at this gestation, the mechanisms are not related to accelerated white matter maturation inferred from fractional anisotropy.
Funding
This study was funded by a project grant from the Health Research Council of New Zealand (HRC 14/153).
Keywords: Antenatal magnesium sulphate, Fetal neuroprotection, Cerebral palsy, Preterm birth, Brain development, Diffusion tensor magnetic resonance imaging
Research in context.
Evidence before this study
Antenatal magnesium sulphate is recommended in clinical practice guidelines worldwide for women at risk of very preterm birth for neuroprotection of their fetus. Our recent individual participant data meta-analysis, that included five, relevant, randomised trials with a total of 5493 women and 6131 babies, found antenatal magnesium sulphate given prior to preterm birth reduces the risk of cerebral palsy and death or cerebral palsy. What has remained uncertain is how magnesium exerts these neuroprotective effects, and whether magnesium protects parts of the brain that are important for learning and behavior as well as those that control movement and posture.
Added value of this study
MagNUM is a prospective, multicentre, cohort study, recruiting participants from five, tertiary, maternity hospitals in New Zealand and Australia, designed to compare brain white matter microstructure at term equivalent age between babies whose mothers were randomised to receive either antenatal magnesium sulphate or placebo.
We wished to assess whether magnesium sulphate would have neuroprotective effects on the white matter tracts that subserve motor and cognitive function. To our knowledge this is the first comparative study to explore neuroprotective mechanisms for antenatal magnesium sulphate using diffusion tensor magnetic resonance imaging.
Our results show that babies exposed to magnesium sulphate compared to babies not exposed had lower fractional anisotropy (FA) and higher radial diffusivity (RD) in key white matter tracts at term-equivalent age not consistent with greater fibre coherence and maturation, or improved myelination. We also found lower FA and higher RD within fibres that subserve cognitive processes.
Implications of all the available evidence
Our findings show that antenatal magnesium sulphate prior to preterm birth after 30 weeks’ gestation does not promote white matter development in pathways affecting motor and cognitive function. These data suggest that if magnesium sulphate is found to be neuroprotective at gestational ages beyond 30 weeks, the mechanism of action is not related to accelerated white matter maturation inferred from fractional anisotropy.
Alt-text: Unlabelled box
Introduction
Babies born preterm compared with those born at term have a higher chance of dying in the first few weeks of life. Those who survive have a greater risk of neurologic impairments, including cerebral palsy, cognitive dysfunction, educational difficulties and psychiatric disorders in adulthood, with increased educational and societal costs.1, 2, 3
Antenatal magnesium sulphate is recommended for neuroprotection of the fetus in women at risk of very preterm birth,4 having been shown in individual participant data meta-analysis of relevant randomised trials to reduce the risk of death and cerebral palsy.5 How magnesium exerts this protective effect, and whether magnesium protects parts of the brain that are important for learning and behavior as well as those that control movement and posture, remains uncertain.
Dysmaturation of developing white matter is an important neuropathological substrate of adverse neurological outcome after preterm birth.6 It arises from upstream insults of hypoxia, ischaemia, and inflammation, which lead to primary injury or death of key cellular elements followed by secondary dysmaturation. The cells that are most susceptible are pre-myelinating oligodendrocytes, but axons within white matter, and subplate, thalamic and late migrating GABAergic neuronal populations are also affected. The end result of injurious and maldevelopmental processes affecting this range of cell types is a constellation of features, collectively termed the ‘encephalopathy of prematurity’, that are apparent on neonatal MRI as alterations in white and grey matter microstructure, impaired cortical folding and disturbances to regional brain growth.6
Diffusion tensor magnetic resonance imaging (DTI) has provided valuable insights into the effects of maturational and injurious processes in the developing brain.7 This is rooted in the premise that water movement is restricted by the presence of axons, neuronal cell bodies, glial cells and macromolecules, and this allows inference about brain water content, axonal density, axonal calibre, myelination, dendritic arborization and synapse formation. Commonly used DTI parameters are fractional anisotropy (FA), which describes the directional dependence of random water motion, mean diffusivity (MD) a measure of the magnitude of water motion, axial diffusivity (AD), the largest eigenvalue of the diffusion tensor in each voxel, potentially indicative of water diffusion parallel to axons, and radial diffusivity (RD), the average of the two remaining eigenvalues, potentially indicative of water diffusion perpendicular to axons. A consistent finding is that FA increases and MD decreases with increasing maturation of the preterm brain,8 reflecting decreasing water content and increasing complexity of white matter. Lower FA and higher MD are seen in the white matter of preterm infants at term equivalent age compared with healthy infants born at term.9, 10, 11
Tract-Based Spatial Statistics (TBSS) enables unbiased group-wise analysis of FA within the white matter derived from DTI data.9,12 TBBS has been used to map microstructural alterations in neonatal white matter tracts associated with preterm birth,13 intrauterine inflammation,14 maternal opioid use,15 and early life nutrition,16 and has proven useful for investigating neuroprotective treatments in newborns including erythropoietin for preterm brain injury, and therapeutic hypothermia and inhaled xenon for hypoxic-ischaemic encephalopathy.17,18
In this paper, we report TBSS results from the Magnesium for Neuroprotection: Understanding Mechanisms (MagNUM) Study which employed DTI to compare brain white matter microstructure at term-equivalent age between babies whose mothers were randomised to receive antenatal magnesium sulphate and those randomised to receive placebo. Our hypothesis was that magnesium sulphate would have neuroprotective effects in the white matter tracts that subserve motor and cognitive function. Since brain white matter microstructure is also influenced by gestational age, sex, multiple pregnancy, breast milk intake and serious neonatal illness such as bronchopulmonary dysplasia and necrotising enterocolitis, we also explored the effect of these factors on our findings using subgroup and sensitivity analyses.
Methods
Study design and participants
The MagNUM Study was nested within the multicentre Magnesium Sulphate at 30 to <34 weeks’ Gestational age Neuroprotection Trial (MAGENTA) comparing magnesium sulphate (magnesium sulphate heptahydrate: 8 mmol magnesium ions) with placebo (saline) in women at risk of imminent (within 24 h) preterm birth at 30 to <34 weeks’ gestation for the prevention of death or cerebral palsy, the primary outcome19 (Australian New Zealand Clinical Trials Registry ACTRN12611000491965). The central randomisation service stratified by collaborating centre, gestational age (30 to <32 weeks; 32 to <34 weeks’ gestation) and number of fetuses (1 or 2).
Ethics
Babies born to mothers enrolled in the MAGENTA Trial at Auckland City Hospital, Middlemore Hospital, and Christchurch Women's Hospital, New Zealand, and Women's and Children's Hospital and Flinders Medical Centre, Adelaide, Australia, were eligible for enrolment in the MagNUM Study. Exclusion criteria were known congenital or genetic disorders likely to affect brain structure, baby too unwell to have an MRI scan safely, or the family lived more than a one-hour drive from the MRI centre. Written informed consent was obtained from the caregiver of eligible babies. The MagNUM Study was approved by the New Zealand Northern B Health and Disability Ethics Committees LRS/12/06/021/AM02 and by the South Australian Human Research Ethics Committee HREC/16/WCHN/196.
Procedures
MRI was conducted at term-equivalent age (38 to 42 weeks’ postmenstrual age) on a 3 Tesla Siemens Skyra system at the Auckland Centre for Advanced MRI and the South Australia Medical Research Institute in Adelaide, and a 3 Tesla General Electric HDxt system at Pacific Radiology in Christchurch, using a 32 channel adult head coil. Babies were scanned in a neonate MRI beanbag evacuated for stabilization during natural sleep following a feed and swaddling.
To ensure the validity and robustness of inter-site DTI comparisons, the MRI protocol was standardised to acquire whole brain diffusion-weighted and high resolution T1 - and T2 - weighted anatomical MRI data with the same number of baseline and diffusion encoding gradient directions, b-values, slice locations and voxel dimensions (Table 1). A detailed written protocol was provided to the radiographers at all sites.
Table 1.
Summary table of DTI parameters used in the scanners at the study sites.
| Siemens Skyra (Auckland and Adelaide) | GE HDxT (Christchurch) | |
|---|---|---|
| T2 weighted MRI | Sampling perfection with application optimized contrasts using a different flip angle evolution (SPACE) | GE SPACE (“CUBE”) |
| Repetition time | 3200 ms | 2500 ms |
| Echo time | 405 ms | 100 ms |
| Voxels | 0·9 × 0·9 × 1 mm | 1 × 1 × 1 mm |
| Field of view | 180 mm | 180 mm |
| Diffusion weighted MRI | ||
| Repetition time | 7300 ms | 7300 ms |
| Echo time | 106 ms | 97 ms |
| Voxels | 2 mm3 | 2 mm3 |
| Field of view | 256 mm | 256 mm |
| Diffusion weighted directions | 64 | 64 |
| b-value | 750 | 750 |
| b0 images | 11 | 11 |
| Phase-encoding | Right to left | Right to left |
Outcomes
The primary outcome was regional group differences in FA throughout the cerebral white matter skeleton measured using TBSS. Secondary outcomes were group differences in MD (average diffusion along the three main axes of the diffusion tensor), axial diffusivity (AD, parallel to the white matter tract) and radial diffusivity (RD, perpendicular to the main axis of the diffusion tensor). Based on computational modelling20 and precedent from the TOBY-Xenon neonatal neuroprotective hypothermia randomised trial18 a study of 60 infants in each treatment group was estimated to be able to detect a 10% difference in FA with 80% power and two-sided 5% significance.
Statistical analysis
Analyses followed a statistical analysis plan prepared prior to any data analyses. Pre-specified reasons for participants to be excluded from TBSS analyses were if there was significant brain injury defined as parenchymal injury or brain abnormality identified on structural MRI by clinical reviewers and considered likely to confound image registration; if there was no diffusion MRI, scanner error, or excessive motion; if more than 10 diffusion weighted MRI volumes had slice dropout; or if registration failed. Application of these exclusion criteria was done blind to treatment group allocation to reduce the risk of selection bias in deriving the MagNUM Study per-protocol population. Researchers were blind to treatment group allocation until analyses were completed.
DTI data were analysed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library, version 5.0.10. (https://fsl.fmrib.ox.ac.uk/fsl)21 and DTI-ToolKit (v2.3.1 www.dti-tk. sourceforge.net) (DTI-TK).22 Data were corrected for phase encoding distortions, eddy-induced distortions and motion using the topup-eddy algorithm,21 using T2 structural volumes rigidly registered to b0 maps and assuming a bandwidth of zero (no phase-encoding).23 A diffusion tensor model was fitted to each voxel and the eigenvalues and eigenvectors were used to convert the corrected diffusion weighted images into diffusion tensor volumes (DTI-TK).
Tensor-based image registration (DTI-TK) was used to produce a population-specific diffusion tensor template. From this template the mean FA volume was derived and thinned by perpendicular non-maximum suppression to create the mean white matter tract skeleton, thresholded at FA > 0·15 to exclude peripheral tracts.9 All participants’ diffusion tensor volumes were registered to the diffusion tensor template and FA, MD, AD and RD were extracted and projected onto the white matter tract skeleton.
TBSS12 was used to compare voxel-wise statistics across the white matter skeleton between treatment groups, using a general linear model adjusting for postmenstrual age at birth and at MRI, and scan site. Significance was set as P < 0·05, following family-wise error rate correction and threshold-free cluster enhancement. TBSS results were reported as maps overlaying the skeleton where voxels with p-values <0.05 are highlighted using a colour bar to show the range of significant values.
Pre-specified sensitivity analyses included only babies who were exposed to at least some of the treatment allocated at randomisation to the MAGENTA Trial (magnesium sulphate or saline placebo); scanned at the largest MRI site; singletons; born before 34 weeks’ gestation; without bronchopulmonary dysplasia or necrotising enterocolitis; and exclusively received breast milk at the time of scanning. Pre-specified subgroup analyses assessed gestation at trial entry (30 to <32 weeks and 32 to <34 weeks), and boys and girls separately, and were not adjusted for scan site. For the sensitivity and subgroup analyses, where smaller sample sizes were expected to limit power, a pre-specified sequential analysis was undertaken. Magnesium sulphate and placebo groups were initially compared using TBSS analysis. If no significant differences were detected, a region-of-interest analysis was undertaken to determine if the direction of effect was consistent with the primary analysis. Voxels with significant between group differences in the primary analysis were used as the region of interest. Regions of interest were defined separately for FA, MD, AD and RD. The mean FA, MD, AD and RD within the respective to region-of-interest were compared using a two-sample t-test.
Role of funders
The funders of the study had no role in study design, data collection, analysis, interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
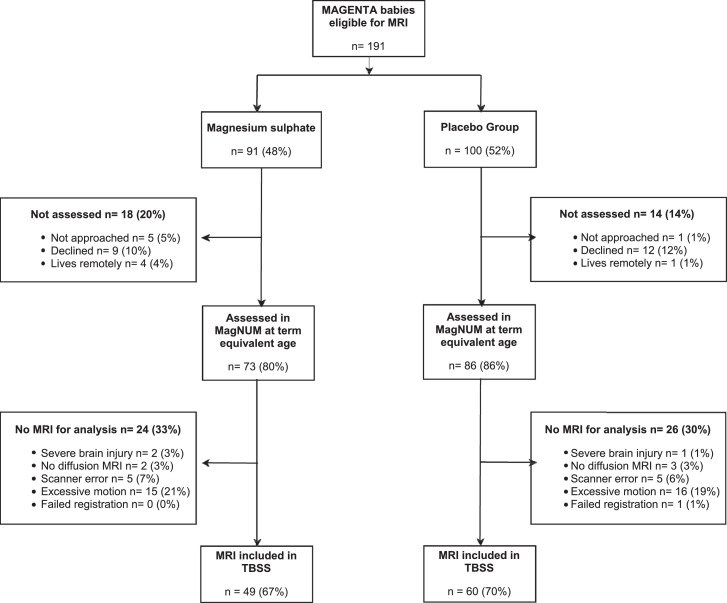
Of 191 babies eligible for the MagNUM Study, 159 babies were recruited for MRI at term equivalent age between 22 October 2014 and 25 October 2017 and, after exclusions, DTI data from 109 babies (49 in the magnesium sulphate group and 60 in the placebo group) were included (Figure 1). Babies and their mothers included in the MRI analyses were similar to the babies and their mothers not included (see Supplementary Table). The per-protocol MagNUM Study population of mothers and babies were similar in the magnesium sulphate and placebo groups in their demographics at entry into the MAGENTA Trial and after birth (Table 2).
Figure 1.
MagNUM Study recruitment and inclusion in Tract-Based Spatial Statistics (TBSS) analysis.
Table 2.
Characteristics of mothers and babies in the MagNUM Study
| Characteristics | Magnesium Sulphate | Placebo | p value |
|---|---|---|---|
| Mothers | n = 45 | n = 52 | |
| Age (years) | 30·9 (7·1) | 32·1 (5·3) | 0·36 |
| Nulliparous | 27 (60·0) | 28 (53·8) | 0·54 |
| Ethnicity | 0·43 | ||
| . Caucasian | 17 (37·8) | 24 (46·2) | |
| . Asian | 7 (15·6) | 12 (23·1) | |
| . Aboriginal or Torres Strait Islander | 0 (0·0) | 1 (1·9) | |
| . Polynesian | 2 (4·4) | 3 (5·8) | |
| . Māori | 7 (15·6) | 5 (9·6) | |
| . Other | 12 (26·7) | 7 (13·5) | |
| BMI (kg/m2) | 26·4 (5·2) | 26·9 (7·2) | 0·70 |
| Gestation at trial entry (weeks) | 31·9 (1·2) | 31·9 (1·0) | 0·73 |
| Main reason at risk of preterm birth: | |||
| . Antepartum haemorrhage | 5 (11·1) | 6 (11·5) | 0·95 |
| . Preterm prelabour rupture of membranes | 16 (35·6) | 16 (30·8) | 0·62 |
| . Preterm labour | 19 (42·2) | 24 (46·2) | 0·70 |
| . Pre-eclampsia | 8 (17·8) | 9 (17·3) | 0·95 |
| . Fetal compromise | 5 (11·1) | 11 (21·2) | 0·18 |
| . Other | 10 (22·2) | 11 (21·2) | 0·90 |
| Received treatment allocated | 44 (97·8) | 51 (98·1) | 0·92 |
| Babies | n=49 | n=60 | |
| Gestation at birth (weeks) | 32·2 (1·6) | 31·9 (0·9) | 0·28 |
| Twins | 0·53 | ||
| . Singleton | 38 (77·6) | 41 (68·3) | |
| . Twin 1 | 5 (10·2) | 10 (16·7) | |
| . Twin 2 | 6 (12·2) | 9 (15·0) | |
| MRI site | 0·15 | ||
| . Auckland | 33 (67·3) | 33 (55·0) | |
| . Christchurch | 13 (26·5) | 16 (26·7) | |
| . Adelaide | 3 (6·1) | 11 (18·3) | |
| Post-menstrual age at MRI (weeks) | 40·2 (1·3) | 40·1 (1·5) | 0·75 |
| Birth weight (g) | 1821 (536) | 1663 (362) | 0·08 |
| Birth weight (z score) | 0·25 (1·09) | -0·04 (1·04) | 0·16 |
| Bronchopulmonary dysplasia | 2 (4·1) | 6 (10·0) | 0·24 |
| Necrotising enterocolitis | 0 | 0 | |
| Exclusive breast milk feeding at time of MRI | 36 (73·5) | 39 (65·0) | 0·41 |
| Sex (Female) | 23 (46·9) | 27 (45·0) | 0·84 |
Data are mean (standard deviation) or n (%). Bronchopulmonary dysplasia was defined as oxygen requirement at 36 week's post-menstrual age. Z scores were calculated using WHO standards.40
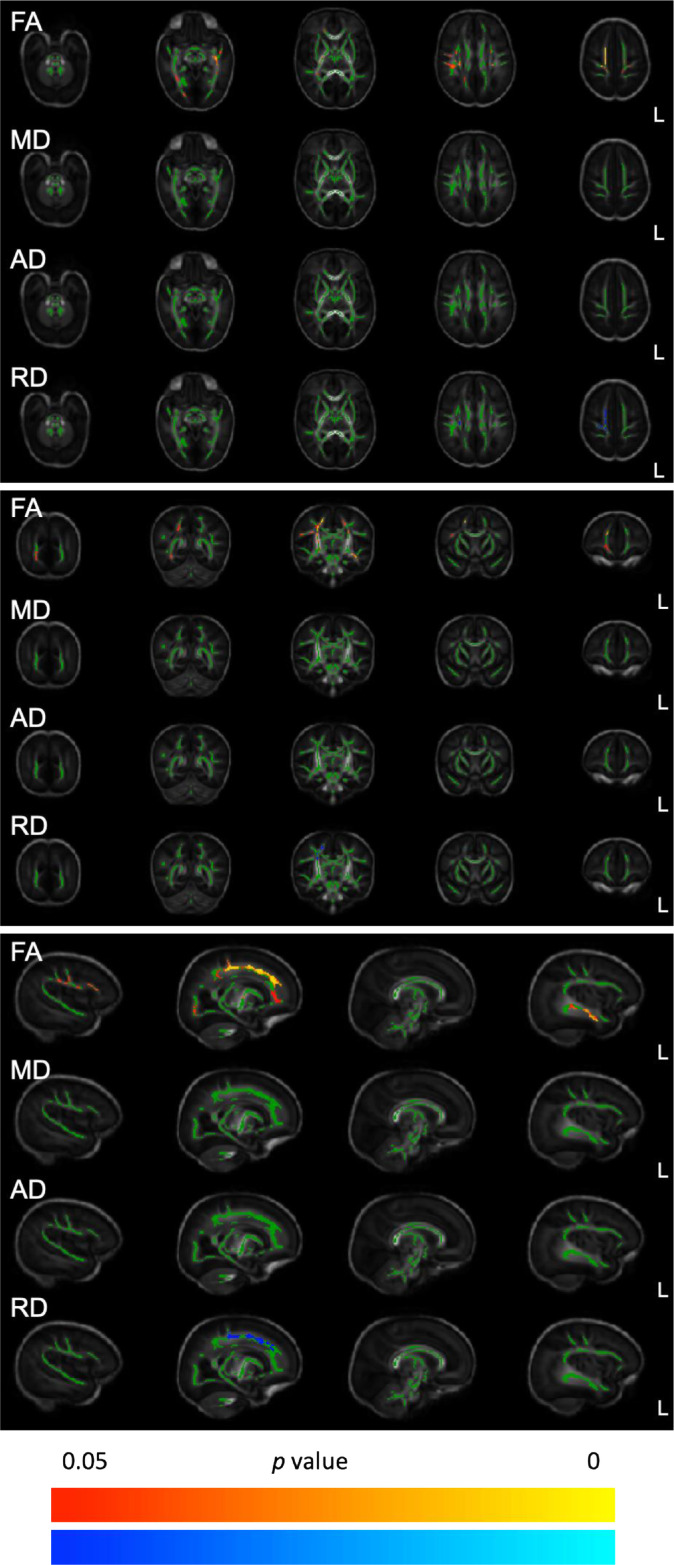
Babies whose mothers were randomised to antenatal magnesium sulphate compared with babies whose mothers were randomised to placebo had significantly lower FA including in the superior portions of the corticospinal tracts and the inferior fronto-occipital fasciculi bilaterally, and in the right corona radiata, the right superior longitudinal fasciculus, the right precentral and postcentral gyri, the right posterior limb of the internal capsule, and the left inferior longitudinal fasciculus (Figure 2). The magnesium sulphate group had increased RD in the superior corona radiata and the right precentral gyrus. There were no differences in MD or AD between the treatment groups in any region (Figure 2).
Figure 2.
Tract-based spatial statistics comparing the brain white matter skeletons at term equivalent age of babies exposed in utero to magnesium sulphate or placebo
Footnote: A group-specific template underlies each axial (top four rows) coronal (middle four rows) and sagittal (bottom four rows) slices, with the white matter tract skeleton shown in green. Regions where the magnesium sulphate group (n = 49) had a significantly lower diffusion metric than the placebo group (n = 60) are shown in red-yellow (family-wise error corrected, P < 0·05), while regions where the magnesium sulphate group had significantly higher measures than the placebo group are in blue.
Sensitivity analyses
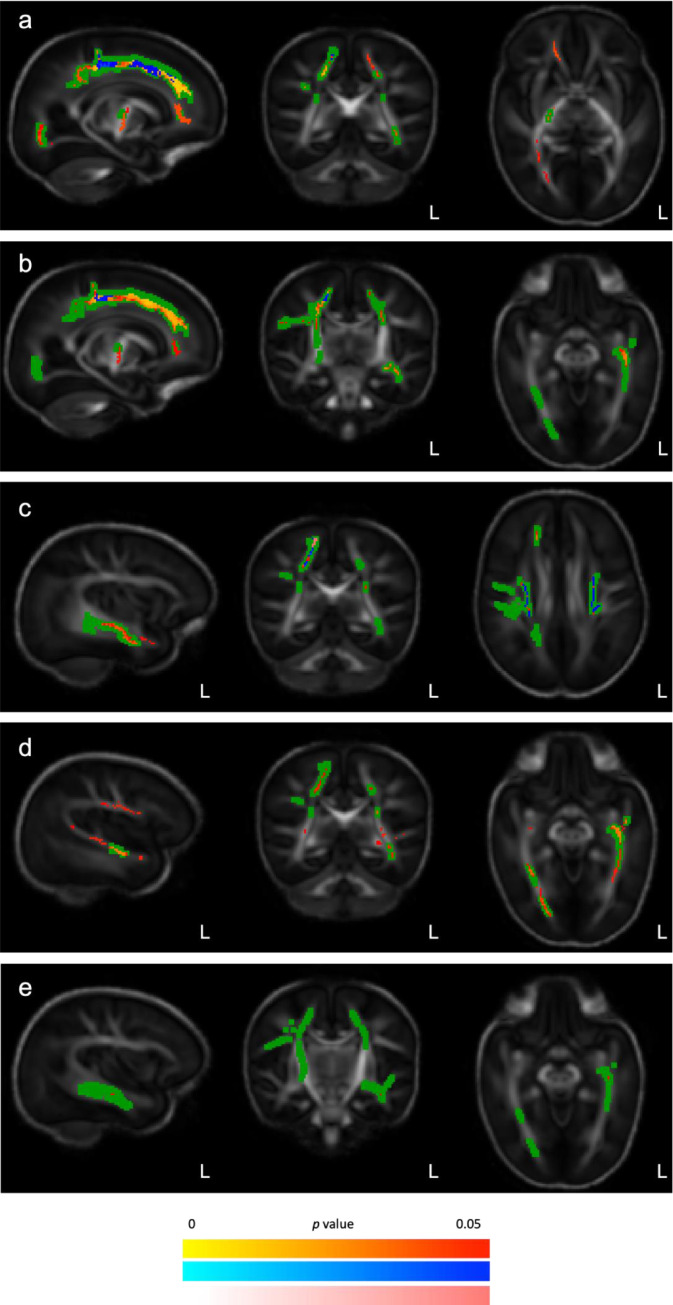
When only babies whose mother received at least some of the allocated treatment were included (n = 107; 48 magnesium sulphate, 59 placebo) the findings were similar to the primary analysis (Figure 3a).
Figure 3.
Sensitivity and subgroup tract-based spatial statistics comparing the white matter skeletons of babies at term equivalent age of babies exposed in utero to magnesium sulphate or placebo
Footnote: A group-specific template underlies each axial (right) coronal (middle) and sagittal (left) slices, with significant primary analysis differences between the magnesium and placebo groups indicated in green. Regions where babies who received magnesium sulphate had a significantly (family-wise error corrected, (P < 0·05)) lower FA (red-yellow), higher RD (blue) or higher MD (pink) than babies who received placebo are layered over the template.
a: Babies whose mother received at least some of their allocated treatment;
b: Babies born <34 weeks’ gestation;
c: Babies who did not have bronchopulmonary dysplasia or necrotising enterocolitis;
d: Babies who were exclusively fed breast milk at the time of MRI;
e: Babies who were 32 to <34 weeks’ gestation at trial entry.
When including only babies who were scanned at the largest MRI site (n = 66; 33 magnesium sulphate, 33 placebo) there were no significant differences between groups on whole skeleton TBSS analysis. On region-of-interest analysis, the magnesium sulphate group had marginally lower FA (mean ± standard deviation; 0·23 ± 0·02) than the placebo group (0·25 ± 0·02, P = 0·02) but RD did not differ (magnesium sulphate group 1·24 ± 0·07 × 10−3 mm2 s−1, placebo group 1·21 ± 0·08 × 10−3 mm2 s−1, P = 0·08). When including only babies born before 34 weeks’ gestation (n = 106; 46 magnesium sulphate, 60 placebo) the findings were similar to the primary analysis (Figure 3b).
When only singletons were included (n = 79; 38 magnesium sulphate, 41 placebo), there were no differences between groups on whole skeleton TBSS analysis. On region-of-interest analysis, the magnesium sulphate group had slightly lower FA (0·24 ± 0·03) than the placebo group (0·25 ± 0·02, P = 0·03) but there was no difference in RD (1·22 ± 0·09 × 10−3 mm2 s−1 vs 1·18 ± 0·09 × 10−3 mm2 s−1, P = 0·10).
When only babies without bronchopulmonary dysplasia were included (n = 101; 47 magnesium sulphate, 54 placebo), the findings were similar to the primary analysis but there were no differences in the superior longitudinal fasciculus or the inferior fronto-occipital fasciculus. However, the magnesium sulphate group had higher MD in the precentral gyrus, and more extensive clusters with significantly higher RD in both the left and right corona radiata (Figure 3c). There were no differences between groups in AD.
When only babies who were exclusively receiving breast milk at the time of MRI were included (n = 75; 36 magnesium sulphate, 39 placebo), FA results were similar to the primary analysis, but with more differences between groups in the left hemisphere. The magnesium sulphate group had lower FA in the left superior longitudinal fasciculus, the left and right optic radiations, the left and right corticospinal tracts (including the left posterior limb of the internal capsule), and precentral gyri (Figure 3d). There were no differences between groups in MD, AD or RD.
Subgroup analyses
In the subgroup of babies randomised at 30 to <32 weeks’ gestation (n = 56; 26 magnesium sulphate, 30 placebo) there were no differences between groups in any regions of the white matter skeleton. On region-of-interest analysis, there were also no differences between groups in FA (0.24 ± 0·02 vs 0.24 ± 0·02, P = 0·13) or RD (1·24 ± 0·09 × 10−3 mm2 s−1 vs 1·21 ± 0·07 × 10−3 mm2 s−1, P = 0·16). In the subgroup of babies randomised at 32 to <34 weeks’ gestation (n = 53; 23 magnesium sulphate, 30 placebo), the magnesium sulphate group had lower FA in a cluster of the left inferior longitudinal fasciculus (Figure 3e). There were no significant differences between groups in MD, AD or RD.
When babies of each sex were analysed separately (50 girls; 23 magnesium sulphate, 27 placebo, and 59 boys; 26 magnesium sulphate, 33 placebo) there were no differences between treatment groups on TBSS analysis. On region-of-interest analysis, boys in the magnesium sulphate group had lower FA than those in the placebo group (0·24 ± 0·03 vs 0·25 ± 0·02, P = 0·01) and higher RD (1·24 ± 0·09 vs 1·19 ± 0·08 × 10−3 mm2 s−1, P = 0·03). For girls, there were no differences in the region-of-interest analyses for FA (0.24 ± 0·02 vs 0.25 ± 0·02, P = 0·13) or for RD (1·21 ± 0·06 × 10−3 mm2 s−1 vs 1·16 ± 0·09 × 10−3 mm2 s−1, P = 0·08).
Discussion
Neuroprotective benefits, including lower rates of cerebral palsy, are observed after exposure to antenatal magnesium sulphate before 30 weeks’ gestation,5 but there has been uncertainty about whether the neuroprotective effects extend to later gestational ages4,24, 25, 26, 27, 28 sufficient to justify further clinical trials.19 In this study, we found that antenatal magnesium sulphate administered to mothers at risk of imminent preterm birth from 30 to <34 weeks’ gestation did not appear to protect white matter development in their babies. We had hypothesised that magnesium sulphate would improve white matter development, with TBBS findings indicating greater fibre coherence and maturation along the axis of greatest water molecule diffusion and improved myelination, but our findings did not support this. Rather, at term-equivalent age, key white matter tracts of babies exposed to antenatal magnesium sulphate had lower FA and higher RD than those babies not exposed. In particular, the findings suggested delay, rather than acceleration, of white matter maturation in corticospinal tracts, which is of concern given that reduced FA of the corticospinal tract at term-equivalent age in babies born preterm independently predicts later motor delay and cerebral palsy.29
The reasons for these findings are not clear. It is possible that magnesium sulphate administered at this gestation does not have the same neuroprotective effect as it does at earlier gestations. In the pre-specified subgroup analysis of gestation at trial entry, we found that in babies randomised at 30 to <32 weeks (51% of the cohort) there were no differences between magnesium exposed and placebo groups in any white matter region, whereas in the babies randomised at 32 to <34 weeks (49% of the cohort) there was lower FA in the magnesium exposed group, although this was restricted to the left inferior longitudinal fasciculus. These findings suggest that magnesium sulphate may have different effects on neuroprotective pathways at different gestational ages.
The four randomised trials of use of antenatal magnesium sulphate before 34 weeks’ gestation for fetal neuroprotection included in the Cochrane review24 all specified different upper limits of gestational ages for eligibility. Whilst the overall effect was for reduced cerebral palsy, of note is the suggestion of increased risk of death and cerebral palsy30 reported in the one small trial that recruited women at risk of preterm birth up to 34 weeks’ gestation.25 Our study was nested within a randomised controlled trial19 of mothers recruited at 30 to <34 weeks’ gestation that has yet to report on neurodevelopmental finding of the trial participants at two years’ corrected age, and those findings will be critical in interpreting the MRI findings reported here.
The effects of antenatal magnesium sulphate on FA and RD were not confined to motor pathways and extended to the superior longitudinal fasciculus, inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus. These association fibres subserve cognitive processes including regulation of motor behavior, visually guided behaviors, language, and working memory, and all are implicated in the cerebral phenotype of preterm brain injury.23,31,32 Furthermore, alterations in DTI parameters have been associated with cognitive deficits in children and adults born preterm.33,34 Our findings therefore suggest that children in the magnesium sulphate group may have altered cognitive as well as motor outcomes, but the findings are in the reverse direction from that expected. Participants in the MAGENTA Trial19 have been assessed at two years of age for the primary outcome, which will enable further assessment of the neuroprotective effect of magnesium sulphate and outcome across a broad range of developmental domains.
As this was a nested study within a multicentre, randomised trial, we used three different MRI facilities to maximise recruitment. To ameliorate the possible confounding effects of scanner variation, image acquisition sequences were matched as closely as possible, scan site was included as a covariate in primary analysis, and we carried out a sensitivity analysis using data only from the largest site (60% of all MRI scans). There were no significant differences between magnesium sulphate and placebo groups on TBSS analysis, and in the region-of-interest analysis the direction of effect was maintained. These results were consistent with the primary analysis.
There were several notable findings from the sensitivity analyses. When only singletons were included (73% of the cohort) there were no differences between groups on TBBS analysis, and the direction of effect was maintained on region-of-interest analysis. This is consistent with previous findings which indicate that the clinical effects of antenatal magnesium sulphate for neuroprotection are similar in singleton and multiple pregnancies.5
Breastmilk intake can affect early brain development and later neurodevelopment after preterm birth, and was therefore a potential confounder. In very preterm babies, the proportion of early enteral intake from breastmilk is associated with neonatal white matter connectivity.16,35 In the subgroup of babies who were exclusively receiving breastmilk at the time of scanning (74% of the cohort), overall findings were similar to those of the primary analysis, although lower FA was seen in the optic radiations, associated with visual function, in the magnesium sulphate exposed group.
In sensitivity analyses, we also investigated a group without bronchopulmonary dysplasia because of its consistent association with atypical brain development,9 and again found that the findings were similar to the primary analysis in babies without this comorbidity of preterm birth.
Male sex is associated with diffuse white matter injury after preterm birth.36 In extremely preterm babies who underwent MRI at term equivalent age, boys were reported to have delayed myelination,37 and lower FA in the splenium of the corpus callosum38 compared with girls. Although we found no differences in whole skeleton analysis between the magnesium sulphate and placebo groups in girls or boys separately, we did observe a modest decrease in FA and higher RD in region-of-interest analysis, which was significant only for boys. This raises the possibility that boys have a different response to magnesium sulphate exposure as a neuroprotective agent to girls. This warrants further investigation.
A major strength of the MagNUM Study is that it was nested within a randomised controlled trial that is still to report on neurodevelopmental finding of the children at two years’ corrected age. Assessment of the relationships between the MRI changes observed at term-equivalent age and later developmental outcomes will be needed. A possible weakness was the sample size, partly due to motion artifact that resulted in exclusion of some babies from the analyses, which reduced power to detect small effects both in the main group and in subgroups of interest including those for gestational age at birth. However, in all subgroups the direction of the differences in FA and RD were consistent with those of the primary analysis. Further imaging studies with larger sample sizes that include assessments of white matter using biophysical models such as neurite orientation dispersion and density imaging (NODDI)39 and cerebral cortical architecture are required to investigate further the effects of this treatment on the development of the infant brain.
In babies born preterm after 30 weeks’ gestation, antenatal magnesium sulphate exposure did not promote development of white matter microstructure in pathways affecting motor and cognitive functions inferred from fractional anisotropy.
Contributors
CAC, JEH conceived the study and developed the study design with BT, JPB, MEB and JA. TP, JA, GD, JEH, CAC were involved in data acquisition. TP and BT verified and analysed the data. TP and CAC wrote the initial draft of the original manuscript, CAC and JEH prepared the initial draft of the revised manuscript and all authors contributed to interpretation of the data, critical revision of the manuscript and approved the final version.
The MagNUM study group
Steering Committee: Caroline Crowther, Ben Thompson, Gerard Deib, Jane Alsweiler, Jane Harding.
Co-investigators: James Boardman, Mark Bastin.
Research Management Team: Liggins Institute: Debbie Samuel, Jane Yates, Khan Safayet Hossin, Rashedul Hasan, Thach Tran, Sabine Huth, Eleanor Kennedy.
Collaboration by sites: (total number of babies recruited)
Auckland, New Zealand (102): Centre for Advanced MRI, University of Auckland: Anna Lydon, Gerard Deib; Auckland City Hospital Jane Alsweiler, David Perry; Middlemore Hospital: David Hou, Jen Schroder.
Christchurch, New Zealand (42): Pacific Radiology Group: Ross Keenan, Scott Wells, Tracy Melzer; Christchurch Women's Hospital: Nicola Austin, Nicola Ellis, Trish Graham, Jo Gullam, Di Leishman.
Adelaide, Australia (15): South Australia Medical Research Institute, Jones and Partners Radiology: Andrew Dwyer, Angela Walls; Women's and Children's Hospital: Pat Ashwood, Andrew McPhee; Flinders Medical Centre: Scott Morris.
Data sharing statement
Data and associated documentation are available to other users under the data sharing arrangements provided by the Maternal and Perinatal Research Hub, based at the Liggins Institute, University of Auckland (https://wiki.auckland.ac.nz/researchhub). A publicly created dataset is not available. Researchers can request a de-identified dataset through the Data Access Committee of the Liggins Institute (researchhub@auckland.ac.nz). Data will be shared with researchers who provide a methodologically sound proposal and have appropriate ethical approval, where necessary, to achieve the research aims in the approved proposal. Data requestors are required to sign a Data Access Agreement that includes a commitment to using the data only for the specified proposal, not to attempt to identify any individual participant, a commitment to secure storage and use of the data, and to destroy or return the data after completion of the project.
Declaration of interests
We declare no competing interests.
Acknowledgments
We thank the families who participated in the MagNUM Study for their support and commitment.
This study was funded by a project grant from the Health Research Council of New Zealand (HRC 14/153).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103923.
Appendix. Supplementary materials
References
- 1.Johnson S., Marlow N. Early and longterm outcome for infants born preterm. Arch Dis Child. 2017;102:97–102. doi: 10.1136/archdischild-2015-309581. [DOI] [PubMed] [Google Scholar]
- 2.Twilhaar S.W., Wade R.M., de Kieviet J.F., et al. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors. A meta-analysis and meta-regression. JAMA Pediatr. 2018;172(4):361–367. doi: 10.1001/jamapediatrics.2017.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrou S., Yiu H.H., Kwon J. Economic consequences of preterm birth: a systematic review of the recent literature (2009–2017) Arch Dis Child. 2019;104(5):456–465. doi: 10.1136/archdischild-2018-315778. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva: 2015. Recommendations on Interventions to Improve Preterm Birth Outcomes.http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/preterm-birth-guideline [PubMed] [Google Scholar]
- 5.Crowther C.A., Middleton P.F., Voysey M., et al. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis. PLoS Med. 2017;14(10) doi: 10.1371/journal.pmed.1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpe J.J. Dysmaturation of premature brain: importance, cellular mechanisms, and potential interventions. Pediatr Neurol. 2019;95:42–66. doi: 10.1016/j.pediatrneurol.2019.02.016. Jun. [DOI] [PubMed] [Google Scholar]
- 7.Boardman J.P., Counsell J.P. Factors associated with atypical brain development in preterm infants: insights from magnetic resonance imaging. Neuropathol Appl Neurobiol. 2019 doi: 10.1111/nan.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrova R., Pietsch M., Christiaens D., et al. Heterogeneity in brain microstructural development following preterm birth. Cereb Cortex. 2020:1–11. doi: 10.1093/cercor/bhaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball G., Counsell S.J., Anjari M., et al. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage. 2010;53(1):94–102. doi: 10.1016/j.neuroimage.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 10.Boardman J.P., Craven C., Valappil S., et al. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage. 2010;52(2):409–414. doi: 10.1016/j.neuroimage.2010.04.261. [DOI] [PubMed] [Google Scholar]
- 11.Counsell S.J., Shen Y., Boardman J.P., et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117(2):376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- 12.Smith S.M., Jenkinson M., Johansen-Berg H., et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Barnett M.L., Tusor N., Ball G., et al. Exploring the multiple-hit hypothesis of preterm white matter damage using diffusion MRI. Neuroimage Clin. 2017;17:596–606. doi: 10.1016/j.nicl.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anblagan D., Pataky R., Evans M.J., et al. Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci Rep. 2016;6:37932. doi: 10.1038/srep37932. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monnelly V.J., Ablagan D., Quigley A., et al. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin. 2017;18:9–14. doi: 10.1016/j.nicl.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blesa M., Sullivan G., Anblagan D., et al. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage. 2019;184:431–439. doi: 10.1016/j.neuroimage.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 17.O'Gorman R.L., Bucher H.U., Held U., et al. Tract-based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain. 2015;138(2):388–397. doi: 10.1093/brain/awu363. [DOI] [PubMed] [Google Scholar]
- 18.Azzopardi D., Robertson N.J., Bainbridge A., et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 2016;15(2):145–153. doi: 10.1016/S1474-4422(15)00347-6. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowther C.A., Middleton P.F., Wilkinson D., et al. Magnesium sulphate at 30 to 34 weeks’ gestational age: neuroprotection trial (MAGENTA) - study protocol. BMC Pregnancy Childbirth. 2013;13(1):91. doi: 10.1186/1471-2393-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball G., Boardman J.P., Arichi T., et al. Testing the sensitivity of tract-based spatial statistics to simulated treatment effects in preterm neonates. PLoS One. 2013;8(7):e67706. doi: 10.1371/journal.pone.0067706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H., Yushkevich P.A., Alexander D.C., et al. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal. 2016;10(5):764–785. doi: 10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Batalle D., Hughes E.J., Zhang H., et al. Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage. 2017;149:379–392. doi: 10.1016/j.neuroimage.2017.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle L.W., Crowther C.A., Middleton P., Marret S., Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD004661. Art. No.: CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 25.The Antenatal Magnesium Sulphate for Neuroprotection Guideline Development Panel . The University of Adelaide; 2010. Antenatal Magnesium Sulphate Prior to Preterm Birth for Neuroprotection of the Fetus, Infant and Child: National Clinical practice guidelines. Adelaide<https://www.sahmri.org/m/downloads/cp128_mag_sulphate_child.pdf>. [Google Scholar]
- 26.Magee L.A., De Silva D.A., Sawchuck D., Synnes A., von Dadelszen P. Magnesium sulphate for fetal neuroprotection. J Obstet Gynaecol Can. 2019;41(4):505–522. doi: 10.1016/j.jogc.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 27.ACOG Committee on obstetric practice & SMFM committee opinion no 652: magnesium sulfate use in obstetrics. Obstet Gynaecol. 2016;127(1):e52–e53. doi: 10.1097/AOG.0000000000001267. [DOI] [PubMed] [Google Scholar]
- 28.Preterm Labour and Birth. NICE guideline, No. 25, National Institute for Health and Care Excellence, London. 2015 <https://www.nice.org.uk/guidance/ng25>.
- 29.De Bruïne F.T., Wezel-Meijler G.V., Leijser L.A., et al. Tractography of white-matter tracts in very preterm infants: a 2-year follow-up study. Dev Med Child Neurol. 2013;55:427–443. doi: 10.1111/dmcn.12099. [DOI] [PubMed] [Google Scholar]
- 30.Mittendorf R., Dambrosia J., Pryde P.G., et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–1118. doi: 10.1067/mob.2002.123544. [DOI] [PubMed] [Google Scholar]
- 31.Telford E.J., Simon R., Fletcher-Watson S., et al. A latent measure explains substantial variance in white matter microstructure across the newborn human brain. Brain Struct Funct. 2017;222:4023–4033. doi: 10.1007/s00429-017-1455–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galdi P., Blesa M., Stoye D.Q., et al. Neonatal morphometric similarity mapping for predicting brain age and characterizing neuroanatomic variation associated with preterm birth. Neuroimage Clin. 2020;25 doi: 10.1016/j.nicl.2020.102195. Epub 2020 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldman H.M., Lee E.S., Yeatman J.D., Yeom K.W. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50(14):3348–3362. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvan P., Froudist Walsh S., Allin M.P.G., et al. Road work on memory lane-functional and structural alterations to the learning and memory circuit in adults born very preterm. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Belfort M.B., Anderson P.J., Nowak V.A., et al. Breast milk feeding, brain development, and neurocognitive outcomes: a 7-year longitudinal study in infants born at less than 30 weeks’ gestation. J Pediatr. 2016;177:133–139. doi: 10.1016/j.jpeds.2016.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnett M.L., Tusor N., Ball G., et al. Exploring the multiple-hit hypothesis of preterm white matter damage using diffusion MRI. Neuroimage Clin. 2017;17:596–606. doi: 10.1016/j.nicl.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skiöld B., Alexandrou G., Padilla N., et al. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr. 2014;164(5):1012–1018. doi: 10.1016/j.jpeds.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 38.Rose J., Butler E.E., Lamont L.E., et al. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol. 2007;49:745–750. doi: 10.1111/j.1469-8749.2008.03231.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H., Schneider T., Wheeler-Kingshott C.A., DC A. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2021;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 40.Cole T.J., Williams A.F., Wright CM. Revised birth centiles for weight, length and head circumference in the UK-WHO growth charts. Ann Hum Biol. 2011;38(1):7–11. doi: 10.3109/03014460.2011.544139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.