Abstract
Maternal immune activation (MIA) is a risk factor for schizophrenia in the offspring. MIA in pregnant rodents can be induced by injection of synthetic polyriboinosinic-polyribocytidilic acid (Poly I:C), which causes decreased striatal dopamine D2 receptor (D2R) expression and behavioral dysfunction mediated by the dopaminergic system in the offspring. However, previous studies did not determine whether Poly I:C induced cortical dopamine D2R abnormality in an MIA rat model. In this study, we performed micro-positron emission tomography (micro-PET) in vivo imaging and ex vivo neurochemical analyses of cortical D2Rs in MIA. In the micro-PET analyses, the anterior cingulate cortex (ACC) region in the offspring showed significantly reduced binding potential for [11C]FLB457, a high affinity radio-ligand toward D2Rs. Neurochemical analysis showed reduction of D2Rs and augmentation of dopamine turnover in the ACC of the rat offspring. Thus, MIA induces dopaminergic dysfunction in the ACC of offspring, similar to the neuronal pathology reported in patients with schizophrenia.
Keywords: Maternal immune activation, Schizophrenia, Dopamine D2 receptors, Dopamine, Anterior cingulate cortex, Poly I:C, Positron emission tomography, In vivo imaging, [11C]FLB457, MIA rat model
Highlights
-
•
Maternal immune activation (MIA) is a risk factor for schizophrenia.
-
•
Improving extra-striatal Dopamine D2 receptors(D2Rs) thought to be important for the treatment of schizophrenia.
-
•
In vivo imaging showed that the anterior cingulate cortex region in MIA model rat had reduced D2Rs density.
-
•
The findings were similar to those of several publications regarding patients with schizophrenia.
1. Introduction
Emerging literature from human epidemiological studies has provided evidence for the contribution of maternal infection to the etiology of schizophrenia. Several studies have reported correlations between influenza infection during pregnancy and an increased risk of psychiatric disorders in offspring (Barr et al., 1990; Brown et al., 2004; Brown and Meyer, 2018; Kendell and Kemp, 1989; Mednick et al., 1988; Westergaard et al., 1999). Similar to influenza infection, other maternal infectious diseases have been also shown to increase this risk (Blomstrom et al., 2012; Brown and Susser, 2002; Suvisaari et al., 1999), suggesting that maternal immune activation (MIA) rather than a specific viral infection increases the risk of schizophrenia in offspring.
MIA in pregnant experimental animals can be induced by injection of synthetic polyriboinosinic-polyribocytidilic acid (Poly I:C) or lipopolysaccaride (LPS), and these injections have been shown to cause neurobehavioral abnormalities in the offspring (Brown and Meyer, 2018; Hsiao et al., 2012; Hsiao et al., 2013; Meyer, 2013, 2014; Meyer et al., 2005; Patterson, 2009, 2011). These abnormalities were reportedly ameliorated by antipsychotic administration (Casquero-Veiga et al., 2019; Ozawa et al., 2006; Piontkewitz et al., 2012; Roenker et al., 2011; Romero et al., 2007; Scarborough et al., 2020; Zuckerman et al., 2003). Dopamine D2 receptor (D2R) alterations have been reported in MIA model rodents (Aguilar-Valles et al., 2020), and these alterations may represent meaningful neuronal abnormalities, because almost all antipsychotics have an inhibitory effect on D2Rs. Nevertheless, the complete picture of D2R alterations in the MIA model has not been clarified to date.
In vivo imaging studies using positron emission tomography (PET) have shown disturbances of extra-striatal D2Rs in patients with schizophrenia, and improvements in these disturbances are thought to be important for the treatment of schizophrenia (Takahashi et al., 2006). In this study, we investigated extra-striatal D2R abnormalities in a Poly I:C-induced MIA rat model by performing micro-positron emission tomography (micro-PET) with affinity D2R radio-ligands such as [11C]FLB457 and ex vivo neurochemical analyses.
2. Materials and methods
Described in Supplemental data.
3. Results
3.1. Localization of D2R dysfunction in the extra-striatal region of MIA rats
Extra-striatal D2Rs in the MIA (n = 5) and control (n = 5) offspring rats (postnatal weeks 12–13; see Supplemental data) were imaged by PET with [11C]FLB457, a radioligand for extra-striatal D2Rs (Halldin et al., 1995). PET was performed during stable anesthetization of the rats. Specific binding (BPND) of [11C]FLB457 in the extra-striatal region was estimated by using a simplified reference tissue model with the cerebellum as the reference region (Lammertsma and Hume, 1996) (Fig. S1), and parametric images of BPND were generated. Voxel-wise statistical parametric mapping (SPM) analysis revealed a significant decrease in [11C]FLB457 binding in a small cluster in MIA rats (uncorrected, P < 0.01) (Fig. 1A), which corresponded to a part of Brodmann's area 24 (BA24) of ACC (Hoover and Vertes, 2007). The reduction in BPND was 39% in comparison with the control rats (t(8) = 4.19, P = 0.003) (Fig. 1B). No BPND alterations were observed in any regions other than BA24. Morphological comparisons based on MR images showed no significant volume reduction in the ACC regions of the MIA rats (Fig. S2), suggesting that the BPND reduction in the ACC regions of the MIA brains was not accompanied by significant volume changes.
Fig. 1.
Micro-PET with [11C]FLB457 analysis in the control and MIA rats. (A) MRI images of the standard rat brain are shown in grey scale, on which SPM images are overlaid in color to show the reduction in [11C]FLB457 binding in MIA rats in comparison with that in control rats. The color bar in the panel shows the scale of the SPM images, and a lighter color indicates a greater probability of a reduction in [11C]FLB457 binding. (B) [11C]FLB457 binding potentials in the ACC of MIA rats were about 39% lower than those in the control. ∗∗in the figure indicates P < 0.01. Error bars denote mean ± SEM. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Reduction of D2R protein and up-regulation of DA turnover in the ACC of MIA rats
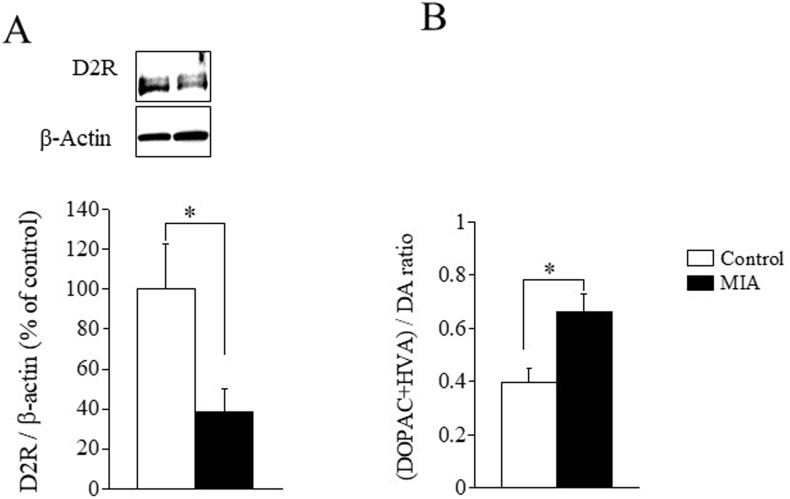
Our PET data suggested a marked reduction in D2R expression in the ACC of MIA rats. To verify this, we performed Western blot analysis to assess the level of D2R protein expression in ACC. The upper four panels of Fig. 2A show western blots of D2R proteins (top row) together with β-actin as controls (bottom row) in the ACC of control (left lane in the panel) and MIA (right lane in the panel) samples. The bar graph in Fig. 2A shows D2R protein levels normalized by the β-actin level of the MIA samples (filled bars; n = 6) in the ACC in MIA rats was about 40% of that in the controls (open bars; n = 6), and the difference was statistically significant (t-test; t(10) = 2.4, P = 0.037).
Fig. 2.
Reduction of the D2R expression level and abnormal DA turnover in MIA rats. (A) The upper two panels show western blots of D2R proteins together with β-actin as controls in the ACC of the control and MIA samples. The D2R- and β-actin-specific bands were at 75k and 38k, respectively, on the SDS-PAGE gel. The concentration ratios of D2R to β-actin indexed to healthy controls show a reduction in the ACC of MIA rats. Data are expressed as percentage of the value in the control rats. (B) The ratio of the metabolites (DOPAC + HVA)/DA in the ACC and TC in MIA rats was significantly greater than that in the control. ∗ in the figure indicates P < 0.05. Error bars denote mean ± SEM.
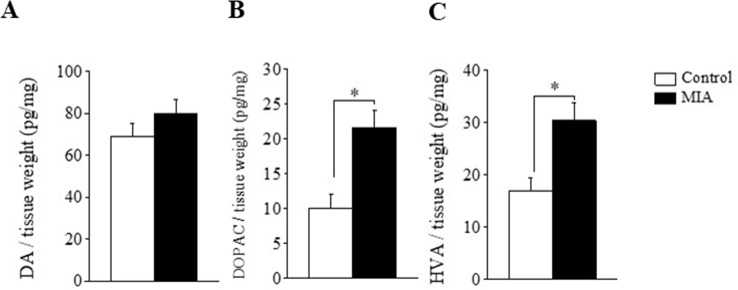
We also assessed DA turnover, a biochemical correlate of DA release (Murphy et al., 1996), by estimating the ratio of DA metabolites to DA in vitro. We measured the amount of DA and its two major DA metabolites, DOPAC and HVA, in ACC tissues excised from the two groups of rats. The DA turnover in the ACC (i.e., the (DOPAC + HVA)/DA ratio) in MIA rats was significantly higher than that in the controls (t-test, t (8) = 3.0, P < 0.05, n = 5 each, Fig. 2B). Both DOPAC and HVA concentrations were also significantly higher in MIA rats (Fig. S3). These results suggest that DA turnover is upregulated in the ACC of MIA rats.
4. Discussion
All three kinds of experimental data in this study, i.e., (1) in vivo imaging with micro-PET of MIA rat brain ACC, (2) Western blot analysis of D2R proteins in MIA ACC, (3) HPLC-ECD measurement of dopamine turnover in MIA ACC, unanimously point toward the presence of D2R dysfunction in MIA ACC.
We identified BA24 of ACC as a single specific brain region in which MIA induced reduction of [11C]FLB457 binding without volume reduction. [11C]FLB457 binding was considered to reflect both D2 and D3 receptor density (Halldin et al., 1995). In the rat cortical region, the D3R mRNA density was much lower than that of D2R mRNA, and the D2R mRNA density was as low as that of D3R mRNA in the cerebellum (Bouthenet et al., 1991); thus, [11C]FLB457 binding in the ACC mainly reflects D2R density. Reduction of the [11C]FLB457 signal in BA24 in the MIA rat could be due to signal spillover from the striatum where the signal was very high. However, the results of western blotting support a decrease in [11C]FLB457 binding in the ACC, reflecting a reduction in D2R expression in MIA rats.
HPLC-ECD measurement of dopamine and its metabolites showed that dopamine turnover was enhanced in MIA rat ACC, suggesting that DA transmission is up-regulated. Pyramidal neurons in BA24 can show excitatory activity in the ventral tegmental area (VTA) through direct connections (Gabbott et al., 2005; Hoover and Vertes, 2007). Pharmacological stimulation of D2R in the medial prefrontal cortex, including BA24, inhibits the excitatory glutamate drive on DA neurons in the VTA (Harte and O'Connor, 2004). Thus, a reduction of D2R in BA24 of MIA rat may cause disinhibition of DA neurons in VTA and thereby induce upregulation of DA release in the cortical regions. This possibility should be investigated in future studies. Immunoreactivity of D2 receptors is reduced in the pre-frontal cortex of MIA mice and increased in the nucleus accumbens (Meyer et al., 2008; Vuillermot et al., 2010). D2 receptor binding is reduced in the striatum of MIA mice (Ozawa et al., 2006). Prenatal Poly IC injection at gestational day (GD) 15 produced enhanced release of DA from rat striatal explants (Zuckerman et al., 2003). Mouse model studies showed that DA and its metabolites were also altered in other brain regions (i.e., pre-frontal cortex, globus pallidus, and nucleus accumbens) (Giovanoli et al., 2013; Winter et al., 2009). The present results, together with the findings of previous studies in MIA rodent models described above, indicate an outline of the pathophysiology in this model—enhanced DA transmission brains.
D2R alterations in the cortical regions of patients with schizophrenia have been investigated by PET/SPECT studies using high-affinity D2R radio-ligands such as [11C]FLB457, [123I]epidepride, or [18F]fallypride; the results showed alterations of radio-ligand binding in various regions (Buchsbaum et al., 2006; Glenthoj et al., 2006; Suhara et al., 2002; Talvik et al., 2003; Tuppurainen et al., 2003; Yasuno et al., 2004). Our MIA rat model findings are similar to those described in several reports that noted reduction of D2R binding in the ACC of patients with schizophrenia by PET studies using [11C]FLB457 and [18F]fallypride (Buchsbaum et al., 2006; Suhara et al., 2002).
Because D2R is abundant in the interneurons in ACC (Xu and Zhang, 2015), reduced D2R binding may reflect an alteration in the interneurons in this region. Indeed, MIA produces GABAergic interneuronal dysfunction in the rodent model forebrain (Canetta et al., 2016; Dickerson et al., 2014; Nakamura et al., 2019). Interestingly, administration of anti-psychotic drugs (i.e,. haloperidol) has been reported to lead to an enlarged and elongated GABAergic terminal on pyramidal neurons in the medial prefrontal cortex (mPFC) of rats (Vincent et al., 1994). Although behavioral improvements in the abnormalities in the MIA rodent model by administration of anti-psychotic drugs (Ozawa et al., 2006; Zuckerman et al., 2003) has been thought to be mediated by normalization of dopamine excess in the striatum, restoration of GABAergic interneuronal dysfunction in ACC could also contribute to it.
We conclude that MIA causes a reduction in D2 receptors with abnormal dopaminergic transmission in the ACC in the rodent model. The micro-PET findings were similar to the in vivo imaging findings for patients with schizophrenia. Therefore, D2 receptor alteration in the ACC may be an important finding for cortical pathology and for understanding the pathogenesis of schizophrenia.
Declaration of competing interest
A. O-N. is CEO & CTO of RESVO Inc. and owns more than 5% of the shares of RESVO Inc., but had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This disclosure does not alter our adherence to Brain, behavior and immunity publication policy. The other authors have no competing interests to declare.
Acknowledgements
We are grateful to late Prof. P. Patterson (Caltech) and Dr. N. Suzuki (Kitasato Univ.) for their comments on an earlier version of the manuscript, H. Tomizawa (Chiba Univ.) for technical assistance, and J. Ko (Caltech) for technical advice. This work was supported by KAKENHI 23700432 (to A.O-N), Japan Advanced Molecular Imaging Program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. A part of this work was carried out under the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) by MEXT, Japan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100446.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
References
- Aguilar-Valles A., Rodrigue B., Matta-Camacho E. Maternal immune activation and the development of dopaminergic neurotransmission of the offspring: relevance for schizophrenia and other psychoses. Front. Psychiatr. 2020;11:852. doi: 10.3389/fpsyt.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr C.E., Mednick S.A., Munk-Jorgensen P. Exposure to influenza epidemics during gestation and adult schizophrenia. A 40-year study. Arch. Gen. Psychiatr. 1990;47:869–874. doi: 10.1001/archpsyc.1990.01810210077012. [DOI] [PubMed] [Google Scholar]
- Blomstrom A., Karlsson H., Wicks S., Yang S., Yolken R.H., Dalman C. Maternal antibodies to infectious agents and risk for non-affective psychoses in the offspring--a matched case-control study. Schizophr. Res. 2012;140:25–30. doi: 10.1016/j.schres.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Bouthenet M.L., Souil E., Martres M.P., Sokoloff P., Giros B., Schwartz J.C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Brown A.S., Begg M.D., Gravenstein S., Schaefer C.A., Wyatt R.J., Bresnahan M., Babulas V.P., Susser E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatr. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown A.S., Meyer U. Maternal immune activation and neuropsychiatric illness: a translational research perspective. Am. J. Psychiatr. 2018;175:1073–1083. doi: 10.1176/appi.ajp.2018.17121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S., Susser E.S. In utero infection and adult schizophrenia. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- Buchsbaum M.S., Christian B.T., Lehrer D.S., Narayanan T.K., Shi B., Mantil J., Kemether E., Oakes T.R., Mukherjee J. D2/D3 dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophr. Res. 2006;85:232–244. doi: 10.1016/j.schres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Canetta S., Bolkan S., Padilla-Coreano N., Song L.J., Sahn R., Harrison N.L., Gordon J.A., Brown A., Kellendonk C. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol. Psychiatr. 2016;21:956–968. doi: 10.1038/mp.2015.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casquero-Veiga M., Garcia-Garcia D., MacDowell K.S., Perez-Caballero L., Torres-Sanchez S., Fraguas D., Berrocoso E., Leza J.C., Arango C., Desco M., Soto-Montenegro M.L. Risperidone administered during adolescence induced metabolic, anatomical and inflammatory/oxidative changes in adult brain: a PET and MRI study in the maternal immune stimulation animal model. Eur. Neuropsychopharmacol. 2019;29:880–896. doi: 10.1016/j.euroneuro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Dickerson D.D., Overeem K.A., Wolff A.R., Williams J.M., Abraham W.C., Bilkey D.K. Association of aberrant neural synchrony and altered GAD67 expression following exposure to maternal immune activation, a risk factor for schizophrenia. Transl. Psychiatry. 2014;4:e418. doi: 10.1038/tp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott P.L., Warner T.A., Jays P.R., Salway P., Busby S.J. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Giovanoli S., Engler H., Engler A., Richetto J., Voget M., Willi R., Winter C., Riva M.A., Mortensen P.B., Feldon J., Schedlowski M., Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- Glenthoj B.Y., Mackeprang T., Svarer C., Rasmussen H., Pinborg L.H., Friberg L., Baare W., Hemmingsen R., Videbaek C. Frontal dopamine D(2/3) receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol. Psychiatr. 2006;60:621–629. doi: 10.1016/j.biopsych.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Halldin C., Farde L., Hogberg T., Mohell N., Hall H., Suhara T., Karlsson P., Nakashima Y., Swahn C.G. Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J. Nucl. Med. 1995;36:1275–1281. [PubMed] [Google Scholar]
- Harte M., O'Connor W.T. Evidence for a differential medial prefrontal dopamine D1 and D2 receptor regulation of local and ventral tegmental glutamate and GABA release: a dual probe microdialysis study in the awake rat. Brain Res. 2004;1017:120–129. doi: 10.1016/j.brainres.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Hoover W.B., Vertes R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hsiao E.Y., McBride S.W., Chow J., Mazmanian S.K., Patterson P.H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., Patterson P.H., Mazmanian S.K. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendell R.E., Kemp I.W. Maternal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatr. 1989;46:878–882. doi: 10.1001/archpsyc.1989.01810100020004. [DOI] [PubMed] [Google Scholar]
- Lammertsma A.A., Hume S.P. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Mednick S.A., Machon R.A., Huttunen M.O., Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatr. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meyer U. Developmental neuroinflammation and schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U. Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatr. 2014;75:307–315. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Schedlowski M., Yee B.K. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci. Biobehav. Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U., Nyffeler M., Schwendener S., Knuesel I., Yee B.K., Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- Murphy B.L., Arnsten A.F., Goldman-Rakic P.S., Roth R.H. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura J.P., Schroeder A., Hudson M., Jones N., Gillespie B., Du X., Notaras M., Swaminathan V., Reay W.R., Atkins J.R., Green M.J., Carr V.J., Cairns M.J., Sundram S., Hill R.A. The maternal immune activation model uncovers a role for the Arx gene in GABAergic dysfunction in schizophrenia. Brain Behav. Immun. 2019;81:161–171. doi: 10.1016/j.bbi.2019.06.009. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Hashimoto K., Kishimoto T., Shimizu E., Ishikura H., Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol. Psychiatr. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Patterson P.H. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Patterson P.H. Maternal infection and immune involvement in autism. Trends Mol. Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y., Bernstein H.G., Dobrowolny H., Bogerts B., Weiner I., Keilhoff G. Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav. Immun. 2012;26:353–363. doi: 10.1016/j.bbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Roenker N.L., Gudelsky G., Ahlbrand R., Bronson S.L., Kern J.R., Waterman H., Richtand N.M. Effect of paliperidone and risperidone on extracellular glutamate in the prefrontal cortex of rats exposed to prenatal immune activation or MK-801. Neurosci. Lett. 2011;500:167–171. doi: 10.1016/j.neulet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero E., Ali C., Molina-Holgado E., Castellano B., Guaza C., Borrell J. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology. 2007;32:1791–1804. doi: 10.1038/sj.npp.1301292. [DOI] [PubMed] [Google Scholar]
- Scarborough J., Mueller F., Arban R., Dorner-Ciossek C., Weber-Stadlbauer U., Rosenbrock H., Meyer U., Richetto J. Preclinical validation of the micropipette-guided drug administration (MDA) method in the maternal immune activation model of neurodevelopmental disorders. Brain Behav. Immun. 2020;88:461–470. doi: 10.1016/j.bbi.2020.04.015. [DOI] [PubMed] [Google Scholar]
- Suhara T., Okubo Y., Yasuno F., Sudo Y., Inoue M., Ichimiya T., Nakashima Y., Nakayama K., Tanada S., Suzuki K., Halldin C., Farde L. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch. Gen. Psychiatr. 2002;59:25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- Suvisaari J., Haukka J., Tanskanen A., Hovi T., Lonnqvist J. Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am. J. Psychiatr. 1999;156:1100–1102. doi: 10.1176/ajp.156.7.1100. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Higuchi M., Suhara T. The role of extrastriatal dopamine D2 receptors in schizophrenia. Biol. Psychiatr. 2006;59:919–928. doi: 10.1016/j.biopsych.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Talvik M., Nordstrom A.L., Olsson H., Halldin C., Farde L. Decreased thalamic D2/D3 receptor binding in drug-naive patients with schizophrenia: a PET study with [11C]FLB 457. Int. J. Neuropsychopharmacol. 2003;6:361–370. doi: 10.1017/S1461145703003699. [DOI] [PubMed] [Google Scholar]
- Tuppurainen H., Kuikka J., Viinamaki H., Husso-Saastamoinen M., Bergstrom K., Tiihonen J. Extrastriatal dopamine D 2/3 receptor density and distribution in drug-naive schizophrenic patients. Mol. Psychiatr. 2003;8:453–455. doi: 10.1038/sj.mp.4001334. [DOI] [PubMed] [Google Scholar]
- Vincent S.L., Adamec E., Sorensen I., Benes F.M. The effects of chronic haloperidol administration on GABA-immunoreactive axon terminals in rat medial prefrontal cortex. Synapse. 1994;17:26–35. doi: 10.1002/syn.890170104. [DOI] [PubMed] [Google Scholar]
- Vuillermot S., Weber L., Feldon J., Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J. Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard T., Mortensen P.B., Pedersen C.B., Wohlfahrt J., Melbye M. Exposure to prenatal and childhood infections and the risk of schizophrenia: suggestions from a study of sibship characteristics and influenza prevalence. Arch. Gen. Psychiatr. 1999;56:993–998. doi: 10.1001/archpsyc.56.11.993. [DOI] [PubMed] [Google Scholar]
- Winter C., Djodari-Irani A., Sohr R., Morgenstern R., Feldon J., Juckel G., Meyer U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int. J. Neuropsychopharmacol. 2009;12:513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- Xu L., Zhang X.H. Distribution of D1 and D2-dopamine receptors in calcium-binding-protein expressing interneurons in rat anterior cingulate cortex. Sheng Li Xue Bao. 2015;67:163–172. [PubMed] [Google Scholar]
- Yasuno F., Suhara T., Okubo Y., Sudo Y., Inoue M., Ichimiya T., Takano A., Nakayama K., Halldin C., Farde L. Low dopamine d(2) receptor binding in subregions of the thalamus in schizophrenia. Am. J. Psychiatr. 2004;161:1016–1022. doi: 10.1176/appi.ajp.161.6.1016. [DOI] [PubMed] [Google Scholar]
- Zuckerman L., Rehavi M., Nachman R., Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.