Abstract
Background
Management of chronic recurrent medical conditions (CRMCs), such as migraine headaches, chronic pain, and anxiety/depression, remains a major challenge for modern providers. Our team has developed an edge-based, semiautomated mobile health (mHealth) technology called iMTracker that employs the N-of-1 trial approach to allow self-management of CRMCs.
Objective
This study examines the patterns of adoption, identifies CRMCs that users selected for self-application, and explores barriers to use of the iMTracker app.
Methods
This is a feasibility pilot study with internet-based recruitment that ran from May 15, 2019, to December 23, 2020. We recruited 180 patients to pilot test the iMTracker app for user-selected CRMCs for a 3-month period. Patients were administered surveys before and after the study.
Results
We found reasonable usage rates: a total of 73/103 (70.9%) patients who were not lost to follow-up reported the full 3-month use of the app. Most users chose to use the iMTracker app to self-manage chronic pain (other than headaches; 80/212, 37.7%), followed by headaches in 36/212 (17.0%) and mental health (anxiety and depression) in 27/212 (12.8%). The recurrence rate of CRMCs was at least weekly in over 93% (169/180) of patients, with 36.1% (65/180) of CRMCs recurring multiple times in a day, 41.7% (75/180) daily, and 16.1% (29/180) weekly. We found that the main barriers to use were the design and technical function of the app, but that use of the app resulted in an improvement in confidence in the efficiency and safety/privacy of this approach.
Conclusions
The iMTracker app provides a feasible platform for the N-of-1 trial approach to self-management of CRMCs, although internet-based recruitment provided limited follow-up, suggesting that in-person evaluation may be needed. The rate of CRMC recurrence was high enough to allow the N-of-1 trial assessment for most traits.
Keywords: mHealth, patient-specific modeling, chronic disease, smartphone, implementation and deployment, facilitators and barriers
Introduction
Chronic recurrent medical conditions (CRMCs) encompass a major proportion of the modern health care burden, accounting for significant costs in the form of both management and lost productive time [1]. For example, chronic migraine headaches affect about 2% of the global population [2], and in the United States alone, cost more than US $20 billion annually [1] to manage. Chronic low back pain accounts for over 5 hours/week in lost productivity by workers, resulting in over US $10 billion in lost revenue per year [1]. Mental health disorders, including depression and anxiety, accounted for 183.9 million disability-adjusted life years and 175.3 million years lived with disability worldwide [3], with an increase of 37.6% over the years from 1990 to 2010 [3].
On a more granular level, CRMCs create a major challenge for today’s busy clinician. Although widely variable across providers and practices, the time available for a face-to-face encounter with patients continues to trend downward, despite an increase in the number of clinical items needing to be addressed [4]. As a result, providers have less time available to focus on the range of triggers and contributing factors for any given CRMC. This trend is unfortunate, as for many CRMCs the number and complexity of environmental and lifestyle triggers can be quite robust. For example, sleep changes have been described in about 50% of patients with migraine headaches, although 75% of patients also chose to sleep due to the migraine headache [5]. In addition, a study of 1207 patients with migraine headache identified no less than 16 possible triggers present in at least 5% of these individuals [6]. A similar scale in triggers has also been noted for depression [7], anxiety [8], and chronic low back pain flares [9]. As such, tailored management of patients with these and other CRMCs often requires the provider to take a detailed, longitudinal history with attention to temporal relationships—an approach that fits poorly with the practical constraints of modern clinical practice.
Despite these limitations, there is evidence that an individualized approach to self-management of CRMCs using mobile health (mHealth) apps has potential to improve clinical outcomes. Specifically, the N-of-1 approach to care has been applied to study various interventions for pain [10-14], depression [15-17], anxiety [18,19], and migraine headaches [20-22], and has been incorporated into mHealth technology [23-25]. In 2017, our team developed a prototype semiautomated iOS mHealth app called the iMTracker (Figure 1), which incorporates the N-of-1 platform for patient-entered data to log recurrences of a given CRMC, as well as the opportunity to log possible triggers or suppressors of the CRMC. The iMTracker provides edge-based analysis of symptom correlations, in which data are stored and analyzed on each user’s device, without the need to transfer or store data to a central server. However, it is unknown which specific patients with CRMCs would be most likely to use the iMTracker for self-management, and whether the rate of recurrence is high enough to maintain a sufficient level of engagement to draw meaningful associations with lifestyle triggers and to evaluate the impact of interventions on recurrence rate. In this feasibility pilot investigation, we aimed to apply an internet-based recruitment approach to enroll patients to trial the iMTracker app. Our goal in this study was to examine the 3-month adoption rate of the iMTracker app by patients with CRMCs, to understand the patterns and characteristics of the possible CRMCs and users, and to identify design and functional barriers to the use of iMTracker prior to its use. Additionally, we planned to examine the strengths and limitations of the internet-based recruitment approach to development and testing of mHealth apps, and identify areas to address for future prospective studies aimed at improving outcomes.
Figure 1.
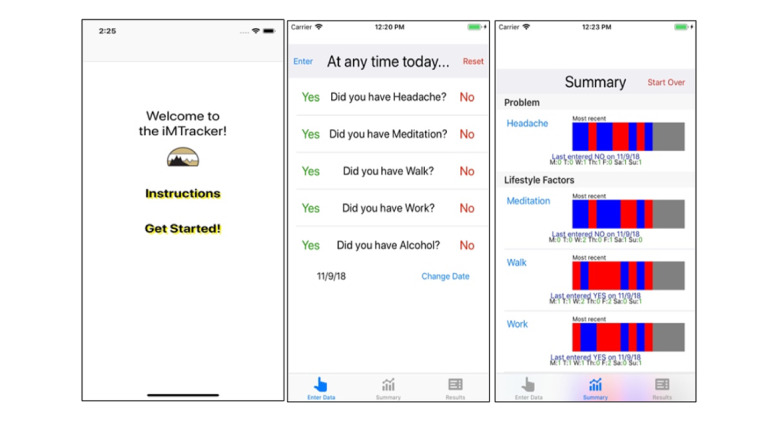
Screenshots of iMTracker.
Methods
Patients
From May 15, 2019, to December 23, 2020, we recruited 180 patients to test the iMTracker app for iPhone for self-management of their CRMCs using an internet-based study design. Inclusion criteria were aged 18 or older, presence of a CRMC, and use of an iPhone. There were no official exclusion criteria, although based on study design and app functionality, patients generally needed to be English speaking and familiar with the use of iPhone apps, as well as the use of email and internet. We started with advertising on social media, such as Twitter, campus-based fliers, and provider word-of-mouth, but found limited recruitment, by which only 2 patients were recruited. We then employed the TrialFacts patient-recruitment company [26] (San Diego, CA, USA) to assist with internet-based recruitment. Patients were provided a small financial stipend for participation, which was paid on enrollment only (nothing additional for follow-up). Written informed consent was obtained for all patients.
Ethics Approval
The study protocol was approved by the University of Colorado Institutional Review Board (Protocol #18-1000).
mHealth App (iMTracker)
The platform of iMTracker was designed based on an automated N-of-1 trial approach (Figure 2) that includes both hypothesis generation and hypothesis testing, which can be built into the logic of an mHealth app. The iMTracker allows the user to select any problem (outcome) and any potential lifestyle factors (risk factors) or intervention that the user would like to test for an association with the outcome. Through iteration between hypothesis generation (ie, “Is there an association between risk factor A and occurrence of my condition?”) and hypothesis testing (ie, “Does changing risk factor A improve the rate of occurrence of my condition?”), the user is able to self-manage his or her condition toward an overall goal of reducing recurrence. The platform thus provides a semiautomated approach to self-management, in which the analysis provides potential lifestyle/behavioral factors that are associated with the CRMC, but allows users to select which factor to intervene upon to examine impact on CRMC recurrence rate. Importantly, the overarching design of the iMTracker app has been focused on application of edge computing [27,28] strategies that run on the mobile device itself, to allow complete usage of the iMTracker app without the need for transfer or storage of data on a server, which provides patients with a level of privacy and data security [29,30].
Figure 2.
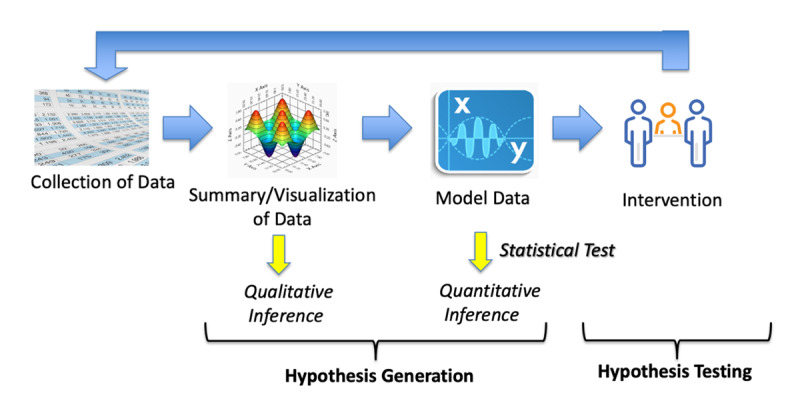
The semiautomated N-of-1 approach motivating the iMTracker design.
Data are manually entered by the user, presented in a visual format, and then modeled for correlations between the selected outcome (problem) and potential risk factors (Figure 1). Through built-in notifications, the iMTracker app prompts the user to input data daily, and keeps a running summary of the inputs. This analysis includes correlation between the outcome and risk factors on a daily basis and with 1-day lag to identify risk factors that could potentially cause the outcome on the following day, using the phi statistic for correlation between discrete variables [31]. Although analysis is performed after only 3 days of data collection, users are informed that the accuracy of the correlation is higher with greater amounts of data collection (Multimedia Appendix 1). Once enough data have been collected to form hypotheses about causative associations, users are directed to reset the data collection and select an intervention in the form of a lifestyle modification, from which future data will examine the role of that intervention in reducing recurrences of the outcome.
Survey
Our team designed a brief survey instrument with several goals in mind. First, we wanted to identify which specific CRMCs users selected for self-management using the iMTracker app. These diagnoses were self-provided, and we did not perform a separate validation with either treating clinicians or chart review. Second, we sought to collect information about the typical pattern of CRMCs—that is, frequency of recurrence—to understand the burden of disease of a possible user of the iMTracker app, and also to guide future work in automating analyses toward sufficient statistical power to detect associated lifestyle conditions and the effect of interventions. Third, we included questions aimed at detecting prior experience with regular data collection (eg, “How often do you weigh yourself?”), information sharing (eg, “How often do you post on social media?”), and electronic engagement with providers using email or secure messaging. Broadly, these questions helped to frame the users’ motivation for using this type of technology for self-management of CRMCs. Fourth, we inquired about concerns of using technology for self-management of conditions; specifically, we asked users to rank concerns related to data security, privacy, efficiency (time demand), and efficacy. Finally, we inquired about specific concerns with the iMTracker app, and asked for qualitative input about the design and function. In addition, we collected basic demographic information about categories of age, education, race, and ethnicity.
The follow-up survey was designed to obtain information about the app itself, as well as the process of the N-of-1 approach to self-management of their selected condition, and to assess the 3-month adoption rate to provide a baseline for future clinical trials. Patients were sent a link to the poststudy survey 3 months after the date of enrollment. Patients that provided no answer for the 3-month adoption, but who completed the postsurvey were assumed to have not completed the 3-month adoption. The postsurvey questions are provided in Multimedia Appendix 2.
The study was conducted remotely using email and phone calls with patients. After informed consent was obtained, patients were given a link to the online survey using a REDCap database. Patients were guided through download and use of the iMTracker mHealth app for iPhone (iOS) by a member (AM) of the research team, and given the opportunity to provide qualitative feedback about the app design, outside the survey data. The postuse survey was deployed after 3 months of use (Multimedia Appendix 3).
Analysis
All study data were collected in a REDCap database. Statistical tests of proportions were based on Fisher exact test. The analysis was performed using Stata IC, version 16.1 (StataCorp, Inc.).
Results
Of the 180 patients who completed the preuse survey, 103 also completed the postuse survey (57.2%). Only 2 patients were recruited by the study team outside of use of the TrialFacts company referrals. A total of 172 patients (95.6%) were under the age of 65, with the predominant age range being 31-45 (80 patients, 44.4%; Table 1). Most patients had at least some college (171/180, 95.0%), and most were White (144/180, 80.0%) and non-Hispanic/Latino (161/180, 89.4%).
Table 1.
Demographics of iMTracker users.
| Demographics | Value, n (%) | |
| Age (years; n=180) |
|
|
|
|
Under 30 | 38 (21.1) |
|
|
31-45 | 80 (44.4) |
|
|
46-55 | 32 (17.8) |
|
|
56-65 | 22 (12.2) |
|
|
66-75 | 8 (4.4) |
|
|
Over 75 | 0 (0) |
| Education (n=177) |
|
|
|
|
Grade school only | 1 (0.6) |
|
|
High-school diploma/general educational development | 5 (2.8) |
|
|
Some college | 50 (28.2) |
|
|
College degree | 66 (37.3) |
|
|
Master’s degree | 48 (27.1) |
|
|
Doctorate degree | 7 (3.9) |
| Race (n=180) |
|
|
|
|
White | 144 (80.0) |
|
|
African American | 14 (7.8) |
|
|
Asian | 9 (5.0) |
|
|
American Indian or Alaskan Native | 5 (2.8) |
|
|
More than 1/unknown | 8 (4.4) |
| Ethnicity (n=180) |
|
|
|
|
Hispanic/Latino | 17 (9.4) |
|
|
Not Hispanic/Latino | 161 (89.4) |
|
|
Unknown | 2 (1.1) |
The most common CRMCs (self-reported) for which patients planned to use the iMTracker app to self-manage were pain (80/212, 37.7%), including low back pain and other musculoskeletal pain syndromes; headaches (36/212, 17.0%), including migraines; gastrointestinal symptoms (17/212, 8.0%), including inflammatory bowel disease flares and irritable bowel disease; and mental health conditions, including anxiety (12/212, 5.7%) and depression (15/212, 7.1%; Table 2). A total of 19/180 patients (10.6%) planned to use the app to monitor more than 1 CRMC. For most CRMCs, frequency was daily (75/180, 41.7%) or multiple times a day (65/180, 36.1%), with few occurring less often than monthly (5/180, 2.8%; Table 3). Patients were allowed to apply the iMTracker app to more than 1 condition, which is why there are 212 listed in Table 2.
Table 2.
Groups of chronic recurrent medical conditions for which patients planned to use the iMTracker app (n=212).
| Condition group | Frequency, n (%) |
| Chronic pain | 80 (37.7) |
| Headaches | 36 (17.0) |
| Gastrointestinal symptoms | 17 (8.0) |
| Depression | 15 (7.1) |
| Anxiety | 12 (5.7) |
| Palpitations | 7 (3.3) |
| Hypertension | 7 (3.3) |
| Dizziness | 5 (2.4) |
| Other | 33 (15.6) |
Table 3.
Frequency of recurrence (n=180).
| Recurrence rate | Frequency, n (%) |
| Multiple times/day | 65 (36.1) |
| Daily | 75 (41.7) |
| Weekly | 29 (16.1) |
| Monthly | 6 (3.3) |
| Every few months | 3 (1.7) |
| Less than every few months | 2 (1.1) |
To assess overall patterns of self-management and use of media, patients were asked about lifestyle and technology savviness. About one-sixth (28/180, 15.6%) weighed themselves daily, 58/180 (32.2%) weighed themselves at least weekly, 42/180 (23.3%) weighed themselves monthly, and 52/180 (28.9%) weighed themselves rarely or not at all. A total of 49/180 (27.2%) posted on social media multiple times a day, 54/180 (30.0%) posted daily, 49/180 (27.2%) posted weekly, 12/180 (6.7%) posted monthly, 14/180 (7.8%) posted rarely or never, and 2/180 (1.1%) were not on social media; 64/178 (36.0%) communicated with their primary physician regularly using messaging/technology, 66/178 (37.1%) communicated rarely, and 13/178 (7.3%) preferred not to communicate using technology/messaging and only in person. Prior to the study, 74/180 patients (41.1%) said they were very likely to use an mHealth app to self-manage CRMCs, 63/180 (35.0%) were somewhat likely, and 6/180 (3.3%) said they were unlikely to use an mHealth app to self-manage CRMCs. After using the iMTracker app, all patients who said they were unlikely to use an mHealth app changed their answers to neutral (2/6, 33%) or somewhat likely (4/6, 67%).
Patients were asked about concerns for using an mHealth app for self-management of CRMCs both before and after use of the iMTracker. As shown in Table 4, patients were generally more likely to have concerns about effectiveness after using the app, and less likely to have concerns about privacy, data safety/security, or time requirements, after use.
Table 4.
Change in concerns about mHealth apps for self-management of chronic recurrent medical conditions (n=103).
| Concern | More likely, n (%) | Less likely, n (%) | Significance (P value) |
| Effectiveness | 28 (27.2) | 10 (9.7) | .001 |
| Privacy | 8 (7.8) | 11 (10.7) | <.001 |
| Data safety/security | 5 (4.9) | 18 (17.5) | .001 |
| Time demands | 11 (10.7) | 29 (28.2) | .005 |
Of the 103 patients who completed the postuse survey, 73 (70.9%; Table 5) said they used the iMTracker app for the planned 3-month period; among those who stopped beforehand, 2/16 (13%) used it for 2 months, 5/16 (31%) used it for 1 month, and 9/16 (56%) used it for less than a month. Among those completing the 3-month use period, only 3/103 (2.9%) failed to enter data on over 50% of days, and 22/103 (21.4%) reported missing less than 5% of days entering data. These patients reported reviewing their data summary weekly or every few weeks in 13/22 (59%) cases, and daily in 3/22 (14%) cases. Finally, 27/103 (26.2%) patients said they were likely or very likely to use the iMTracker app again to self-manage their CRMCs, and 58/103 (56.3%) said they were unlikely to use it without modifications. There was a potential signal for increased levels of education being statistically associated with increased 3-month adoption rate (P=.04; Table 5), although the association did not reach a level of statistical significance after adjustment for multiple comparisons (Bonferroni P for significance [α]=0.05/4=.0125). Among the subjective reasons for not continuing to use the iMTracker app, issues with data sharing and ease of use were most cited, followed by design/display limitations.
Table 5.
Poststudy survey.
| Characteristicsa | Completed follow-up, n/N (%) | 3-month adoption, n/N (%)b | |
| Age category (n=103/180) |
|
|
|
|
|
Under 30 | 19/38 (50) | 13/19 (68) |
|
|
31-45 | 46/80 (58) | 29/46 (63) |
|
|
46-55 | 20/32 (63) | 15/20 (75) |
|
|
56-65 | 13/22 (59) | 11/13 (85) |
|
|
66-75 | 5/8 (63) | 5/7 (71) |
|
|
P value | .87 | .37 |
| Education (n=101/180) |
|
|
|
|
|
Grade school only | 0/4 (0) | N/Ac |
|
|
High school diploma/general educational development | 1/5 (20) | 1/1 (100) |
|
|
Some college | 22/50 (44) | 17/22 (77) |
|
|
College degree | 39/66 (59) | 28/39 (72) |
|
|
Master’s degree | 34/48 (71) | 22/34 (65) |
|
|
Doctorate degree | 5/7 (71) | 3/5 (60) |
|
|
P value | .04 | .83 |
| Race (n=103/180) |
|
|
|
|
|
Caucasian | 84/144 (58) | 60/84 (71) |
|
|
African American | 9/14 (64) | 8/9 (89) |
|
|
Asian | 2/9 (22) | 2/2 (100) |
|
|
American Indian or Alaskan Native | 3/5 (60) | 3/3 (100) |
|
|
More than 1/unknown | 5/8 (63) | 3/5 (60) |
|
|
P value | .37 | .05 |
| Ethnicity (n=103/180) | N=103 |
|
|
|
|
Hispanic/Latino | 10/17 (59) | 7/10 (70) |
|
|
Not Hispanic/Latino | 92/161 (57) | 65/92 (71) |
|
|
Unknown | 1/2 (50) | 1/1 (100) |
|
|
P value | >.99 | >.99 |
aN=103 for age category, race, and ethnicity, and 101 for education.
bN=73 for age category and ethnicity, 71 for education, and 76 for race.
cN/A: not applicable.
Discussion
Principal Findings
In this internet-based, pilot study of predominantly young and middle-age, educated, White patients, using a semiautomated, edge-based, mHealth app that uses individualized data for tailored management of CRMCs, we made several key observations with regard to both the internet-based recruitment approach to the study of mHealth apps, as well as the specific usage rates and patterns of use by participants. First, we found that while an internet-based recruitment approach was superior to “grass-roots” local methods of recruiting participants on our campus using fliers and word-of-mouth, the 3-month follow-up rates were only slightly above 50% (103/180), indicating that future studies using this type of methodology targeted to achieve a prespecified degree of statistical power will need to account for a high number of dropouts. Second, we found that among those with complete follow-up, the 3-month adoption rate of the iMTracker app was about 70.9% (73/103), with the most common CRMCs that users chose to self-apply the app being chronic pain, headaches, and mental health conditions. This information not only provides conditions and clinical settings in which to target future clinical trials, but also indicates that there may be a need for better tools to manage these conditions beyond what is presently available in clinical practice. Importantly, we found that on completion of this study, more patients had increased perceptions of the safety, privacy, and time demands with the use of an mHealth app for self-management of CRMCs. Finally, we found that the main barrier to use, based on both subjective and quantitative feedback, was related to the design and workflow of iMTracker itself, which was reflected in the decrease in perception of efficacy noted on completion, and has important implications for future development and testing of this app as well as other mHealth technologies. In other words, this finding indicates that patients are likely to be receptive to the semiautomated N-of-1 trial methodology employed by the iMTracker app, but that greater attention to design and function is needed before moving forward with clinical testing targeted toward improvement in outcomes.
mHealth apps have increased significantly in frequency over the years, with iOS apps including health and fitness groups increasing from 43,000 in 2013 to 98,000 in 2015 [32]. Unfortunately, these tools do not consistently employ best practices for self-management [33], and many of these approaches have failed to reach any meaningful level of adoption across the medical community [32,34], likely due to a lack of formal clinical testing. Our finding that there was an increased concern among patients about the effectiveness of iMTracker is consistent with prior studies of mHealth app for self-management of CRMCs. In a previous investigation, it was found that only 3.4% of apps on the iTunes and Google Play stores promoted for management of depression and anxiety had research to justify their claims of effectiveness, with only 30.4% having expert input in development [35]. A study by Devan et al [36] of 19 apps available commercially for self-management of pain found that only 2 had been validated to improve health outcomes. A similar lack of scientific support for commercially available mental health–targeted [37-39] and pain [25,40] apps has been reported by other investigators. Although we did not inquire about prior use of mHealth apps for self-management, one can infer that most participants in this study had tried prior apps without success. Clinical validation of any mHealth app should be required before integration into the clinical care process, and our study further suggests that while users are optimistic that self-management using an app is possible, follow-up clinical studies will be needed.
Among the characteristics of the specific CRMCs that patients identified for use of an mHealth app, recurrent pain, headaches, and mental health were highly represented. While these diagnoses were self-identified by users, and not validated with clinicians or clinical data (ie, chart review), it does help to identify potential clinics and providers for testing mHealth apps, as would be needed before an app such as the iMTracker could be incorporated into routine clinical care. In addition, the majority of patients noted a high frequency of recurrence of their condition, which is key in determining the number of patients needed for a prospective study to demonstrate efficacy, as a condition that occurred less frequently, for example, once or twice a year, would require an extended amount of time to identify correlation of episodes with other lifestyle and behaviors, or assess the impact of an intervention.
Although our study did not specifically examine clinical efficacy of the iMTracker app in terms of a reduction in the primary CRMC, we did note that there was enthusiasm about future uses, particularly if barriers related to design and function could be addressed. This finding is in line with prior work, such as that by Neuhauser et al [41,42], who noted that participatory methods linked with traditional health communication theory and methods can create effective health communication using artificial intelligence, highlighting the role of design science theory in the development and refinement of mHealth apps. Such insights highlight the challenge that is unique to mHealth, and other health IT apps, in which consideration of user-based preferences and desires must be merged with information and guidance grounded in biology and evidence-based medicine principles. In terms of design life cycles, this requires an integrated design approach with features of both top-down (ie, waterfall) strategies and bottom-up, user-driven design (eg, agile) strategies. Our team has already begun efforts to improve the design and function of the iMTracker app, and future studies will examine the improvement in these changes to make the app more in line with the level of commercial design that many patients have come to expect from all mobile apps, in addition to mHealth.
Despite results to suggest a high degree of potential for clinical application of the iMTracker app, there were several key limitations in our study. First, we generally had a limited amount of follow-up of patients, with roughly half of the patients who enrolled being lost to follow-up. We suspect that this limitation highlighted the challenges of using the internet for recruitment, and the trade-off between use of network-based recruitment methods (ie, online) and in-person clinic recruitment. As an extreme example of the potential of the former, the Apple Watch study recruited over 400,000 participants in an 8-month period using online methods, although only 450 actually returned the confirmatory patches in follow-up [43]. As such, future studies of mHealth technology should consider that the potential benefit in terms of recruitment numbers using internet-based recruitment may accompany a relatively high degree of dropouts. A second limitation was that the population we studied were primarily White, educated, and young/middle age adults; these were individuals who engage regularly with providers using technology, post to social media, and perform self-management with regular weight checks. Missing from our population are older patients, those with less education, and those from underrepresented populations—the type of populations that have also been shown to have less close clinical follow-up for their conditions [44-46], and who might stand to benefit the most from an app that allows self-management. This population bias is critical in considerations of further app development as the design and functionality changes that would typically guide app development would be needed for successful integration with clinical care. Further work is needed on methods to include less represented populations in mHealth studies. Among the issues with biased recruitment was the omission of sex from the baseline variables we collected. Qualitatively (based on the first names of the participants), we suspect recruitment was not heavily imbalanced toward 1 specific sex, although formal assessment would have been beneficial in terms of statistical analysis. In future studies, we plan to examine sex along with other user characteristics in terms of both usage patterns and study participation. Finally, a key limitation of the study was the inability to confirm diagnoses or response to therapy among users of iMTracker. While we have ongoing studies to examine the app prospectively toward clinical outcomes, the findings from this investigation provided important information about which specific conditions, and which types of clinics, to target for recruitment. This finding was critical as the iMTracker was originally developed toward treatment of recurrent cardiac symptoms (ie, palpitations), and yet we identified other conditions that included chronic pain, headaches, and mental health as being more heavily favored by patients in this investigation.
In future studies we plan to examine the impact of specific design and function improvements on iMTracker, including an examination of the use of industry-standard Agile development life cycles to make iterative improvements to the user interface within the Scrum methodology [47]. Our team has already employed a newer user interface and data entry design based on emojis, although additional work is needed to ensure all users of all levels of education and medical literacy can use the app comfortably. Following this step, we plan to target clinical trials of iMTracker to primary care, neurology, and psychiatry clinics to assess the impact of N-of-1 management on recurrence of chronic pain, headaches, and mental health conditions. Finally, our team has continued work on integration of more sophisticated data management approaches using federated and distributive learning, as well as Bayesian-based analytical frameworks, and we plan to examine the improvement in accuracy with these innovations. Ultimately, much work is needed before the iMTracker app can be used routinely in clinics to manage CRMCs, although feedback from this study has helped target efforts toward high-yield conditions and modifications to improve the chances of success.
Conclusions
In conclusion, in this feasibility pilot study using internet-based recruitment, we found that the primary barrier to investigation was study follow-up, but that among those who were not lost to follow-up, there was generally good adherence to use of the iMTracker app. We identified design and function barriers as being of foremost concern among users, but also noted that the frequency of recurrence of the selected CRMCs should provide ample opportunity to identify a clinical benefit for future studies. We also identified population bias in the patients enrolled using internet-based recruitment alone, and note that additional efforts will be needed to ensure that future studies enroll sufficient numbers of underrepresented populations, specifically older, non-White, and less education populations.
Acknowledgments
This work was supported by funding from the National Institutes of Health and the National Heart, Lung, and Blood Institute (grant numbers R01 HL146824 and K23 HL127296).
Abbreviations
- CRMC
chronic recurrent medical condition
- mHealth
mobile health
Screenshot from Results presentation of iMTracker.
Postuse survey questions.
Sample of feedback about iMTracker from users on postsurvey.
Footnotes
Conflicts of Interest: The iMTracker app is a licensed intellectual property of the University of Colorado. The authors have no other conflicts of interest to disclose.
References
- 1.Stewart WF, Ricci Judith A, Chee Elsbeth, Morganstein David, Lipton Richard. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003 Nov 12;290(18):2443–54. doi: 10.1001/jama.290.18.2443.290/18/2443 [DOI] [PubMed] [Google Scholar]
- 2.Natoli JL, Manack A, Dean B, Butler Q, Turkel CC, Stovner L, Lipton RB. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010 May;30(5):599–609. doi: 10.1111/j.1468-2982.2009.01941.x.CHA1941 [DOI] [PubMed] [Google Scholar]
- 3.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJL, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013 Nov 9;382(9904):1575–86. doi: 10.1016/S0140-6736(13)61611-6.S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- 4.Abbo ED, Zhang Q, Zelder M, Huang ES. The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008 Dec 2;23(12):2058–65. doi: 10.1007/s11606-008-0805-8. http://europepmc.org/abstract/MED/18830762 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005 Jul;45(7):904–10. doi: 10.1111/j.1526-4610.2005.05159.x.HED05159 [DOI] [PubMed] [Google Scholar]
- 6.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007 May 26;27(5):394–402. doi: 10.1111/j.1468-2982.2007.01303.x.CHA1303 [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos GS. Depression in the elderly. The Lancet. 2005 Jun;365(9475):1961–1970. doi: 10.1016/s0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 8.Miloyan B, Bulley A, Suddendorf T. Episodic foresight and anxiety: Proximate and ultimate perspectives. Br J Clin Psychol. 2016 Mar 16;55(1):4–22. doi: 10.1111/bjc.12080. [DOI] [PubMed] [Google Scholar]
- 9.Shiri R, Falah-Hassani Kobra, Heliövaara Markku, Solovieva S, Amiri S, Lallukka T, Burdorf A, Husgafvel-Pursiainen Kirsti, Viikari-Juntura Eira. Risk Factors for Low Back Pain: A Population-Based Longitudinal Study. Arthritis Care Res (Hoboken) 2019 Feb 29;71(2):290–299. doi: 10.1002/acr.23710. [DOI] [PubMed] [Google Scholar]
- 10.Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, Sansom C. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1' studies. Anaesthesia. 2004 May;59(5):440–52. doi: 10.1111/j.1365-2044.2004.03674.x. https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0003-2409&date=2004&volume=59&issue=5&spage=440 .ANA3674 [DOI] [PubMed] [Google Scholar]
- 11.Germini F, Coerezza A, Andreinetti L, Nobili A, Rossi PD, Mari D, Guyatt G, Marcucci M. N-of-1 Randomized Trials of Ultra-Micronized Palmitoylethanolamide in Older Patients with Chronic Pain. Drugs Aging. 2017 Dec 5;34(12):941–952. doi: 10.1007/s40266-017-0506-2.10.1007/s40266-017-0506-2 [DOI] [PubMed] [Google Scholar]
- 12.Joy TR, Monjed A, Zou GY, Hegele RA, McDonald CG, Mahon JL. -of-1 (Single-Patient) Trials for Statin-Related Myalgia. Ann Intern Med. 2014 Mar 04;160(5):301-310. doi: 10.7326/m13-1921. [DOI] [PubMed] [Google Scholar]
- 13.Green AL, Shad A, Watson R, Nandi D, Yianni J, Aziz TZ. N-of-1 Trials for Assessing the Efficacy of Deep Brain Stimulation in Neuropathic Pain. Neuromodulation. 2004 Apr;7(2):76–81. doi: 10.1111/j.1094-7159.2004.04010.x.S1094-7159(11)60282-1 [DOI] [PubMed] [Google Scholar]
- 14.Yelland MJ, Poulos CJ, Pillans PI, Bashford GM, Nikles CJ, Sturtevant JM, Vine N, Del Mar CB, Schluter PJ, Tan M, Chan J, Mackenzie F, Brown R. N-of-1 randomized trials to assess the efficacy of gabapentin for chronic neuropathic pain. Pain Med. 2009 May 01;10(4):754–61. doi: 10.1111/j.1526-4637.2009.00615.x.PME615 [DOI] [PubMed] [Google Scholar]
- 15.Kronish IM, Hampsey M, Falzon L, Konrad B, Davidson KW. Personalized (N-of-1) Trials for Depression. J Clin Psychopharmacol. 2018;38(3):218–225. doi: 10.1097/jcp.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen IH, Olde Rikkert M G, Hulsbos HA, Hoefnagels WH. Toward individualized evidence-based medicine: five "N of 1" trials of methylphenidate in geriatric patients. J Am Geriatr Soc. 2001 May 21;49(4):474–6. doi: 10.1046/j.1532-5415.2001.49092.x. [DOI] [PubMed] [Google Scholar]
- 17.Cook DJ, Guyatt GH, Davis C, Willan A, McIlroy W. A diagnostic and therapeutic N-of-1 randomized trial. Can J Psychiatry. 1993 May 01;38(4):251–4. doi: 10.1177/070674379303800405. [DOI] [PubMed] [Google Scholar]
- 18.Malboeuf-Hurtubise C, Lacourse E, Herba C, Taylor G, Amor LB. Mindfulness-based Intervention in Elementary School Students With Anxiety and Depression: A Series of n-of-1 Trials on Effects and Feasibility. J Evid Based Complementary Altern Med. 2017 Oct 30;22(4):856–869. doi: 10.1177/2156587217726682. https://journals.sagepub.com/doi/10.1177/2156587217726682?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaus W, Högel J. Studies on the efficacy of unconventional therapies. Problems and designs. Arzneimittelforschung. 1995 Jan;45(1):88–92. [PubMed] [Google Scholar]
- 20.Haas DC, Sheehe PR. Dextroamphetamine pilot crossover trials and n of 1 trials in patients with chronic tension-type and migraine headache. Headache. 2004 Nov 10;44(10):1029–37. doi: 10.1111/j.1526-4610.2004.04199.x.HED4199 [DOI] [PubMed] [Google Scholar]
- 21.Weng H, Cohen AS, Schankin C, Goadsby PJ. Phenotypic and treatment outcome data on SUNCT and SUNA, including a randomised placebo-controlled trial. Cephalalgia. 2018 Aug 02;38(9):1554–1563. doi: 10.1177/0333102417739304. https://journals.sagepub.com/doi/10.1177/0333102417739304?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos C, Weaver DF. Topically applied linoleic/linolenic acid for chronic migraine. J Clin Neurosci. 2018 Dec;58:200–201. doi: 10.1016/j.jocn.2018.10.013.S0967-5868(18)30648-9 [DOI] [PubMed] [Google Scholar]
- 23.Kravitz RL, Schmid CH, Marois M, Wilsey B, Ward D, Hays RD, Duan N, Wang Y, MacDonald S, Jerant A, Servadio JL, Haddad D, Sim I. Effect of Mobile Device-Supported Single-Patient Multi-crossover Trials on Treatment of Chronic Musculoskeletal Pain: A Randomized Clinical Trial. JAMA Intern Med. 2018 Oct 01;178(10):1368–1377. doi: 10.1001/jamainternmed.2018.3981.2698146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitney RL, Ward DH, Marois MT, Schmid CH, Sim I, Kravitz RL. Patient Perceptions of Their Own Data in mHealth Technology-Enabled N-of-1 Trials for Chronic Pain: Qualitative Study. JMIR Mhealth Uhealth. 2018 Oct 11;6(10):e10291. doi: 10.2196/10291. https://mhealth.jmir.org/2018/10/e10291/ v6i10e10291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pombo N, Garcia N, Bousson K, Spinsante S, Chorbev I. Pain Assessment--Can it be Done with a Computerised System? A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2016 May 13;13(4):415. doi: 10.3390/ijerph13040415. https://www.mdpi.com/resolver?pii=ijerph13040415 .ijerph13040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TrialFacts. [2022-03-25]. https://trialfacts.com/
- 27.Vega-Barbas M, Diaz-Olivares J, Lu K, Forsman M, Seoane F, Abtahi F. P-Ergonomics Platform: Toward Precise, Pervasive, and Personalized Ergonomics using Wearable Sensors and Edge Computing. Sensors (Basel) 2019 Mar 11;19(5):1225. doi: 10.3390/s19051225. https://www.mdpi.com/resolver?pii=s19051225 .s19051225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamb Z, Agrawal D. Analysis of Mobile Edge Computing for Vehicular Networks. Sensors (Basel) 2019 Mar 15;19(6):1303. doi: 10.3390/s19061303. https://www.mdpi.com/resolver?pii=s19061303 .s19061303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi S, Kane T, Paton C. The Privacy and Security Implications of Open Data in Healthcare. Yearb Med Inform. 2018 Aug 22;27(1):41–47. doi: 10.1055/s-0038-1641201. http://www.thieme-connect.com/DOI/DOI?10.1055/s-0038-1641201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabriel MH, Noblin A, Rutherford A, Walden A, Cortelyou-Ward K. Data breach locations, types, and associated characteristics among US hospitals. Am J Manag Care. 2018 Feb;24(2):78–84. https://www.ajmc.com/pubMed.php?pii=87440 .87440 [PubMed] [Google Scholar]
- 31.Jackson RA. Interpretation of research data: selected statistical procedures. Am J Hosp Pharm. 1980 Dec;37(12):1673–80. [PubMed] [Google Scholar]
- 32.Aitken M. IQVIA. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2015. Sep, [2022-02-11]. Patient adoption of mHealth: use, evidence and remaining barriers to mainstream acceptance. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/patient-adoption-of-mhealth.pdf . [Google Scholar]
- 33.Rathbone AL, Prescott J. The Use of Mobile Apps and SMS Messaging as Physical and Mental Health Interventions: Systematic Review. J Med Internet Res. 2017 Aug 24;19(8):e295. doi: 10.2196/jmir.7740. https://www.jmir.org/2017/8/e295/ v19i8e295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shortliffe EH. Strategic action in health information technology: why the obvious has taken so long. Health Aff (Millwood) 2005;24(5):1222–33. doi: 10.1377/hlthaff.24.5.1222.24/5/1222 [DOI] [PubMed] [Google Scholar]
- 35.Marshall JM, Dunstan DA, Bartik W. The Digital Psychiatrist: In Search of Evidence-Based Apps for Anxiety and Depression. Front Psychiatry. 2019;10:831. doi: 10.3389/fpsyt.2019.00831. doi: 10.3389/fpsyt.2019.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devan H, Farmery D, Peebles L, Grainger R. Evaluation of Self-Management Support Functions in Apps for People With Persistent Pain: Systematic Review. JMIR Mhealth Uhealth. 2019 Feb 12;7(2):e13080. doi: 10.2196/13080. https://mhealth.jmir.org/2019/2/e13080/ v7i2e13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen ME, Huckvale K, Nicholas J, Torous J, Birrell L, Li E, Reda B. Using science to sell apps: Evaluation of mental health app store quality claims. NPJ Digit Med. 2019;2:18. doi: 10.1038/s41746-019-0093-1. doi: 10.1038/s41746-019-0093-1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen ME, Nicholas J, Christensen H. Quantifying App Store Dynamics: Longitudinal Tracking of Mental Health Apps. JMIR Mhealth Uhealth. 2016 Aug 09;4(3):e96. doi: 10.2196/mhealth.6020. https://mhealth.jmir.org/2016/3/e96/ v4i3e96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grist R, Porter J, Stallard P. Mental Health Mobile Apps for Preadolescents and Adolescents: A Systematic Review. J Med Internet Res. 2017 May 25;19(5):e176. doi: 10.2196/jmir.7332. https://www.jmir.org/2017/5/e176/ v19i5e176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosser BA, Eccleston C. Smartphone applications for pain management. J Telemed Telecare. 2011;17(6):308–12. doi: 10.1258/jtt.2011.101102.jtt.2011.101102 [DOI] [PubMed] [Google Scholar]
- 41.Neuhauser L, Kreps GL, Morrison K, Athanasoulis M, Kirienko N, Van Brunt Deryk. Using design science and artificial intelligence to improve health communication: ChronologyMD case example. Patient Educ Couns. 2013 Aug;92(2):211–7. doi: 10.1016/j.pec.2013.04.006.S0738-3991(13)00164-X [DOI] [PubMed] [Google Scholar]
- 42.Neuhauser L, Kreps GL. Integrating design science theory and methods to improve the development and evaluation of health communication programs. J Health Commun. 2014 Dec;19(12):1460–71. doi: 10.1080/10810730.2014.954081. [DOI] [PubMed] [Google Scholar]
- 43.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP, Apple Heart Study Investigators Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019 Nov 14;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. http://europepmc.org/abstract/MED/31722151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wools A, Dapper E, de Leeuw J R J. Colorectal cancer screening participation: a systematic review. Eur J Public Health. 2016 Mar 14;26(1):158–68. doi: 10.1093/eurpub/ckv148.ckv148 [DOI] [PubMed] [Google Scholar]
- 45.Hodgkinson S, Godoy L, Beers LS, Lewin A. Improving Mental Health Access for Low-Income Children and Families in the Primary Care Setting. Pediatrics. 2017 Jan;139(1):e20151175. doi: 10.1542/peds.2015-1175. http://europepmc.org/abstract/MED/27965378 .peds.2015-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pimple SA, Mishra GA. Global strategies for cervical cancer prevention and screening. Minerva Ginecol. 2019 Aug;71(4):313–320. doi: 10.23736/s0026-4784.19.04397-1. [DOI] [PubMed] [Google Scholar]
- 47.Wilson K, Bell C, Wilson L, Witteman H. Agile research to complement agile development: a proposal for an mHealth research lifecycle. NPJ Digit Med. 2018;1:46. doi: 10.1038/s41746-018-0053-1. doi: 10.1038/s41746-018-0053-1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Screenshot from Results presentation of iMTracker.
Postuse survey questions.
Sample of feedback about iMTracker from users on postsurvey.


