Abstract
Biomarker testing is recommended for the accurate and timely diagnosis of Alzheimer's disease (AD). Using illustrative case narratives we consider how cerebrospinal fluid (CSF) biomarker tests may be used in different presentations of cognitive impairment to facilitate timely and differential diagnosis, improving diagnostic accuracy, providing prognostic information, and guiding personalized management in diverse scenarios. Evidence shows that (1) CSF ratios are superior to amyloid beta (Aβ)1‐42 alone; (2) concordance of CSF ratios to amyloid positron emission tomography (PET) is better than Aβ1‐42 alone; and (3) phosphorylated tau (p‐tau)/Aβ1‐42 ratio is superior to p‐tau alone. CSF biomarkers are recommended for the exclusion of AD as the underlying cause of cognitive impairment, diagnosis of AD at an early stage, differential diagnosis of AD in individuals presenting with other neuropsychiatric symptoms, accurate diagnosis of AD in an atypical presentation, and for clinical trial enrichment.
Highlights
Cerebrospinal fluid (CSF) Alzheimer's disease (AD) biomarker testing may be underused outside specialist centers.
CSF biomarkers improve diagnostic accuracy, guiding personalized management of AD.
CSF ratios (amyloid beta [Aβ]1‐42/Aβ1‐40 and phosphorylated tau/Aβ1‐42) perform better than single markers.
CSF ratios produce fewer false‐negative and false‐positive results than individual markers.
CSF biomarkers should be included in diagnostic work‐up of AD and mild cognitive impairment due to AD.
Keywords: Alzheimer's disease, cerebrospinal fluid biomarkers, diagnosis, mild cognitive impairment
1. INTRODUCTION
Diagnosis of mild cognitive impairment (MCI) and Alzheimer's disease (AD) has evolved from a traditional description based on a clinical syndrome, to encompass a more detailed assessment of etiology, and biologic characterization of the disease using specific biomarkers (amyloid beta [Aβ] and tau), as described in the most recent criteria from the National Institute on Aging–Alzheimer's Association (NIA‐AA) and the International Working Group (IWG). 1 , 2 , 3 Aβ and tau biomarkers can be measured in the cerebrospinal fluid (CSF); however, use of testing may be limited outside specialist and academic centers 4 because of barriers, such as reluctance to use a perceived invasive procedure like lumbar puncture, unfamiliarity with interpretation of test results, and skepticism about the clinical value of knowing an individual's biomarker status. 2 , 5 Ideally, physicians and their patients would like clear negative or positive results from diagnostic testing, but guidelines and uniformity in reporting test results from laboratory to physician and from physician to patients are still under development. 6 , 7 Studies of CSF biomarkers in regular clinical practice are under way. 8 , 9
Previous reviews have indicated advantages and disadvantages of different biomarker strategies in dementia. 10 , 11 In this paper, we consider whether and how physicians should use CSF biomarkers in memory and dementia clinics beyond the academic and research settings. We present clinical cases to examine how CSF biomarkers could inform clinical practice decisions, by facilitating timely and accurate diagnosis, providing prognostic information, and guiding personalized management. We also consider the limitations of current biomarkers and look to future innovations.
2. METHODS
We searched PubMed in February 2021 using the terms: “dementia,” “Alzheimer's disease,” “prodromal Alzheimer's disease,” “mild cognitive impairment,” and “subjective cognitive decline”; “biomarker,” “amyloid,” “amyloid beta,” “tau,” “tau/amyloid beta ratio”; “amyloid beta42/amyloid beta40 ratio”; “lumbar puncture,” “complications,” and “safety”; “neuropsychology,” “diagnosis,” “differential diagnosis,” “communication,” “clinical decline,” and “conversion to dementia.” The search objective was to identify practical applications and limitations of biomarker tests in clinical practice to develop illustrative case studies based on experiences with real‐life subjects in memory clinics. Background information and clinical details have been changed to protect anonymity while maintaining clinical authenticity.
3. WHICH CSF BIOMARKERS ARE CURRENTLY RECOMMENDED?
CSF AD biomarkers provide a continuous, quantitative measure of AD‐related proteins; the three core CSF biomarkers currently used for AD diagnostics are the 42 amino acid‐long amyloid‐beta peptide (Aβ1‐42), total tau protein (t‐tau), and tau phosphorylated at threonine 181 (p‐tau181). 12 CSF biomarker testing has been improved by international quality programs 13 and immunoassays that run on fully automated analyzers that can measure multiple biomarkers (e.g., Elecsys® and Lumipulse®). 14 , 15
RESEARCH IN CONTEXT
Systematic Review: We searched PubMed and other literature sources to evaluate the practical application and limitations of cerebrospinal fluid (CSF) biomarker tests in Alzheimer's disease (AD) or dementia so that we could provide relevant case studies to illustrate key issues hampering adequate application in clinical practice.
Interpretation: Although some physicians may be reluctant to use CSF biomarker tests routinely in patients with cognitive impairment, we illustrate with case descriptions how the advantages of testing outweigh possible disadvantages and skepticism about the value of knowing biomarker status.
Future Directions: Use of CSF biomarker tests optimizes patient management, including precise selection for novel disease‐modifying drugs. New biomarker development should focus on minimizing invasive procedures and improving the detection of specific pathologic processes more accurately.
3.1. International guidelines recommend use of clinical symptoms and CSF biomarkers
Neuropsychological exam is the cornerstone for clinical diagnosis and can determine whether individuals have normal cognition, cognitive impairment, and/or dementia. 1 Table 1 summarizes current international guidelines for using CSF biomarkers as part of the diagnosis and classification of AD. 3 , 13 , 16 , 17 Core CSF biomarkers (Aβ1‐42, t‐tau, and p‐tau) provide objective information about underlying disease pathology to support clinical work‐up, and are recommended for timely and accurate diagnosis, for differential diagnosis, and to predict the risk and rate of clinical decline. 18 , 19
TABLE 1.
Recommendations from international guidelines for the use of biomarkers in the diagnosis of suspected AD a
| Guideline | Diagnostic criteria for AD | Appropriate use criteria for CSF biomarkers in the differential diagnosis of cognitive impairment |
|---|---|---|
| IWG 20211 |
AD diagnosis is restricted to people who have positive biomarkers together with specific AD phenotypes Biomarker‐positive cognitively unimpaired individuals should be considered only at risk for progression to AD |
Biological requirements: Aβ marker (CSF or PET) and tau marker (CSF or PET) |
| NIA‐AA research framework 3 |
AD should be defined as a biologic construct that is identified by biomarkers in living people Only biomarkers that are specific for hallmark AD proteinopathies (i.e., Aβ and pathologic tau) should be considered potential biomarker definitions of the disease |
A: Aβ biomarkers determine whether an individual is in the AD continuum. T: Pathologic tau biomarkers determine whether someone who is in the AD continuum has AD. A and T indicate specific neuropathologic changes that define AD Neurodegenerative/neuronal injury biomarkers (N) and cognitive symptoms (C) are not specific to AD |
| Alzheimer's Association 16 | CSF biomarker testing is appropriate for specific clinical indications | Appropriate use of LP and CSF testing in the diagnosis of AD:
|
| WFSBP Task Force 13 | The potential role of CSF biomarkers in early (predementia) diagnosis, differential diagnosis, prognosis, and selection for clinical trials is noted |
|
| BIOMARKAPD 17 | CSF AD biomarkers are recommended as a supplement to clinical evaluation |
|
All guidelines also include recommendations for core clinical criteria and neuroimaging evidence to be used in the diagnosis of AD, which is not included here.
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; BIOMARKAPD, Biomarkers for AD and Parkinson's disease; CSF, cerebrospinal fluid; IWG, International Working Group; LP, lumbar puncture; MCI, mild cognitive impairment; NIA‐AA, National Institute on Aging and Alzheimer's Association; SCD, subjective cognitive decline; WFSBP, World Federation of Societies of Biological Psychiatry.
Importantly, recent iterations of criteria from the IWG and NIA‐AA research framework include recommendations for using clinical findings and abnormal biomarkers (Aβ and tau; Table 1). 1 , 3 These biomarkers define AD biologically throughout the disease continuum. 1 , 3 Biomarker testing is recommended for clinically symptomatic individuals where neuropsychological workup is indicative of cognitive decline. Biomarker testing is not routinely indicated for individuals with a normal neuropsychological examination because of the difficulty in classifying cognitively unimpaired biomarker‐positive cases. 1
3.2. CSF biomarker ratios improve agreement with amyloid imaging
Diagnostic criteria for AD recognize both CSF biomarkers obtained by lumbar puncture and positron emission tomography (PET) imaging, which measures amyloid in cortical brain tissue and can detect deposition in different brain regions. 11 Table 2 summarizes positive and negative agreement values between core CSF biomarker results and amyloid PET imaging in studies using fully automated assay platforms. 14 , 15 , 20 , 21 , 22 , 23 , 24 Overall, 90% or more of subjects with a positive CSF Aβ test were also positive using amyloid PET imaging, whereas the degree of agreement between those testing negative both with CSF biomarkers and PET varied between 51% and 81%. These findings support CSF biomarkers as a convenient and cheaper alternative to amyloid PET imaging. 1
TABLE 2.
CSF PET agreement in studies reporting individual biomarkers and biomarker ratios using automated assay platforms
| Individual CSF biomarkers | CSF biomarker ratios | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Study objective | Platform | Measure | p‐tau | t‐tau | Aβ1‐42 | Aβ1‐40 | p‐tau/Aβ42 | t‐tau/ Aβ42 | Aβ1‐42/Aβ1‐40 |
| Schindler et al. 201823 | To measure relationship between CSF biomarkers and amyloid PET | Elecsys | PPA | 82% | 68% | 90% | 60% | 92% | 92% | 96% |
| NPA | 76% | 83% | 73% | 58% | 89% | 85% | 82% | |||
| OPA | 78% | 79% | 77% | 59% | 89% | 87% | 86% | |||
| AUC | 0.84 | 0.81 | 0.85 | 0.60 | 0.96 | 0.95 | 0.93 | |||
| Doecke et al. 202014 | To measure concordance between CSF biomarkers and pathological AD via PET imaging | Elecsys | PPA | 81% | 86% | 81% | 90% | 83% | 90% | |
| NPA | 77% | 66% | 81% | 91% | 97% | 90% | ||||
| OPA | 79% | 75% | 81% | 91% | 91% | 90% | ||||
| AUC | 0.84 | 0.81 | 0.86 | 0.94 | 0.94 | 0.94 | ||||
| Willemse et al. 2020 (abstract) 24 | To measure CSF biomarkers compared to amyloid PET imaging | Elecsys | PPA | 91% | 96% | 96% | ||||
| NPA | 75% | 89% | 80% | |||||||
| OPA | ||||||||||
| AUC | ||||||||||
| To measure CSF biomarkers compared to amyloid PET imaging | Lumipulse | PPA | 91% | 97% | 99% | |||||
| NPA | 73% | 91% | 83% | |||||||
| OPA | ||||||||||
| AUC | ||||||||||
| Keshaven et al. 202021 | To measure concordance between CSF biomarkers and PET imaging | Lumipulse | PPA | 100% | 54% | 100% | 100% | 92% | 100% | |
| NPA | 66% | 82% | 74% | 94% | 90% | 94% | ||||
| OPA | ||||||||||
| AUC | 0.879 | 0.665 | 0.891 | 0.966 | 0.955 | 0.966 | ||||
| Alcolea et al. 201920 | To determine cut‐offs between PET and CSF biomarkers | Lumipulse | PPA | 80% | 75% | 95% | 93% | 81% | 88% | |
| NPA | 83% | 83% | 51% | 80% | 83% | 77% | ||||
| OPA | 81% | 78% | 79% | 88% | 82% | 84% | ||||
| AUC | 0.84 | 0.80 | 0.76 | 0.59 | 0.88 | 0.87 | 0.86 | |||
| Kaplow et al. 202015 | To determine concordance of CSF biomarker ratios with amyloid PET (test cohort A/B) | Lumipulse | PPA | 74.1% | 98.8% | 97.5% | ||||
| NPA | 89.8% | 75.5% | 89.8% | |||||||
| OPA | 80.0% | 90.0% | 94.6% | |||||||
| AUC | 0.87 | 0.92 | 0.95 | |||||||
| Moon et al. 202122 | To evaluate concordance of CSF biomarkers and PET imaging | Lumipulse | PPA | 79.5% | 59.0% | 79.5% | 84.6% | 84.6% | 84.6% | |
| NPA | 78.6% | 89.3% | 88.1% | 92.9% | 88.1% | 91.7% | ||||
| OPA | ||||||||||
| AUC | 0.839 | 0.791 | 0.857 | 0.840 | 0.842 | 0.856 | ||||
Notes:
PPA: positive percent agreement (defined as the percent of PET‐positive individuals also positive by a CSF biomarker measure).
NPA: negative percent agreement (defined as the percent of PET‐negative individuals also negative by a CSF biomarker measure).
OPA: overall percent agreement (defined as the sum of the PET‐positive individuals also positive by a CSF biomarker measure and the PET‐negative individuals also negative by a CSF biomarker measure divided by the entire cohort size.
AUC: area under the receiver operator curve for PPA on the Y axis and 1 – NPA on the X axis; AUC 1 represents a ‘perfect test,’ where there is 100% agreement between CSF biomarker and PET imaging results.
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; CSF, cerebrospinal fluid; LP, lumbar puncture; PET, positron emission tomography; p‐tau, phosphorylated tau; t‐tau, total tau.
Interestingly, studies found CSF biomarker ratios (Aβ1‐42/1‐40, p‐tau/Aβ1‐42, and t‐tau/Aβ1‐42) performed better than individually measured values. 14 , 20 , 21 , 22 , 23 For example, Schindler et al. used the fully automated Elecsys assays to compare CSF biomarker ratios to amyloid PET imaging in samples from 200 individuals enrolled in studies of normal aging and dementia. 23 The overall percent agreement ([PET‐positive and CSF biomarker‐positive individuals] plus [PET‐negative and CSF biomarker‐negative individuals]/total number of individuals) values were better for CSF ratios (86% for Aβ1‐42/1‐40, 89% for p‐tau/Aβ1‐42, and 87% for t‐tau/Aβ1‐42) than for the individual marker values (77% for Aβ1‐42, 78% for p‐tau, and 79% for t‐tau).
From a clinical perspective, these results mean fewer false‐negative and false‐positive results using CSF ratios than individual markers. An evidence‐based review concluded that the CSF Aβ1‐42/1‐40 ratio, rather than the absolute value of CSF Aβ1‐42, should be used when analyzing CSF AD biomarkers to improve the percentage of appropriately diagnosed patients. 11 Similarly, CSF p‐tau/Aβ1‐42 or t‐tau/Aβ1‐42 ratios perform better than p‐tau or t‐tau alone, respectively, 14 , 23 and Aβ1‐42 alone in terms of PET concordance, 19 , 24 potentially allowing detection of tau accumulation at an earlier stage than with PET imaging in individuals positive for Aβ. 25 CSF Aβ1‐42/1‐40 and CSF p‐tau/Aβ1‐42 or t‐tau/Aβ1‐42 ratios appear to perform equivalently. 11
Abnormalities in core CSF and PET AD biomarkers are caused by the hallmark AD pathologies of amyloid plaque formation and neurofibrillary tangles. An increased amyloid burden is indicated by decreased CSF Aβ1‐42 levels or by uptake of specific PET tracers. On the other hand, an increase in levels of CSF tau reflects neuronal injury and neurodegeneration, while an increase of tau PET indicates tangles pathology. These biomarker changes, which begin many years before clinical symptoms, do not necessarily occur simultaneously. 26 Aβ deposition detected by PET provides different information than Aβ processing reflected by CSF biomarkers. CSF biomarker changes may precede PET biomarker changes; for example, elevation of p‐tau in the CSF may precede PET‐tracer positivity in the progression of AD pathogenesis and related cognitive decline. 27 This may explain some of the discordance between CSF and PET biomarkers 27 , 28 that are found in ≈12% of cases. 15 Differences between biomarker tests may reflect disease pathology at specific time points and the likely evolution of disease, although discrepancies may also result from inter‐individual variability in the production and clearance of Aβ, and variance in different measures rather than reflecting real discordance. 11 , 27 Interestingly, CSF ratios (Aβ1‐42/1‐40 or Aβ1‐42/1‐38) may correct for inter‐individual differences in total Aβ levels. 11
Using information both from CSF biomarkers and PET imaging may be helpful in some unclear cases. For example, a mismatch between the primary clinical diagnosis and CSF Aβ1‐42/tau ratio was the main reason for requesting a subsequent Aβ PET scan. 29 Other indications for a PET scan after CSF biomarker testing include incongruent magnetic resonance imaging (MRI) findings, an unusual clinical presentation, and young age at presentation. 29
Combining results from CSF Aβ and tau assays may be more informative than individual CSF biomarker results because the ratios provide a more detailed snapshot of different underlying disease process. 23 p‐tau is the most helpful biomarker for differential diagnosis of AD from non‐AD dementia. 30 Furthermore, CSF Aβ1‐42/1‐40 and tau/Aβ1‐42 ratios are reliable markers of AD, at both the preclinical and clinical stages of disease, and can differentiate between AD and non‐AD causes of cognitive decline. 31 Importantly, brain pathologies not specific to AD may be associated with reduced Aβ1‐42 levels in the CSF, whereas biomarker ratios are not similarly affected. 11 As CSF AD biomarker ratios correct for the potentially confounding influence of variance in CSF protein turnover between individuals, they may add relevant information in the differential diagnosis of neurodegenerative diseases associated with low levels of Aβ1‐42. For example, CSF amyloid ratio (Aβ1‐42/Aβ1‐40) is superior to Aβ1‐42 to differentiate patients with AD from those with vascular dementia, dementia with Lewy bodies, and non‐AD dementia, 31 while CSF p‐tau/Aβ1‐42 ratio performed better than p‐tau or Aβ1‐42 in differentiating AD from other types of dementia. 32 CSF p‐tau/Aβ1‐42 ratio may be useful in clinical practice to exclude underlying AD pathology in the differential diagnosis of frontotemporal lobar degeneration. 33 In addition, CSF ratios can predict the risk of progression from MCI to AD. 18
Despite evidence‐based recommendations for CSF biomarkers in the diagnostic workup of suspected AD, there remains a degree of reluctance to use them in routine clinical practice. 5 The following authentic case narratives show how CSF biomarkers may be used in different clinical scenarios to improve the accuracy of diagnosis and to inform clinical management decisions.
4. CASE 1: THE MAN WITH A LARGE HEAD
4.1. Background
Mr. B is 61 years old and works in an abattoir. He never learned to read or write. Although he has no cognitive complaints and his activities of daily living appear normal, his wife, who has always arranged household, finances, and clothing, is concerned because he has become forgetful over the last 2 to 3 years and often tells the same stories twice. His personality has also changed, he is often agitated, talks to strangers, and has become dysfunctional at work.
4.2. What did the initial assessment show and why did the physician order specialist tests?
Neurological examination showed Mr. B has a large head circumference (>97th percentile reference); however, initial cognitive tests and further neuropsychological testing (Table 3) were deemed unreliable because of his limited education. Computed tomography (CT) showed enlarged ventricles, probably long standing, considering the large skull. There was no evidence of hippocampal atrophy, although definitive assessment was difficult because of enlarged ventricles. As diagnosis was uncertain from initial assessments, additional tests were done to explore different possible causes of cognitive decline including hydrocephalus, depression, other psychiatric disorders, or AD.
TABLE 3.
Summary of biomarker test findings in case studies
| Case | CSF test findings | PET test findings | Neuropsychology testing | AD diagnosis |
|---|---|---|---|---|
| #1 Mr B | Not done | Aβ negative |
MMSE 19/30 CAMCOG 67 (cut‐off >84) Frontal assessment battery 12/18 |
AD excluded |
| #2 Mr C |
Glucose 4.0mmol/L Proteins 0.40g/L t‐tau 594ng/L (cut‐off <360) a p‐tau 81ng/L (cut‐off <60) a Aβ1‐42 611ng/L (cut‐off >450) a t‐tau/Aβ1‐42 0.972 (cut‐off <0.28) p‐tau/Aβ1‐42 0.133 (cut‐off <0.02) |
Not done | Examination abandoned because patient was distracted and anxious | MCI due to AD |
| #3 Mrs N |
Aβ1‐42 498 pg/ml (cut‐off >1000) t‐tau 635 pg/ml (cut‐off <235) p‐tau‐181 73 pg/ml (cut‐off <19) t‐tau/Aβ1‐42 1.275 (cut‐off <0.28) p‐tau/Aβ1‐42 0.147 (cut‐off <0.02) |
Not done |
MMSE 26/30 Deficits in memory tasks |
MCI due to AD |
| #4 Mr G |
Aβ1‐42 567 pg/ml (cut off >1000) t‐tau 364 pg/ml (cut off <235) p‐tau‐181 36 pg/ml (cut off <19) t‐tau/Aβ1‐42 0.642 (cut‐off <0.28) p‐tau/Aβ1‐42 0.063 (cut‐off <0.02) |
Aβ + |
MMSE 26/30 CAMCOG 91 (cut‐off >84) |
PCA due to AD |
| #5 Mr T |
Aβ1‐42/1‐40 (cut‐off >0.046) 0.037 at 62 years 0.037 at 66 years p‐tau181/ Aβ1‐42 ratio (cut‐off <0.038) 0.042 at 62 years 0.045 at 66 years |
Aged 62 years: Aβ+ (centiloid of 91.6) Aged 70 years: Aβ + (centiloid of 113.1) Tau + |
Aged 62‐72: MMSE 30/30 Aged 72: MMSE 30/30 Delayed recall (Rey Auditory Verbal Learning Test and the Logical Memory I and II subtests of the Wechsler Memory Scale‐Revised) |
Aged 62 and 66 years: At risk for AD Aged 72 years: MCI |
ELISA assay.
Abbreviations: CAMCOG, Cambridge Cognitive Examination; PCA, posterior cortical atrophy.
4.3. How did biomarker and other tests improve diagnostic accuracy?
PET scanning using the Pittsburgh compound B (PiB) tracer showed no evidence of Aβ take‐up and AD was excluded (Table 3). CSF biomarkers could have been used to test for Aβ to exclude AD, but PET was used in this case because of the presence of enlarged ventricles. In such cases, individual biomarker levels could be decreased overall, which may result in a false‐negative normal (low) Aβ level, whereas CSF AD biomarker ratios could be useful because they control for this confounding finding.
4.4. How did tests help to guide management of the patient?
After exclusion of AD, further psychiatric evaluation revealed prior psychotrauma related to childhood sexual abuse that the patient and caregiver were not able to discuss during the first visit. The final diagnosis was post‐traumatic stress disorder (PTSD). Treatment with antidepressants and psychotherapy markedly improved cognitive function. Mr. B's case shows biomarker testing can rule out AD as the primary cause of cognitive deterioration, providing a clear direction for subsequent treatment, and even improvement of his cognitive complaints. Many cases of dementia are not caused by AD and may be reversible with adequate treatment.
The diagnostic value of biomarkers is increased in cases in which medical history provides limited objective clinical information, for example, in individuals with low education, a previous psychotic disease, no informant, or with language barriers.
5. CASE 2: PROFESSIONAL BURNOUT
5.1. Background
Mr. C is the 61‐year‐old son of working‐class parents. After compulsory schooling, he completed an apprenticeship as a craftsman, a profession he practiced for a few years before training as a policeman. He has been in this profession for 12 years and is responsible for a team of 12 people. He lives with his wife and son. He enjoys cooking, walking, watching TV, playing computer games, and reading the newspaper. His parents are alive and there is no family history of dementia nor known history of cognitive impairment in close relatives. His father has memory problems, but no diagnosis has been made. Mr. C sought medical help for evaluation of memory impairment after professional burnout, linked to overwork, a chronic shortage of staff, and an increasing workload.
5.2. What did the initial assessment show and why did the doctor order specialist tests?
During the first consultation by dementia specialists Mr. C spontaneously described professional overwork, feeling “confused in his head” and slowed down, while being less efficient doing certain tasks. He described himself as very stressed in the workplace, with the feeling that he is performing less well than before, “putting pressure on himself,” and “being apprehensive of doing wrong.” He mentioned occasional forgetfulness about where he puts his things (misplacing his glasses). His wife is worried about him and confirmed these problems. Outside work, he is self‐sufficient in all activities of everyday life. He drives and makes his payments on the internet without difficulties. Until recently Mr. C had a stable and resilient personality, with no evidence of anxiety or depressive episodes, and no need for psychological/psychiatric therapy or psychotropic drugs.
On a scale assessing change in cognitive functioning (IQ‐CODE = 3.25, not significant), his wife described a slight decline in his ability to learn new things, recall recent events, and remember where things are stored. Behaviorally, she finds him much calmer. These disorders first appeared about 2 years previously, after a malaise, possibly associated with an event diagnosed as a transient ischemic attack. Mr. C also had two episodes of malaise 4 hours apart 3 years earlier, although no CT scan was done then. Mr. C is in good physical health overall and laboratory tests a year ago were normal. He is currently on aspirin, rosuvastatin, and irbesartan.
Neurological examination was unremarkable. The neurologist's MRI report was within the normal range, except for very discrete white matter signal abnormalities at the supratentorial level reportedly of ischemic origin. During neuropsychological evaluation and psychiatric assessment Mr. C was distractible, verbalizing, and showing significant anxiety related to the testing situation, which led to several tasks being abandoned and significantly limited the examination. He expressed a lack of confidence in his own abilities, apprehension of doing the wrong thing, and low self‐esteem related to overwork. There was no evidence of psychotic features.
Mr. C was given a diagnosis of cognitive impairment due to anxiety and lack of confidence in his cognitive abilities in the context of professional burnout. The dementia specialists recommended adopting a healthier lifestyle (via diet, sleep, smoking cessation, and physical activity), psychological care focused on stress management, greater involvement in stimulating cognitive and social activities, and regular monitoring of vascular risk factors. However, his wife was not convinced that his condition was entirely caused by anxiety, as the neuropsychologist and medical specialist had implied, and she asked for a second opinion. Biomarker testing was therefore done to help answer outstanding questions: is the MRI normal and can the cognitive impairment be due only to anxiety or is there AD pathology?
5.3. How did biomarker and other tests improve diagnostic accuracy?
Closer examination of the MRI scans showed a posterior atrophy pattern with milder medial temporal involvement. Microvascular changes were clinically irrelevant. CSF biomarkers showed abnormalities in Aβ1‐42 and t‐tau (Table 3).
Based on clinical symptoms and abnormalities in Aβ1‐42 level and p‐tau/Aβ1‐42 and t‐tau/Aβ1‐42 ratios, Mr. C was diagnosed with MCI due to AD (also known as prodromal AD 1 ).
5.4. How did tests help to guide management of the patient?
Mr. C's prognosis changed after diagnosis. He and his family were informed that cognitive impairment was due to a neurodegenerative disease and would progress in the coming months and years. A biomarker‐confirmed diagnosis of MCI due to AD and worsening of Mr. C's condition supported consideration of drug therapy for symptomatic management. 34
The possibility of participating in a clinical trial of a novel disease‐modifying drug was discussed because CSF biomarkers and clinical conditions showed that he met the eligibility criteria.
6. CASE 3: WORRYING SIGNS OF DEPRESSION
6.1. Background
Mrs. N is a 63‐year‐old retired secondary school teacher. After retiring at age 60, she enjoyed 2 years adjusting to her new routine and taking the opportunity to read more books. During the third year of retirement, her partner noticed that she had missed important appointments and was finding it difficult to follow or discuss the books that she was reading. She started to experience more notable memory problems 6 months before seeking medical help, at which point she was showing signs of depression. Her partner confirmed that progressive amnesia was affecting her moods and ability to enjoy retirement.
6.2. What did the initial assessment show and why did the physician order specialist tests?
Neuropsychological examination showed deficits in memory tasks (Table 3), indicative of amnestic MCI. MRI scan showed a medial temporal atrophy (MTA) score between 0 and 1, indicating only a minor degree of atrophy, normal for her age (MTA score ranges from 0 [no atrophy] to 4 [severe atrophy] and may be used to predict AD in patients with MCI). There was no vascular damage evident on the MRI. As the initial tests proved inconclusive, additional investigation with CSF biomarkers was indicated to make a differential diagnosis between AD and depression as the underlying cause of cognitive impairment.
6.3. How did biomarker and other tests improve diagnostic accuracy?
CSF biomarker results showed abnormal levels of Aβ1‐42, t‐tau, and t‐tau/Aβ1‐42, consistent with a diagnosis of AD (Table 3).
One year later, Mrs. N had deteriorated and was having problems using the telephone and finding her way in her home village. Neuropsychological examination showed Mini‐Mental State Examination (MMSE) was 23/30 and MRI revealed progression of hippocampal atrophy (MTA score between 1 and 2). Mrs. N was diagnosed with dementia due to AD. In this case, both CSF biomarker abnormalities and progressive hippocampal atrophy occurring within 1 year of her first presentation indicated AD as the underlying neurodegenerative process. Importantly, changes in CSF biomarkers identified AD a year before neurodegeneration was shown by hippocampal atrophy.
6.4. How did tests help to guide management of the patient?
Mrs. N's case shows how biomarkers may inform the diagnosis and management of patients presenting with cognitive impairment with mood changes and no dementia. Late‐life depression and cognitive impairment are commonly encountered neuropsychiatric disorders, and may coexist with AD, making diagnosis challenging for physicians. 35 In Mrs. N's case, CSF biomarkers identified AD pathology as the underlying cause for cognitive decline, allowing her management to be adapted appropriately.
7. CASE 4: THREE NEW PAIRS OF GLASSES IN 2 YEARS
7.1. Background
Mr. G is 58 years old and was recently dismissed from his job as an electrical engineer because of poor performance. He became worried about progressive impairments in spatial orientation, which started 2 years before presentation and increased after he experienced retinal detachment. He was also concerned about his eyesight because he needed to buy three new pairs of glasses in 2 years. In addition, he noticed problems with planning, reading, and driving.
Mrs. G confirmed his medical history and reported that her husband was showing more dependent behavior than before his health problems started. However, Mr. and Mrs. G found it difficult to describe his cognitive complaints, so clinicians were unable to develop a complete picture of his medical history.
7.2. What did the initial assessment show and why did the physician order specialist tests?
Neurological examination showed Mr. G's memory and language were intact; however, he exhibited slight dyspraxia affecting his physical co‐ordination; severe spatial and visuo‐perceptive problems, shown by an inability to draw a clock or pentagons; and an inability to perceive multiple objects simultaneously (simultanagnosia). He underwent a full neuropsychological examination (Table 3).
A clinical diagnosis of posterior cortical atrophy (PCA) was made. In most cases PCA is caused by underlying AD; however, other neurodegenerative diseases, such as Lewy body dementia or Creutzfeldt‐Jakob disease, may also cause this clinical syndrome. 36
7.3. How did biomarker and other tests improve diagnostic accuracy?
Amyloid PET imaging using the PiB tracer confirmed amyloid pathology and Mr. G was diagnosed with PCA due to AD.
For research purposes, CSF biomarkers were measured and confirmed abnormalities consistent with a diagnosis of AD (Table 3). There was good agreement between the different approaches, in line with studies showing concordance between amyloid PET and CSF biomarkers, particularly when CSF biomarker ratios are measured (see Table 2).
7.4. How did tests help to guide management of the patient?
Biomarker tests are particularly useful in atypical presentations of AD. Biomarker tests provide useful information about prognosis, 36 allowing the physician to counsel the patient about what to expect in terms of increasing cognitive dysfunction and other cognitive domains over the coming years.
8. CASE 5: AN INCREASED RISK FOR DEMENTIA
8.1. Background
Mr. T, a 72‐year‐old, former office manager fills his retirement time traveling, playing golf, and socializing. He presented with a subjective complaint of memory loss. His father had been diagnosed with AD aged 75 years, so Mr. T was increasingly concerned about his forgetfulness. Ten years previously, Mr. T had joined a long‐term registry study because he felt at increased risk of dementia. At the start of the study, he had no memory or cognitive complaints nor major health issues besides being moderately overweight (body mass index 27) and mildly hypertensive. He was identified as apolipoprotein E ε4 positive (3/4). He attended his first study visit for neuropsychological assessment and diagnostic tests soon after his 62nd birthday.
8.2. What did the initial assessment show and why did the physician order specialist tests?
Routine neuropsychological assessments on previous visits, when Mr. T was aged 62, 64, and 66 years, had not shown evidence of cognitive abnormalities of concern. On this occasion, aged 72 years, testing revealed psychometric evidence of decline from the previously measured higher level of cognitive functioning. Although his MMSE was 30/30 he performed in the impaired range on tests of delayed recall for word lists and stories (Table 3). Other aspects of cognition were in the high average to superior range. This pattern of performance together with the subjective complaint in the context of otherwise intact cognition and no substantial functional impairment indicated a diagnosis of MCI.
8.3. How did biomarker and other tests improve diagnostic accuracy?
Biomarkers and PET imaging were done as part of the research study. Mr. T had lumbar puncture procedures when aged 62 and 66 years, which were analyzed using an exploratory multi‐panel of CSF biomarkers (Neurotoolkit; Van Hulle et al. 49 ). Interestingly, Aβ1‐42/1‐40 ratios were positive for both lumbar punctures (Table 3). p‐tau181/Aβ1‐42 ratios were also positive, whereas p‐tau181 alone was negative and neurofilament light and neurogranin were normal at all time points.
Early evidence of biomarker changes was also seen on brain imaging (Figure 1). Amyloid PET with [C‐11]PiB was positive and increased progressively from his first PiB scan at age 62 years to his most recent PiB scan at age 70 years (Table 3). He was also tau PET positive using the radioligand [F‐18]MK6240 at 70 years (Figure 1). The signal was most evident in the bilateral amygdala, with additional signal in the entorhinal cortex and fusiform more left than right. At his second scan the angular gyrus on the right was also focally positive (Figure 1).
FIGURE 1.
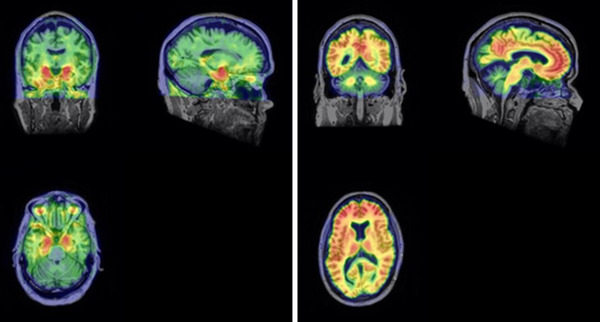
Tau positron emission tomography imaging (left set of orthogonal views) and amyloid imaging (right set of orthogonal views) were taken when Mr. T was aged 70 years. He had prior amyloid imaging at age 62 years (not pictured) together with cerebrospinal fluid sampling and both demonstrated amyloid positivity at least 10 years before his diagnosis of mild cognitive impairment at age 72
8.4. How did tests help to guide management of the patient?
Mr. T's MCI was first apparent on clinical findings at age 72 years, while his CSF was positive for amyloid 10 years earlier. This case illustrates the potential of CSF biomarkers to assess risk of AD based on early detection of specific pathologic changes associated with AD before clinical symptoms become apparent. 1 This is compelling for researchers but how does the clinician use this information to help their patient when proven disease‐modifying therapies are not widely available?
A timely diagnosis provides an opportunity for physicians to encourage lifestyle changes and cognitive training to improve overall health. 37 A Lancet Commission report suggests that modifying 12 risk factors might prevent or delay up to 40% of dementias. 38 In addition, CSF biomarker testing provides a useful tool for clinical trial enrichment. 2 However, the impact on risk stratification of biomarker positivity is beyond the scope of this paper. Risk profiling in individuals with a family history is not currently recommended in the clinic and guidelines do not advocate routine testing of individuals like Mr. T.
9. CSF AD BIOMARKERS IN TRIAL ENRICHMENT AND TREATMENT SELECTION
CSF biomarkers are promising for enrichment of clinical trials with subjects showing AD pathology and, in the future, it is likely that accurate diagnostic tests will be needed to select appropriate patients for novel therapies based on specific target pathologies, for example the presence of amyloid or tau aggregates. 2 Recently, the US Food and Drug Administration (FDA) conditionally approved aducanumab (a monoclonal antibody therapy that targets Aβ) for selected patients with MCI or mild AD dementia. The benefit of this novel disease‐modifying therapy is based on a surrogate endpoint (reduction of Aβ plaque in the brain). We recommend that careful patient selection using biomarkers will be crucial in providing access to this drug.
10. BARRIERS AND LIMITATIONS TO CSF AD BIOMARKERS IN CLINICAL PRACTICE
Clear guidelines and uniform reporting are required to facilitate appropriate use of CSF AD biomarkers in clinical practice so physicians can communicate the potential benefits. 5 Physicians need to counsel their patients about whether they would like to know their prognosis or would prefer to “wait and see.” In addition, it is important to provide adequate psycho‐education both for caregivers and the patients. 39
CSF biomarker tests involve a perceived invasive procedure (lumbar puncture) that may limit participation in clinical research and reduce acceptance in clinical practice, particularly among some ethnic groups. 4 , 40 The procedure is easy and safe when performed following state of the art protocols 41 and collection of up to 30mL of CSF appears well tolerated. 42 The most common side effects of lumbar puncture are back pain and headache, which are easily managed, while serious complications (infections, spinal and subdural cerebral hematoma, and cerebral venous thrombosis) are very rare (prevalence <0.01%). 41 , 42 , 43 Guidelines have been developed to reduce the risks of complications when lumbar puncture is used in daily neurological practice and highlight the need to use atraumatic needle tips. 41 Providing up‐to‐date education and audio‐visual information to physicians and patients can allay common misconceptions about the acceptability and safety of lumbar puncture. 16 , 44
Previously, different methodologies and lack of standardization added to the complexity of performing and interpreting CSF biomarker tests. 45 Today, uniform protocols and standards have been agreed, 46 while fully automated testing procedures are now available. These advances mean that CSF biomarker tests can be considered for use in routine clinical practice. 45 The tests provide simple positive or negative results for amyloid isoforms and (p)tau based on standardized cut‐off values, which can be used alongside neuropsychological evaluation consistent with the latest international clinical diagnostic criteria. 1 , 3 While a positive biomarker test supports the diagnosis of AD, it is important to remember that a biomarker profile that is negative for AD does not exclude other dementias.
Experts recommend CSF ratios to improve the timeliness and accuracy of diagnosis of AD, and to predict progression from MCI to AD. 3 , 11 Importantly, CSF biomarkers provide information on both amyloid and tau biomarkers. The additional financial cost of using CSF ratio tests is considerably lower (10–15 times) than PET imaging; 11 however, it may take time to obtain CSF analysis results from a clinical chemistry laboratory.
11. NOVEL BIOMARKERS IN DEVELOPMENT
The rationale for new biomarkers includes minimizing invasive procedures, making tests more widely available, and improving the ability to detect specific pathologic processes more accurately. 47
Blood‐based biomarkers may be attractive in primary care because of the familiarity and ease of obtaining blood and plasma samples in this setting. 13 In the first steps of the diagnostic process, blood‐based biomarkers could be used alongside cognitive testing to improve referral to specialists. Plasma Aβ and tau biomarkers could indicate a need for testing in specialized clinics, including CSF or PET biomarkers to confirm amyloid positivity. Additional research is warranted to scale‐up clinical use of blood‐based biomarkers for AD, particularly in relation to validation with existing large sample sets and ethnically diverse populations. 48 Similarly, biomarkers measured in other fluids (eyes, mouth, ears, and nose) offer relatively non‐invasive methods, but their potential remains to be realized.
Novel biomarkers that track different aspects of AD pathology, including synaptic dysfunction, neuro‐inflammation, and glial activation, 49 may be useful for early diagnosis of MCI and AD, and differential diagnosis of AD from other neurodegenerative diseases (see supporting information). Digital tools may also contribute to screening and diagnostic pathways in AD. 50
12. CONCLUSIONS
We recommend that CSF AD biomarkers should be part of the standard of care for the work‐up of MCI and dementia patients. The case narratives show how CSF AD biomarkers may be useful at different stages of AD diagnosis and across different ages (see Box A).
Box A. Uses of CSF AD biomarker testing as shown by the case narratives
In the clinic
Exclusion of AD as the underlying cause of dementia, helping Mr. B to receive appropriate therapy for a psychiatric condition.
Diagnosis of AD at an early stage (MCI due to AD/prodromal AD), enabling Mr. C to start symptomatic treatment for cognitive impairment and planning for future life changes (early retirement).
Accurate and prompt diagnosis in individuals such as Mr. G with relatively young‐onset AD.
Differential diagnosis of AD in individuals presenting with other neuropsychiatric symptoms, and accurate diagnosis of AD despite an atypical presentation, allowing Mrs. N and Mr. G, respectively, to benefit from appropriate clinical management plans.
For research
Detection of therapeutic target for future treatments (anti‐amyloid immunotherapies).
Determination of prevention or lifestyle interventions, for example, Mr. T, whose heightened risk state could be assessed in the context of a research program, where amyloid changes could be detected 10 years before clinical diagnosis.
In regular clinical practice, standardized reporting of CSF AD biomarker tests should be an important part of the diagnostic pathway and help to answer patients’ questions about what their cognitive complaints mean for them. Tests revealing normal Aβ and tau levels show there is no evidence of AD pathology, whereas abnormal levels indicate a risk of AD independently of clinical stage. CSF Aβ1‐42 assays have good agreement with amyloid PET imaging, while Aβ1‐42/1‐40 and tau/Aβ1‐42 ratios have superior performance to Aβ1‐42 alone.
While timely and precise diagnosis can improve clinical management of patients with suspected AD, the negative predictive value of CSF AD biomarkers is also clinically important and can be psychologically reassuring for patients and clinicians. Biomarker tests are important for patients because by being better informed they can take steps to plan their future lives. In the future, appropriate use of biomarker testing will be essential for the careful selection of patients to receive disease‐modifying therapies, such as aducanumab, which was approved based on surrogate benefits.
CONFLICTS OF INTEREST
Femke Bouwman received a grant from Optina Dx (Canada), honoraria from Roche, and provided expert testimony for Biogen; all payments were made to the institution. Giovanni B. Frisoni has no competing interests to declare. Sterling C. Johnson received a research grant from Cerveau Technologies paid to the institution, participated in advisory boards for Roche Diagnostics and Prothena, and received an equipment grant from Roche Diagnostics paid to the institution. Xiaochun Chen has no competing interests to declare. Sebastiaan Engelborghs received grants from Janssen Pharmaceutica and ADx Neurosciences paid to the institution; consulting fees from icometrix (paid to institution), Biogen, Roche, and Novartis; and honoraria from Eisai/Pfizer. Takeshi Ikeuchi received grants AMED JP21dk0207049, AMED JP21dk0207045, and AMED JP21ek0109545 paid to the institution, and honoraria from Eisai, Daiichi‐Sankyo, Fuji Film RI Pharma, Ajinomoto, Novartis, and Chugai Pharmaceutical. Claire Paquet is on advisory boards for Roche, Lilly, and Biogen laboratories. Craig Ritchie received consulting fees from Roche, Biogen, Eisai, Sygnature, Actinogen, Eli Lilly, MSD, Signant Health, and Alchemab, and honoraria from Biogen, Roche, and Eisai. Charlotte Teunissen has collaboration contracts with ADx Neurosciences and Quanterix, and grants from Axon NeuroSciences, Biogen, Boehringer, Brainstorm Therapeutics, EIP farma, Esai, Janssen prevention center, Roche, Toyama, and Vivoryon. Sasha Bozeat and Frances‐Catherine Quevenco are paid employees of Roche Diagnostic Solutions. Article processing charges were paid by Roche Diagnostics International Ltd.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
Medical writing support was provided by Tim Kelly, Medi‐Kelsey Ltd, funded by Roche Diagnostics International Ltd, Rotkreuz, Switzerland. ELECSYS is a registered trademark of Roche.
Bouwman FH, Frisoni GB, Johnson SC, et al. Clinical application of CSF biomarkers for Alzheimer's disease: From rationale to ratios. Alzheimer's Dement. 2022;14:e12314. 10.1002/dad2.12314
REFERENCES
- 1. Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer's disease: recommendations of the International Working Group. Lancet Neurol. 2021;20:484‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol. 2017;16:661‐676. [DOI] [PubMed] [Google Scholar]
- 3. Jack CR, Jr. , Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Judge D, Roberts J, Khandker RK, Ambegaonkar B, Black CM. Physician practice patterns associated with diagnostic evaluation of patients with suspected mild cognitive impairment and Alzheimer's disease. Int J Alzheimers Dis. 2019;2019:4942562. 10.1155/2019/4942562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teunissen CE, Otto M, Engelborghs S, et al. White paper by the Society for CSF Analysis and Clinical Neurochemistry: overcoming barriers in biomarker development and clinical translation. Alzheimers Res Ther. 2018;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fruijtier AD, Visser LNC, Bouwman FH, et al. What patients want to know, and what we actually tell them: the ABIDE project. Alzheimers Dement (NY). 2020;6:e12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Visser LNC, Pelt SAR, Kunneman M, et al. Communicating uncertainties when disclosing diagnostic test results for (Alzheimer's) dementia in the memory clinic: the ABIDE project. Health Expect. 2020;23:52‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niemantsverdriet E, Feyen BFE, Le Bastard N, et al. Added diagnostic value of cerebrospinal fluid biomarkers for differential dementia diagnosis in an autopsy‐confirmed cohort. J Alzheimers Dis. 2018;63:373‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paquet C, Magnin E, Wallon D, et al. Utility of CSF biomarkers in psychiatric disorders: a national multicentre prospective study. Alzheimers Res Ther. 2016;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blennow K, Zetterberg H. Fluid biomarker‐based molecular phenotyping of Alzheimer's disease patients in research and clinical settings. Prog Mol Biol Transl Sci. 2019;168:3‐23. [DOI] [PubMed] [Google Scholar]
- 11. Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid beta (Abeta) 42/40 ratio in the diagnosis of Alzheimer's Disease. Alzheimers Res Ther. 2019;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bjerke M, Engelborghs S. Cerebrospinal fluid biomarkers for early and differential Alzheimer's disease diagnosis. J Alzheimers Dis. 2018;62:1199‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewczuk P, Riederer P, O'Bryant SE, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the Consensus Of The Task Force On Biological Markers In Psychiatry Of The World Federation Of Societies Of Biological Psychiatry. World J Biol Psychiatry. 2018;19:244‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doecke JD, Ward L, Burnham SC, et al. Elecsys CSF biomarker immunoassays demonstrate concordance with amyloid‐PET imaging. Alzheimers Res Ther. 2020;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplow J, Vandijck M, Gray J, et al. Concordance of Lumipulse cerebrospinal fluid t‐tau/Abeta42 ratio with amyloid PET status. Alzheimers Dement. 2020;16:144‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaw LM, Arias J, Blennow K, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2018;14:1505‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simonsen AH, Herukka SK, Andreasen N, et al. Recommendations for CSF AD biomarkers in the diagnostic evaluation of dementia. Alzheimers Dement. 2017;13:274‐284. [DOI] [PubMed] [Google Scholar]
- 18. Blennow K, Shaw LM, Stomrud E, et al. Predicting clinical decline and conversion to Alzheimer's disease or dementia using novel Elecsys Abeta(1‐42), pTau and tTau CSF immunoassays. Sci Rep. 2019;9:19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid‐beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14:1470‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alcolea D, Pegueroles J, Munoz L, et al. Agreement of amyloid PET and CSF biomarkers for Alzheimer's disease on Lumipulse. Ann Clin Transl Neurol. 2019;6:1815‐1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keshavan A, Wellington H, Chen Z, et al. Concordance of CSF measures of Alzheimer's pathology with amyloid PET status in a preclinical cohort: a comparison of Lumipulse and established immunoassays. Alzheimers Dement (Amst). 2020;12:e12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moon S, Kim S, Mankhong S, et al. Alzheimer's cerebrospinal biomarkers from Lumipulse fully automated immunoassay: concordance with amyloid‐beta PET and manual immunoassay in Koreans: CSF AD biomarkers measured by Lumipulse in Koreans. Alzheimers Res Ther. 2021;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schindler SE, Gray JD, Gordon BA, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimers Dement. 2018;14:1460‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willemse EAJ, Tijms BM, Van Berckel BNM, et al. Using cerebrospinal fluid amyloid‐beta (1‐42) in the memory clinic: concordance with PET and use of biomarker ratios across immunoassays. Alzheimers Dement. 2020;16:e045128. [Google Scholar]
- 25. Guo T, Korman D, La Joie R, et al. Normalization of CSF pTau measurement by Abeta40 improves its performance as a biomarker of Alzheimer's disease. Alzheimers Res Ther. 2020;12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo J, Agboola F, Grant E, et al. Sequence of Alzheimer disease biomarker changes in cognitively normal adults: a cross‐sectional study. Neurology. 2020;95:e3104‐e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyer PF, Pichet Binette A, Gonneaud J, Breitner JCS, Villeneuve S. Characterization of Alzheimer disease biomarker discrepancies using cerebrospinal fluid phosphorylated tau and AV1451 positron emission tomography. JAMA Neurol. 2020;77:508‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reimand J, de Wilde A, Teunissen CE, et al. PET and CSF amyloid‐beta status are differently predicted by patient features: information from discordant cases. Alzheimers Res Ther. 2019;11:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reimand J, Groot C, Teunissen CE, et al. Why is amyloid‐beta PET requested after performing CSF biomarkers? J Alzheimers Dis. 2020;73:559‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Struyfs H, Niemantsverdriet E, Goossens J, et al. Cerebrospinal fluid P‐Tau181P: biomarker for improved differential dementia diagnosis. Front Neurol. 2015;6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Struyfs H, Van Broeck B, Timmers M, et al. Diagnostic accuracy of cerebrospinal fluid amyloid‐beta isoforms for early and differential dementia diagnosis. J Alzheimers Dis. 2015;45:813‐822. [DOI] [PubMed] [Google Scholar]
- 32. Santangelo R, Dell'Edera A, Sala A, et al. The CSF p‐tau181/Abeta42 ratio offers a good accuracy “in vivo” in the differential diagnosis of Alzheimer's dementia. Curr Alzheimer Res. 2019;16:587‐595. [DOI] [PubMed] [Google Scholar]
- 33. Goldhardt JP, Weinberger F, Müller‐Sarnowski J, et al. Elecsys CSF assays accurately distinguish AD from frontotemporal lobar degeneration. J Prev Alzheimers Dis. 2020;7:S83 (P050). [DOI] [PubMed] [Google Scholar]
- 34. Petersen RC, Thomas RG, Aisen PS, et al. Randomized controlled trials in mild cognitive impairment: sources of variability. Neurology. 2017;88:1751‐1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF Biomarkers analysis can differentiate Alzheimer's disease from late‐life depression. Front Aging Neurosci. 2018;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong B, Lucente DE, MacLean J, et al. Diagnostic evaluation and monitoring of patients with posterior cortical atrophy. Neurodegener Dis Manag. 2019;9:217‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McGrattan AM, McEvoy CT, McGuinness B, McKinley MC, Woodside JV. The effect of diet, lifestyle and/or cognitive interventions in Mild Cognitive Impairment: a systematic review. Proc Nutr Soc. 2017;76:E114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Visser LNC, Kunneman M, Murugesu L, et al. Clinician‐patient communication during the diagnostic workup: the ABIDE project. Alzheimers Dement (Amst). 2019;11:520‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blazel MM, Lazar KK, Van Hulle CA, et al. Factors associated with lumbar puncture participation in Alzheimer's disease research. J Alzheimers Dis. 2020;77:1559‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Engelborghs S, Niemantsverdriet E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement (Amst). 2017;8:111‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Monserrate AE, Ryman DC, Ma S, et al. Factors associated with the onset and persistence of post‐lumbar puncture headache. JAMA Neurol. 2015;72:325‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cognat E, Koehl B, Lilamand M, et al. Preventing post‐lumbar puncture headache. Ann Emerg Med. 2021;78:443‐450. [DOI] [PubMed] [Google Scholar]
- 44. Babapour Mofrad R, Fruijtier AD, Visser LNC, et al. Lumbar puncture patient video increases knowledge and reduces uncertainty: an RCT. Alzheimers Dement (NY). 2021;7:e12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Herukka SK, Simonsen AH, Andreasen N, et al. Recommendations for cerebrospinal fluid Alzheimer's disease biomarkers in the diagnostic evaluation of mild cognitive impairment. Alzheimers Dement. 2017;13:285‐295. [DOI] [PubMed] [Google Scholar]
- 46. Hansson O, Batrla R, Brix B, et al. The Alzheimer's Association international guidelines for handling of cerebrospinal fluid for routine clinical measurements of amyloid beta and tau. Alzheimers Dement. 2021;17:1575‐1582. [DOI] [PubMed] [Google Scholar]
- 47. Alawode DOT, Heslegrave AJ, Ashton NJ, et al. Transitioning from cerebrospinal fluid to blood tests to facilitate diagnosis and disease monitoring in Alzheimer's disease. J Intern Med. 2021;290:583‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chong JR, Ashton NJ, Karikari TK, et al. Blood‐based high sensitivity measurements of beta‐amyloid and phosphorylated tau as biomarkers of Alzheimer's disease: a focused review on recent advances. J Neurol Neurosurg Psychiatry. 2021;92:1231‐1241. [DOI] [PubMed] [Google Scholar]
- 49. Van Hulle C, Jonaitis EM, Betthauser TJ, et al. An examination of a novel multipanel of CSF biomarkers in the Alzheimer's disease clinical and pathological continuum. Alzheimers Dement. 2021;17:431‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sabbagh MN, Boada M, Borson S, et al. Rationale for early diagnosis of mild cognitive impairment (MCI) supported by emerging digital technologies. J Prev Alzheimers Dis. 2020;7:158‐164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


