Abstract
Protocols for DNA electroporation in Leishmania promastigote cells are well established. More recently, in vitro culture of axenic Leishmania amastigotes became possible. We have established conditions for DNA transformation of axenically grown Leishmania infantum amastigotes. Parameters for DNA electroporation of Leishmania axenic amastigotes were systematically studied using luciferase-mediated transient transfection. Cell lines expressing stable luciferase activity were then selected, and their ability to be used in an in vitro drug screening procedure was determined. A model was established, using axenic amastigotes expressing luciferase activity, for rapidly determining the activity of drugs directly against both axenic and intracellular amastigotes. For intracellular amastigotes, the 50% effective concentrations of pentamidine, sodium stibogluconate (Pentostam), meglumine (Glucantime), and potassium antimonyl tartrate determined with the luciferase assay were 0.2 μM (0.12 μg/ml), 55 μg/ml, 95 μg/ml, and 0.12 μg/ml, respectively; these values are in agreement with values determined by more labor-intensive staining methods. We also showed the usefulness of luciferase-expressing parasites for analyzing drug resistance. The availability of luciferase-expressing amastigotes for use in high-throughput screening should facilitate the search for new antileishmanial drugs.
Leishmaniasis is a significant cause of morbidity and mortality in several countries of the world (19). A vertebrate host is infected with flagellated extracellular promastigote forms via the bite of a sand fly. Promastigotes are rapidly transformed into nonflagellated amastigotes, which divide actively within the mononuclear phagocytes of the vertebrate host.
The basic treatment for leishmaniasis consists of the administration of sodium stibogluconate (Pentostam), meglumine (Glucantime), or pentamidine. Treatment failure, especially in kala-azar, mucosal leishmaniasis, and diffuse cutaneous leishmaniasis, is becoming a common problem in many areas where the diseases are endemic. There are now strong indications that treatment failure may be partly due to the drug resistance of the parasite (15, 18, 21, 25). In addition, numerous cases of relapse or unresponsiveness have been reported during the treatment of patients coinfected with human immunodeficiency virus and Leishmania spp. (1). Although rapid assays for drug screening of Leishmania promastigotes have been devised (5), promastigotes are usually less sensitive to various drugs than amastigotes (11, 29). Although animal models are well established for drug testing, they are not suitable for large-scale primary drug screens. The development of new drugs has been impeded by the lack of a simple, reliable, and rapid evaluation system allowing the simultaneous determination of drug activity at the mammalian stage under both axenic and intracellular conditions. The development of a system for the in vitro cultivation of amastigotes under axenic conditions enabled the development of models for in vitro drug screening directly on the clinically relevant stage of the parasite (7, 34). However, these systems present some limitations, including the absence of information on the influence of macrophages on drug activity. In order to delineate the potential role played by macrophages in drug toxicity, time-consuming experiments must be carried out using macrophage models like THP-1 (17, 32) or human or mouse monocyte-derived macrophages (3, 4).
The drug screening for several other intracellular pathogens is also complicated by the difficulties in assessing the number of pathogens remaining after drug treatment. The use of reporter genes such as the firefly luciferase, β-galactosidase, or green fluorescent protein gene has considerably facilitated the screening of antimicrobial agents against intracellular pathogens such as Mycobacterium tuberculosis (10, 22), Trypanosoma cruzi (6), and Toxoplasma gondii (26). The same strategy should be of great interest while testing new antileishmanial agents. Protocols for DNA electroporation of Leishmania promastigote cells are well established (9), but until now there has been no report of DNA electroporation into axenic Leishmania amastigotes. In this study, we have established conditions for DNA transformation of Leishmania infantum axenic amastigotes. The use of axenic amastigotes stably expressing the firefly luciferase reporter gene for quickly and simultaneously testing drug activity against both axenic and intracellular amastigotes was also evaluated.
MATERIALS AND METHODS
Materials.
Meglumine (Glucantime; batch number 331-2), which does not contain m-chlorocresol as a preservative, was supplied by Rhône Poulenc Specia. Sodium stibogluconate (Pentostam; batch number B4131A) from Glaxo-Wellcome was kindly supplied by Chris Carter, University of Strathclyde. Potassium antimonyl tartrate trihydrate, neomycin, hygromycin, phorbol myristate acetate (PMA), 3-(4,5-dimethlythinzol-2-yl)-2,5-diphenyltetrazolium (MTT), and pentamidine isethionate were supplied by Sigma.
Parasites and cultures.
A cloned line of L. infantum (MHOM/MA/67/ITMAP-263) was used in all experiments. Axenically grown amastigote forms of L. infantum were maintained at 37°C with 5% CO2 by weekly subpassages in a cell-free medium called MAA/20 (medium for axenically grown amastigotes) in 25-ml flasks, as previously described (34). From a starting inoculum of 5 × 105 amastigotes/ml, a cell density of about 5 × 107 parasites/ml was obtained on day 7. MAA/20 consisted of modified medium 199 (Gibco BRL) with Hanks' balanced salts supplemented with 0.5% soya trypto-casein (Pasteur Diagnostics, Marne la Coquette, France), 0.01 mM bathocuproine disulfonic acid, 3 mM l-cysteine, 15 mM d-glucose, 5 mM l-glutamine, 4 mM NaHCO3, 0.023 mM bovine hemin, 25 mM HEPES (final pH, 6.5), and 20% fetal calf serum (FCS).
Viability test.
To estimate the 50% inhibitory concentration of drugs, the MTT micromethod was used as previously described (34). Briefly, axenically grown amastigotes were seeded in a volume of 100 μl in 96-well flat-bottom microtrays. Drugs were added, and after 72 h of incubation 10 μl of MTT (10 mg/ml) was added to each well and plates were further incubated for 4 h. The reaction was then stopped by the addition of 100 μl of 50% isopropanol–10% sodium dodecyl sulfate. The plates were incubated for an additional 30 min under agitation before reading the optical density at 570 nm.
DNA construct and transfection.
The pSPαLUC vector used in transient-transfection experiments was made by subcloning the intergenic region of the α-tubulin gene (24) as an EcoRI-BamHI fragment into pSP72 (Promega). The LUC gene was amplified by PCR and subcloned into the BamHI site of vector pSPα filled in by Klenow polymerase to yield pSPαLUC. The vector PGMαNEOαLUC has been described before (30).
Drug efficacy assay in THP-1.
The growth of the luciferase-expressing amastigotes of L. infantum in a human leukemia monocyte cell line (THP-1 cells) was evaluated according to the method described by Gebre-Hiwot et al. (17), with modifications. Briefly, THP-1 cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mM glutamine, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml. THP-1 cells in the log phase of growth were differentiated by incubation for 2 days in medium containing 20 ng of PMA/ml (Sigma), which induced differentiation and caused the cells to become adherent. THP-1 cells treated with PMA were washed with prewarmed medium and then infected with stationary-phase extracellular amastigotes in 96-microwell plates (Nunc) at a parasite/macrophage ratio of 2:1 for 2 h at 37°C with 5% CO2. Noninternalized parasites were removed. Serial dilutions of each drug were made in the RPMI medium supplemented with 10% FCS and were dispensed in wells. After 5 days of drug exposure, wells containing adherent differentiated THP-1 cells were washed, and luciferase activity was determined. Alternatively, Leishmania cells were counted by Giemsa staining as described elsewhere (33).
Luciferase assay.
The luciferase activity of the LUC-recombinant parasites was determined essentially as described elsewhere (30). Values were expressed as relative light units (RLU) and were transformed by the formula RLU index [100 − (RLU of untreated wells/RLU of treated wells)] × 100%.
RESULTS
DNA transformation in axenic amastigotes.
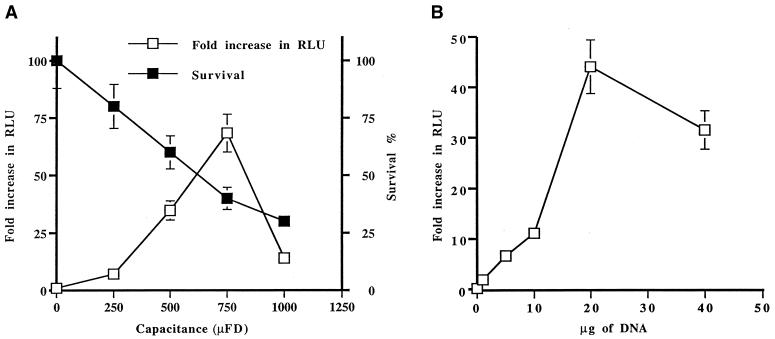
Transformation of the insect stage of a number of kinetoplastid parasites has already been reported (9). It is also possible to transform Trypanosoma brucei bloodstream-form parasites with DNA (8), but no work has been reported yet on DNA transformation of Leishmania amastigotes. We have therefore set up the technique to introduce DNA into axenic amastigotes. First, we have attempted to optimize parameters involved in DNA transformation. We used a constant voltage of 450 V, a voltage that we use routinely to transform promastigotes. Using this constant voltage, we introduced by electroporation the plasmid pSPαLUC into the L. infantum axenic strain while varying the capacitance (Fig. 1A). Immediately after the shock, cells were put in culture in MAA/20 medium, and after 24 h luciferase activity was measured and the optimal transient-transfection efficiency was found at 750 μF. The transfection efficiency was in close correlation with the survival of the parasites following electroporation (Fig. 1A), with the maximum transfection efficiency observed under conditions leading to 50% survival. We also tested the correlation between the amount of DNA and transformation efficiency, and we found a maximum efficiency while using 20 μg of DNA (Fig. 1B).
FIG. 1.
Effect of capacitance variation and DNA concentration on efficiency of transient transfection of L. infantum axenic amastigotes. The efficiency of transfection was determined by measuring luciferase activity. Briefly, 2 × 108 axenic amastigotes were transformed with the plasmid pSP72αLuc. The luciferase activity of 107 axenic amastigotes was determined 24 h after the transfection. Results are expressed as fold increase in luciferase activity versus the capacitance (A) or the DNA concentration (B). The viability of the transfected parasites was measured by the MTT test.
Stable transformation of axenic amastigotes.
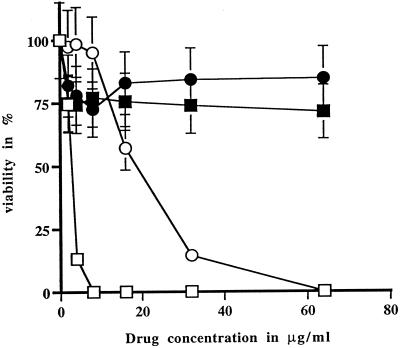
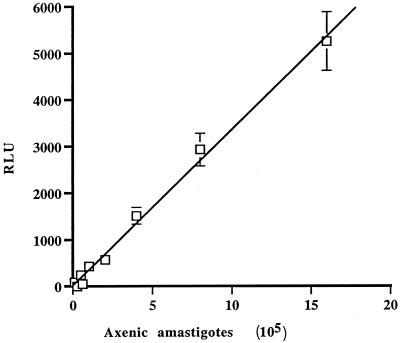
In order to have stable DNA transformation, we tested whether L. infantum amastigotes were susceptible to the antibiotics G418 and hygromycin B, the two most widely used drugs selective for Leishmania transformation. The cells were found to be sensitive to the two antibiotics, with 50% effective concentration (EC50) values of 4 μg/ml for G418 and 17 μg/ml for hygromycin B (Fig. 2). Using optimized conditions established by transient transfection, we have electroporated the plasmid pGMαNEOαLUC (30), which contains the selectable marker for neomycin phosphotransferase (NEO), and the cosmid vector CL-Hyg (31), which contains the hygromycin phosphotransferase (HYG) gene. The selection with the drugs was initiated 24 h after electroporation. The growth of cells highly resistant to G418 was observed after 14 to 20 days, while hygromycin B-resistant cells appeared as quickly as 10 days after the initial electroporation. The transfectants were clearly resistant to the selective drug (Fig. 2). Southern blot analysis of these amastigote transfectants confirmed that both the plasmid pGMαNEOαLUC and the cosmid CL-Hyg were present in several copies (data not shown). Since the pGMαNEOαLUC plasmid contains the luciferase gene, it permitted us to observe a linear correlation between the number of amastigote cells and luciferase activity (Fig. 3). By increasing the concentration of the selective drug, we could increase the copy number of the vector, which led to an even better sensitivity in the detection of the parasite (data not shown).
FIG. 2.
Susceptibility of axenic amastigotes to neomycin and hygromycin B. Results are expressed as the mean of triplicate experiments. □, wild type with G418; ■, WT-pGMαNEOαLUC with G418; ○, wild type with hygromycin B; ●, WT-CLHyg with hygromycin B.
FIG. 3.
Relationship between number of axenic amastigotes and luciferase activity. Results are given as mean values for three experiments. Amastigotes were counted using a hemacytometer, while the number of RLU was determined as described in Materials and Methods.
Use of luciferase-expressing axenic amastigotes in drug screens.
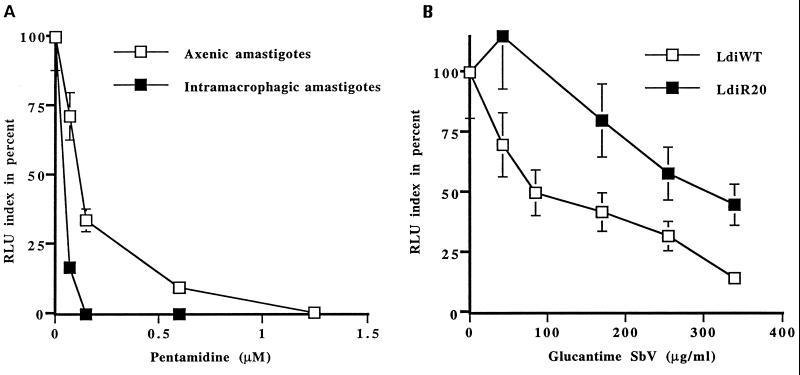
For several drugs, the use of axenic amastigotes in drug screens has been found to be superior to the use of promastigotes (7, 34). The linearity between luciferase activity and the number of amastigotes (Fig. 3) prompted us to test whether measuring luciferase activity would permit a rapid determination of drug activity with axenic amastigotes and, even more importantly, with intracellular amastigotes in macrophages. The activity of pentamidine was determined against axenic and intracellular amastigotes, and the EC50s were 1.5 and 0.2 μM, respectively (Fig. 4A). Those values are similar to values reported in the literature while using a laborious staining method for counting the parasites in macrophages (Table 1). Using the luciferase-expressing parasites, we could determine rapidly the EC50 of potassium antimonyl tartrate [Sb(III)] and two pentavalent antimonial compounds, sodium stibogluconate and meglumine for axenic and intracellular amastigotes (Table 1). Values obtained were consistent with values reported in the literature for other methods (Table 1).
FIG. 4.
Use of luciferase-expressing parasites to monitor drug activity. The toxicity of drugs for intracellular amastigotes was assessed by infecting human cell lines with luciferase-expressing amastigotes at a cell/parasite ratio of 1:2 in medium supplemented with 10% FCS after differentiation with PMA. Infected cells were exposed to drugs for 5 days, after which luciferase activity was measured. The toxicity of pentamidine for axenic amastigotes was done by seeding axenic amastigotes in 96-well plates. After 5 days, parasites were washed and the luciferase activity was determined. Results are expressed as the mean of three experiments, each carried out in triplicate. (A) Pentamidine toxicity in axenic and intracellular amastigotes; (B) toxicity of meglumine (Glucantime) against wild-type and Sb(III)-resistant L. infantum.
TABLE 1.
EC50 determinations in Leishmania sp. axenic and intracellular amastigotes
| Drug | EC50
|
|||
|---|---|---|---|---|
| Axenic amastigotes
|
Intracellular amastigotes
|
|||
| Luc assay | Other methods (source)a | Luc assay | Staining (source)a | |
| Pentamidine | 1.50 ± 0.52 μM | 1–3 μM (7, 34) | 0.20 ± 0.05 μM | 0.7–1 μM (4, 7, 33) |
| Sodium stibogluconate | 80 μg Sb/ml | 24–134 μg/ml (7, 14, 34) | 55 ± 10 μg Sb/ml | 35 ± 10 μg/ml (this study) |
| Sodium meglumine | >400 μg Sb/ml | 1,800 μg/ml (32) | 95 ± 15 μg Sb/ml | 25–50 μg/ml (7, 15, 32) |
| Sb(III) | NDb | 4.70 ± 2.4 μg/ml (this study) | 0.12 ± 0.09 μg/ml | 0.20 ± 0.1 μg/ml (33) |
Reference(s) or source of EC50 is shown within parentheses.
ND, not determined.
In another application of the luciferase marker, we also introduced the pGMαNEOαLUC plasmid into L. infantum R20 (LdiR20), a mutant cell line selected for resistance to potassium antimonyl tartrate (32). We have already shown that this mutant was cross-resistant to meglumine once inside macrophages but not in axenic culture (32). Using the luciferase assay we could easily determine that the LdiR20 amastigote cells were clearly cross-resistant to meglumine once inside macrophages, with an increase in the EC50 from 95 to 330 μg/ml (Fig. 4B).
DISCUSSION
The use of reporter genes in a number of intracellular pathogenic microorganisms has facilitated antimicrobial drug discovery and testing (6, 10, 22, 26). Recently, models of axenic Leishmania amastigotes have been introduced and proposed for drug testing, instead of promastigotes (7, 13, 34). In certain models, preservative-free pentavalent antimony was found to be more active against intracellular Leishmania than against axenic amastigotes (32). The evaluation of parasite number in macrophages or in animals is laborious and renders difficult the screening of several drugs at a time. Luciferase had already been transfected transiently (16), and more recently stably (30), in Leishmania promastigotes. Leishmania promastigotes stably transfected with reporter genes have been used to look at infections of macrophages or animals (27, 30). The possibility of using luciferase-expressing Leishmania amastigotes for drug testing was investigated in this study. In order to achieve this goal, we set up conditions to transfect efficiently DNA into Leishmania axenic amastigotes. Indeed, although Leishmania promastigotes can routinely be transfected, no successful DNA electroporation of Leishmania axenic amastigotes has been reported. By monitoring capacitance and quantity of DNA, we found that we could indeed successfully transform amastigote cells. Conditions differed slightly from those for electroporation of promastigotes, which is usually carried out at 500 μF.
Toxicity of first- (antimonials) and second-line (pentamidine) drugs was determined for both axenic and intracellular amastigotes expressing the luciferase gene. We found that pentamidine was toxic for axenic amastigotes at concentrations in the range of low micromolar concentrations and in the concentration range potentially achieved during chemotherapy (Table 1). This result was in substantial agreement with those previously found using other protocols, like the MTT test (34). Independently of the method used for assessing the number of parasites, pentamidine was found to be significantly less toxic for axenic amastigotes than for intracellular ones. Moreover, the 50% inhibitory concentration for pentamidine was in the same range regardless of whether its determination was done using the luciferase or conventional staining techniques in a number of different cell types (2, 34). Sodium stibogluconate was toxic for both axenic and intracellular amastigotes at concentrations below 100 and 55 μg of Sb/ml, respectively. Using a viability-based test, it has been previously shown that sodium stibogluconate is toxic for axenic amastigotes of L. infantum at concentrations from 24 to 134 μg of Sb/ml (Table 1). We confirmed that intracellular amastigotes were more susceptible to sodium stibogluconate than were extracellular ones, at a concentration of 55 μg/ml in the luciferase assay (Table 1). To compare more adequately those values obtained with staining using the same cell line and the same drug lot, we determined the EC50 by using Giemsa staining to be 35 μg/ml (Table 1). These values are slightly higher than this laboratory's previous determination of 10 μg/ml (32) and other values available in the literature, which are in the range of 10 to 30 μg/ml (20, 29). The second antimony-containing drug in clinical use, meglumine, was also toxic for intracellular amastigotes at a concentration (95 μg/ml) which was slightly higher than those previously found using other models or methodologies (15, 33). As reported previously (32), meglumine at concentrations as high as 400 μg/ml was not toxic for axenic amastigotes of L. infantum, as previously shown for the same species using counting methods (32). The activity of the trivalent antimony-containing drug potassium antimonyl tartrate was found to be highly toxic for intracellular amastigotes of L. infantum, at a concentration below 1 μg/ml.
Collectively, all these data show that most well-known leishmaniacidal agents, whose activities have been demonstrated in vitro with a macrophage model and are in clinical use, were toxic for both axenic and intracellular amastigotes expressing luciferase activity. This in vitro model presents numerous advantages over the traditional drug-screening procedure: (i) interpretation of the results is easier; and (ii) since the same method is used to measure drug activity against both axenic and intramacrophagic amastigotes, the influence of macrophages on drug activity can now be directly analyzed. The main advantage of the use of luciferase-expressing parasites is the possibility of determining intracellular infection with and without drugs at a fraction of the time required when using staining methods. This model, which permits us to directly evaluate the toxicity of new compounds directly against the mammalian stage of the parasites and to evaluate the role of macrophages in the toxicity has the potential to be automated in 96-well formats for high-throughput screening of drugs. There are, nonetheless, some differences in EC50 determinations, depending on whether the determinations are done using luciferase or staining. One difference is that the luciferase method measures the total number of parasites present, while the staining methods provide an approximation of the macrophages that were counted. Thus, the new methodology may explain the differences between our EC50 determinations, notably for Sb(V) drugs, and the values available in the literature. Differences in host cell lines, in Leishmania species, and in the batch of drug used may also influence the EC50 values. Nonetheless, the results indicate that all antileishmanial drugs tested are toxic against intracellular luciferase-expressing amastigotes, and therefore these cells can be useful for large-scale drug screening experiments. Other Leishmania strains or species which are frequently used in drug screens could also be transfected with the luciferase gene.
Our ability to transfect axenic amastigotes will be useful for drug screening procedures, but it also has several other potential uses. One use could be in studying drug resistance, as shown in Fig. 4B. We are now using this system to analyze the role of isolated drug resistance genes that we have isolated in resistant promastigotes (28) by introducing them into amastigotes. We are planning to introduce the LUC marker into field strains unresponsive to treatment to test whether we can detect resistance in in vitro models and in animals. The ability to transfect CL-Hyg in amastigotes (Fig. 2) will permit functional cloning approaches in the amastigote stage of the parasite. Using functional cloning in Leishmania promastigotes, drug resistance genes were isolated (12, 23, 35), and recently we succeeded in isolating two antimony resistance genes in amastigotes by functional cloning (unpublished results). Overall, the possibility of growing amastigotes axenically and now transfecting Leishmania amastigotes will facilitate experiments on the stage of the parasite that is present in infected individuals and in contact with drugs during chemotherapy.
ACKNOWLEDGMENTS
This work was supported in part by a group grant from the Canadian Institutes of Health Research (CIHR) to M.O. and B.P. and by a collaborative Wellcome Trust-Burroughs Wellcome Fund grant in infectious diseases. D.S. was partially supported by an institutional FRSQ postdoctoral fellowship. B.P. is an FRSQ Scholar, and M.O. is an MRC Scientist and a Burroughs Wellcome Fund New Investigator in Molecular Parasitology.
The CL-Hyg vector was kindly provided by S. Beverley, Washington University in St. Louis.
REFERENCES
- 1.Alvar J, Canavate C, Gutierrez-Solar B, Jimenez M, Laguna F, Lopez-Velez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman J D, Gallalee J V. Semiautomated assessment of in vitro activity of potential antileishmanial drugs. Antimicrob Agents Chemother. 1985;28:723–726. doi: 10.1128/aac.28.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman J D, Lee L S. Activity of antileishmanial agents against amastigotes in human monocyte-derived macrophages and in mouse peritoneal macrophages. J Parasitol. 1984;70:220–225. [PubMed] [Google Scholar]
- 4.Berman J D, Wyler D J. An in vitro model for investigation of chemotherapeutic agents in leishmaniasis. J Infect Dis. 1980;142:83–86. doi: 10.1093/infdis/142.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Bodley A L, McGarry M W, Shapiro T A. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J Infect Dis. 1995;172:1157–1159. doi: 10.1093/infdis/172.4.1157. [DOI] [PubMed] [Google Scholar]
- 6.Buckner F S, Verlinde C L, La Flamme A C, Van Voorhis W C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callahan H L, Portal A C, Devereaux R, Grogl M. An axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41:818–822. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carruthers V B, van der Ploeg L H, Cross G A. DNA-mediated transformation of bloodstream-form Trypanosoma brucei. Nucleic Acids Res. 1993;21:2537–2538. doi: 10.1093/nar/21.10.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clayton C E. Genetic manipulation of kinetoplastida. Parasitol Today. 1999;15:372–378. doi: 10.1016/s0169-4758(99)01498-2. [DOI] [PubMed] [Google Scholar]
- 10.Collins L A, Torrero M N, Franzblau S G. Green fluorescent protein reporter microplate assay for high-throughput screening of compounds against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:344–347. doi: 10.1128/aac.42.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coombs G H, Hart D T, Capaldo J. Leishmania mexicana: drug sensitivities of promastigotes and transforming amastigotes. J Antimicrob Chemother. 1983;11:151–162. doi: 10.1093/jac/11.2.151. [DOI] [PubMed] [Google Scholar]
- 12.Cotrim P C, Garrity L K, Beverley S M. Isolation of genes mediating resistance to inhibitors of nucleoside and ergosterol metabolism in leishmania by overexpression/selection. J Biol Chem. 1999;274:37723–37730. doi: 10.1074/jbc.274.53.37723. [DOI] [PubMed] [Google Scholar]
- 13.Ephros M, Bitnun A, Shaked P, Waldman E, Zilberstein D. Stage-specific activity of pentavalent antimony against Leishmania donovani axenic amastigotes. Antimicrob Agents Chemother. 1999;43:278–282. doi: 10.1128/aac.43.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ephros M, Waldman E, Zilberstein D. Pentostam induces resistance to antimony and the preservative chlorocresol in Leishmania donovani promastigotes and axenically grown amastigotes. Antimicrob Agents Chemother. 1997;41:1064–1068. doi: 10.1128/aac.41.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, Faugere B, Dumon H. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:827–830. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gay L S, Wilson M E, Donelson J E. The promoter for the ribosomal RNA genes of Leishmania chagasi. Mol Biochem Parasitol. 1996;77:193–200. doi: 10.1016/0166-6851(96)02594-7. [DOI] [PubMed] [Google Scholar]
- 17.Gebre-Hiwot A, Tadesse G, Croft S L, Frommel D. An in vitro model for screening antileishmanial drugs: the human leukaemia monocyte cell line, THP-1. Acta Trop. 1992;51:237–245. doi: 10.1016/0001-706x(92)90042-v. [DOI] [PubMed] [Google Scholar]
- 18.Grogl M, Thomason T N, Franke E D. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992;47:117–126. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
- 19.Herwaldt B L. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim M E, Hag-Ali M, el-Hassan A M, Theander T G, Kharazmi A. Leishmania resistant to sodium stibogluconate: drug-associated macrophage-dependent killing. Parasitol Res. 1994;80:569–574. doi: 10.1007/BF00933004. [DOI] [PubMed] [Google Scholar]
- 21.Jackson J E, Tally J D, Ellis W Y, Mebrahtu Y B, Lawyer P G, Were J B, Reed S G, Panisko D M, Limmer B L. Quantitative in vitro drug potency and drug susceptibility evaluation of Leishmania spp. from patients unresponsive to pentavalent antimony therapy. Am J Trop Med Hyg. 1990;43:464–480. doi: 10.4269/ajtmh.1990.43.464. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs W R, Jr, Barletta R G, Udani R, Chan J, Kalkut G, Sosne G, Kieser T, Sarkis G J, Hatfull G F, Bloom B R. Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of luciferase reporter phages. Science. 1993;260:819–822. doi: 10.1126/science.8484123. [DOI] [PubMed] [Google Scholar]
- 23.Kündig C, Haimeur A, Légaré D, Papadopoulou B, Ouellette M. Increased transport of pteridines compensates for mutations in the high affinity folate transporter and contributes to methotrexate resistance in the protozoan parasite Leishmania tarentolae. EMBO J. 1999;18:2342–2351. doi: 10.1093/emboj/18.9.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laban A, Tobin J F, Curotto de Lafaille M A, Wirth D F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990;343:572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- 25.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, Sacks D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180:564–567. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- 26.McFadden D C, Seeber F, Boothroyd J C. Use of Toxoplasma gondii expressing β-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob Agents Chemother. 1997;41:1849–1853. doi: 10.1128/aac.41.9.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misslitz A, Mottram J C, Overath P, Aebischer T. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in leishmania amastigotes. Mol Biochem Parasitol. 2000;107:251–261. doi: 10.1016/s0166-6851(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 28.Ouellette M, Légaré D, Haimeur A, Grondin K, Roy G, Brochu C, Papadopoulou B. ABC transporters in Leishmania and their role in drug resistance. Drug Resist Updates. 1998;1:43–48. doi: 10.1016/s1368-7646(98)80213-6. [DOI] [PubMed] [Google Scholar]
- 29.Roberts W L, Rainey P M. Antileishmanial activity of sodium stibogluconate fractions. Antimicrob Agents Chemother. 1993;37:1842–1846. doi: 10.1128/aac.37.9.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy G, Dumas C, Sereno D, Wu Y, Singh A K, Tremblay M J, Ouellette M, Olivier M, Papadopoulou B. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol. 2000;110:195–206. doi: 10.1016/s0166-6851(00)00270-x. [DOI] [PubMed] [Google Scholar]
- 31.Ryan K A, Dasgupta S, Beverley S M. Shuttle cosmid vectors for the trypanosomatid parasite Leishmania. Gene. 1993;131:145–150. doi: 10.1016/0378-1119(93)90684-u. [DOI] [PubMed] [Google Scholar]
- 32.Sereno D, Cavaleyra M, Zemzoumi K, Maquaire S, Ouaissi A, Lemesre J L. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob Agents Chemother. 1998;42:3097–3102. doi: 10.1128/aac.42.12.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sereno D, Holzmuller P, Lemesre J L. Efficacy of second line drugs on antimonyl-resistant amastigotes of Leishmania infantum. Acta Trop. 2000;74:25–31. doi: 10.1016/s0001-706x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 34.Sereno D, Lemesre J L. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother. 1997;41:972–976. doi: 10.1128/aac.41.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasudevan G, Carter N S, Drew M E, Beverley S M, Sanchez M A, Seyfang A, Ullman B, Landfear S M. Cloning of leishmania nucleoside transporter genes by rescue of a transport-deficient mutant. Proc Natl Acad Sci USA. 1998;95:9873–9878. doi: 10.1073/pnas.95.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]