Abstract
Rationale & Objective
This study investigated the effects on patients’ outcomes of using medium cutoff (MCO) versus high-flux (HF) dialysis membranes.
Study Design
A retrospective, observational, multicenter, cohort study.
Setting & Participants
Patients aged greater than 18 years receiving hemodialysis at the Baxter Renal Care Services dialysis network in Colombia. The inception of the cohort occurred from September 1, 2017, to November 30, 2017, with follow-up to November 30, 2019.
Exposure
The patients were divided into 2 cohorts according to the dialyzer used at the inception: (1) MCO membrane or (2) HF membrane.
Outcomes
Primary outcomes were the hospitalization rate from any cause and hospitalization days per patient-year. Secondary outcomes were acute cardiovascular events and mortality rates from any cause and secondary to cardiovascular causes. Laboratory parameters were assessed throughout the 2-year follow-up period.
Analytical Approach
Descriptive statistics were used to report population characteristics. Inverse probability of treatment weighting was applied to each group before analysis. All categorical variables were compared using Pearson’s χ2 test, and continuous variables were analyzed with the t test. Baseline differences between groups with a value of >10% were considered clinically meaningful. Laboratory variables were measured at 5 consecutive time points. A between-patient effect was analyzed using a split-plot factorial analysis of variance.
Results
The analysis included 1,098 patients, of whom 564 (51.3%) were dialyzed with MCO membranes and 534 (48.7%) with HF membranes. Patients receiving hemodialysis with MCO membranes had a lower all-cause hospitalization incidence rate (IR) per patient-year (IR = 0.93; 95% CI, 0.82-1.03) than those receiving hemodialysis with HF membranes (IR = 1.13; 95% CI, 0.96-1.30), corresponding to a significant incident rate ratio (MCO/HF) of 0.82 (95% CI, 0.68-0.99; P = 0.04). The frequency of nonfatal cardiovascular events showed statistical significance, with a lower incidence in the MCO group (incident rate ratio = 0.66; 95% CI, 0.46-0.96; P = 0.03). No statistically significant differences in all-cause time until death were observed (P = 0.48). Albumin levels were similar between the 2 dialyzer cohorts.
Limitations
Despite the robust statistical analysis, there remains the possibility that unmeasured variables may still generate residual imbalance and, therefore, skew the results.
Conclusions
The incidences of hospitalization and cardiovascular events in patients receiving hemodialysis were lower when dialyzed with MCO membranes than HF membranes. A randomized controlled trial would be desirable to confirm these results.
Trial Registration
Clinical Trials.gov, ISRCTN12403265.
Index Words: Clinical outcomes, dialysis membranes, hemodialysis, high-flux, medium cutoff
Visual Abstract
Plain-Language Summary.
Improving dialysis technology is important for reducing complications in patients with kidney failure. The present study evaluated clinical outcomes in 2 cohorts, 1 using medium cutoff membranes and the other using high-flux membranes. Decreases in the frequency of hospitalization events and nonfatal cardiovascular events were observed in the medium cutoff group. No differences were found between the groups in serum albumin levels, survival rates, or hospital stays. Randomized controlled trials would be desirable to confirm these results.
Advances in technologies, pharmacotherapeutics, and health care in the field of chronic hemodialysis (HD) over the last half century have led to improvements in patients’ survival and reduced morbidity.1, 2, 3 Despite these advances, the outcomes for the chronic HD population are still far from optimal. The scientific community, therefore, continues to pursue routes to improve these outcomes.4,5 One of the paths identified is to improve the clearance of uremic toxins and, therefore, reduce their adverse effects on various biological systems in patients with kidney failure.6 The hemodialysis study demonstrated that HD with high-flux (HF) membranes was associated with lower mortality and hospitalization for cardiovascular causes.7 A recent literature review focused on the potential benefit of increased removal of large, middle molecules by reducing the effects of these on adverse health outcomes, such as chronic inflammation, secondary immunodeficiency, atherosclerosis, and left ventricular hypertrophy.8
An important step forward in this improvement journey was the development of hemodiafiltration that combines convection and diffusion to increase the clearance of middle molecules.9,10 More recently, advances in bioengineering have allowed a new class of membranes called medium cutoff (MCO) membranes to be developed. These MCO membranes improve the clearance of large, middle molecules with sizes of ≥25 kDa, a fact that constitutes a step toward optimization of clinical outcomes.11, 12, 13, 14, 15, 16
In this sense, some studies have demonstrated the safety of this new type of dialyzer in maintaining albumin levels and the absence of adverse events.17, 18, 19 Some reports have shown a relationship between the use of the MCO membranes and improved patient-reported outcome measures, demonstrating improvements in measures of quality of life and symptoms such as restless legs syndrome.20,21 To date, there are no randomized controlled trials that have evaluated the influence of these MCO membranes on outcomes such as mortality, hospitalizations, or the development of cardiovascular events. Given the scarcity of this type of study with long follow-up times, an option is observational studies based on real-life registries and statistical analysis techniques that control the studied cohorts' imbalances.22
This study aimed to determine whether there are differences in chronic HD patients' clinical outcomes when treated with the MCO membranes compared with HF membranes in Colombia.
Methods
Study Design and Patients
This is a retrospective, observational, multicenter, cohort study of prevalent patients undergoing HD (defined as having received HD for 90 days). Patients were included from the Baxter Renal Care Services network of renal clinics that met water-quality standards established by the Association for the Advancement of Medical Instrumentation. The inception of the cohort was performed from September 1, 2017, to November 30, 2017, with follow-up to November 30, 2019. The inception occurred immediately for those patients in the MCO cohort once switched to this new membrane type. The inclusion criteria included participants aged greater than 18 years receiving either expanded HD using an MCO membrane (Theranova, Baxter) or conventional HD using an HF membrane for a minimum of 4 hours, 3 times per week. Patients in both cohorts were included from the same dialysis clinics. There were no clinical criteria to indicate the type of dialyzer given to specific patients, that is, its use depended on the availability of the dialyzer in renal clinics. The exclusion criteria were a life expectancy of less than 6 months, active infection, metastatic disease, or a Charlson comorbidity index score of greater than 8. The patients were divided into 2 cohorts according to the dialyzer used at the inception: (1) MCO membrane or (2) HF membrane. Censored events were a kidney transplant, loss of follow-up, suspension of dialysis therapy, change of dialysis provider, change of dialysis modality, change of type of membrane, and recovered kidney function. Once a patient had been included in 1 of the 2 cohorts, the clinical teams were instructed not to change the membrane type unless determined by a medical decision or patient request.
Patients who had 13 or more dialysis sessions during follow-up with a different type of dialyzer than that from study initiation were censured because of protocol deviation.
The study protocol was approved by the Cardioinfantil Foundation clinical research ethics committee (May 6, 2020, Minute, item number: 15-2020), which exempted informed consent, as this study does not contain identifiable information and is an observational study.
Baseline Patient Characteristics
Demographic and clinical baseline variables included age; sex; race; dialysis vintage; Charlson comorbidity index score; Karnofsky scale score; body mass index; research site location; chronic kidney disease etiology; history of cardiovascular disease, hypertension, or diabetes; normalized protein catabolic rate; serum levels of hemoglobin, phosphorus, albumin, potassium, and parathyroid hormone; Kt/V single pool value; and urine output in milliliter per day. Additionally, we collected the data regarding vascular access, dialysis flow rate, and blood flow rate.
The exposure variable of interest was the type of dialysis therapy at the inception of the cohort (MCO or HF). The analysis approach was an intention-to-treat analysis (type of dialyzer: MCO vs HF) at the cohort’s inception. Patients with more than 13 consecutive sessions with a different type of dialyzer than the original were censured. All data were obtained from the electronic medical records of Baxter Renal Care Services called Versia and recorded in a REDCAP electronic data capture database. An external audit carried out a data quality assurance process by the National University of Colombia.
Study Outcomes
The primary outcomes were the hospitalization rate from any cause and hospitalization days per patient-year. The secondary outcomes were nonfatal cardiovascular events (defined as any hospitalization event for causes predefined in the International Classification of Diseases, Tenth Revision, as cardiovascular). A time-to-death survival analysis was evaluated for death from any cause. Also, changes in serum albumin levels were evaluated during follow-up in the 2 groups. All outcomes were systematically defined in the electronic medical record and validated by the data auditor.
Statistical Analysis
Descriptive statistics were used to report the population characteristics as the mean and standard deviation for normally distributed variables and median and interquartile range for nonnormally distributed variables. Additionally, all categorical variables were compared between HD groups using Pearson’s χ2 test, and continuous variables were analyzed with the t test. Baseline differences between the groups were compared using standardized differences (for which a value of >10% was considered clinically meaningful).
Laboratory variables were measured at 5 consecutive time points for each of the 2 treatment groups. We considered the between-patient effect (related to the type of dialyzer: MCO or HF) in the analysis. This effect was analyzed using a split-plot factorial analysis of variance.
Considering the potential for confounding related to the nonexperimental design, for comparing some of the outcomes between groups (MCO vs HF), we used inverse probability of treatment weighting with a propensity score to control differences between groups. The propensity score for each participant was calculated from a logistic regression model that included a number of clinical and demographic variables as predictors of the exposure status such as age; sex; race; dialysis vintage; chronic kidney disease cause; hypertension history; diabetes history; cardiovascular disease history; Charlson comorbidity index score; Karnofsky scale score; urine output in milliliter per day; body mass index; measurements of serum albumin levels, hemoglobin levels, levels of parathormone intact, phosphorous levels, potassium levels, Kt/V single pool, and normalized protein catabolic rate; and the research site location.
In accordance with recommendations for the analysis of propensity score weighting with clustered data by Li et al23 and Arpino and Mealli,24 we included fixed effects of “research site locations” in the logistic regression model. The inverse probability of treatment weighting for each individual participant was then calculated. The balance between exposed and unexposed groups in the weighted sample was evaluated on the basis of descriptive methods (standardized differences, with a target value of <0.1, and variance ratios) and inferential methods (overidentification test for covariate balance), and the effect of the exposure on each outcome was estimated using robust standard errors. For the outcomes of hospitalization rate and days, negative binomial regression models were used for the violation of the overdispersion assumption, and Akaike and Bayesian information criteria were used to compare the models; the association was estimated with an incidence rate ratio. Survival time was estimated in the full sample and the weighed population according to membrane use; the log-rank test was used to compare the equality of the survival functions in the full sample; for comparison in the weighed population, we used Cox regression. To confirm the direction of the effect, negative binomial regression models in the full sample were performed (Tables S2-S4). Additionally, the hospitalization causes were reported as frequencies and percentages. Stata 16 (Release 16, StataCorp LLC) was used in the statistical analyses.
Results
Patients
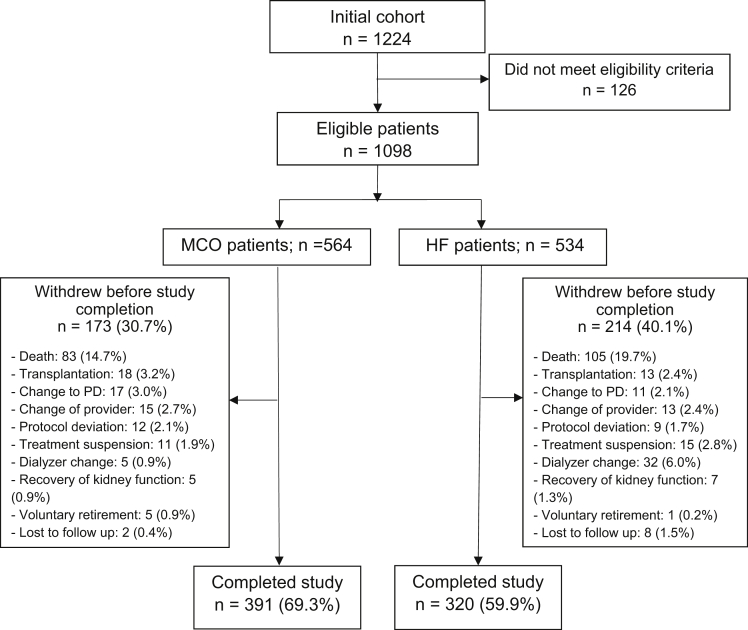
A total of 1,098 patients were enrolled in this study (564 in the MCO group and 534 in the HF group; Fig 1), and 711 patients completed the total follow-up time (391 in the MCO group and 320 in the HF group). The mean age of patients in the MCO group was 60.3 ± 14.9 years, and 65.2% of the patients were men; in the HF group, the mean age of the patients was 60.8 ± 15.0 years and 59.6% were men. The proportions of patients with diabetes mellitus were similar in the 2 groups (43.6% for the MCO group vs 40.1% for the HF group; P = 0.23). The baseline albumin levels were 4.0 g/dL for the MCO group versus 4.0 g/dL for the HF group; more details of the sociodemographic and clinical characteristics of the 2 populations are presented in Table 1.
Figure 1.
Flowchart of patients in the study. Of the 1,224 originally recruited patients, 126 did not meet the eligibility criteria. Among the 711 patients who completed the study, 391 were in the MCO group and 320 were in the HF group. Abbreviations: HF, high-flux; MCO, medium cutoff; PD = peritoneal dialysis.
Table 1.
Baseline Characteristics of the Full Study Population According to Dialyzer Membrane
| Characteristics | Full Sample |
MCO Membrane |
HF Membrane |
P Value |
|---|---|---|---|---|
| N = 1,098 | n = 564 | n = 534 | ||
| Age, y, mean (SD) | 60.6 (14.9) | 60.8 (15.0) | 60.4 (14.9) | 0.66 |
| Sex, n (%) | ||||
| Male | 684 (62.3) | 336 (59.6) | 348 (65.2) | 0.06 |
| Female | 414 (37.7) | 228 (40.4) | 186 (34.8) | — |
| Race, n (%) | ||||
| African American | 83 (7.6) | 28 (5.0) | 55 (10.3) | <0.01 |
| Mestizo | 1015 (92.4) | 536 (95.0) | 479 (89.7) | — |
| Dialysis vintage, n (%) | ||||
| <1 y | 159 (14.5) | 70 (12.4) | 89 (16.7) | 0.13 |
| 1-3 y | 279 (25.4) | 148 (26.2) | 131 (24.5) | — |
| >3 y | 660 (60.1) | 346 (61.4) | 314 (58.8) | — |
| CKD etiology, n (%) | ||||
| Hypertension | 320 (29.1) | 168 (29.8) | 152 (28.4) | 0.49 |
| Diabetes | 409 (37.3) | 217 (38.5) | 192 (36.0) | — |
| Glomerular disease | 85 (7.7) | 44 (7.8) | 41 (7.7) | — |
| Obstructive | 72 (6.6) | 35 (6.2) | 37 (6.9) | — |
| Polycystic kidney disease | 30 (2.7) | 16 (2.8) | 14 (2.6) | — |
| Unknown | 128 (11.7) | 64 (11.4) | 64 (12.0) | — |
| Other | 54 (4.9) | 20 (3.5) | 34 (6.4) | — |
| Hypertension history, n (%) | 1031 (93.9) | 540 (95.7) | 491 (91.9) | 0.01 |
| Diabetes history, n (%) | 460 (41.9) | 246 (43.6) | 214 (40.1) | 0.23 |
| Cardiovascular disease history, n (%) | 207 (18.8) | 121 (21.5) | 86 (16.1) | 0.02 |
| Charlson comorbidity index, n (%) | ||||
| 0-2 | 588 (53.6) | 291 (51.6) | 297 (55.6) | 0.33 |
| 3-5 | 469 (42.7) | 249 (44.1) | 220 (41.2) | — |
| >5 | 41 (3.7) | 24 (4.3) | 17 (3.2) | — |
| Karnofsky scale, mean (SD) | 77.1 (14.9) | 78.5 (13.8) | 75.6 (15.9) | 0.01 |
| Body mass index, mean (SD) | 24.5 (4.3) | 24.9 (4.4) | 24.0 (4.1) | <0.01 |
| Urine output, n (%) | ||||
| <150 mL/d | 804 (73.2) | 412 (73.1) | 392 (73.4) | 0.89 |
| ≥150 mL/d | 294 (26.8) | 152 (26.9) | 142 (26.6) | — |
| Albumin, g/dL, mean (SD) | 4.0 (0.4) | 4.0 (0.3) | 4.0 (0.5) | <0.01 |
| Hemoglobin, g/dL, mean (SD) | 11.7 (1.8) | 11.9 (1.7) | 11.6 (1.9) | <0.01 |
| Potassium, mEq/L, mean (SD) | 5.2 (0.8) | 5.2 (0.8) | 5.2 (0.8) | 0.19 |
| Phosphorus, mg/dL, mean (SD) | 4.6 (1.5) | 4.6 (1.4) | 4.6 (1.5) | 0.44 |
| Parathormone intact, pg/mL, median (IQR) | 329 (166, 629) | 325 (166, 610) | 335 (162, 662) | 0.49 |
| Kt/V single pool, mean (SD) | 1.6 (0.4) | 1.7 (0.4) | 1.6 (0.3) | <0.01 |
| nPCR, g/kg/d, mean (SD) | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.2) | 0.04 |
| Dialyzer membrane, n (%) | ||||
| Revaclear | 525 (47.8) | 0 | 525 (98.3) | <0.01 |
| Polyflux | 5 (0.5) | 0 | 5 (0.9) | — |
| Xenium | 4 (0.4) | 0 | 4 (0.8) | — |
| Theranova 400 | 557 (50.7) | 557 (98.8) | 0 | — |
| Theranova 500 | 7 (0.6) | 7 (1.2) | 0 | — |
| Vascular access, n (%) | ||||
| Catheter | 195 (17.8) | 77 (13.7) | 118 (22.1) | <0.01 |
| Arteriovenous fistula | 903 (82.2) | 487 (86.4) | 416 (77.9) | — |
| QD, mL/min, mean (SD) | 484 (55.3) | 488 (61.5) | 480 (47.7) | 0.02 |
| QB, mL/min, mean (SD) | 340 (49.3) | 349 (52.1) | 330 (43.9) | <0.01 |
| Research site location, n (%) | ||||
| Site 1 | 490 (44.6) | 223 (39.5) | 267 (50.0) | <0.01 |
| Site 2 | 310 (28.2) | 190 (33.7) | 120 (22.4) | — |
| Site 3 | 138 (12.6) | 41 (7.3) | 97 (18.2) | — |
| Site 4 | 160 (14.6) | 110 (19.5) | 50 (9.4) | — |
| Follow-up, mo, mean (SD) | 19.1 (8.1) | 20.0 (7.4) | 18.3 (8.6) | <0.01 |
Note: Categorical variables were compared with Pearson’s χ2 test, and continuous variables were analyzed with the t test.
Abbreviations: CKD, chronic kidney disease; HF, high-flux; IQR, interquartile range; MCO, medium cutoff; nPCR, normalized protein catabolic rate; QB, blood flow rate; QD, dialysate flow rate; SD, standard deviation.
Outcomes
Comparison of Laboratory Data and Dialysis Adequacy
Table 2 displays serum levels of albumin, hemoglobin, phosphate, potassium, parathyroid hormone, and the single-pool Kt/V at baseline and at 6, 12, 18, and 24 months of follow-up. There are no differences between the 2 groups in any laboratory values evaluated with the split-plot analysis of variance test, except for hemoglobin levels (P < 0.01) and Kt/V (P < 0.01). Figure 2 shows the serum albumin levels during follow-up in each group.
Table 2.
Time Trends of Laboratory Parameters and Dialysis Adequacy According to the Type of Dialyzer Membrane
| Laboratory Parameters | Membrane | Baseline |
6 Mo |
12 Mo |
18 Mo |
24 Mo |
Split-Plot ANOVA |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Albumin, g/dL | N | 1,098 | 944 | 835 | 772 | 710 | F(1, 3,550) = 0.57; P = 0.45 |
| MCO | 4.0 (0.3) | 4.0 (0.4) | 4.0 (0.4) | 4.0 (0.4) | 4.0 (0.4) | ||
| HF | 4.0 (0.4) | 4.0 (0.5) | 4.0 (0.4) | 4.0 (0.4) | 4.0 (0.5) | ||
| Hemoglobin, g/dL | N | 1,098 | 945 | 838 | 773 | 711 | F(1, 3,621) = 13.15; P < 0.01 |
| MCO | 11.9 (1.7) | 11.7 (1.8) | 11.9 (1.7) | 11.8 (1.6) | 11.4 (1.6) | ||
| HF | 11.6 (1.9) | 11.6 (1.9) | 11.7 (1.8) | 11.5 (1.8) | 11.2 (1.8) | ||
| Phosphorus, mg/dL | N | 1,096 | 945 | 838 | 772 | 711 | F(1, 3,599) = 0.46; P = 0.49 |
| MCO | 4.6 (1.4) | 4.6 (1.4) | 4.7 (1.4) | 4.5 (1.4) | 4.5 (1.4) | ||
| HF | 4.6 (1.5) | 4.5 (1.4) | 4.6 (1.4) | 4.5 (1.4) | 4.6 (1.4) | ||
| Potassium, mEq/L | N | 1,098 | 945 | 838 | 773 | 711 | F(1, 3,602) = 0.29; P = 0.59 |
| MCO | 5.2 (0.8) | 5.1 (0.8) | 5.1 (0.7) | 5.1 (0.7) | 5.1 (0.8) | ||
| HF | 5.2 (0.8) | 5.2 (0.7) | 5.1 (0.8) | 5.2 (0.7) | 5.1 (0.7) | ||
| Parathormone, pg/mL | N | 1,067 | 945 | 838 | 773 | 711 | F(1, 3,588) = 2.33; P = 0.13 |
| MCO | 510 (573) | 501 (563) | 471 (491) | 469 (533) | 412 (428) | ||
| HF | 578 (745) | 552 (699) | 565 (699) | 551 (717) | 513 (620) | ||
| Kt/V single pool | N | 1,096 | 945 | 837 | 773 | 710 | F(1, 3,594) = 30.46; P < 0.01 |
| MCO | 1.7 (0.4) | 1.7 (0.4) | 1.7 (0.4) | 1.7 (0.4) | 1.7 (0.4) | ||
| HF | 1.6 (0.3) | 1.6 (0.3) | 1.6 (0.3) | 1.6 (0.3) | 1.6 (0.4) |
Note: The P value corresponds to the group effect in ANOVA.
Abbreviations: ANOVA, analysis of variance; HF, high-flux; MCO, medium cutoff; SD, standard deviation.
Figure 2.
Time trends in serum albumin according to the type of dialyzer membrane. Abbreviations: HF, high-flux; MCO, medium cutoff.
Weighted Analysis
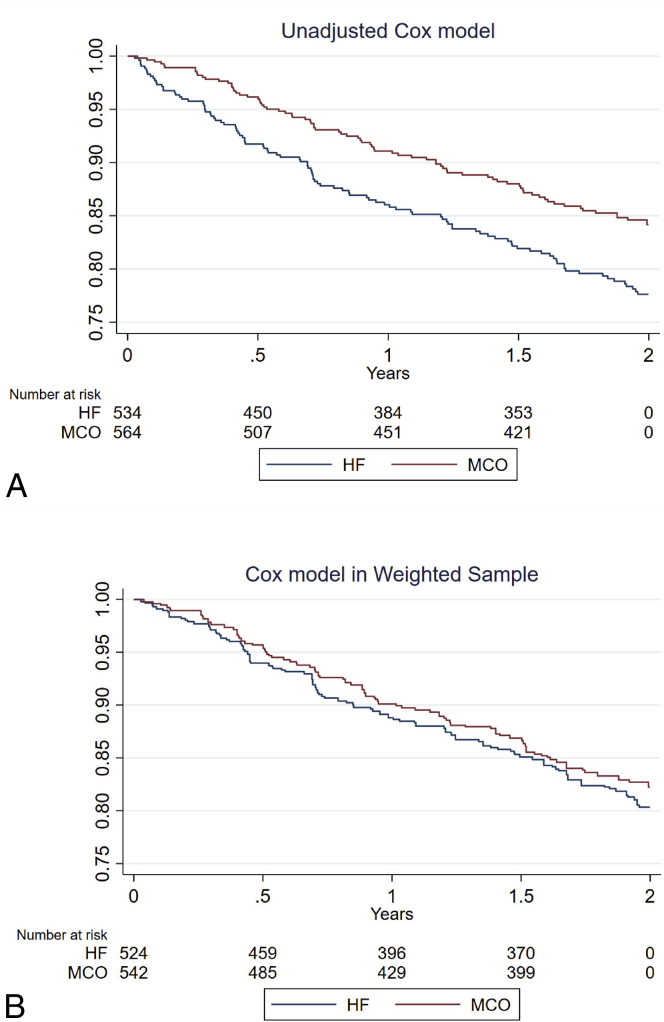
After the process of cohort weighting using inverse probability of treatment weighting, no statistically significant differences were observed between the MCO and HF groups with regard to baseline characteristics (Table S1). In the weighted sample, the MCO dialyzer was associated with fewer all-cause hospitalization events rate per patient-year (incidence rate = 0.93 [95% confidence interval {CI}, 0.82-1.03] vs incidence rate = 1.13 [95% CI, 0.96-1.30] for the HF dialyzer), corresponding to a significant incidence rate ratio MCO/HF of 0.82 (95% CI, 0.68-0.99; P = 0.04; Table 3). There was not a significant difference when comparing the hospital days between groups (P = 0.73). The nonfatal cardiovascular event rate per patient-year was lower in the MCO group (incidence rate = 0.18 [95% CI, 0.14-0.22] vs incidence rate = 0.28 [95% CI, 0.19-0.36] for the HF group), corresponding to a significant incidence rate ratio MCO/HF of 0.66 (95% CI, 0.46-0.96; P = 0.03). There was no significant difference in the survival function values between the MCO and HF dialyzers (hazard ratio = 0.88; 95% CI, 0.64-1.23; P = 0.48; Table 3; Fig 3). Table 4 displays hospitalization details, with cardiovascular events as the most frequent cause.
Table 3.
Clinical Outcomes in Full Sample and Weighted Samples
| Clinical Outcomes | Crude Estimate (95% CI) | P Value | Adjusted Estimate With IPTW (95% CI) | P Value |
|---|---|---|---|---|
| Hospitalization eventsa | ||||
| HF membrane | 1.07 (0.99-1.14) | — | 1.13 (0.96-1.30) | — |
| MCO membrane | 0.79 (0.73-0.84) | — | 0.93 (0.82-1.03) | — |
| IRR | 0.74 (0.67-0.82) | <0.01 | 0.82 (0.68-0.99) | 0.04 |
| Hospital daysa | ||||
| HF membrane | 10.18 (9.96-10.4) | — | 13.21 (10.47-15.95) | — |
| MCO membrane | 6.45 (6.29-6.62) | — | 12.47 (9.36-15.59) | — |
| IRR | 0.63 (0.61-0.66) | <0.01 | 0.94 (0.68-1.30) | 0.73 |
| Nonfatal cardiovascular eventsa | ||||
| HF membrane | 0.25 (0.21-0.28) | — | 0.28 (0.19-0.36) | — |
| MCO membrane | 0.16 (0.13-0.18) | — | 0.18 (0.14-0.22) | — |
| IRR | 0.64 (0.52-0.80) | <0.01 | 0.66 (0.46-0.96) | 0.03 |
| Time to deathb | ||||
| HR MCO/HF membrane | 0.66 (0.50-0.89) | 0.01 | 0.88 (0.64-1.23) | 0.48 |
Note: The IRR was defined as MCO/HF.
Abbreviations: CI, confidence interval; HF, high-flux; HR, hazard ratio; IPTW, inverse probability of treated weighting; IRR, incidence rate ratio; MCO, medium cutoff.
Negative binomial regression and expressed as hospital days per patient-years.
Cox proportional regression.
Figure 3.
Survival curves according to the type of dialyzer membrane. (A) Compares the survival functions in the full sample (crude), finding a statistically significant difference in favor of the MCO membrane. (B) Compares the survival functions in weighted samples, for which no statistically significant difference is observed. Abbreviations: HF, high-flux; MCO, medium cutoff.
Table 4.
Description of the Cause of Hospitalization According to Type of Membrane
| Hospitalization Causes | Full Sample |
MCO Membrane |
HF Membrane |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Bacteremia, septicemia, or infections | 111 | 7.0 | 46 | 6.3 | 65 | 7.6 |
| Cardiovascular and cerebrovascular disease | 473 | 29.9 | 207 | 28.5 | 266 | 31.1 |
| Genitourinary diseases | 80 | 5.1 | 33 | 4.5 | 47 | 5.5 |
| Central nervous system diseases | 22 | 1.4 | 10 | 1.4 | 12 | 1.4 |
| Musculoskeletal diseases | 36 | 2.3 | 15 | 2.1 | 21 | 2.5 |
| Respiratory system diseases | 161 | 10.2 | 80 | 11.0 | 81 | 9.5 |
| Hematopoietic diseases | 53 | 3.4 | 14 | 1.9 | 39 | 4.6 |
| Endocrine and metabolic diseases | 97 | 6.1 | 47 | 6.5 | 50 | 5.9 |
| Digestive system diseases | 180 | 11.4 | 97 | 13.3 | 83 | 9.7 |
| Others | 192 | 12.1 | 97 | 13.3 | 95 | 11.1 |
| Skin and subcutaneous tissue disorders | 60 | 3.8 | 23 | 3.2 | 37 | 4.3 |
| Mental disorders | 11 | 0.7 | 5 | 0.7 | 6 | 0.7 |
| Traumatic lesions | 66 | 4.2 | 35 | 4.8 | 31 | 3.6 |
| Tumor or neoplasia | 32 | 2.0 | 16 | 2.2 | 16 | 1.9 |
| Unknown | 7 | 0.4 | 2 | 0.3 | 5 | 0.6 |
| Total | 1,581 | 100 | 727 | 100 | 854 | 100 |
Abbreviations: HF, high-flux; MCO, medium cutoff.
A negative binomial regression model for nonfatal cardiovascular events showed a statistically significant difference comparing MCO versus HF dialyzers (incidence rate ratio = 0.66; 95% CI, 0.49-0.90; P = 0.01; Table S2). We did not observe significant differences for the outcomes of hospitalizations and hospital days (Tables S3 and S4).
Discussion
The results of our cohort study in patients treated with MCO versus HF membranes showed that the former had lower rates of hospitalizations and cardiovascular events. These findings are of importance because both patients and health systems seek to understand which outcomes are improved by novel health innovations. Outcomes such as mortality, cardiovascular disease, fatigue, and vascular access are frequently of interest in populations receiving dialysis.25,26 In the absence of evidence from randomized controlled trials, observational studies from registry data can provide valuable information on new medical interventions' effectiveness and safety.27, 28, 29 Furthermore, a statistically significant difference was not observed in the number of hospital days per patient-year. These data support the hypothesis that the increased clearance of proinflammatory middle molecules could reduce occurrences of chronic inflammation, atherosclerosis, structural heart disease, and immunodeficiency and, by this route, reduce the frequency of hospitalization events, particularly for cardiovascular causes.8,30 As mentioned, robust statistical methods were used to estimate the intervention's (MCO or HF use) causal effect on the outcomes, a fact that confers more confidence in the results.31,32 However, although inverse probability of treatment weighting controls for differences between cohorts, residual confounding may persist. These findings, therefore, need to be confirmed by a randomized controlled trial.
Our study did not demonstrate a difference in all-cause mortality or cardiovascular mortality. This could, however, be explained by the short period of follow-up, of only 2 years. The most frequently observed cause of hospitalization was cardiovascular events, which were less frequent in the group dialyzed with MCO membranes.
The evaluation of albumin levels in the 2 cohorts is an important aspect of the safety analysis of this study. There was no statistically significant difference identified between 2 cohorts; this finding corroborates the results of other studies.14,16,19
Interestingly, the nadir of the mean serum albumin levels during follow-up in the MCO group was 4.0 g/dL, which is above the safety level of 3.8 g/dL that has recently been highlighted in an in-depth literature review of this topic.33,34 Stabilization of serum albumin levels was observed in the MCO group during the follow-up, which others have also reported.19 It may be associated with no differences in mortality between the cohorts.
This study's strength lies in the large numbers of patients reported on, which is a magnitude greater than previous studies assessing the use of MCO membranes in chronic HD cohorts. The study allowed for standardized collection of information with robust quality controls. Statistical analysis methods were used to deal with the confounding inherent in this type of observational study.
The main weakness of the present study is its observational nature. Despite the robust statistical analysis, there remains the possibility that unmeasured variables may still generate a residual imbalance, skewing the results. This is an important point to consider when evaluating a new HD membrane, as selection bias could favor the new device being used by a healthier patient population. Therefore, despite the statistical approach employed to deal with the confounding phenomenon, a randomized controlled trial could confirm the findings.
In conclusion, this large cohort study demonstrated that there are lower incidences of hospitalization events and cardiovascular events in chronic HD patients dialyzed with MCO membranes than those treated with HF membranes. These results should be corroborated with randomized controlled trials.
Article Information
Authors’ Full Names and Academic Degrees
Alejandra P. Molano, MD, Colin A. Hutchison, PhD, Ricardo Sanchez, MSc, Angela S. Rivera, MD, Giancarlo Buitrago, PhD, María P. Dazzarola, MD, Mario Munevar, MD, Mauricio Guerrero, MD, Jasmín I. Vesga, MSc, and Mauricio Sanabria, MSc.
Authors’ Contributions
Research idea and study design: APM, CAH, RS, ASR, GB, MPD, MM, MG, JIV, MS; data acquisition: JIV, MS; data interpretation: APM, RS, ASR, GB, MPD, MM, MG, JIV, MS; statistical analysis: RS, GB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Funding provided by Baxter Healthcare. The funder of this study had no role in the study design, data collection, data analysis or interpretation, writing of the report, or the decision to submit the report for publication.
Financial Disclosure
Dr Molano, Dr Dazzarola, Dr Munevar, and Dr Guerrero were full-time employees of Baxter Renal Care Services–Colombia. Dr Rivera is an employee of Baxter Healthcare Corporation, Deerfield, Illinois. Ms Vesga is a full-time employee of Baxter Renal Care Services–Colombia, Bucaramanga, Colombia. Dr Sanabria is a full-time employee of Baxter Renal Care Services–Latin America, Bogotá, Colombia. Dr Sanchez and Dr Buitrago were employees of Clinical Research Institute, School of Medicine, Universidad Nacional de Colombia, Bogotá, and they have received honorarium for consultancy and statistical analysis from Baxter Renal Care Services–Colombia.
Acknowledgements
The authors express their gratitude to all patients and clinical teams who participated in the study.
Peer Review
Received September 15, 2021. Evaluated by 2 external peer reviewers, with direct editorial input by a Statistical Editor and the Editor-in-Chief. Accepted in revised form December 16, 2021.
Data Sharing
A secure database that meets the requirements of confidentiality and that safeguards patient privacy is maintained as part of the study protocol. To ensure that patient privacy is maintained, the availability of data is subject to restrictions. The protocol is available at https://doi.org/10.1186/ISRCTN12403265.
Footnotes
Complete author and article information provided before references.
Table S1: Covariate balance evaluation with standardized differences.
Table S2: Negative binomial regression model for nonfatal cardiovascular events.
Table S3: Negative binomial regression model for hospitalization events.
Table S4: Negative binomial regression model for hospital days.
Descriptive Text for Online Delivery
Supplementary Material
Tables S1-S4.
References
- 1.Saran R., Bragg-Gresham J.L., Levin N.W., et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69(7):1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M., Vanholder R., Himmelfarb J. Health policy for dialysis care in Canada and the United States. Clin J Am Soc Nephrol. 2020;15(11):1669–1677. doi: 10.2215/CJN.14961219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaf J. Current trends in European renal epidemiology. Clin Kidney J. 2017;10(2):149–153. doi: 10.1093/ckj/sfw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson L.W. Dialysis in the 21st century. Am J Kidney Dis. 1996;28(6):951–957. doi: 10.1016/s0272-6386(96)90400-x. [DOI] [PubMed] [Google Scholar]
- 5.Oreopoulos D.G. Beyond KT/V: redefining adequacy of dialysis in the 21st century. Int Urol Nephrol. 2002;34(3):393–403. doi: 10.1023/a:1024426003688. [DOI] [PubMed] [Google Scholar]
- 6.Vanholder R., De Smet R., Glorieux G., et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63(5):1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 7.Eknoyan G., Beck G.J., Cheung A.K., et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 8.Wolley M., Jardine M., Hutchison C.A. Exploring the clinical relevance of providing increased removal of large middle molecules. Clin J Am Soc Nephrol. 2018;13(5):805–814. doi: 10.2215/CJN.10110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S., Kazama J.J., Wakamatsu T., et al. Removal of uremic toxins by renal replacement therapies: a review of current progress and future perspectives. Ren Replace Ther. 2016;2(1):1–8. [Google Scholar]
- 10.Krieter D.H., Hackl A., Rodriguez A., et al. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol Dial Transplant. 2010;25(1):212–218. doi: 10.1093/ndt/gfp437. [DOI] [PubMed] [Google Scholar]
- 11.Ronco C. The rise of expanded hemodialysis. Blood Purif. 2017;44(2):I–VIII. doi: 10.1159/000476012. [DOI] [PubMed] [Google Scholar]
- 12.Ronco C., Marchionna N., Brendolan A., Neri M., Lorenzin A., Martínez Rueda A.J. Expanded haemodialysis: from operational mechanism to clinical results. Nephrol Dial Transplant. 2018;33(suppl 3):iii41–iii47. doi: 10.1093/ndt/gfy202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirsch A.H., Lyko R., Nilsson L.G., et al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant. 2017;32(1):165–172. doi: 10.1093/ndt/gfw310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maduell F., Rodas L., Broseta J.J., et al. Medium cut-off dialyzer versus eight hemodiafiltration dialyzers: comparison using a global removal score. Blood Purif. 2019;48(2):167–174. doi: 10.1159/000499759. [DOI] [PubMed] [Google Scholar]
- 15.Belmouaz M., Bauwens M., Hauet T., et al. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: a randomized clinical trial. Nephrol Dial Transplant. 2020;35(2):328–335. doi: 10.1093/ndt/gfz189. [DOI] [PubMed] [Google Scholar]
- 16.Weiner D.E., Falzon L., Skoufos L., et al. Efficacy and safety of expanded hemodialysis with the Theranova 400 dialyzer: a randomized controlled trial. Clin J Am Soc Nephrol. 2020;15(9):1310–1319. doi: 10.2215/CJN.01210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnasamy R., Hawley C.M., Jardine M.J., et al. A trial evaluating mid cut-off value membrane clearance of albumin and light chains in hemodialysis patients: a safety device study. Blood Purif. 2020;49(4):468–478. doi: 10.1159/000505567. [DOI] [PubMed] [Google Scholar]
- 18.Schepers E., Glorieux G., Eloot S., et al. Assessment of the association between increasing membrane pore size and endotoxin permeability using a novel experimental dialysis simulation set-up. BMC Nephrol. 2018;19(1):1. doi: 10.1186/s12882-017-0808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunch A., Sanchez R., Nilsson L.G., et al. Medium cut-off dialyzers in a large population of hemodialysis patients in Colombia: COREXH registry. Ther Apher Dial. 2021;25(1):33–43. doi: 10.1111/1744-9987.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim J.H., Park Y., Yook J.M., et al. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci Rep. 2020;10(1):7780. doi: 10.1038/s41598-020-64622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alarcon J.C., Bunch A., Ardila F., et al. Impact of medium cut-off dialyzers on patient-reported outcomes: COREXH registry. Blood Purif. 2021;50(1):110–118. doi: 10.1159/000508803. [DOI] [PubMed] [Google Scholar]
- 22.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F., Zaslavsky A.M., Landrum M.B. Propensity score weighting with multilevel data. Stat Med. 2013;32(19):3373–3387. doi: 10.1002/sim.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arpino B., Mealli F. The specification of the propensity score in multilevel observational studies. Comp Stat Data Anal. 2011;55(4):1770–1780. [Google Scholar]
- 25.Tong A., Manns B., Hemmelgarn B., et al. Establishing core outcome domains in hemodialysis: report of the standardized outcomes in nephrology-hemodialysis (SONG-HD) consensus workshop. Am J Kidney Dis. 2017;69(1):97–107. doi: 10.1053/j.ajkd.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra S., Kharbanda K. Effects of expanded hemodialysis therapy on clinical outcomes. Contrib Nephrol. 2017;191:188–199. doi: 10.1159/000479267. [DOI] [PubMed] [Google Scholar]
- 27.Concato J., Shah N., Horwitz R.I. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trotter J.P. Patient registries: a new gold standard for “real world” research. Ochsner J. 2002;4(4):211–214. [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman S.L. From randomized controlled trials to observational studies. Am J Med. 2009;122(2):114–120. doi: 10.1016/j.amjmed.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 30.Florens N., Juillard L. Expanded haemodialysis: news from the field. Nephrol Dial Transplant. 2018;33(suppl 3):iii48–iii52. doi: 10.1093/ndt/gfy203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin P.C. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655. doi: 10.1002/sim.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaysen G.A., Johansen K.L., Cheng S.C., Jin C., Chertow G.M. Trends and outcomes associated with serum albumin concentration among incident dialysis patients in the United States. J Ren Nutr. 2008;18(4):323–331. doi: 10.1053/j.jrn.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K., Ficociello L.H., Bazzanella J., Mullon C., Anger M.S. Slipping through the pores: hypoalbuminemia and albumin loss during hemodialysis. Int J Nephrol Renovasc Dis. 2021;14:11–21. doi: 10.2147/IJNRD.S291348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4.