Abstract
Finding therapies that can protect and expand functional β-cell mass is a major goal of diabetes research. Here, we generated β-cell–specific conditional knockout and gain-of-function mouse models and used human islet transplant experiments to examine how manipulating Nrf2 levels affects β-cell survival, proliferation, and mass. Depletion of Nrf2 in β-cells results in decreased glucose-stimulated β-cell proliferation ex vivo and decreased adaptive β-cell proliferation and β-cell mass expansion after a high-fat diet in vivo. Nrf2 protects β-cells from apoptosis after a high-fat diet. Nrf2 loss of function decreases Pdx1 abundance and insulin content. Activating Nrf2 in a β-cell–specific manner increases β-cell proliferation and mass and improves glucose tolerance. Human islets transplanted under the kidney capsule of immunocompromised mice and treated systemically with bardoxolone methyl, an Nrf2 activator, display increased β-cell proliferation. Thus, by managing reactive oxygen species levels, Nrf2 regulates β-cell mass and is an exciting therapeutic target for expanding and protecting β-cell mass in diabetes.
Introduction
A characteristic of all forms of diabetes is insufficient functional β-cell mass. Therefore, to therapeutically expand β-cell mass, several approaches have been explored, including induced self-replication of β-cells and protection of existing β-cells (1). Oxidative stress is a major mediator of β-cell glucotoxicity and plays an essential role in the development of type 2 diabetes (2). Indeed, chronic exposure of β-cells to high levels of reactive oxygen species (ROS) results in reduction of functional β-cell mass. If severe enough, ROS stimulates β-cell apoptosis through the mitochondrial “intrinsic” pathway by activation of the proapoptotic members of the Bcl-2 family and by recruitment of cytochrome C–mediated caspase 3 (3). Increased ROS may also lead to defective glucose-stimulated insulin secretion (GSIS) in β-cells by inhibiting glycolytic enzymes and impairing the activity of potassium channels (4,5). Additionally, glucotoxic ROS decreases the expression of the insulin gene by inhibiting the activity and levels of the duodenal homeobox factor 1 (Pdx1) and v-Maf musculoaponeurotic fibrosarcoma oncogene family, protein A (MafA), transcription factors (6). Moreover, increased ROS activates the transcription factor FoxO1, which acts to inhibit β-cell proliferation under these conditions (7). Therefore, balancing ROS levels is critical for maintaining functional β-cell mass in patients with diabetes.
Nuclear factor erythroid 2–related factor (Nrf2) is a transcription factor that confers cell protection against xenobiotic and oxidative stresses (8). During resting conditions, Nrf2 is bound to its repressor, the Kelch-like ECH-associated protein 1 (Keap1), which through the cullin3-E3 ubiquitin ligase complex targets Nrf2 to proteosomal degradation. Upon oxidative stress, critical cysteines in Keap1 are oxidized, leading to disruption of Keap1-Keap1 homodimerization and Keap1-cullin3 interaction, all of which disrupt the ability of Keap1 to target Nrf2 for degradation. As a result, Nrf2 is phosphorylated at Ser40 and translocates to the nucleus where it binds to antioxidant response element sequences in the regulatory regions of antioxidant target genes (8). Activation of Nrf2 protects β-cells against a variety of hazardous conditions, including damage induced by oxidative stress, streptozotocin, glucotoxicity, cholesterol, and cytokines. In addition, Nrf2 regulates β-cell mitochondrial biogenesis and function (8). These findings suggest that loss of Nrf2 function might play an important role in the development of diabetes. In agreement with this notion, genome-wide association study analysis uncovered several mutations in the Nrf2 pathway that are associated with type 2 diabetes (9–11). Recently, we found that Nrf2 stimulates β-cell proliferation in both rat insulinoma INS-1 cells and in primary pancreatic human islets (12). We therefore explored the role of Nrf2 in preserving and expanding β-cell mass under diabetogenic-related stress conditions, and tested whether activation of Nrf2 leads to increased proliferation and expansion of β-cell mass in vivo.
Here, we show that Nrf2 is rapidly activated by glucose in vitro and necessary for glucose-regulated β-cell proliferation. In addition, depletion of Nrf2 in an inducible β-cell–specific knockout (KO) mouse model results in impaired high-fat diet (HFD)–induced adaptive β-cell expansion due to increased β-cell death and decreased β-cell proliferation and insulin content. Conversely, genetic and pharmacological Nrf2 gain of function increases mouse β-cell proliferation and mass and improves glucose tolerance. Importantly, pharmacological Nrf2 gain of function stimulates human β-cell proliferation both ex vivo and in vivo. We conclude that the antioxidant Nrf2 pathway is a novel target for preserving and increasing functional β-cell mass for the treatment of diabetes.
Research Design and Methods
Cell Culture
An INS-1–derived 832/13 rat insulinoma cell line was cultured as previously described (12). Cells were grown in a 37°C incubator under a humidified atmosphere containing 5% CO2.
RT-PCR
Total RNA was extracted from isolated β-cells using the RNeasy Micro Kit (no. 74004; QIAGEN), and real-time (RT)-PCR was performed as previously described (12). The sequences of primers used for quantitative real-time PCR are listed in Supplementary Table 1.
Mouse Lines
βNrf2KO and βKeap1KO male mice were generated by crossing MIP-CreERTAM mice (13) (Resource Research Identifier [RRID]: IMSR_JAX:024709) with Nrf2lox/lox (14) (RRID: IMSR_JAX:025433) or Keap1lox/lox (15) (RRID: MGI:4948839) mice, respectively. Keap1lox/lox-Nrf2lox/lox male mice were generated by crossing Nrf2lox/lox and Keap1lox/lox mice. βNrf2KO, βKeap1KO, and MIP-CreERTAM male mice were injected intraperitoneally for 5 consecutive days with 75 μg/g tamoxifen (Tam) (no. T5648; Sigma-Aldrich) dissolved in corn oil. All studies were performed with the approval of and in accordance with guidelines established by the institutional animal care and use committee of the Icahn School of Medicine at Mount Sinai.
Adenoviruses and Reagents
Keap1lox/lox- Nrf2lox/lox and Keap1lox/lox-Nrf2lox/lox mouse islets were dispersed using 0.05% Trypsin-EDTA (no. 25300-054; Gibco), after which they were transduced with adenoviral vectors encoding for Cre or LacZ at a multiplicity of infection of 150 in serum-free RPMI media for 2 h. After 2 h, the culture media were replaced with serum-containing media as previously described (12). Cells were treated with N-acetyl cysteine (NAC) (no. A9165; Sigma-Aldrich), bardoxolone methyl (CDDO-Me) (no. SMB00376; Sigma-Aldrich), sulforaphane (SFN) (no. S4441; Sigma-Aldrich), or brusatol (no. AK128303; Ark Pharm).
Human and Mouse Islets
Mouse islets were isolated after collagenase P (no. 11213865001; Sigma-Aldrich) injection through the pancreatic duct, followed by digestion and separation by density gradient using Histopaque-1077 (no. 10771; Sigma-Aldrich), as previously reported (16). Cadaveric human donor islets were received from the Integrated Islet Distribution Program (https://iidp.coh.org/overview.aspx) and cultured as previously described (12). Specific details of human islet donors are provided in Supplementary Table 2. Both mouse and human islets were cultured in RPMI medium (no. 11879-020; Gibco) supplemented with 5 or 20 mmol/L d-glucose, 10% FBS (no. 35-011-CV; Corning), and 1% penicillin/streptomycin (no. 15140-122; Gibco).
Immunostaining
For β-cell proliferation, dispersed islets or paraffin-embedded pancreatic sections were immunostained with specific Ki67 and insulin antibodies (Supplementary Table 3). The percentage of Ki67-positive/insulin-positive events was calculated from the total insulin-positive cells. For β-cell mass and islet morphometry, an average of three insulin-stained mouse pancreatic section samples were measured using ImageJ software (National Institutes of Health). For measuring oxidative stress levels in paraffin-embedded pancreatic sections, samples were immunostained with insulin and an antibody that recognizes DNA and RNA oxidative damage (Supplementary Table 3). The percentage of DNA/RNA-positive/insulin-positive events was calculated from the total insulin-positive cells.
Immunoblotting
Mouse islets (400 islet equivalents) were digested using Pierce radioimmunoprecipitation assay buffer (no. 89900; Thermo Fisher Scientific) supplemented with Halt Protease and Phosphatase Inhibitor (no. 78443; Thermo Fisher Scientific), followed by sonication (85% amplitude, 20 s on, 10 s off for a total of 5 min). Forty micrograms of total protein extract from mouse islets was separated on 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (no. IPVH00010; Millipore), and membranes were incubated with primary antibodies (Supplementary Table 3), followed by peroxidase-conjugated secondary antibodies. Bands were visualized with Amersham ECL Western Blotting Detection Reagents (no. RPN2209 for actin) and the more sensitive Amersham Cytiva ECL Prime Blotting Detection Reagents (no. RPN2232) for other proteins. Protein levels were quantified using Image J densitometry and normalized to actin levels.
TUNEL Assay
TUNEL labeling was performed according to the manufacturer instructions, using the DeadEnd Fluorometric TUNEL System (no. G3250; Promega). Samples were then immunostained with insulin antibody (Supplementary Table 3). The percentage of TUNEL-positive/insulin-positive events was calculated from the total insulin-positive cells.
Detection of ROS in Live Cells
The cell-permeant 2′,7′-Dichlorodihydrofluorescein Diacetate (H2DCFDA) (also known as dichlorofluores cin diacetate) Reactive Oxygen Species Detection Kit (no. D399; Molecular Probes, Invitrogen) was used to measure the production of ROS in live cells after exposure to high-glucose concentration. Generation of intracellular ROS was measured by plate reader (excitation/emission 495/520 nm) according to the manufacture’s instructions.
Trypan Blue Viability Assay
Following treatment, INS-1 cells were stained with negative stain Trypan blue (1:2 v/v) for 3 min at room temperature, and live cells were counted under a light microscope.
Acute and Chronic HFD Feeding
Two- to 4-month-old βNrf2KO and βKeap1KO Tam- or vehicle corn oil–injected mice were placed for 1 week or 1 month on a lard-based HFD (41% kcal from fat, no. TD 96001; Harlan Teklad) or a regular diet (RD) (13.1% kcal from fat, Purina PicoLab 5053; LabDiet).
Glucose Homeostasis and Insulin Content
Blood glucose was determined by glucometer and plasma insulin by ELISA (no. 10-1249-01; Mercodia). For intraperitoneal glucose tolerance tests, mice were fasted for 16 h and then intraperitoneally injected with glucose at a dose of 2 g/kg d-glucose. Insulin tolerance tests were performed in nonfasting conditions with intraperitoneally injected human insulin (1.5 units/kg). GSIS and insulin content measurements were performed as previously described (17,18).
Euglycemic Human Islet Transplantation Model
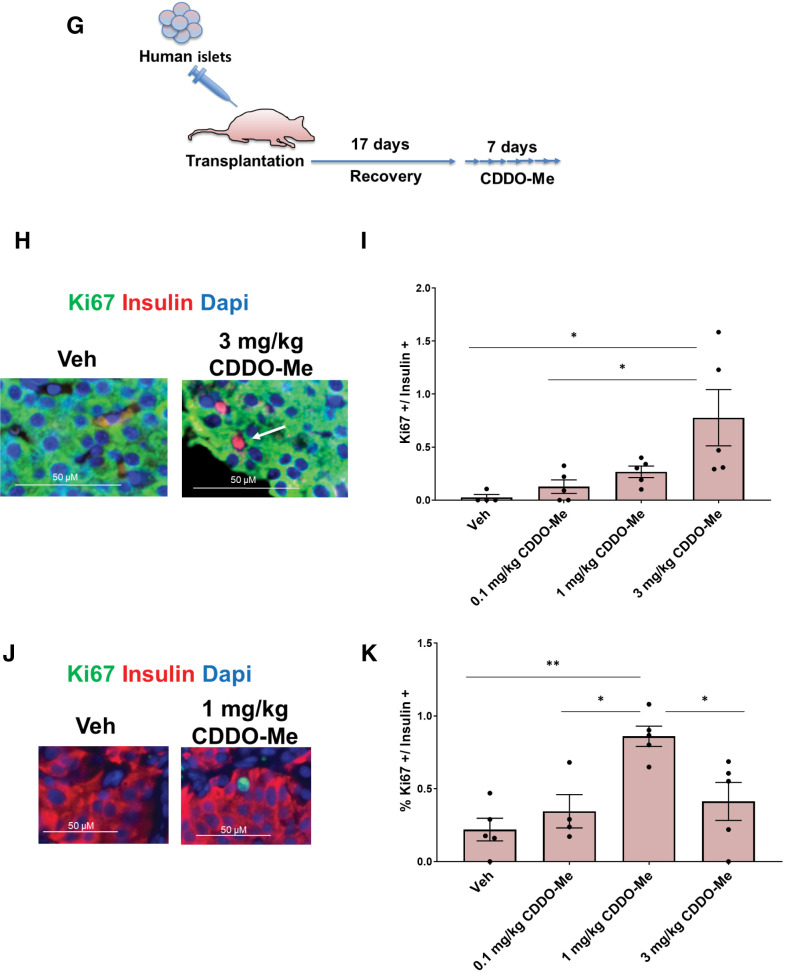
Five hundred human islet equivalents from five different cadaveric donors were transplanted under the renal capsule of five euglycemic 3- to 7-month-old NOD-SCID or Rag1−/− immunodeficient mice as detailed previously (18). Animals were allowed to recover for 17 days and then were randomly selected to be given daily intraperitoneal injections of 0.1, 1.0, or 3.0 mg/kg CDDO-Me or vehicle (DMSO) for 7 days. Animals were then euthanized, and kidneys and pancreata were harvested, fixed, embedded, sectioned, and immunostained for insulin and Ki67 as described above.
Statistical Analysis
Data are presented as means ± SE. Statistical analysis was performed using unpaired two-tailed t test, one-way ANOVA, and two-way ANOVA with Tukey multiple comparison test.
Data and Resource Availability
All data generated or analyzed during this study are included in the published article (and its online supplementary files). The mouse lines generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
Nrf2 Is Activated by Glucose Metabolism and ROS Production in Pancreatic β-Cells
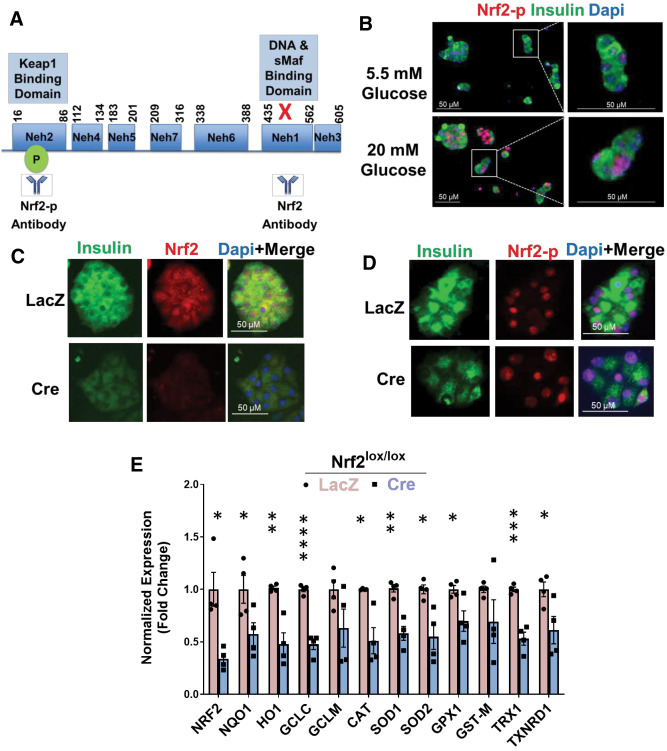
ROS is predominately generated by the mitochondria as a by-product of increased glucose metabolism (8). Cells exposed to high concentrations of glucose form ROS, which stimulate nuclear translocation of Nrf2 and activation of its transcriptional activity (19,20). Once Nrf2 is released from Keap1 binding, it is phosphorylated at Ser40 (localized at the Keap1-binding site) and translocates to the nucleus (8). Therefore, the use of Nrf2-Ser40 (Nrf2-p) antibody (Fig. 1A) can be used to quantify the proportion of nuclear Nrf2 as a surrogate of Nrf2 activation. To our knowledge, the effect of increased glucose metabolism on Nrf2 activation has never been studied in pancreatic β-cells. INS-1 832/13 cells exposed to high concentrations of glucose (20 mmol/L) presented increased ROS generation in <10 min (1.5-fold compared with control) (Supplementary Fig. 1A) and increased nuclear staining of Nrf2-p (Supplementary Fig. 1B). Interestingly, Nrf2 activation persisted even after 48-h incubation in high glucose. However, addition of the antioxidant NAC blocked Nrf2 nuclear recruitment, suggesting that glucose increases Nrf2 nuclear levels by ROS formation (Supplementary Fig. 1C). In addition, high glucose increased expression (sixfold) of NAD(P)H quinone dehydrogenase 1 (Nqo1), a known Nrf2 target in β-cells (12) (Supplementary Fig. 1D). Similar to INS-1 832/13 cells, primary mouse β-cells displayed an increase in Nrf2 nuclear staining in just 5 min of high glucose (Fig. 1B).
Figure 1.
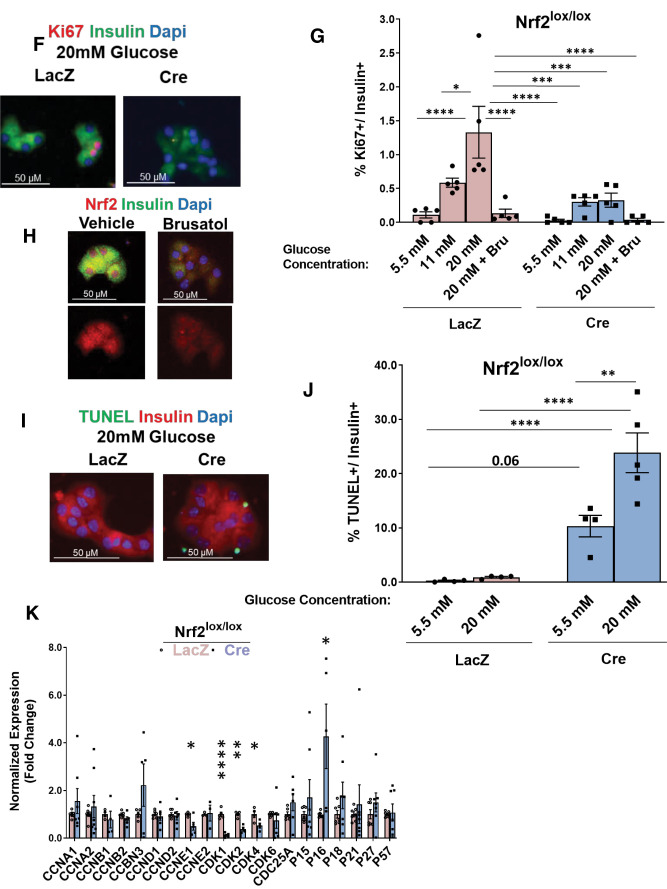
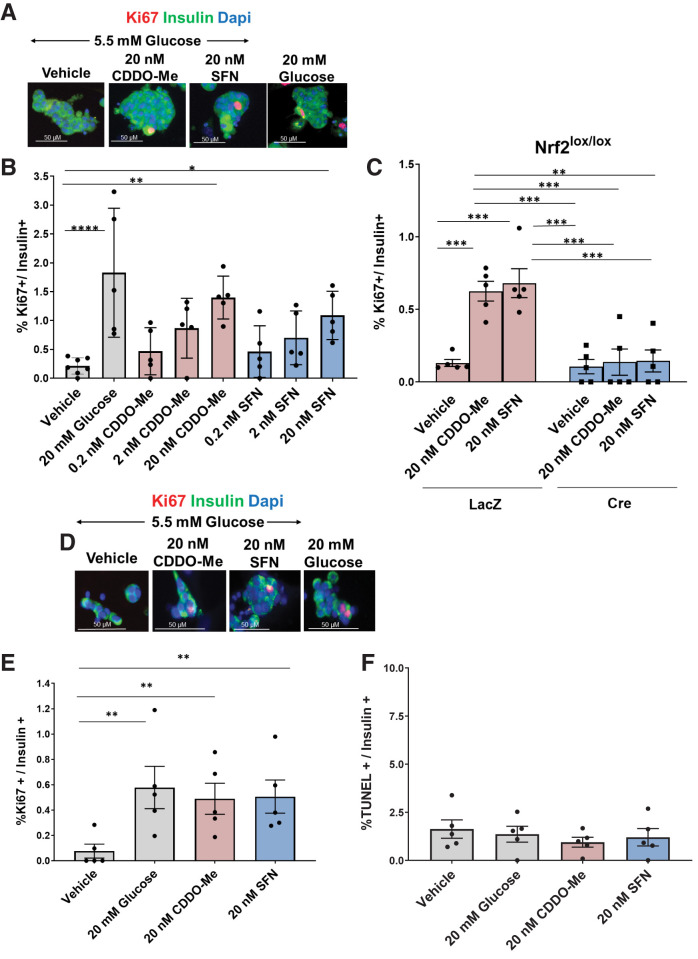
Nrf2 is necessary for glucose-stimulated β-cell proliferation ex vivo. A: Illustration of the Nrf2 protein structure emphasizing the deletion of exon 5, which expresses the Neh1 domain, in Nrf2lox/lox mice. Nrf2 antibody (Nrf2 and Nrf2-p) epitopes are indicated. B: Islets from C57BL/6 mice were isolated, dispersed, and incubated in 5.5 or 20 mmol/L glucose for 5 min, followed by immunostaining with insulin and Nrf2-p antibodies. C and D: Dispersed Nrf2lox/lox mouse islets were transduced with LacZ or Cre adenovirus and cultured in the presence of 20 mmol/L glucose for 48 h, followed by immunostaining using insulin and Nrf2 (C) or insulin and Nrf2-p antibodies (D). E: Dispersed Nrf2lox/lox islets were incubated with 20 mmol/L glucose for 48 h, followed by RNA isolation. F and G: mRNA expression of Nrf2 and known Nrf2 target genes was measured. Dispersed Nrf2lox/lox mouse islets were transduced with LacZ or Cre adenovirus and incubated with 50 nmol/L brusatol (Bru) or vehicle (100% ethanol) in the presence of 5.5, 11, or 20 mmol/L glucose for 48 h, followed by immunostaining using insulin and Ki67 antibodies. Percentage of Ki67-positive and insulin-positive cells was then calculated. H: Mouse islets were incubated with 50 nmol/L Bru or vehicle in the presence of 20 mmol/L glucose for 48 h, followed by immunostaining with insulin and Nrf2 antibodies. I and J: Dispersed Nrf2lox/lox mouse islets were transduced with adenoviruses expressing either LacZ or Cre and cultured in the presence of 20 mmol/L glucose for 48 h, followed by TUNEL assay. The percentage of insulin- and TUNEL-positive cells was calculated. K: Nrf2lox/lox islets transduced with LacZ or Cre adenovirus were incubated in 20 mmol/L glucose for 24 h, followed by RNA extraction. Expression of cell-cycle regulator genes was measured by RT-PCR. Data are mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001.
Nrf2 Loss of Function Decreases Glucose-Stimulated β-Cell Proliferation Ex Vivo
We then tested whether depletion of active Nrf2 affects glucose-stimulated β-cell proliferation. Nrf2lox/lox mice (14) were used, wherein LoxP sites flank Nrf2 exon 5 that encodes the Neh1 domain, which includes sequences necessary for DNA binding and for binding to sMaf, the Nrf2 obligatory heterodimer partner (21). The Neh1 domain contains one nuclear localization signal and one nuclear export signal (22–24). Deletion of exon 5 in Nrf2 (confirmed by loss of fluorescence from an antibody that recognizes the Neh1 domain [Fig. 1A and C]), did not prevent Nrf2 nuclear translocation (detected by Nrf2-p antibody [Fig. 1A and D]). However, the deletion of Nrf2 exon 5 rendered Nrf2 inactive, as we found a 70% decrease in the Neh1 domain (Fig. 1E) and a significant reduction in the expression of known Nrf2 target genes, including Nqo1, heme oxygenase (Ho1), glutamate-cysteine ligase catalytic subunit (Gclc), catalase (Cat), superoxide dismutase (Sod1 and Sod2), glutathione peroxidase (Gpx1), thioredoxin-1 (Trx1), and thioredoxin reductase (Txnrd1) (8,25,26). To test whether Nrf2 regulates the mitogenic effect of glucose in β-cells, isolated Nrf2lox/lox islets were treated with a control adenovirus (LacZ) or Cre adenovirus to remove Nrf2 exon 5 (encoding the DNA-binding domain) and then incubated with 5.5, 11, or 20 mmol/L glucose for 48 h. Incubation with 20 mmol/L glucose increased control β-cell proliferation from ∼0.1% for 5.5 mmol/L glucose to ∼1.3%, an effect that was lost after depletion of active Nrf2 (Fig. 1F and G). This result was confirmed using brusatol, a natural quassinoid that blocks Nrf2 protein translation (27) (Fig. 1G and H and Supplementary Fig. 1E and F). Thus, Nrf2 is necessary for glucose-stimulated β-cell proliferation in mice.
Nrf2 was previously shown to protect β-cells against glucotoxicity (8). Therefore, we investigated whether Nrf2 loss of function decreases β-cell survival under high-glucose concentration. As expected, removal of Nrf2 had 27-fold more TUNEL-positive β-cells compared with control cells cultured in 20 mmol/L glucose (Fig. 1I and J). Thus, the presence of Nrf2 protects β-cells from cell death elicited by the ROS generated from high concentrations of glucose.
To investigate further, mouse Nrf2lox/lox islets were isolated, treated with LacZ or Cre adenovirus, and cultured in 20 mmol/L glucose. After 24 h, RNA was extracted, and the expression of cell-cycle regulators was measured. Depletion of Nrf2 decreased expression of cyclin E1, a cyclin that regulates cell transition from the G1 to S phase during cell division (28), and cyclin-dependent kinase 1 (Cdk1), Cdk2, and Cdk4, which are essential for cell-cycle progression (29) (Fig. 1K). No changes of cyclin E1 were observed at the protein level (data not shown), yet it might be that 24 h of incubation was not the optimal time point for detecting such changes. Together, these correlative results are consistent with a role for Nrf2 in cell-cycle regulation.
Nrf2 Is Necessary for HFD-Mediated Adaptive β-Cell Mass Expansion
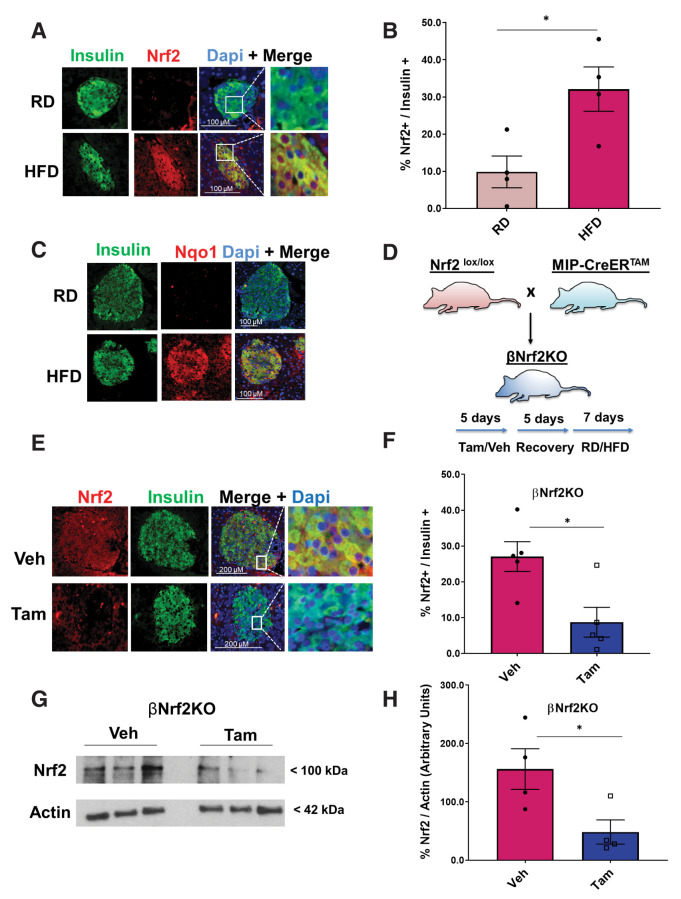
Mice were fed an HFD or RD for 1 week to test whether the Nrf2 pathway is activated in response to hypercaloric conditions. Mice fed an HFD displayed increased Nrf2 levels in β-cells (3.3-fold) compared with RD-fed mice (Fig. 2A and B). Additionally, HFD-fed mice displayed increased expression of the Nrf2 target gene, Nqo1 (Fig. 2C), confirming that Nrf2 is activated in mouse β-cells under hypercaloric conditions in concert with a previous report in rats (30). Interestingly, we saw no change in Keap1 levels when comparing RD- and HFD-fed mice (Supplementary Fig. 2A), suggesting that activation of Nrf2 is not due to changes in Keap1 expression.
Figure 2.
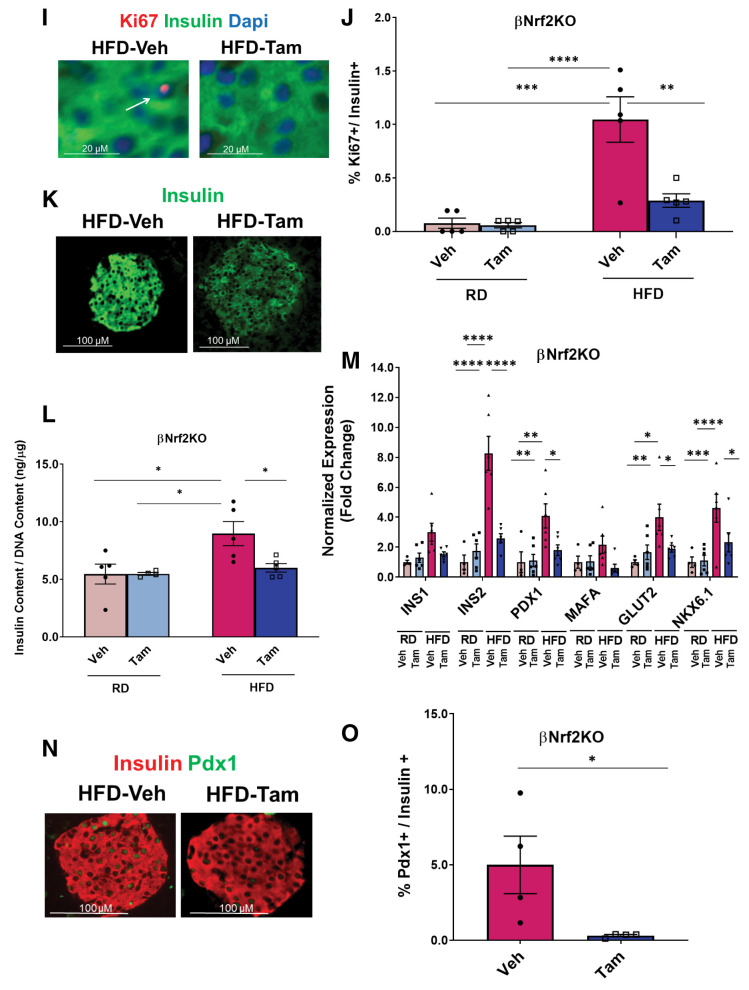
Nrf2 is necessary for HFD-adaptive β-cell proliferation in vivo. A and B: C57BL/6 mice were fed an HFD or RD for 1 week, after which their pancreata were removed, embedded, and immunolabeled with insulin and Nrf2 antibodies (inset magnification 3.5×). Percentage of Nrf2- and insulin-positive cells was calculated. C: Pancreata from C57BL/6 mice fed an RD or HFD for 1 week were immunolabeled with insulin and Nqo1 antibodies. D: MIP-CreERTAM/Nrf2lox/lox mice were crossed to generate βNrf2KO mice, which were injected daily for 5 days with Tam or vehicle (Veh) (corn oil), followed by 5 days of recovery and 1-week feeding with HFD or RD. E and F: Pancreata from βNrf2KO mice fed an HFD for 1 week immunolabeled with insulin and Nrf2 antibodies (inset magnification 7.5x). Percentage of Nrf2- and insulin-positive cells was calculated. G and H: Islet protein extracts from βNrf2KO mice fed an HFD for 1 week were immunoblotted against Nrf2 and actin antibodies. Nrf2 levels were then quantified using densitometry. I and J: Pancreata from βNrf2KO mice fed an RD or HFD for 1 week were immunolabeled with insulin and Ki67 antibodies. Percentage of proliferation in insulin-positive cells was then calculated. K: Pancreata from βNrf2KO mice fed an HFD or RD for 1 week were immunolabeled with insulin. L: Islets from βNrf2KO mice fed an RD or HFD for 1 week were assayed for insulin content normalized to DNA content. M: Islets were isolated from βNrf2KO mice fed an HFD or RD for 1 week, RNA was extracted, and expression of β-cell identity genes was measured. N and O: Pancreata from βNrf2KO mice fed an HFD for 1 week were immunolabeled with insulin and Pdx1 antibodies. Percentage of Pdx1 nuclear-positive/insulin-positive cells was calculated. Data are mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001.
To test the role of Nrf2 in HFD-mediated adaptive β-cell proliferation, we established a Tam-inducible β-cell–specific Nrf2 loss-of-function mouse model by crossing MIP-CreERTAM mice (13) with Nrf2lox/lox mice (14), named here βNrf2KO mice. βNrf2KO mice were injected with corn oil (vehicle) or Tam, followed by a recovery period after which they were fed for 1 week with RD or HFD (Fig. 2D). Immunostaining of the pancreata from HFD-fed βNrf2KO mice using Nrf2 and insulin antibodies (Fig. 2E and F) showed that Tam-injected, HFD-fed mice had a 60% reduction in Nrf2 levels in β-cells compared with vehicle control mice, confirming the deletion of Nrf2 exon 5 in β-cells. Immunoblotting verified this deletion at the protein level, showing a reduction of 3.2-fold compared with vehicle control (Fig. 2G and H).
Mice fed an HFD for 1 week have increased β-cell proliferation as an adaptive response to increased insulin demand (31). As expected, feeding control mice an HFD for 1 week increased β-cell proliferation eightfold compared with RD-fed mice (Fig. 2I and J). Similar results were observed in MIP-CreERTAM control mice (Supplementary Fig. 2B). However, this adaptive increase of β-cell proliferation was abolished in Tam-injected βNrf2KO mice (Fig. 2I and J), demonstrating the necessity of Nrf2 for HFD-stimulated β-cell proliferation.
Our results showed that reducing Nrf2 in β-cells under metabolic stress resulted in decreased insulin staining intensity compared with control (Fig. 1C, D, F, H, and I and Supplementary Fig. 3A and B), raising the possibility that Nrf2 depletion affects β-cell insulin content. To test this hypothesis, mouse Nrf2lox/lox islets were isolated and cultured in 20 mmol/L glucose for 48 h. Cells were harvested, and insulin content was measured. Cre-expressing Nrf2lox/lox islets had a 40% reduction in insulin content (Supplementary Fig. 3C). Concordantly, Tam-injected βNrf2KO mice, which were fed an HFD for 1 week, presented with a 35% decrease in insulin content compared with vehicle control mice (Fig. 2K and L). The same results were found using a different source of insulin antibody (data not shown). On the basis of these findings, we analyzed the expression of β-cell identity genes in βNrf2KO islets and found a significant reduction in the expression of Ins2, Pdx1, Glut2, and Nkx6.1 in Tam-injected βNrf2KO mice compared with vehicle control mice fed an HFD (Fig. 2M). Reduced levels of Pdx1 were also observed in pancreata after depletion of Nrf2 (Fig. 2N and O). Similar to βNrf2KO, in Nrf2lox/lox islets, a reduction in Pdx1 expression was observed in Cre-expressing islets compared with control (Supplementary Fig. 3D). Thus, deletion of Nrf2 in β-cells under metabolic stress leads to decreased insulin expression and content.
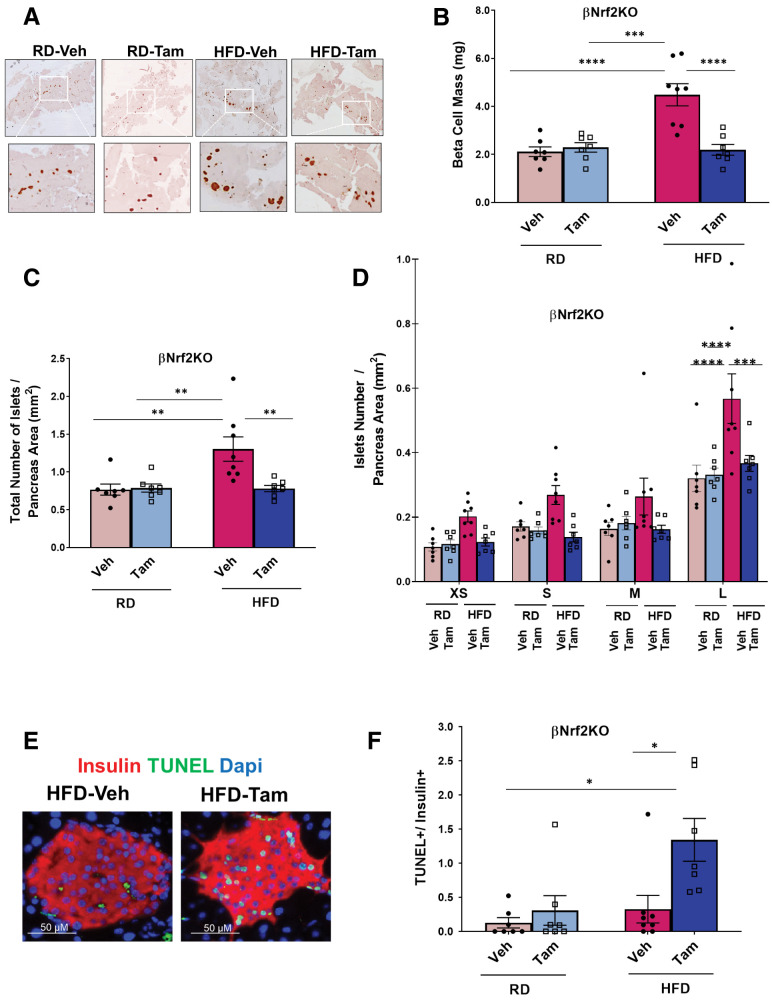
Adaptive proliferation in response to an HFD leads to significantly increased β-cell mass after 2–4 weeks (32). To test whether depletion of Nrf2 affects adaptive β-cell mass expansion, vehicle or Tam-injected βNrf2KO mice were placed on an HFD or RD for 29 days, and β-cell mass was measured. Depletion of Nrf2 impaired adaptive expansion of β-cell mass (Fig. 3A and B). Furthermore, Tam-injected βNrf2KO mice did not display an increase in total islet number (1.7-fold) seen when control mice were fed an HFD (Fig. 3C). Moreover, Nrf2-depleted HFD-fed mice displayed a 35% decrease in the largest islets, with no change in the number of smaller islets, compared with control mice (Fig. 3D). Thus, Nrf2 is required for the expansion of larger islets after an HFD.
Figure 3.
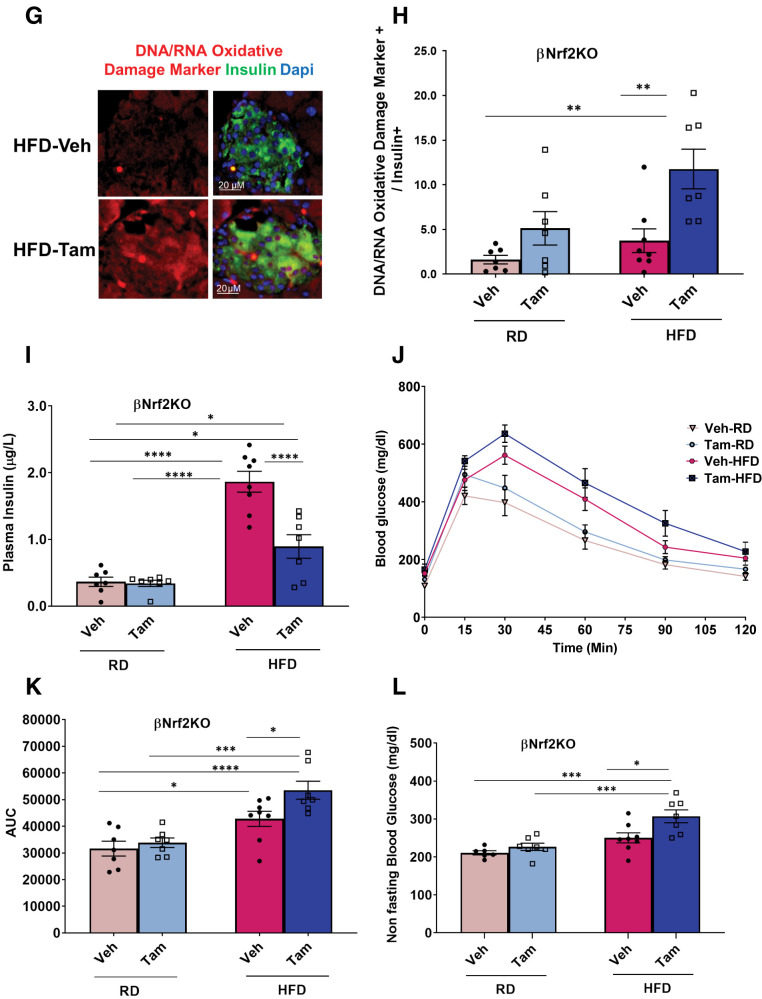
Nrf2 is necessary for HFD-mediated β-cell mass expansion. Pancreata from βNrf2KO mice fed an RD or HFD for 29 days were immunolabeled for insulin. A and B: β-Cell mass and islet morphometry were determined (magnification 10×, inset 30×). C: Total islet number per pancreas area. D: Islet size was divided into four groups, including extrasmall (XS) (<1,000 μm2), small (S) (1,001–2,200 μm2), medium (M) (2,201–4,400 μm2), and large (L) (>4,400 μm2), and islet numbers per pancreas area were calculated. E and F: Percentage of apoptotic insulin-positive cells was calculated using a TUNEL assay in pancreata from HFD-fed βNrf2KO mice. G and H: Pancreata from βNrf2KO mice were immunolabeled for insulin and DNA/RNA oxidative damage markers. DNA/RNA oxidative damage marker–positive/insulin-positive cells were calculated. I: Plasma insulin was measured from βNrf2KO mice after 29 days of HFD or RD feeding. J and K: Intraperitoneal glucose tolerance test performed in βNrf2KO mice after an overnight fast. Area under the curve (AUC) was calculated. L: Nonfasting blood glucose. Data are mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001. Veh, vehicle.
Nrf2 promotes β-cell survival under various stresses (8). Therefore, we investigated whether Nrf2 loss of function attenuates HFD-adaptive β-cell expansion by decreasing β-cell survival. Tam-injected βNrf2KO mice on an HFD had 18.8-fold more TUNEL-positive β-cells compared with control mice on an HFD (Fig. 3E and F). In addition, immunostaining using an antibody against the DNA/RNA oxidative damage marker showed a 6.2-fold increase in oxidative stress in β-cells depleted of Nrf2 compared with control mice fed an HFD (Fig. 3G and H). Thus, the presence of Nrf2 protects β-cells from the increased oxidative stress and cell death elicited by an HFD.
Vehicle control βNrf2KO mice fed an HFD had increased plasma insulin levels (5.1-fold) compared with mice fed an RD (Fig. 3I). However, the increase in insulin levels was attenuated in HFD-fed Nrf2-depleted βNrf2KO mice. Accordingly, a significant reduction in glucose tolerance (Fig. 3J and K) and an increase in nonfasting blood glucose levels (Fig. 3L) were observed in Tam-injected mice compared with control mice fed an HFD. No significant changes in body weight or fasting blood glucose were observed in Nrf2-depleted mice compared with control mice on an HFD (Supplementary Fig. 4A and B). No differences were observed in GSIS, suggesting that β-cell function of HFD-fed Nrf2-depleted βNrf2KO mice was not hampered (Supplementary Fig. 4C). Additionally, no significant changes in insulin sensitivity were observed (Supplementary Fig. 4D and E), indicating that the effects on glucose tolerance were not due to defective insulin signaling. Thus, Nrf2 is necessary for adaptive expansion of β-cell mass, which is necessary for maintaining glucose homeostasis after an HFD.
Nrf2 Gain of Function Increases β-Cell Proliferation Ex Vivo
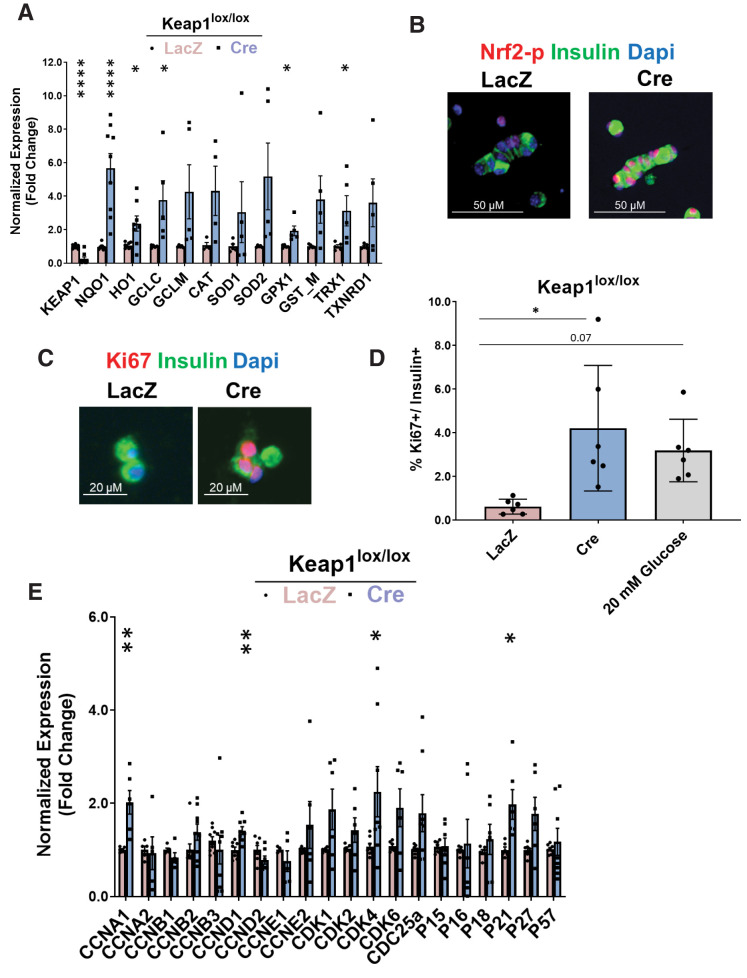
We tested whether Nrf2 gain of function stimulates β-cell proliferation. Under nonstressful conditions, Nrf2 levels are low through interaction with Keap1, which targets Nrf2 to proteosomal degradation (33). Therefore, to increase Nrf2 levels, we used Keap1lox/lox mice in which LoxP sites flank Keap1 exons 2 and 3. In this model, Cre-mediated recombination results in a nonfunctional Keap1 protein (15). Islets from Keap1lox/lox were isolated, cultured in 5.5 mmol/L glucose, and transduced with adenoviruses expressing either LacZ (control) or Cre recombinase. Cre-expressing islets had a 90% decrease in Keap1 RNA expression concomitant with increased expression of Nrf2 target genes (Fig. 4A) and increased levels of nuclear Nrf2-p (Fig. 4B), consistent with Nrf2 activation. The same islets displayed a nearly sevenfold increase in β-cell proliferation, comparable to treatment with 20 mmol/L glucose (Fig. 4C and D).
Figure 4.
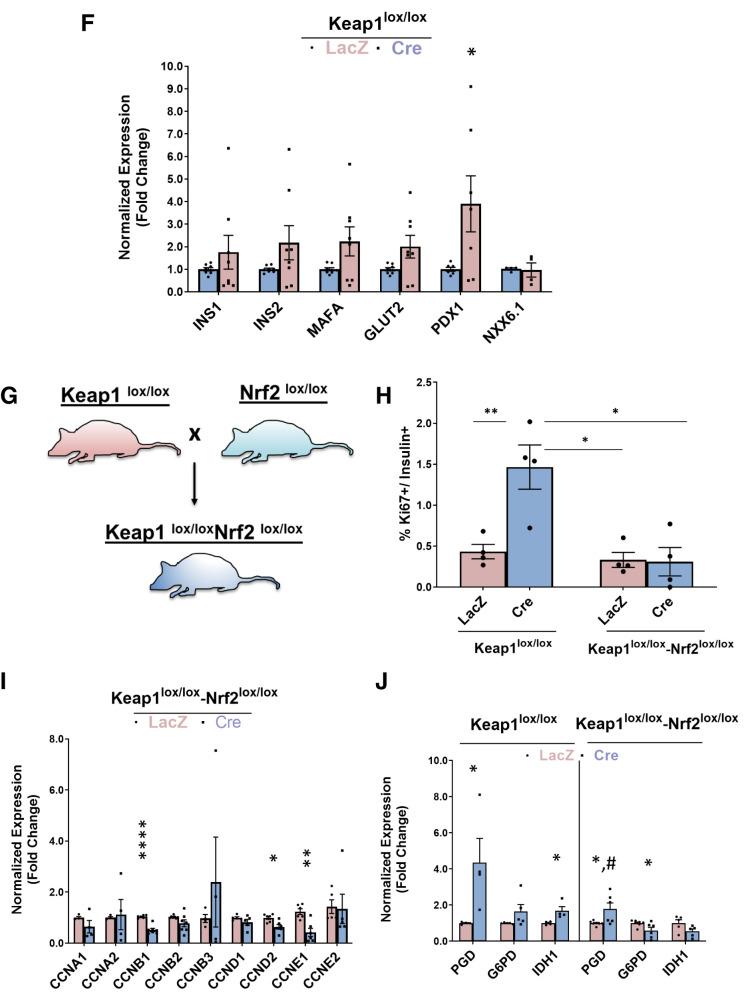
Genetic Nrf2 gain of function increases β-cell proliferation ex vivo. Dispersed Keap1lox/lox islets were transduced with Cre- or LacZ-expressing adenoviruses. A: After 72 h, RNA was isolated, and mRNA for Keap1, Nrf2, and known Nrf2 target genes were measured. B and C: Keap1lox/lox islets were transduced with LacZ- or Cre-expressing adenoviruses and immunolabeled with insulin and Nrf2-p or insulin and Ki67 antibodies. D: Percentage of proliferating insulin-positive cells was calculated. E and F: Keap1lox/lox islets were isolated and transduced with Cre or LacZ adenoviruses. After 24 h, RNA was extracted, and the expression of cell-cycle regulators or β-cell identity genes was measured. G: Keap1lox/lox mice were crossed with Nrf2lox/lox to make double-KO Keap1lox/lox-Nrf2lox/lox mice. H–J: Keap1lox/lox-Nrf2lox/lox or Keap1lox/lox islets were isolated, transduced with Cre or LacZ adenoviruses, and stained for insulin and Ki67 (H) or were extracted for RNA and measured for expression of cyclins (I) and Nrf2 target gene (J) that produce NADPH. Percentage of Ki67-positive and insulin-positive cells was calculated. Data are mean ± SEM. *P < 0.05, **P < 0.005, ****P < 0.0001 compared with LacZ controls; #P < 0.0001 Cre-treated Keap1lox/lox-Nrf2lox/lox compared with Cre-treated Keap1lox/lox.
To investigate further, RNA was extracted from LacZ- or Cre-transduced Keap1lox/lox islets, and expression of cell-cycle regulators was measured. Keap1 deletion significantly increased expression of cyclins A1, D1, and Cdk4, in concordance with increased β-cell proliferation. Keap1 deletion also significantly increased expression of the cell-cycle inhibitor p21 (Fig. 4F), which under certain conditions promotes cell proliferation (34). Additionally, Keap1 deletion resulted in increased Pdx1 expression (Fig. 4F).
Although Keap1 is the canonical inhibitor of Nrf2, several other proteins bind Keap1 (35–38). Therefore, to confirm that Nrf2 activation was Keap1 specific, we generated double-KO mice (Keap1lox/lox-Nrf2lox/lox) in which both Keap1 and Nrf2 are deleted by Cre recombinase (Fig. 4G). As expected, deletion of Keap1 resulted in increased β-cell proliferation (4.1-fold), but there was no increased proliferation when both Keap1 and Nrf2 were deleted (Fig. 4H). Additionally, the increase of cyclins A1 and D1 seen in the Cre-transduced Keap1lox/lox islets was blunted after the additional deletion of Nrf2 and was accompanied by a significant reduction in expression of cyclins B1, D2, and E1 (Fig. 4I). Thus, deletion of Keap1 increases β-cell proliferation because of activation of the Nrf2 pathway.
In cancer cells, Nrf2 supports cell proliferation by inducing genes that produce NADPH and drive purine nucleotide synthesis, both needed for dividing cells (39–41). These genes include glucose-6-phosphate dehydrogenase (G6pd) and phosphogluconate dehydrogenase (Pgd) of the pentose phosphate pathway, and isocitrate dehydrogenase 1 (Idh1). We found that depletion of Keap1 increased expression of Pgd (4.3-fold) and Idh1 (1.6-fold), which was significantly lower when both Keap1 and Nrf2 were deleted (Fig. 4J). Taken together, these results suggest that Nrf2 supports β-cell proliferation in part by increasing NADPH levels.
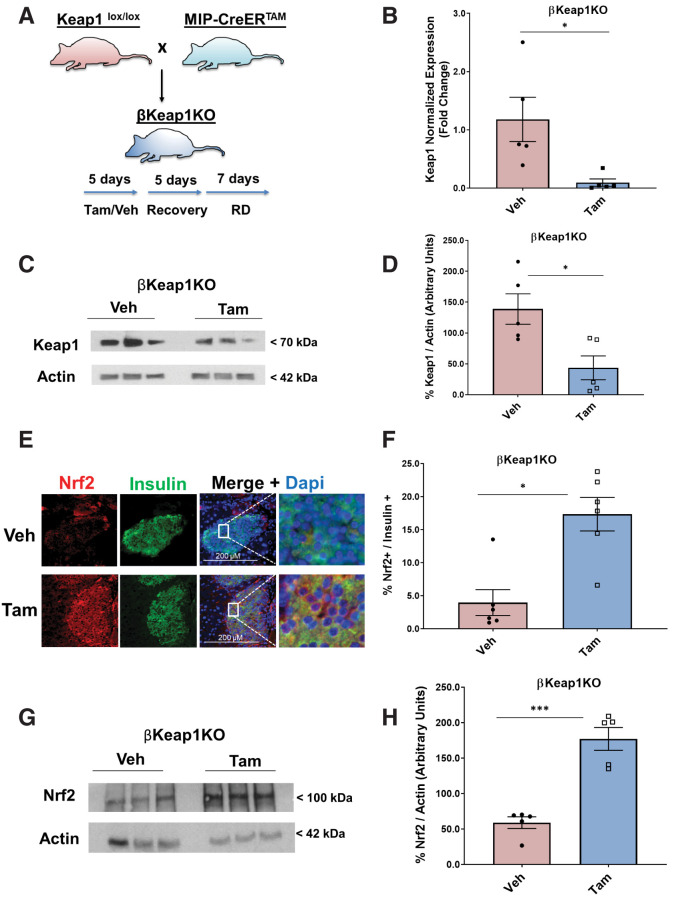
Nrf2 Gain of Function Increases β-Cell Mass In Vivo
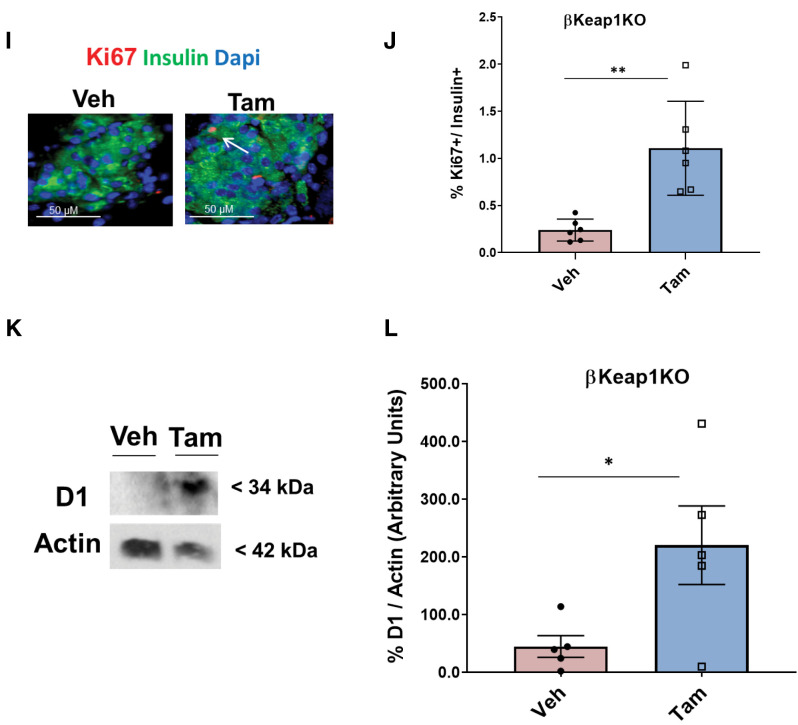
Conditional β-cell–specific Keap1 KO mice were generated by crossing MIP-CreERTAM mice with Keaplox/lox mice to obtain βKeap1KO mice (13,15) (Fig. 5A). Deletion of Keap1 in Tam-injected mice resulted in a 92% decrease of Keap1 mRNA and (Fig. 5B) and 68% decrease in Keap1 protein levels (Fig. 5C and D). In contrast, Keap1 deletion led to a 4.4-fold increase in β-cell Nrf2 immunostaining levels (Fig. 5E and F) and a threefold increase in Nrf2 protein levels (Fig. 5G and H) compared with control mice. Additionally, Tam-injected βKeap1KO mice had a 4.6-fold increase in β-cell proliferation compared with control mice (Fig. 5I and J) and 4.9-fold increase in cyclin D1 protein levels (Fig. 5K and L). Thus, increasing Nrf2 abundance stimulates β-cell proliferation both ex vivo and in vivo.
Figure 5.
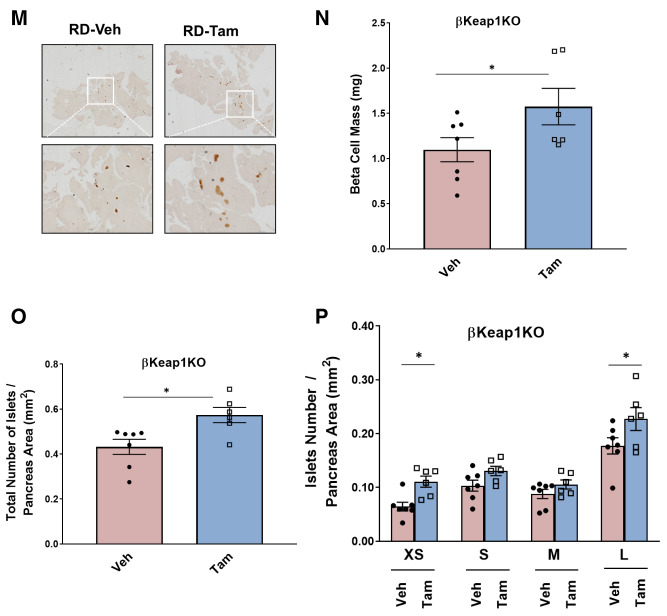
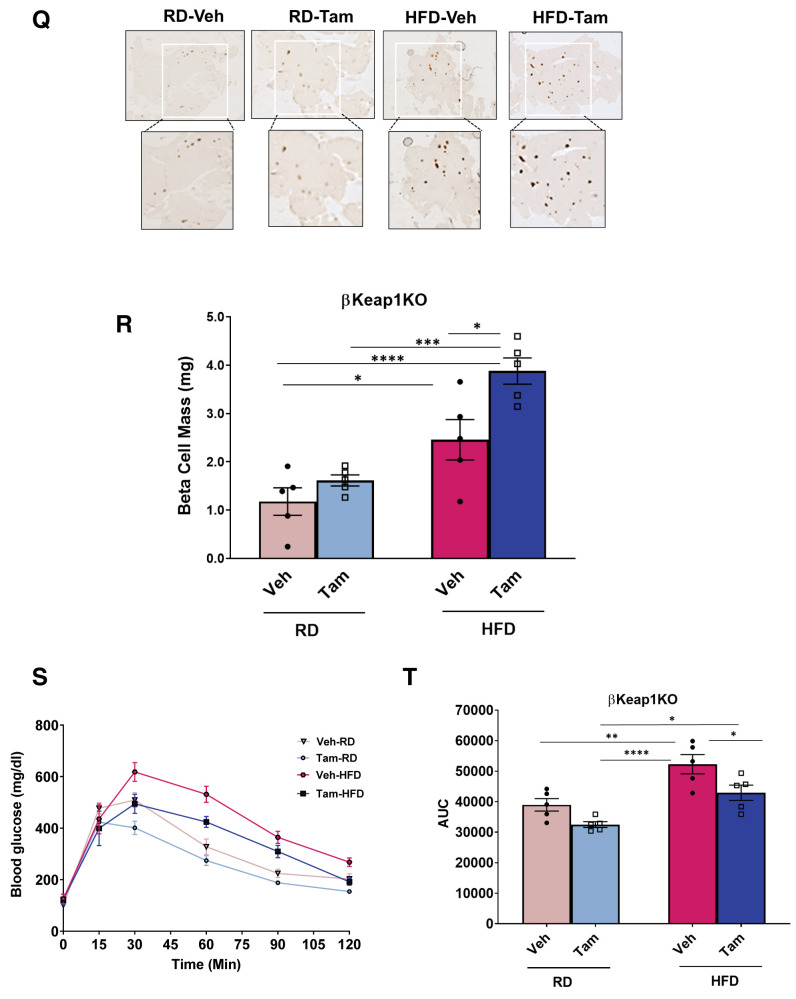
Genetic Nrf2 gain of function increased β-cell proliferation and mass in vivo. A: MIP-CreERTAM and Keap1lox/lox mice were crossed to generate βKeap1KO mice, which were injected daily for 5 days with Tam or vehicle (Veh) (corn oil), followed by 5 days of recovery and 1 week on an RD. B: Islets were isolated from βKeap1KO mice, RNA was extracted, and Keap1 expression was measured. C and D: Islet protein extracts from βKeap1KO mice were immunoblotted against Keap1 and actin antibodies. Keap1 levels were then quantified using densitometry. E and F: Pancreata from βKeap1KO mice were removed, embedded, and immunolabeled with insulin and Nrf2 antibodies (inset magnification 7.5×). Percentage of Nrf2-positive/insulin-positive cells was calculated. G and H: Islet protein extracts from βKeap1KO mice were immunoblotted against Nrf2 and actin antibodies. Nrf2 levels were then quantified using densitometry. I and J: Pancreata from βKeap1KO were immunolabeled with insulin and Ki67 antibodies, and the percentage of cells positive for both Ki67 and insulin was calculated. K and L: Islet protein extracts from βKeap1KO mice were immunoblotted against cyclin D1 and actin antibodies. Cyclin D1 levels were then quantified using densitometry. M–P: βKeap1KO pancreata from Tam- or Veh-treated mice were immunolabeled with insulin. β-Cell mass and islet morphometry (total islet numbers per pancreatic area) were determined (magnifications 10×, inset 50×). Q and R: βKeap1KO mice were fed an RD or HFD for 29 days. Their pancreata were immunolabeled for insulin, and β-cell mass was calculated (magnification 10×, inset 15×). S and T: Intraperitoneal glucose tolerance testing was performed in βKeap1KO mice fed an RD or HFD for 29 days after an overnight fast. Area under the curve (AUC) was calculated. Data are mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001. L, large; M, medium; S, small; XS, extrasmall.
To test whether Nrf2 gain of function in vivo increases β-cell mass, pancreata from βKeap1KO mice were immunostained with an insulin antibody to perform β-cell histomorphometry. Deletion of Keap1 for 1 month resulted in increased β-cell mass (1.5-fold), increased total number of islets (1.3-fold), and increased numbers of extrasmall (1.7-fold) and large (1.3-fold) islets (Fig. 5M–P). These findings indicate that Nrf2 gain of function increases β-cell mass by increasing β-cell proliferation and increasing the number of islets.
To test whether Nrf2 gain of function in vivo improves glucose homeostasis after an HFD, βKeap1KO mice were placed on an HFD or RD for 29 days, and β-cell mass was measured. Deletion of Keap1 significantly increased the expansion of β-cell mass in mice fed an HFD (1.6-fold) (Fig. 5Q and R). Furthermore, Tam-injected βKeap1KO mice displayed increased glucose tolerance (Fig. 5S and T). No differences were observed in blood glucose, plasma insulin, body weight, or insulin sensitivity (Supplementary Fig. 5A–F). These findings indicate that by increasing β-cell mass, Nrf2 gain of function improves glucose tolerance after an HFD.
Pharmacological Stimulation of Nrf2 Increases β-Cell Proliferation In Vitro and In Vivo
The synthetic triterpenoid CDDO-Me disrupts Keap1:Nrf2 interaction and activates the Nrf2 antioxidant pathway (42). Interestingly, CDDO-Me time and dose dependently increased INS-1 832/13 cell number compared with vehicle control (Supplementary Fig. 6A). Additionally, 20 nmol/L CDDO-Me increased Nrf2-p immunostaining in INS-1 832/13 cells after 50 min of incubation (Supplementary Fig. 6B) and increased the expression of Nrf2 target genes in primary C57BL/6 mouse islet cells (Supplementary Fig. 6C), validating Nrf2 activation under these conditions. Interestingly, similar to Keap1 deletion, addition of CDDO-Me to primary C57BL/6 mouse islets increased the expression of the β-cell identity gene Pdx1 (Supplementary Fig. 6D).
These findings prompted us to test whether pharmacological activators of Nrf2 would stimulate primary murine and human β-cell proliferation. Like CDDO-Me, the broccoli-extracted isothiocyanate SFN is also an Nrf2 activator that targets Keap1 (43). Treatment with either 20 nmol/L CDDO-Me or 20 nmol/L SFN in 5.5 mmol/L glucose increased mouse β-cell proliferation compared with vehicle control (6.5- and 5.1-fold, respectively) to levels comparable to high (20 mmol/L) glucose (Fig. 6A and B). Moreover, deletion of Nrf2 in Nrf2lox/lox mouse islets blocked CDDO-Me– and SFN-stimulated β-cell proliferation (Fig. 6C), indicating that these compounds stimulate β-cell proliferation by activation of Nrf2. Importantly, cadaveric human islets treated with either 20 nmol/L CDDO-Me or 20 nmol/L SFN also displayed increased β-cell proliferation (6.4- and 6.6-fold, respectively), similar to high glucose (Fig. 6D and E). CDDO-Me and SFN at these concentrations did not induce human β-cell death, as seen by TUNEL assay (Fig. 6F).
Figure 6.
Pharmacological gain of Nrf2 function increases mouse and human β-cell proliferation. A and B: C57BL/6 mouse islets were isolated, dispersed, and incubated with increasing doses of CDDO-Me or SFN for 72 h, followed by immunostaining with insulin and Ki67 antibodies. Percentage of Ki67 and insulin double-positive cells were calculated. Islet cells treated with 20 mmol/L glucose were used as a positive control. C: Dispersed Nrf2lox/lox mouse islets were incubated with 20 nmol/L CDDO-Me or 20 nmol/L SFN for 72 h, followed by immunostaining using insulin and Ki67 antibodies. Percentage of proliferation in insulin-positive cells was calculated. D and E: Human islets were incubated with 20 nmol/L CDDO-Me or 20 nmol/L SFN for 72 h, followed by immunostaining with insulin and Ki67 antibodies. Percentage of insulin- and Ki67-positive cells was calculated. F: A TUNEL assay was performed on human islets treated as shown. Percentage of cells positive for both TUNEL and insulin was calculated. G: Immunodeficient mice were transplanted with 500 human islets under the kidney capsule. After a 17-day recovery, daily intraperitoneal injections were made with the indicated doses of CDDO-Me for 1 week. H–K: The pancreata (H and I) and kidney grafts (J and K) were immunostained with insulin and Ki67. Percentage of insulin- and Ki67-positive cells was calculated. Data are mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001.
We next tested the effect of a systemic in vivo administration of CDDO-Me on both mouse and human β-cell proliferation. For this purpose, euglycemic immunodeficient mice were transplanted with 500 human islets under the kidney capsule. After 17 days of recovery, mice were injected intraperitoneally daily with increasing doses of CDDO-Me for 7 days, after which mouse pancreata and kidneys containing the grafts were harvested (Fig. 6G). Daily injection with 3 mg/kg CDDO-Me stimulated mouse β-cell proliferation (29.5-fold) (Fig. 6H and I). More importantly, daily injection with 1 mg/kg CDDO-Me significantly and markedly stimulated human β-cell proliferation (3.9-fold) compared with vehicle-treated transplanted mice (Fig. 6J and K). These concentrations of CDDO-Me did not induce human β-cell death as seen by TUNEL assay (Supplementary Fig. 6E). Thus, pharmacological activation of Nrf2 leads to rodent and human β-cell proliferation in vivo.
Discussion
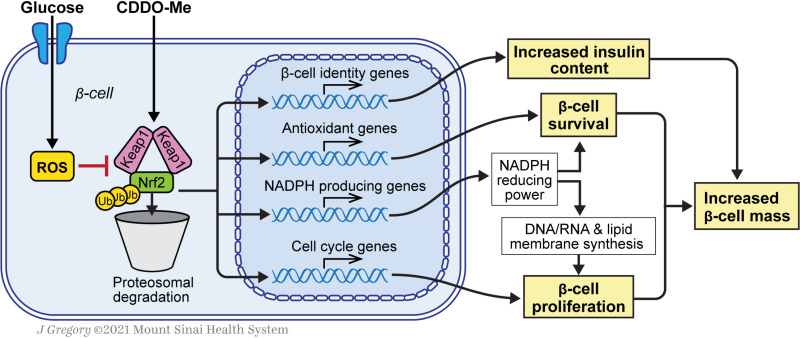
Nrf2 is a master regulator of transcriptional programs designed to protect cells from oxidative stress. Whereas it is well established that activation of the Nrf2 pathway protects β-cells from a variety of metabolic stresses (8), here we add to these observations by demonstrating a pivotal role for Nrf2 in the regulation of β-cell mass. Our study reveals a number of novel findings, including that 1) glucose rapidly activates Nrf2 in INS1 cells and primary β-cells; 2) Nrf2 is necessary for glucose-stimulated β-cell proliferation and for adaptive β-cell proliferation, adaptive expansion of β-cell mass, and protection from cell death in mice fed an HFD; 3) Nrf2 is necessary to maintain insulin content; and 4) activation of Nrf2 is sufficient to increase β-cell proliferation and β-cell mass and improve glucose tolerance after an HFD in mice and human β-cell proliferation in vitro and in vivo. Thus, our data support the notion that Nrf2 is crucial for the regulation of β-cell mass (Fig. 7).
Figure 7.
Nrf2 increases β-cell mass by increasing survival and promoting β-cell proliferation. Glucose metabolism generates ROS in β-cells, which changes Keap1 conformation, decreases Nrf2:Keap1 interaction and proteolytic degradation, and increases Nrf2 abundance and transcriptional activity. CDDO-Me also activates Nrf2 by inhibiting Keap1. Nrf2 target genes include antioxidant enzymes and enzymes that produce NADPH, which is used by antioxidant enzymes to protect β-cells from ROS and as reducing equivalents for synthesis of RNA, DNA, and membrane in proliferating cells. Activation of Nrf2 is also associated with increased expression of cell-cycle regulators (mainly cyclin D1) and increased insulin content. Thus, the combined actions of Nrf2, protection from apoptosis, and increased proliferation lead to increased β-cell mass. Ub, ubiquitin. Reprinted with permission from J. Gregory (Mount Sinai Health System).
We found that exposure of β-cells to high glucose results in increased Nrf2 nuclear localization and transcriptional activation. Thus, Nrf2 joins a growing list of glucose-responsive transcription factors in β-cells that increase transactivation capacity in response to increased glucose metabolism, including ChREBP, MondoA, and Myc (44). ChREBP and MondoA are activated by binding metabolites of glycolysis, likely glucose-6-phosphate (45), and Myc is stabilized by phosphorylation and phosphatase cascades initiated from glucose metabolism (44). Nrf2 is activated by increased production of ROS, which is generated mostly in the mitochondria as a by-product of increased glucose metabolism (8). Bollong et al. (46) found that methylglyoxal, a metabolite of glycolysis, forms covalent modifications on Keap1 cysteine C151, leading to dimerization and Nrf2 activation. We cannot rule out methylglyoxal as a mediator of Nrf2 activation in β-cells. However, methylglyoxal itself can also generate ROS in β-cells (47). Nonetheless, increased glycolysis activates Nrf2.
By use of a β-cell–specific conditional KO model, we found that Nrf2 is necessary for glucose-stimulated β-cell proliferation in vitro and for adaptive proliferation and expansion of β-cell mass in response to an HFD in vivo. These results are consistent with and extend our previous results demonstrating that Nrf2 is activated in response to overexpression of ChREBPα, leading to increased anabolic metabolism, mitochondrial activity, and glucose-stimulated β-cell proliferation (12). Adaptive β-cell proliferative expansion is a result of a sustained, increased demand for insulin, resulting in increased metabolic workload (48). As a master transcriptional regulator of redox balance, Nrf2 is uniquely positioned to support β-cell proliferation. Target genes of Nrf2 include antioxidant enzymes that protect β-cells from the increased oxidative stress brought about by increased mitochondrial activity and anabolic metabolism necessary for manufacturing the biomass needed to create daughter cells in the process of cell division (8). It follows that we found an increase in the expression of antioxidant enzyme genes and genes that produce NADPH, providing reducing power for the antioxidant enzymes and for producing nucleotide and membrane phospholipid synthesis (40,49). Thus, Nrf2 promotes adaptive proliferation by orchestrating programs of gene expression that support the anabolic workload of dividing cells as well as providing protection from the oxidative stress derived from a hypercaloric diet and cellular replication.
Chronic exposure of β-cells to high levels of ROS results in β-cell malfunction and apoptotic cell death (8). Several researchers have found that activation of Nrf2 protects or reverses damage caused by oxidative stress in β-cells (8). For example, Abebe et al. (30) placed female fatty Zucker rats on an HFD for 9 days, resulting in robust oxidative damage and the beginnings of β-cell failure and dysregulated glucose homeostasis. By returning the animals to a standard chow diet, the β-cells repaired themselves, with evidence of a sharp upregulation and expression of Nrf2 and its antioxidant target genes. Similarly, Yagishita et al. (50) used a β-cell–specific inducible nitric oxide synthase 2–expressing mouse model to create oxidative stress, resulting in β-cell dysfunction and glucose intolerance. Activation of Nrf2 by deletion of Keap1 in a β-cell–specific manner reversed the phenotype. Furthermore, the same group found that a global Keap1+/− mouse crossed to db/db diabetic mice or administration of CDDO-Im, an Nrf2 activator, attenuates diabetes in db/db mice (25). In concordance with these studies, we found that β-cell–specific depletion of Nrf2 in mice fed an HFD resulted in increased oxidative stress and apoptosis of β-cells. Thus, Nrf2 defends β-cells from the oxidative damage caused by metabolic stress in a variety of model systems.
Interestingly, in agreement with global Nrf2 KO experiments (25), we found that depletion of Nrf2 activity in β-cells reduced insulin immunostaining and insulin content. Our findings correlated with decreased mRNA expression of Pdx1 and decreased immunostaining of Pdx1 in pancreata from β-cell–specific Nrf2 KO mice. Conversely, both Keap1 deletion and CDDO-Me resulted in increased expression of Pdx1. Although the β-cell identity transcription factor Pdx1 is not known to be a direct Nrf2 target, its level and binding activity to the insulin promoter, and therefore subsequent insulin production, are impaired in hyperglycemic conditions, presumably as a result of oxidative stress and ROS generation (6,51–53). Apart from Pdx1, Nrf2 deletion also contributed to reduced mRNA expression of other β-cell identity genes, such as Ins2, Glut2, and Nkx6.1. Similar to Pdx1, these factors are highly sensitive to any changes in redox balance that can reduce their levels and activity (4,52) Thus, the contribution of Nrf2 activity toward building β-cell mass includes maintaining insulin content.
We found that increasing Nrf2 activity by conditionally deleting the Nrf2 inhibitor Keap1 or with the Nrf2 activator CDDO-Me was sufficient to increase rodent and human β-cell proliferation both in vitro and in vivo in this study and others (12). In the Keap1 KO mouse model, we found that 1 month of Nrf2 activation significantly increased β-cell mass and improved glucose tolerance. Histomorphometry analysis revealed significant increases in the largest and smallest quartile of islets. Since the formation of new islets (neogenesis) from pancreatic progenitor cells results in small islets (54), we cannot exclude neogenesis as another mechanism by which Nrf2 promotes β-cell expansion. Lineage-tracing experiments are needed to determine this possibility.
We found that a week-long treatment with CDDO-Me increased both rodent and human β-cell proliferation in the same model of human islet transplantation. This raises the exciting possibility of Nrf2 activators as therapeutic agents for expanding β-cell mass in patients with diabetes. Indeed, treatment of streptozotocin-induced diabetic nude mice with a CDDO-Me derivative, dh404, resulted in lower prevalence of diabetes after islet transplantation (26,55). However, in those studies, the authors did not analyze whether the CDDO derivative could stimulate human β-cell proliferation. CDDO-Me is a synthetic oleanane triterpenoid and a member of the bardoxolone family of drugs, which have been investigated in several clinical trials, including chronic kidney disease in patients with diabetes (8). Although some clinical trials were terminated earlier than expected because of cardiovascular safety concerns, a new phase 2 clinical trial with CDDO-Me has recently been started and excludes at-risk patients to better define the safety and efficacy of CDDO-Me (56,57). Compounds of this drug family increase islet viability, preserve β-cell numbers, improve β-cell insulin content and insulin secretion, accelerate macroautophagy, protect β-cells from oxidative stress, and reduce secretion of proinflammatory cytokines (8), all of which can result in preservation of functional β-cell mass. Our results add β-cell proliferation as a property of Nrf2 activation by CDDO-Me and SFN. Although this effect was examined in β-cells, it is also possible that proliferation in other islet cell types, or throughout the body, might be increased by CDDO-Me. More studies are needed to ensure the safety of systemic Nrf2 activators, and efforts should be made to design β-cell–targeted Nrf2 activators for increasing β-cell mass in patients with diabetes.
In summary, Nrf2 is necessary for maintaining β-cell redox balance and survival and is sufficient when activated to drive rodent and human β-cell proliferation. Thus, Nrf2, by managing cellular redox levels, is a central regulator of β-cell mass, making this pathway a promising candidate for the development of future therapeutics for the treatment of diabetes.
Article Information
Acknowledgments. The authors thank the Developmental Studies Hybridoma Bank, Department of Biology, The University of Iowa (Iowa City, IA), for providing human insulin antibody. The authors also thank the Icahn School of Medicine at Mount Sinai Microscopy Core and the Einstein/Sinai Diabetes Center Human Islet and Adenovirus Core. The authors thank Dr. Geming Lu and Jiamin Zhang (Icahn School of Medicine at Mount Sinai) for help with immunoblotting assays.
Funding. This study was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants DK114338 (to D.K.S.), DK020541, DK105015, and DK126450 (to A.G.-O.) and a Mindich Child Health and Development Institute Pilot and Feasibility Grant, Mindich Postdoctoral Pilot Award (to S.B.-A.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.B.-A., L.S.K., G.B., C.J.-P., Y.L., and I.T. contributed to the investigation. S.B.-A., S.B., A.G.-O., and D.K.S. contributed to the review and editing of the manuscript. S.B.-A., A.G.-O., and D.K.S. acquired funding. S.B.-A and D.K.S. conceptualized the study and wrote the original draft of the manuscript. S.B., A.G.-O., and D.K.S. contributed resources. A.G.-O. and D.K.S. provided supervision. D.K.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19182224.
References
- 1. Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab 2018;27:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal 2017;26:501–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rojas J, Bermudez V, Palmar J, et al. Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J Diabetes Res 2018;2018:9601801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med Cell Longev 2020;2020:8609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mullarky E, Cantley LC. Diverting glycolysis to combat oxidative stress. In Innovative Medicine: Basic Research and Development. Nakao K, Minato N, Uemoto S, Eds. Tokyo, Springer, 2015, pp. 3–23 [PubMed] [Google Scholar]
- 6. Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol 2006;6:615–619 [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Wang H. Oxidative stress in pancreatic beta cell regeneration. Oxid Med Cell Longev 2017;2017:1930261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baumel-Alterzon S, Katz LS, Brill G, Garcia-Ocana A, Scott DK: Nrf2: the master and captain of beta cell fate. Trends Endocrinol Metab 2021;32:7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiménez-Osorio AS, González-Reyes S, García-Niño WR, et al. Association of nuclear factor-erythroid 2-related factor 2, thioredoxin interacting protein, and heme oxygenase-1 gene polymorphisms with diabetes and obesity in Mexican patients. Oxid Med Cell Longev 2016;2016:7367641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matana A, Ziros PG, Chartoumpekis DV, et al. Rare and common genetic variations in the Keap1/Nrf2 antioxidant response pathway impact thyroglobulin gene expression and circulating levels, respectively. Biochem Pharmacol 2020;173:113605. [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Chen H, Liu J, et al. Association between the NF-E2 related factor 2 gene polymorphism and oxidative stress, anti-oxidative status, and newly-diagnosed type 2 diabetes mellitus in a Chinese population. Int J Mol Sci 2015;16:16483–16496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar A, Katz LS, Schulz AM, et al. Activation of Nrf2 is required for normal and ChREBPα-augmented glucose-stimulated β-cell proliferation. Diabetes 2018;67:1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 2010;59:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reddy NM, Potteti HR, Mariani TJ, Biswal S, Reddy SP. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am J Respir Cell Mol Biol 2011;45:1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blake DJ, Singh A, Kombairaju P, et al. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am J Respir Cell Mol Biol 2010;42:524–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocaña A. Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes 2005;54:2090–2102 [DOI] [PubMed] [Google Scholar]
- 17. Lakshmipathi J, Alvarez-Perez JC, Rosselot C, et al. PKCζ is essential for pancreatic β-cell replication during insulin resistance by regulating mTOR and cyclin-D2. Diabetes 2016;65:1283–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang P, Alvarez-Perez JC, Felsenfeld DP, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 2015;21:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crean D, Felice L, Taylor CT, Rabb H, Jennings P, Leonard MO. Glucose reintroduction triggers the activation of Nrf2 during experimental ischemia reperfusion. Mol Cell Biochem 2012;366:231–238 [DOI] [PubMed] [Google Scholar]
- 20. Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 2010;59:850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal 2018;29:1727–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Theodore M, Kawai Y, Yang J, et al. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J Biol Chem 2008;283:8984–8994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol 2010;647:37–74 [DOI] [PubMed] [Google Scholar]
- 24. Li W, Yu SW, Kong AN. Nrf2 possesses a redox-sensitive nuclear exporting signal in the Neh5 transactivation domain. J Biol Chem 2006;281:27251–27263 [DOI] [PubMed] [Google Scholar]
- 25. Uruno A, Furusawa Y, Yagishita Y, et al. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 2013;33:2996–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masuda Y, Vaziri ND, Li S, et al. The effect of Nrf2 pathway activation on human pancreatic islet cells. PLoS One 2015;10:e0131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harder B, Tian W, La Clair JJ, et al. Brusatol overcomes chemoresistance through inhibition of protein translation. Mol Carcinog 2017;56:1493–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazumder S, DuPree EL, Almasan A. A dual role of cyclin E in cell proliferation and apoptosis may provide a target for cancer therapy. Curr Cancer Drug Targets 2004;4:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 2013;140:3079–3093 [DOI] [PubMed] [Google Scholar]
- 30. Abebe T, Mahadevan J, Bogachus L, et al. Nrf2/antioxidant pathway mediates β cell self-repair after damage by high-fat diet-induced oxidative stress. JCI Insight 2017;2:e92854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am J Physiol Endocrinol Metab 2013;305:E149–E159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dinkova-Kostova AT, Kostov RV, Canning P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch Biochem Biophys 2017;617:84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kreis NN, Louwen F, Yuan J. The multifaceted p21 (Cip1/Waf1/CDKN1A) in cell differentiation, migration and cancer therapy. Cancers (Basel) 2019;11:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamberg N, Tahk S, Koit S, et al. Keap1-MCM3 interaction is a potential coordinator of molecular machineries of antioxidant response and genomic DNA replication in metazoa. Sci Rep 2018;8:12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wan ZH, Jiang TY, Shi YY, et al. RPB5-mediating protein promotes cholangiocarcinoma tumorigenesis and drug resistance by competing with NRF2 for KEAP1 binding. Hepatology 2020;71:2005–2022 [DOI] [PubMed] [Google Scholar]
- 37. Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 2010;12:213–223 [DOI] [PubMed] [Google Scholar]
- 38. Lo SC, Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp Cell Res 2008;314:1789–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hayes JD, Ashford ML. Nrf2 orchestrates fuel partitioning for cell proliferation. Cell Metab 2012;16:139–141 [DOI] [PubMed] [Google Scholar]
- 40. Mitsuishi Y, Taguchi K, Kawatani Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012;22:66–79 [DOI] [PubMed] [Google Scholar]
- 41. Fu J, Xiong Z, Huang C, et al. Hyperactivity of the transcription factor Nrf2 causes metabolic reprogramming in mouse esophagus. J Biol Chem 2019;294:327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cleasby A, Yon J, Day PJ, et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS One 2014;9:e98896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci Technol 2017;69(Pt B):257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosselot C, Baumel-Alterzon S, Li Y, et al. The many lives of Myc in the pancreatic β-cell. J Biol Chem 2021;296:100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abdul-Wahed A, Guilmeau S, Postic C. Sweet sixteenth for ChREBP: established roles and future goals. Cell Metab 2017;26:324–341 [DOI] [PubMed] [Google Scholar]
- 46. Bollong MJ, Lee G, Coukos JS, et al. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature 2018;562:600–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bo J, Xie S, Guo Y, et al. Methylglyoxal impairs insulin secretion of pancreatic β-cells through increased production of ROS and mitochondrial dysfunction mediated by upregulation of UCP2 and MAPKs. J Diabetes Res 2016;2016:2029854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 49. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yagishita Y, Fukutomi T, Sugawara A, et al. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 2014;63:605–618 [DOI] [PubMed] [Google Scholar]
- 51. Reimer MK, Ahrén B. Altered beta-cell distribution of pdx-1 and GLUT-2 after a short-term challenge with a high-fat diet in C57BL/6J mice. Diabetes 2002;51(Suppl. 1):S138–S143 [DOI] [PubMed] [Google Scholar]
- 52. Guo S, Dai C, Guo M, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 2013;123:3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Glavas MM, Hui Q, Tudurí E, et al. Early overnutrition reduces Pdx1 expression and induces β cell failure in Swiss Webster mice. Sci Rep 2019;9:3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roostalu U, Lercke Skytte J, Gravesen Salinas C, et al. 3D quantification of changes in pancreatic islets in mouse models of diabetes type I and II. Dis Model Mech 2020;13:dmm045351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li S, Vaziri ND, Masuda Y, et al. Pharmacological activation of Nrf2 pathway improves pancreatic islet isolation and transplantation. Cell Transplant 2015;24:2273–2283 [DOI] [PubMed] [Google Scholar]
- 56. Robledinos-Antón N, Fernández-Ginés R, Manda G, Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev 2019;2019:9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Toto RD. Bardoxolone-the Phoenix? J Am Soc Nephrol 2018;29:360–361 [DOI] [PMC free article] [PubMed] [Google Scholar]