Abstract
Background:
The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) compared an initial invasive treatment strategy (INV) with an initial conservative strategy (CON) in 5,179 participants with chronic coronary disease (CCD) and moderate or severe ischemia. The ISCHEMIA research program included a comprehensive quality of life (QOL) substudy.
Methods:
In 1,819 participants (907 INV, 912 CON), we collected a battery of disease-specific and generic QOL instruments by structured interviews at baseline; at 3, 12, 24, and 36 months post-randomization; and at study close-out. Assessments included angina-related QOL (19-item Seattle Angina Questionnaire [SAQ-19]), generic health status (EQ-5D), depressive symptoms (Patient Health Questionnaire-8), and, for North American patients, cardiac functional status (Duke Activity Status Index [DASI]).
Results:
Median age was 67 years, 19.2% were female, and 15.9% were non-white. The estimated mean difference for the SAQ-19 Summary score favored INV (1.4 points, 95% confidence interval [CI] 0.2, 2.5 over all follow-up). No differences were observed in patients with rare/absent baseline angina (SAQ Angina Frequency [AF] score >80). Among patients with more frequent angina at baseline (SAQ AF score ≤80, 744 patients, 41%), those randomized to INV had a mean 3.7-point higher SAQ-19 Summary score than CON (95% CI 1.6, 5.8) with consistent effects across SAQ subscales: Physical Limitations 3.2 points (95% CI 0.2, 6.1), Angina Frequency 3.2 points (95% CI 1.2, 5.1), Quality of Life/Health Perceptions 5.3 points (95% CI 2.8, 7.8). For the DASI, no difference was estimated overall by treatment, but in patients with baseline SAQ AF scores ≤80, DASI scores were higher for INV (3.2 points, 95% CI 0.6, 5.7), whereas patients with rare/absent baseline angina showed no treatment-related differences. Moderate to severe depression was infrequent at randomization (11.5% to 12.8%) and was unaffected by treatment assignment.
Conclusions:
In the ISCHEMIA comprehensive QOL substudy, patients with more frequent baseline angina reported greater improvements in the symptom, physical functioning, and psychological well-being dimensions of QOL when treated with an invasive strategy, whereas patients who had rare/absent angina at baseline reported no consistent treatment-related QOL differences.
Keywords: angina pectoris, myocardial ischemia, quality of life, health status, coronary angiography, coronary artery bypass, myocardial revascularization, coronary disease
INTRODUCTION
Chronic coronary disease (CCD) adversely affects both length and quality of life (QOL). Clinicians employ a variety of drugs and procedures to relieve ischemia, preserve cardiac function, and prevent future irreversible complications of the disease. The success with which the biologic effects of those interventions translate into perceptible improvements in QOL, as experienced by patients, varies substantially among CCD patients and across different forms of treatment.1–5 Consequently, evidence of improved coronary physiology with relief of ischemia cannot be presumed to serve as an adequate patient-centric outcome. The primary concerns of patients faced with a complex treatment choice involving significant risks center around how the choices they make will affect their life expectancy and their QOL. Directly assessing patient-reported QOL is thus an essential element in developing a full understanding of impact of different therapies on the “patient journey” with CCD.
QOL is a seemingly familiar and intuitive yet elusive idea that is made accessible to research by operationalizing it as a set of modular constructs or domains.6 A common approach used to measure QOL in cardiovascular clinical trials involves assessing three core domains: disease-specific symptoms (e.g., angina), physical functioning (e.g., ability to perform activities reflecting incremental workloads), and emotional/psychological well-being (e.g., anxiety, depression, hedonic adaptation). Generic instruments are used to provide a lower-power survey of the totality of effects of an illness on QOL, while disease-specific instruments attempt to provide a higher-resolution assessment of symptoms and functional limitations that are directly related to the condition and treatments under study.
The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) recently reported that in patients with CCD and moderate or severe ischemia, an initial invasive strategy (INV) did not reduce the risk of adverse ischemic events or death relative to an initial conservative strategy (CON) of guideline-directed medical therapy alone.7 However, angina-related health status was improved with INV relative to CON when assessed using the disease-specific 7-item Seattle Angina Questionnaire (SAQ-7).8 Differences favoring INV were larger among participants who had more frequent angina at baseline.8 The ISCHEMIA research program also prespecified a more comprehensive assessment of health status and QOL in a subgroup of the ISCHEMIA population, including both disease-specific and generic dimensions.9 That evaluation forms the subject of this report.
METHODS
ISCHEMIA was sponsored by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI), and the trial datasets will be made public via the NIH BioLINCC website (https://biolincc.nhlbi.nih.gov/home/).
Patient Population and Primary Clinical Outcomes
ISCHEMIA randomized 5,179 CCD patients with moderate or severe ischemia at 320 sites in 37 countries.7 All sites received approval from their Institutional Review Boards, and all patients provided informed consent to participate in ISCHEMIA, including collection of QOL data. The trial rationale and design, baseline characteristics of the full trial population, details of the randomized treatment strategies, and principal trial results have been published.7, 9, 10
The cumulative event rate for the primary composite endpoint (cardiovascular death, myocardial infarction, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest) was higher in the INV arm at 6 months (5.3% versus 3.4% for CON) but lower at 5 years (16.4% for INV versus 18.2% for CON).7 The overall average hazard ratio (INV:CON) was 0.93 (95% confidence interval [CI] 0.80 to 1.08). Similar patterns were seen for key secondary outcomes. In the INV arm, 79% of patients underwent revascularization, while in the CON arm 21% underwent revascularization (15% before a primary outcome event).
QOL Assessment in ISCHEMIA
At the time of initial funding, plans called for all randomized patients to complete a battery of QOL instruments before randomization and during follow-up. However, for administrative (site data collection burden) and budgetary reasons, that plan was altered early in the trial to require only a brief assessment of angina-related QOL in all randomized patients and to collect the more comprehensive battery of QOL in a substudy. Patients randomized in the US and Canada plus 11 other countries agreed to participate in this comprehensive QOL substudy. Participation in the substudy was decided by each non-North American site in conjunction with their ISCHEMIA country leader on a voluntary basis.
As previously reported, in the full ISCHEMIA cohort, the INV arm had greater improvement than CON using the SAQ-7.8 At baseline, patients reporting more frequent angina (SAQ Angina Frequency score 80 or less) in the past month had sustained, clinically consequential improvements in SAQ-7 scores with randomization to INV relative to CON. When the treatment effect was modeled as a continuous function of baseline SAQ-7 scores, the treatment benefit of INV showed a sharp and progressive attenuation towards no difference starting with SAQ Angina Frequency scores greater than 80 (rare or no angina) and evident from the 3-month assessment onward. The 35% of the trial cohort that reported no angina in the past month at baseline had no treatment-related differences in SAQ-7 scores in follow-up.
Quality of Life Substudy Battery
The survey instruments used in the comprehensive QOL substudy included the 19-item Seattle Angina Questionnaire11 (SAQ-19) assessing 4 dimensions of angina-related QOL (anginal symptom frequency, physical function/limitations, angina-specific quality of life, and treatment satisfaction), the EQ-5D12 0–100 visual analog scale (VAS) assessing self-rated global health, the Rose Dyspnea Scale13 assessing dyspnea symptoms, and the Patient Health Questionnaire-814 (PHQ-8) assessing depressive symptoms and major depression. Sites in Canada and the US also included the Duke Activity Status Index15 (DASI) assessing cardiac functional status, the RAND General Health16 overall health ordinal rating, the Perceived Stress Scale-417, 18 (PSS-4) assessing the extent to which situations in life are perceived as stressful (unpredictable, uncontrollable), and the Life Orientation Test-Revised19 (LOT-R) assessing optimism/pessimism. A more detailed description of each instrument, including interpretive benchmarks, is provided in Supplement Section 1.
The SAQ-19 Summary score was prespecified as the primary endpoint for this substudy, with all other assessments secondary.20
Health-Related QOL Data Collection Methods and Schedule
QOL data were collected by sites before randomization using structured interviews. Site coordinators were specially trained by the Duke Clinical Research Institute (DCRI) investigational team to conduct these assessments. In follow-up, QOL surveys were conducted centrally by the DCRI call center for participants at sites in Canada and the US and by site coordinators elsewhere at months 3, 12, 24, and 36, and at study close-out. In all cases, standardized structured interviewing techniques were used.
Statistical Analyses
Primary Analyses
All analyses were performed with treatment groups defined by the principle of intention-to-treat (ITT) (i.e., as randomized). For each of the QOL measures examined in this study, we used both descriptive and comparative analyses. Descriptive statistics include percentages for discrete variables, and medians with 25th and 75th percentiles or means with standard deviations for continuous variables. Participants were included in the QOL analysis if they completed a baseline plus at least one follow-up assessment.
The QOL endpoints were compared using a repeated-measures mixed model with responses at baseline; months 3, 12, 24, and 36; and study close-out included as outcome variables, and time, treatment, and time by treatment included as fixed effects. Restricted maximum likelihood estimation with an unstructured covariance matrix and Kenward-Roger degrees of freedom approximation were used to model all available data from each patient. No formal imputation procedures for missing scores were used in our analyses because the mixed effects model does not require complete data or the same length of follow-up for each patient.
Point estimates for strategy arm mean differences (invasive minus conservative treatment) with 95% CIs were generated for each time point. The intervention QOL effect size was also averaged across all follow-up time points. The estimated intervention difference and 95% CIs for the models were implemented using the ESTIMATE statement in SAS PROC MIXED.
Where p values are provided, they are intended as interpretive aids supplemental to effect size and precision estimates, providing a heuristic of unexpectedness regarding the consistency of the data with the null hypothesis.21
Post Hoc Analyses
QOL outcomes were compared among patients who had a baseline SAQ Angina Frequency score of 0–80 (more frequent angina) and those who had a score of 81–100 (rare/absent angina). This division is based on earlier results demonstrating a change in the relationship between SAQ-7 Summary score effect size and baseline SAQ Angina Frequency scores with progressive attenuation above 80. Subgroup treatment effect sizes, confidence intervals, and p values were generated using the ESTIMATE statement in SAS PROC MIXED and included an interaction term between treatment and subgroup and a three-way interaction of treatment, subgroup, and interval.
A “magnitude of response” analysis was conducted for the SAQ-19 Summary score and the DASI. This analysis was prespecified but originally proposed baseline severity groups were subsequently modified during analysis, so we include it as a post hoc analysis. We compared the proportion of patients in each treatment group who improved their SAQ Summary score by ≥5, ≥10, ≥15, and ≥20 points, roughly corresponding to values representing from ¼ standard deviation (SD) of baseline score to 1.0 SD.22 This analysis used baseline severity groups categorized as minimal or no burden (SAQ Summary score 81–100), moderate burden (61–80), and moderately severe to severe burden (≤60).
For the DASI, improvements of ≥4, ≥8, and ≥12 were used, with response subgroups for baseline DASI scores defined as mild to no impairment (DASI score 31.5 to 58.2), moderate impairment (13.5 to 31.4), or moderately severe to severe impairment (0 to 13.4).
All analyses were performed using SAS software versions 9.4 or later (SAS Institute, Cary NC).
RESULTS
Baseline Characteristics and QOL Data Collection Rates
Of the 1,940 eligible patients randomized in ISCHEMIA at sites in countries participating in the comprehensive QOL substudy (37% of total trial enrollment), 2 died and 1 withdrew prior to completing a baseline QOL assessment, and 118 were excluded for incomplete data (Figure 1). Of the remaining 1,819 patients (94%), 907 were randomized to the INV strategy and 912 to the CON strategy. Of these, 616 of the INV group and 618 in the CON group were randomized in North America.
Figure 1:
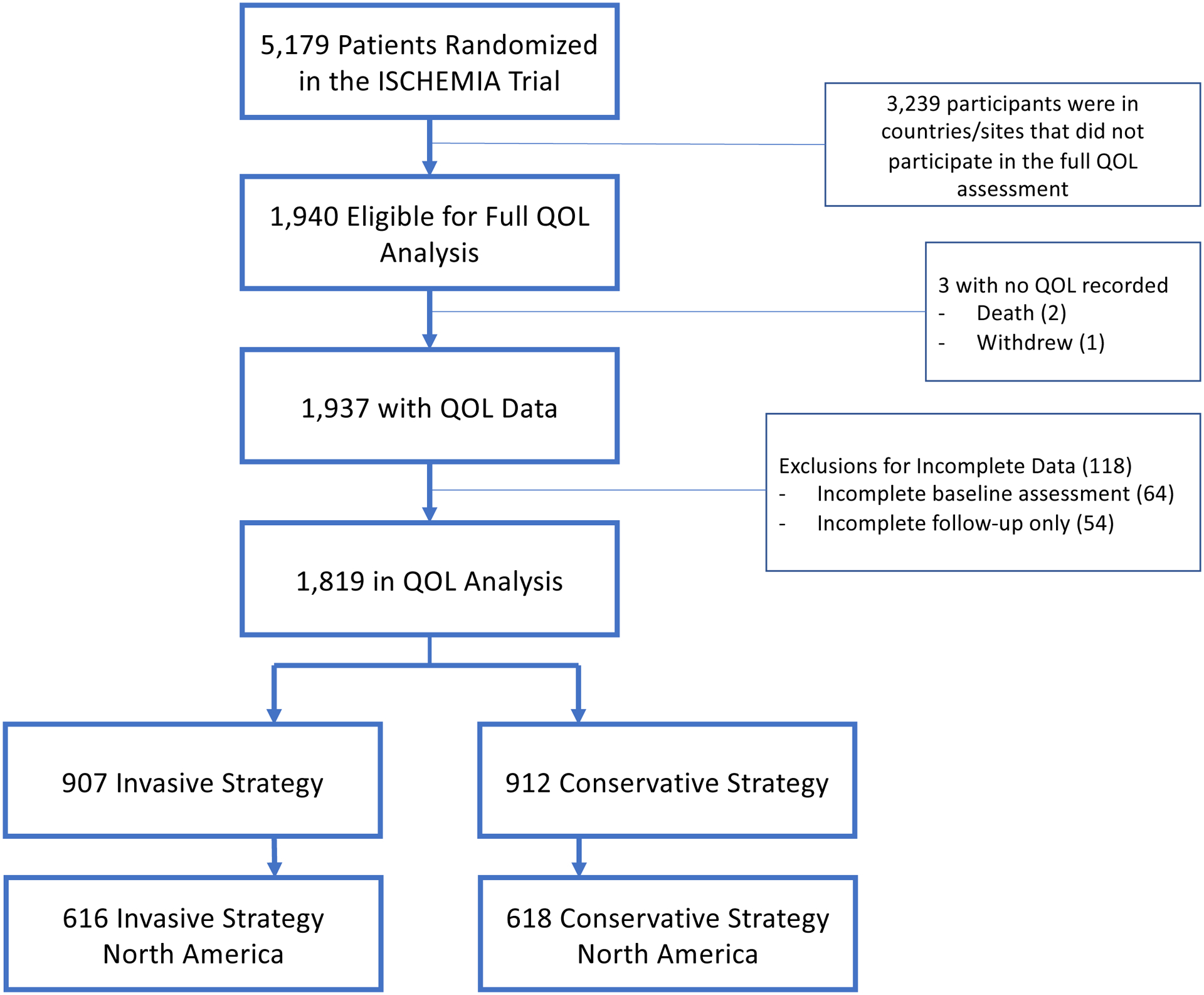
Flow of Participants in the Comprehensive Quality of Life Substudy Cohort in ISCHEMIA
Overall, baseline characteristics were well balanced by treatment in the substudy cohort overall (Table 1), as well as in the North American subgroup (Supplement Table 1). In the overall cohort, median age was 67 years, 19.2% were female, and 15.9% were non-white (Table 1). Severe ischemia was present in 45.6% of participants and moderate ischemia was present in 42.6%. Median ejection fraction was 60%. Among those with a screening coronary computed tomography angiogram at baseline (n=1521), 68.4% had multivessel disease (≥50% stenosis). Of those who could also be assessed for number of diseased vessels, 32.7% had three-vessel disease. According to Canadian Cardiovascular Society (CCS) angina classification, 30.6% had no episodes of angina in the month prior to enrollment, and 17.5% of patients reported never having angina.
Table 1:
Baseline Characteristics in the ISCHEMIA Comprehensive Quality of Life Substudy by Treatment Group
| Characteristic | Overall (N = 1819) |
Invasive (N = 907) |
Conservative (N = 912) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age at Randomization (yrs) | 0.582 | |||
| N | 1819 | 907 | 912 | |
| Median (Q1, Q3) | 67 (61, 72) | 67 (61, 72) | 67 (61, 72) | |
| Sex | 0.511 | |||
| Male | 1,469 / 1,819 (80.8%) | 738 / 907 (81.4%) | 731 / 912 (80.2%) | |
| Female | 350 / 1,819 (19.2%) | 169 / 907 (18.6%) | 181 / 912 (19.8%) | |
| Race | 0.287 | |||
| American Indian or Alaskan Native | 4 / 1,802 (0.2%) | 3 / 901 (0.3%) | 1 / 901 (0.1%) | |
| Asian | 135 / 1,802 (7.5%) | 72 / 901 (8.0%) | 63 / 901 (7.0%) | |
| Native Hawaiian or Other Pacific Islander | 9 / 1,802 (0.5%) | 4 / 901 (0.4%) | 5 / 901 (0.6%) | |
| Black or African American | 132 / 1,802 (7.3%) | 54 / 901 (6.0%) | 78 / 901 (8.7%) | |
| White | 1,516 / 1,802 (84.1%) | 765 / 901 (84.9%) | 751 / 901 (83.4%) | |
| Multiple Races Reported | 6 / 1,802 (0.3%) | 3 / 901 (0.3%) | 3 / 901 (0.3%) | |
| Ethnicity | 0.309 | |||
| Hispanic or Latino | 310 / 1,717 (18.1%) | 145 / 848 (17.1%) | 165 / 869 (19.0%) | |
| Not Hispanic or Latino | 1,407 / 1,717 (81.9%) | 703 / 848 (82.9%) | 704 / 869 (81.0%) | |
| Clinical History | ||||
| Hypertension | 1,427 / 1,817 (78.5%) | 715 / 905 (79.0%) | 712 / 912 (78.1%) | 0.627 |
| Diabetes | 748 / 1,819 (41.1%) | 376 / 907 (41.5%) | 372 / 912 (40.8%) | 0.773 |
| Prior MI | 354 / 1,812 (19.5%) | 176 / 904 (19.5%) | 178 / 908 (19.6%) | 0.942 |
| Prior PCI | 463 / 1,816 (25.5%) | 260 / 905 (28.7%) | 203 / 911 (22.3%) | 0.002 |
| Prior CABG | 98 / 1,819 (5.4%) | 61 / 907 (6.7%) | 37 / 912 (4.1%) | 0.012 |
| Atrial fibrillation/atrial flutter | 100 / 1,818 (5.5%) | 66 / 907 (7.3%) | 34 / 911 (3.7%) | <.001 |
| Non-Cardiac Vascular and Comorbidity History | ||||
| Prior transient ischemia attack | 68 / 1,814 (3.7%) | 37 / 902 (4.1%) | 31 / 912 (3.4%) | 0.431 |
| Prior Stroke | 74 / 1,819 (4.1%) | 41 / 907 (4.5%) | 33 / 912 (3.6%) | 0.330 |
| Prior cerebrovascular disease or peripheral artery disease | 265 / 1,810 (14.6%) | 138 / 902 (15.3%) | 127 / 908 (14.0%) | 0.430 |
| Prior TIA, prior stroke, or prior PAD | 265 / 1,819 (14.6%) | 138 / 907 (15.2%) | 127 / 912 (13.9%) | 0.436 |
|
Qualifying Stress Test Core Lab Interpretation Ischemia Severity by Imaging Modality | ||||
| Stress Imaging Overall (severity) | 0.750 | |||
| Severe | 826 / 1,812 (45.6%) | 406 / 904 (44.9%) | 420 / 908 (46.3%) | |
| Moderate | 772 / 1,812 (42.6%) | 392 / 904 (43.4%) | 380 / 908 (41.9%) | |
| Mild | 124 / 1,812 (6.8%) | 61 / 904 (6.7%) | 63 / 908 (6.9%) | |
| None | 90 / 1,812 (5.0%) | 45 / 904 (5.0%) | 45 / 908 (5.0%) | |
| Exercise Tolerance Test (ETT) | 0.204 | |||
| Severe | 72 / 102 (70.6%) | 36 / 47 (76.6%) | 36 / 55 (65.5%) | |
| Moderate | 19 / 102 (18.6%) | 8 / 47 (17.0%) | 11 / 55 (20.0%) | |
| Mild | 6 / 102 (5.9%) | 1 / 47 (2.1%) | 5 / 55 (9.1%) | |
| None | 5 / 102 (4.9%) | 2 / 47 (4.3%) | 3 / 55 (5.5%) | |
| CCTA Findings | ||||
| Any Obstructive Disease >=50% Stenosis by CCTA | 1,489 / 1,521 (97.9%) | 746 / 760 (98.2%) | 743 / 761 (97.6%) | 0.477 |
| Multi-vessel disease >=50% stenosis by CCTA | 1,040 / 1,521 (68.4%) | 514 / 760 (67.6%) | 526 / 761 (69.1%) | 0.507 |
| Vessels >=50% stenosis by CCTA | 0.174 | |||
| 1 | 270 / 1,521 (17.8%) | 144 / 760 (18.9%) | 126 / 761 (16.6%) | |
| 2 | 380 / 1,521 (25.0%) | 172 / 760 (22.6%) | 208 / 761 (27.3%) | |
| 3+ | 497 / 1,521 (32.7%) | 252 / 760 (33.2%) | 245 / 761 (32.2%) | |
| Angina and Heart Failure History | ||||
| Participant has ever had angina | 1,500 / 1,819 (82.5%) | 757 / 907 (83.5%) | 743 / 912 (81.5%) | 0.264 |
| Angina over the past month | 0.129 | |||
| None | 557 / 1,818 (30.6%) | 265 / 906 (29.2%) | 292 / 912 (32.0%) | |
| I | 563 / 1,818 (31.0%) | 269 / 906 (29.7%) | 294 / 912 (32.2%) | |
| II | 634 / 1,818 (34.9%) | 334 / 906 (36.9%) | 300 / 912 (32.9%) | |
| III | 63 / 1,818 (3.5%) | 37 / 906 (4.1%) | 26 / 912 (2.9%) | |
| IV | 1 / 1,818 (0.1%) | 1 / 906 (0.1%) | 0 / 912 (0.0%) | |
| New Onset of Angina Over the Past 3 Months | 315 / 1,663 (18.9%) | 157 / 825 (19.0%) | 158 / 838 (18.9%) | 0.927 |
| Angina began or became more frequent over the past 3 months | 566 / 1,807 (31.3%) | 284 / 903 (31.5%) | 282 / 904 (31.2%) | 0.907 |
| Prior Heart Failure | 67 / 1,819 (3.7%) | 33 / 907 (3.6%) | 34 / 912 (3.7%) | 0.919 |
| Continuous ejection fraction (%) | 0.954 | |||
| N | 1687 | 845 | 842 | |
| Median (Q1, Q3) | 60 (55, 65) | 60 (55, 65) | 60 (55, 65) | |
| Ejection fraction at peak stress | 0.578 | |||
| N | 1527 | 763 | 764 | |
| Median (Q1, Q3) | 57 (51, 65) | 58 (51, 65) | 57 (50, 65) | |
| NYHA CLASS | 0.895 | |||
| None | 1,092 / 1,819 (60.0%) | 544 / 907 (60.0%) | 548 / 912 (60.1%) | |
| NYHA Class I | 441 / 1,819 (24.2%) | 223 / 907 (24.6%) | 218 / 912 (23.9%) | |
| NYHA Class II | 286 / 1,819 (15.7%) | 140 / 907 (15.4%) | 146 / 912 (16.0%) | |
| Baseline angina frequency | 0.437 | |||
| Daily (score 0–30) | 37 / 1,815 (2.0%) | 21 / 905 (2.3%) | 16 / 910 (1.8%) | |
| Weekly (score 31–60) | 250 / 1,815 (13.8%) | 125 / 905 (13.8%) | 125 / 910 (13.7%) | |
| Monthly (score 61–99) | 699 / 1,815 (38.5%) | 353 / 905 (39.0%) | 346 / 910 (38.0%) | |
| None (score = 100) | 829 / 1,815 (45.7%) | 406 / 905 (44.9%) | 423 / 910 (46.5%) | |
CCS=Canadian Cardiovascular Society, CABG=coronary artery bypass grafting surgery, CCTA=coronary computed tomography angiography, NYHA=New York Heart Association, N=number, PAD=peripheral artery disease, PCI=percutaneous coronary intervention, Q1=quartile 1, Q2=quartile 2, TIA=transient ischemic attack
Compared with North American patients (n=1,234), international patients (n=585) in this substudy had more core laboratory assessed severe ischemia (Supplement Table 2). However, no differences were present in baseline SAQ Summary scores or SAQ Angina Frequency scores.
Compared with the 1,819 patients participating in this substudy, the remaining 3,360 ISCHEMIA patients tended to be younger (median age 63 years versus 67 for participants), more often female (24.3% versus 19.2%), and more often Asian (40.6% versus 7.5%) (Supplement Table 3). Nonparticipants also had a higher prevalence of severe ischemia at baseline testing (59.9% versus 45.6%) but no difference in extent of coronary artery disease (CAD) by coronary computed tomography angiogram. No baseline anginal episodes in the 30 days preceding enrollment were reported using the SAQ Angina Frequency score in 38.9% of nonparticipants and 45.7% of participants (Supplement Table 3).
Quality of Life Outcomes by Intention-to-Treat
QOL Outcomes Available from All Substudy Participants
Participants in both treatment groups reported large improvements in disease-related health status as measured by the median SAQ-19 Summary score through follow-up (Table 2). At 12 months, the invasive strategy showed a 12.0 point mean improvement from baseline while the conservative strategy mean improved by 8.9 points. Mean scores for the INV arm did not change between 12 months and final study assessment, whereas a small additional improvement was observed for the CON patients (Table 2). The adjusted treatment group difference (INV minus CON) was greatest at 12 months (2.3 points, 95% CI 0.9,3.7) and had attenuated by 36 months (0.2 points, 95% CI −1.5, 2.0), with an overall mean difference of 1.4 points (95% CI 0.2, 2.5) (Figure 2).
Table 2:
Quality of Life Measures by Intention-to-Treat in the Comprehensive Quality of Life Substudy in ISCHEMIA
| Invasive Strategy | Conservative Strategy | ||||||
|---|---|---|---|---|---|---|---|
| Median [q1 q3] |
Mean (SD) |
N=907 | Median [q1 q3] |
Mean (SD) |
N=912 | Adjusted Difference (95% CI) |
|
|
SAQ Summary Score Scale: 0–100 such that 0 represents the worst possible health status and 100 the highest | |||||||
| Baseline | 78 [65, 90] | 76.0 (16.9) | 907 | 80 [65, 92] | 76.9 (17.6) | 912 | −0.9 (−2.5, 0.7) |
| 3 month | 92 [79, 97] | 85.2 (16.1) | 863 | 88 [75, 95] | 83.2 (16.1) | 860 | 1.9 (0.4, 3.4) |
| 12 month | 93 [83, 97] | 88.0 (14.3) | 833 | 91 [79, 97] | 85.8 (14.9) | 825 | 2.3 (0.9, 3.7) |
| 24 month | 94 [83, 97] | 88.7 (13.6) | 615 | 92 [82, 97] | 87.0 (14.3) | 601 | 1.7 (0.2, 3.1) |
| 36 month | 94 [83, 97] | 87.9 (14.9) | 411 | 93 [82, 97] | 87.5 (14.5) | 411 | 0.2 (−1.5, 2.0) |
| Close-out | 94 [83, 98] | 88.5 (14.0) | 605 | 93 [82, 97] | 87.6 (14.0) | 619 | 0.7 (−0.8, 2.2) |
| All follow-up | 93 [82, 97] | 87.5 (14.7) | 3327 | 91 [79, 97] | 85.9 (15.0) | 3316 | 1.4 (0.2, 2.5) |
|
Rose Dyspnea Scale Scores range from 0 to 4, where 0 indicates no dyspnea with activity and 4 indicates significant limitations due to dyspnea | |||||||
| Baseline | 1 [0, 2] | 1.1 (1.3) | 888 | 1 [0, 2] | 1.1 (1.3) | 894 | 0 (−0.1, 0.1) |
| 3 month | 0 [0, 1] | 0.8 (1.3) | 820 | 0 [0, 1] | 1.0 (1.3) | 833 | −0.1 (−0.2, 0) |
| 12 month | 0 [0, 1] | 0.7 (1.3) | 808 | 0 [0, 1] | 0.8 (1.2) | 792 | −0.1 (−0.2, 0) |
| 24 month | 0 [0, 1] | 0.8 (1.3) | 593 | 0 [0, 1] | 0.8 (1.2) | 575 | 0.1 (−0.1, 0.2) |
| 36 month | 0 [0, 1] | 0.8 (1.3) | 396 | 0 [0, 1] | 0.9 (1.3) | 391 | 0 (−0.2, 0.1) |
| Close-out | 0 [0, 1] | 0.8 (1.3) | 579 | 0 [0, 1] | 0.9 (1.3) | 580 | −0.1 (−0.3, 0) |
| All follow-up | 0 [0, 1] | 0.8 (1.3) | 3196 | 0 [0, 1] | 0.9 (1.3) | 3171 | −0.1 (−0.2, 0) |
|
EQ-5D-VAS Scale: 0 to 100; higher scores denote better self-reported health state | |||||||
| Baseline | 75 [60, 81] | 71.4 (16.6) | 889 | 75 [60, 80] | 71.9 (17.2) | 895 | −0.5 (−2.1, 1.1) |
| 3 month | 80 [65, 90] | 74.8 (17.1) | 839 | 80 [70, 85] | 75.2 (15.9) | 838 | −0.6 (−2.2, 1.0) |
| 12 month | 80 [70, 90] | 75.6 (17.2) | 816 | 80 [70, 90] | 76.3 (16.8) | 803 | −0.6 (−2.3, 1.0) |
| 24 month | 80 [70, 90] | 76.6 (16.9) | 593 | 80 [70, 90] | 77.2 (16.1) | 586 | −0.4 (−2.1, 1.4) |
| 36 month | 80 [70, 90] | 77.1 (16.8) | 399 | 80 [70, 90] | 76.2 (16.8) | 402 | 0.9 (−1.1, 2.9) |
| Close-out | 80 [70, 90] | 76.8 (16.9) | 588 | 80 [70, 87] | 75.8 (15.7) | 606 | 0.7 (−1.0, 2.5) |
| All follow-up | 80 [70, 90] | 76.0 (17.0) | 3235 | 80 [70, 90] | 76.1 (16.3) | 3235 | 0.0 (−1.3, 1.3) |
|
Patient Health Questionnaire-8 Scale: 0 to 24; higher scores indicating greater severity of depression | |||||||
| Baseline | 2 [1, 6] | 3.9 (4.5) | 847 | 3 [0, 6] | 4.0 (4.7) | 842 | −0.2 (−0.6, 0.2) |
| 3 month | 2 [0, 5] | 3.4 (4.6) | 777 | 2 [0, 5] | 3.2 (3.9) | 778 | 0.2 (−0.2, 0.6) |
| 12 month | 2 [0, 5] | 3.2 (4.3) | 764 | 1 [0, 5] | 3.1 (4.2) | 756 | 0.0 (−0.4, 0.4) |
| 24 month | 2 [0, 4] | 3.1 (4.1) | 565 | 2 [0, 5] | 3.3 (4.3) | 558 | −0.1 (−0.6, 0.3) |
| 36 month | 2 [0, 5] | 3.3 (4.5) | 386 | 2 [0, 4] | 3.1 (4.4) | 385 | 0.1 (−0.4, 0.6) |
| Close-out | 1 [0, 4] | 2.9 (4.0) | 554 | 1 [0, 4] | 2.9 (4.0) | 572 | 0.0 (−0.4, 0.4) |
| All follow-up | 2 [0, 5] | 3.2 (4.3) | 3046 | 2 [0, 4] | 3.1 (4.2) | 3049 | 0.0 (−0.3, 0.4) |
Figure 2:
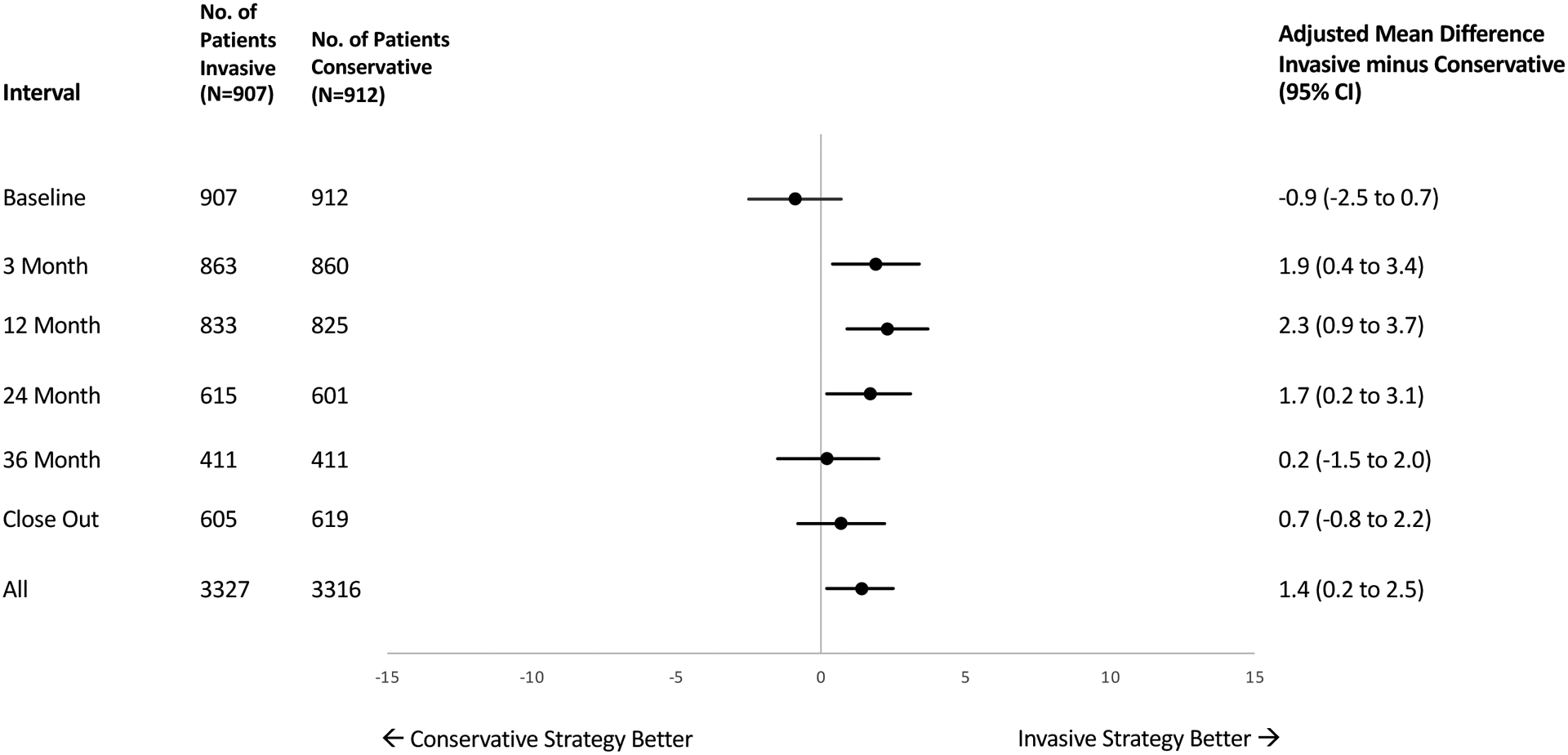
Treatment Differences in Seattle Angina Questionnaire Summary Scores in the ISCHEMIA Comprehensive Quality of Life Substudy
Mean Rose Dyspnea scores at baseline were 1.1 in each treatment group with little change throughout follow-up (Table 2). The difference between treatment groups was consistent over the course of the study and was −0.1 (95% CI −0.2,0) at 3 months, 12 months, and overall.
The EQ-5D Visual Analog Scale mean scores (assessing self-rated global health on 0–100 scale) improved in both treatment groups by 12 months (an average of 4.2 points for INV and 4.4 points for CON) and those improvements were maintained throughout follow-up (Table 2). Differences between treatment groups were small at all follow-up intervals and did not reach statistical or clinical significance (Table 2, Supplement Figure 1).
The frequency of depressive symptoms was low at baseline and was further reduced in follow-up (Table 2). There were no statistically or clinically significant differences between groups throughout follow-up (Table 2, Supplement Figure 2). Moderate/severe depression (PHQ-8 score ≥10) was present at baseline in 11.5% of INV and 12.8% CON (Supplement Table 4). At 12 months and 36 months, the corresponding values were 9.0% and 7.8%, and 9.8% and 9.6%, respectively.
Additional QOL Outcomes Available from North American Participants
Baseline DASI scores showed moderately reduced functional status (mean score 25 in both arms) with small and equivalent improvements over follow-up in both treatment arms. Overall mean differences between groups were not statistically significant at any timepoint (Table 3, Supplement Figure 3).
Table 3:
Quality of Life Measures in the North American Comprehensive Quality of Life Substudy Population
| Invasive Strategy | Conservative Strategy | ||||||
|---|---|---|---|---|---|---|---|
| Median [q1 q3] |
Mean (SD) |
N=606 | Median [q1 q3] |
Mean (SD) |
N=618 | Adjusted Difference (95% CI) |
|
|
DASI Score Scale: 0 to 58.2; higher scores denote greater functional capacity | |||||||
| Baseline | 22 [11, 37] | 24.9 (16.4) | 557 | 21 [11, 37] | 25.2(16.6) | 569 | −0.5 (−2.3, 1.4) |
| 3 month | 23 [13, 37] | 25.7 (16.2) | 549 | 27 [13, 37] | 26.6 (15.4) | 543 | −1.3 (−3.1, 0.5) |
| 12 month | 27 [13, 40] | 27.6 (16.6) | 538 | 27 [13, 40] | 27.4 (16.4) | 537 | 0.3 (−1.6, 2.2) |
| 24 month | 27 [15, 39] | 27.9 (16.4) | 408 | 27 [13, 39] | 27.6 (16.3) | 412 | 0.2 (−1.8, 2.2) |
| 36 month | 27 [13, 37] | 26.5 (16.8) | 277 | 25 [13, 38] | 26.5 (16.6) | 290 | 0.3 (−1.9, 2.5) |
| Close-out | 25 [13, 43] | 27.0 (16.6) | 434 | 26 [13, 37] | 26.3 (15.8) | 445 | 1.2 (−0.7, 3.2) |
| All follow-up | 26 [13, 39] | 26.9 (16.5) | 2206 | 27 [13, 38] | 26.9 (16.0) | 2227 | 0.1 (−1.5, 1.8) |
|
RAND General Health Rating Scale: 0 to 100; higher score indicating greater overall health | |||||||
| Baseline | 50 [25, 75] | 49.1 (22.2) | 593 | 50 [25, 75] | 51.6 (22.6) | 596 | −2.4 (−4.9, 0.1) |
| 3 month | 50 [50, 75] | 54.4 (22.8) | 566 | 50 [50, 75] | 53.5 (22.6) | 565 | 0.6 (−2.0, 3.2) |
| 12 month | 50 [50, 75] | 56.9 (24.0) | 555 | 50 [50, 75] | 54.9 (22.3) | 564 | 1.9 (−0.7, 4.6) |
| 24 month | 50 [50, 75] | 57.2 (24.0) | 433 | 50 [50, 75] | 55.2 (22.9) | 430 | 1.9 (−1.0, 4.8) |
| 36 month | 50 [50, 75] | 55.7 (24.6) | 293 | 50 [50, 75] | 55.9 (23.0) | 312 | −0.7 (−4.1, 2.7) |
| Close-out | 50 [50, 75] | 57.0 (24.3) | 464 | 50 [50, 75] | 54.6 (23.1) | 481 | 2.7 (−0.2, 5.6) |
| All follow-up | 50 [50, 75] | 56.2 (23.8) | 2311 | 50 [50, 75] | 54.7 (22.7) | 2352 | 1.3 (−0.9, 3.5) |
|
Perceived Stress Scale - 4 Scale: 0 to 16; higher values denoting greater perceptions of stress | |||||||
| Baseline | 4 [1, 6] | 4.2 (3.0) | 591 | 4 [1, 7] | 4.4 (3.3) | 594 | −0.2 (−0.6, 0.1) |
| 3 month | 3 [1, 6] | 3.5 (3.1) | 539 | 2 [0, 6] | 3.2 (3.1) | 550 | 0.2 (−0.2, 0.5) |
| 12 month | 2 [1, 6] | 3.4 (3.2) | 539 | 2 [0, 5] | 3.2 (3.2) | 535 | 0.1 (−0.3, 0.5) |
| 24 month | 3 [1, 6] | 3.5 (3.1) | 404 | 2 [0, 6] | 3.4 (3.2) | 409 | 0.0 (−0.4, 0.4) |
| 36 month | 3 [1, 6] | 3.8 (3.3) | 281 | 2 [0, 5] | 3.0 (3.1) | 291 | 0.6 (0.2, 1.1) |
| Close-out | 2 [0, 6] | 3.1 (3.2) | 439 | 2 [0, 5] | 2.7 (3.0) | 440 | 0.3 (−0.1, 0.7) |
| All follow-up | 2 [1, 6] | 3.4 (3.2) | 2202 | 2 [0, 5] | 3.1 (3.1) | 2225 | 0.2 (0.0, 0.5) |
|
Life Orientation Test Score Scale: 0 to 24; higher score indicating greater optimism | |||||||
| Baseline | 18 [14, 21] | 17.4 (4.9) | 582 | 18 [14, 22] | 17.4 (5.0) | 572 | 0.1 (−0.4, 0.7) |
| 3 month | 20 [15, 23] | 18.3 (5.3) | 539 | 19 [15, 23] | 18.4 (4.9) | 549 | 0.0 (−0.6, 0.6) |
| 12 month | 20 [15, 23] | 18.6 (5.1) | 540 | 20 [16, 23] | 19.2 (4.6) | 532 | −0.6 (−1.2, −0.1) |
| 24 month | 20 [15, 23] | 18.6 (4.9) | 408 | 20 [15, 24] | 18.6 (5.1) | 405 | −0.1 (−0.7, 0.6) |
| 36 month | 20 [15, 24] | 18.6 (5.0) | 274 | 21 [16, 24] | 19.2 (5.3) | 287 | −0.5 (−1.2, 0.3) |
| Close-out | 20 [16, 24] | 18.9 (4.9) | 427 | 21 [16, 23] | 19.3 (4.7) | 434 | −0.4 (−1.0, 0.2) |
| All follow-up | 20 [15, 23] | 18.6 (5.1) | 2188 | 20 [16, 24] | 18.9 (4.9) | 2207 | −0.3 (−0.8, 0.2) |
Baseline scores for the PSS-4 averaged 4.2 to 4.4, indicating relatively low overall perceptions of life situations as stressful relative to published population norms. Scores improved (got numerically smaller) in follow-up for both treatment groups with the overall mean follow-up showing no difference between groups (0.2, 95% CI 0.0 to 0.5) (Table 3, Supplement Figure 4). Mean scores for the Life Orientation Test-Revised were in the intermediate range for optimism at baseline (mean scores 17.4 in both arms) and showed little change during follow-up and no incremental treatment effects (Table 3, Supplement Figure 5). The RAND General Health Rating showed no incremental treatment effects (Table 3).
Analyses Comparing QOL Outcomes in Patients According to Baseline Angina Status
Of the 907 patients in the INV group, 533 had baseline SAQ Angina Frequency scores of 81–100 indicating rare or no angina, and 372 had baseline SAQ Angina Frequency scores of 0–80 indicating the presence of more frequent angina in the month prior to enrollment (Supplement Table 5). In the 912 patients randomized to the CON group, 538 had Angina Frequency scores of 81–100 at baseline and 372 had scores of 0–80. Two patients in each treatment group did not report SAQ Angina Frequency scores.
In patients with SAQ Angina Frequency scores of 0–80 at baseline, mean baseline SAQ Summary scores were 62.6 for INV and 62.0 for CON and improved at 12 months by an average of 21.7 points for INV and 17.7 points for CON. The mean adjusted 12-month difference was 4.7 points (95% CI 2.2, 7.2), and the difference averaged over all follow-up was 3.7 points (95% CI 1.6, 5.8) (Figure 3, Supplement Table 5A). In the patients with baseline SAQ Angina Frequency scores of 81–100, mean baseline SAQ Summary scores were 85.3 in INV and 87.1 in CON and improved over time but without any incremental treatment differences (Figure 3, Supplement Table 5B).
Figure 3:
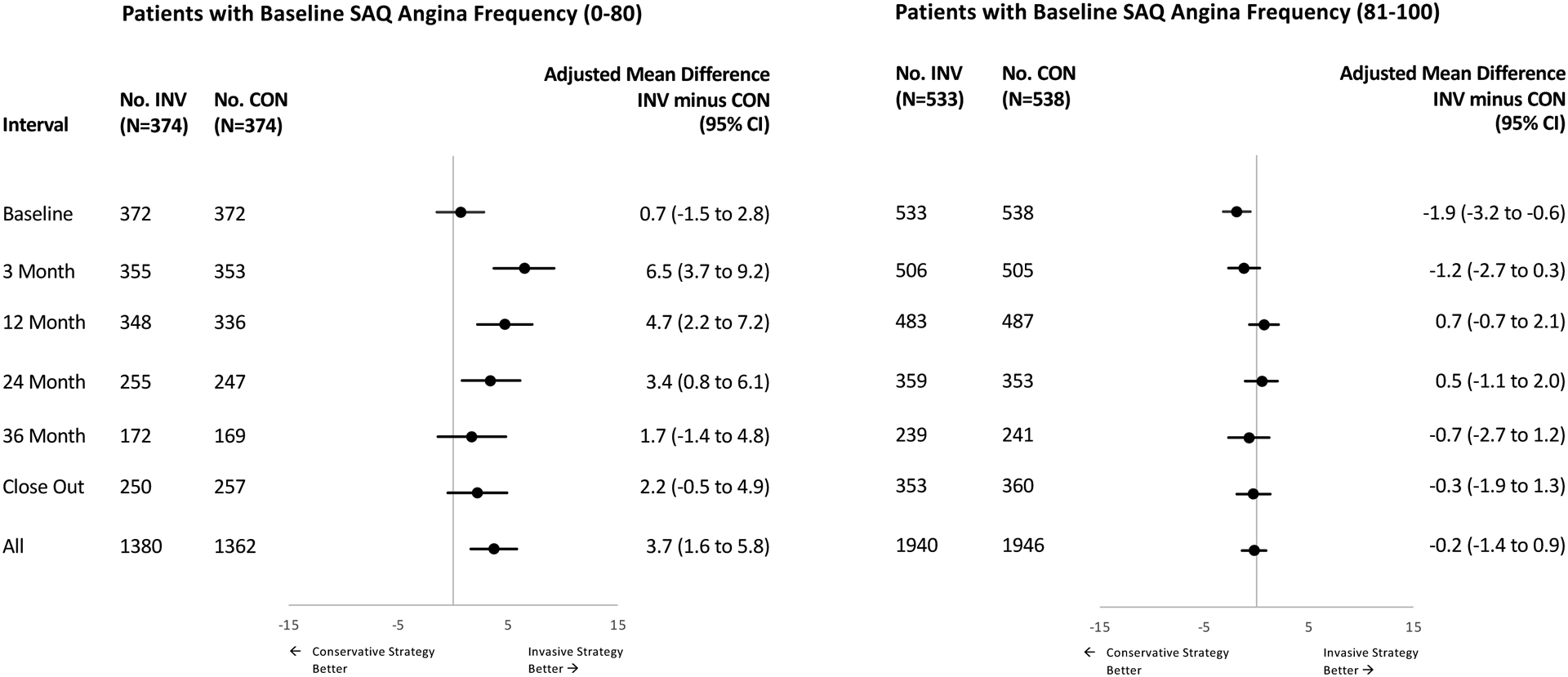
Treatment Differences in Seattle Angina Questionnaire Summary Scores Stratified by Seattle Angina Frequency Scores at Baseline in the ISCHEMIA Comprehensive Quality of Life Substudy
Patterns seen in the SAQ component domain scores were largely concordant with those seen for the SAQ Summary scores (Supplement Table 5). With a baseline SAQ Angina Frequency score 0–80, the three major domains of the SAQ all showed comparable improvement with INV: Physical Limitations 3.2 points (95% CI 0.2, 6.1), Angina Frequency 3.2 points (95% CI 1.2, 5.1), Quality of Life/Health Perceptions 5.3 points (95% CI 2.8, 7.8). With SAQ Angina Frequency scores above 80, none of the SAQ subscales showed clinically consequential treatment effects.
Patients with more frequent angina at baseline had substantially lower DASI scores at baseline (20.3 INV, 18.1 CON) than those with rare or no angina (28.1 INV, 30.0 CON) (Supplement Table 6). Averaged over all follow-up, the INV group improved by 3.2 points (95% CI 0.6, 5.7) more than CON in patients with baseline SAQ Angina Frequency scores 0–80, but no incremental improvement was evident in the DASI for the subgroup with baseline SAQ Angina Frequency scores above 80 (Supplement Table 6).
Mean baseline PHQ-8 scores in patients with rare or no angina were more favorable than those for patients with more frequent angina at baseline, but moderate to severe depression (PHQ-8 ≥10) was infrequent regardless of baseline angina status (data not shown). No evidence for a treatment effect on depressive symptoms was seen according to the amount of baseline angina reported (Supplement Table 6). Similarly, there was little relationship between baseline angina frequency and perceived stress (PSS-4) or optimism (LOT-R) and no impact of baseline angina on treatment differences (Supplement Table 6).
Magnitude of Response Analysis
For a “magnitude of response” (“responder”) analysis, we divided patients for descriptive purposes into three subgroups based on overall disease QOL burden as reflected by different levels of the SAQ Summary score. In patients with a SAQ Summary score above 80 at baseline, signifying low angina-related disease burden, the INV strategy produced more 12-month “responders” when response magnitude (12-month scores – baseline scores) was defined as an improvement of either ≥5 points (39% INV, 30% CON), ≥10 points (17% INV, 13% CON), or ≥15 points (7% INV, 3% CON) (Supplement Figure 6). In patients with moderate baseline angina-related burden (SAQ Summary score 61–80), the INV strategy also produced more responders when response magnitude was defined as improvement ≥5 points (80% INV, 71% CON), ≥10 points (72% INV, 57% CON), ≥15 points (64% INV, 46% CON), and ≥20 points (49% INV, 31% CON) (Supplement Figure 6). For patients with the highest baseline level of angina-related burden (SAQ Summary score ≤60), INV was also associated with more “responders” regardless of the criteria used to define a “response”: ≥5 points (83% INV, 80% CON), ≥10 points (80% INV, 74% CON), ≥15 points (74% INV, 64% CON), and ≥20 points (67% INV, 57% CON). A similar pattern was seen when patients were grouped by baseline angina frequency (Supplement Figure 7), but there was an inconsistent pattern of response magnitude by treatment when patients were grouped by baseline levels of DASI (Supplement Figure 8).
DISCUSSION
This comprehensive examination of QOL domains in ISCHEMIA confirms and expands upon our previous report.8 Four findings from this substudy deserve specific emphasis. First, while several dimensions of quality of life were improved on average by randomization to the initial invasive treatment strategy relative to the initial conservative strategy, these benefits (in the domains of symptoms, physical functioning, and health/disease perceptions) were seen only in patients with more frequent anginal symptoms at baseline (SAQ Angina Frequency scores of 80 or less). Second, patients with CCD and moderate or severe ischemia on stress testing who reported rare or absent angina during the 4 weeks preceding trial randomization (SAQ Angina Frequency scores > 80) did not experience a measurable difference in either functioning (SAQ Physical Limitations, DASI) or well-being (SAQ Disease Perceptions/QOL, EQ-5D) according to treatment assignment. Third, both treatment groups experienced substantial early QOL improvement (by three months) evident in multiple domains that was largely maintained over the duration of the trial follow-up. The magnitude of this baseline to early follow-up improvement seen in both INV and CON arms exceeded the size of the mean adjusted INV-CON treatment differences for all QOL scales showing such differences. Finally, the assigned treatment strategy had little to no effects on generic health status, depression, perceived stress, or optimism.
We have previously reported a comparison of treatment outcomes in the parent ISCHEMIA cohort using the SAQ-7 short form.8 Overall, of the 4,617 who provided SAQ-7 data, 35% reported no angina in the preceding month at baseline (SAQ Angina Frequency score 100). SAQ-7 Summary scores showed the largest improvement with INV relative to CON in the subset of patients with more frequent baseline angina (SAQ Angina Frequency scores of 80 or less). The QOL benefits of an invasive approach, with revascularization of significant obstructive CAD as technically feasible, thus depend on how symptomatic the CAD was at the time of treatment selection. Our results also show that revascularization of ISCHEMIA-eligible patients with moderate to severe ischemia but minimal to no associated angina (SAQ Angina Frequency scores >80, which includes what has been referred to as “silent ischemia”) does not generate consequential QOL benefits in either the short- or longer-term. Since the median ejection fraction in ISCHEMIA was 60% (25th to 75th percentiles 55%, 65%), these conclusions may not apply to minimally symptomatic patients with significant ischemia-related left ventricular dysfunction, for whom revascularization might restore enough ventricular functioning so as to permit a significant increase in functional capacity as well as relief of angina. Most symptomatic CCD patients learn to accommodate to their symptomatic limitations and to keep their daily physical activities below those limits (hedonic adaptation). Many are unaware that they have done so unless challenged by circumstances or exercise testing to exceed those limits. As long as patients keep their activity levels below their symptom-inducing threshold, more effective relief of ischemia may not be accompanied by detectable improvements in patient-reported physical functioning.6 However, we did observe important improvements in physical functioning with INV management when patients reported significant anginal symptoms at baseline.
However, the lack of a relationship between ischemia relief and QOL improvement may not simply be due to avoidance of more challenging activities on the part of patients. The absence of a dose response relationship between myocardial ischemia and QOL impairment was demonstrated in an analysis of the Objective Randomised Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina (ORBITA) trial. While treatment effect sizes (percutaneous coronary intervention versus sham percutaneous coronary intervention) for stress echo scores showed a clear monotonic relationship with lower pre-randomization fractional flow reserve values below a threshold of about 0.8, treatment effect sizes for SAQ Angina Frequency scores had no relationship at all with baseline fractional flow reserve values.23 However, a follow-up analysis from the ORBITA group showed that the placebo-controlled impact of percutaneous coronary intervention on SAQ Angina Frequency Scores varied significantly according to the extent of ischemia as reflected by the baseline stress echo score.24 These results emphasize the complex interrelationships at play in this treatment paradigm and the potential for results to vary according to assessment methods for ischemia and for extent of angina.
As shown in our response magnitude analysis (Supplement Figure 6), the percentage of patients who had 10-point, 15-point, or 20-point improvements at 12 months in SAQ Summary scores relative to their baseline scores showed a clinically relevant advantage for the INV strategy, primarily among those with more angina-related QOL impairment at baseline. For example, among patients with baseline scores between 81 and 100, corresponding to mild QOL impairment, 13% of CON and 17% of INV improved at 12 months by ≥10 points (about ½ SD), and that absolute difference was preserved if the criterion was changed to require a response of ≥15 points. For patients with greater baseline QOL impairment, INV showed a “responder” rate that exceeded CON by 10–18% even when the criterion for response was set at 20 points (equivalent to about 1 SD) (Supplement Figure 6). These patterns are not evident in the mean treatment effect size estimates. Thus, interpretation of the ISCHEMIA QOL treatment effects using the overall results obscures important heterogeneity evident when baseline angina status is accounted for.
While the intention of formal QOL assessment using structured instruments and data collection procedures is to capture a critical aspect of the patient journey that is largely invisible using standard clinical composite outcome measures, the interpretation of the results is often not straightforward. In particular, QOL assessment tools convert clinically familiar concepts to numeric score values that lack obvious intrinsic meaning.6 To aid in interpretability, QOL researchers attempt to establish benchmark values for minimal clinically important differences as well as small, moderate, and large effect sizes. However, those benchmarks apply to patient-level changes and can often be misleading when used to interpret mean trial-level QOL effect sizes, particularly where important heterogeneity of treatment responsiveness exists.25 In the present analysis, the mean effect of INV versus CON on the SAQ Summary score was a 1.6-point improvement. Given that the patient-level minimal clinically important difference for SAQ is generally considered to be ≥5 points, some might conclude from this that INV produces a statistically significant improvement in QOL that was clinically inconsequential. In our view, this would be incorrect for the reasons previously cited.
Thus, two important interpretation insights are provided by the response magnitude analysis. First, mean overall treatment difference magnitudes of QOL scores cannot be assessed in terms of clinical importance with patient-level interpretation benchmarks. Second, both treatment arms had some patients exhibiting quite large QOL score improvements over the first year of trial management. Thus, no simple effect size benchmark can distinguish the “response” to revascularization from that seen with medical management.
The Duke Activity Status Index (DASI) has been used as a primary QOL assessment tool in several randomized trials testing revascularization strategies.3, 26, 27 Although moderately correlated with the SAQ Physical Limitations score, the DASI was developed to provide a questionnaire-based analog of functional capacity as measured by cardiopulmonary testing.11, 15 While the DASI showed no treatment difference in the overall North American cohort in whom it was measured (Table 3), almost 60% of these patients had rare to no angina (SAQ Angina Frequency scores at baseline of 81–100). Among the subset with significant baseline angina, the mean overall improvement in DASI with INV relative to CON was 3.2 points (Supplement Table 6A). This magnitude of improvement equals the difference between being able to do light housework or walk a block or two on level ground with no difficulty versus having significant difficulties with these activities or being unable to perform them.3 The absence of a treatment benefit from INV on DASI scores in patients with rare to no baseline angina provides further support for the findings from the SAQ. Specifically, revascularization of patients likely to fit the designation “silent ischemia” in the context of ISCHEMIA eligibility did not improve patient-reported cardiac physical functioning. The lack of a coherent relationship between treatment effect magnitude and baseline cardiac functional impairment using DASI scores (Supplement Figure 8) further reinforces the pivotal role of angina, rather than overall physical functioning/impairment, in identifying those who can expect a sizeable improvement in QOL with initial invasive management.
Our initial report on QOL outcomes by treatment group in ISCHEMIA noted that effect sizes diminished somewhat over time.8 The posterior mean difference in SAQ Summary scores for a typical patient with a baseline score of about 74 was 4.2 points at 12 months, while at 36 months the mean difference was 2.9 points. Data from the present analysis show that in patients with a baseline SAQ Summary score of ≤80, mean scores showed the largest improvement in the first three months and by 12 months, scores in the INV arm had plateaued with very little change thereafter (Supplement Table 5A). Scores in the CON arm also showed the largest improvement in the first 3 months with a plateau phase starting at about 12 months, but with a modest incremental improvement between 12 months and the study close-out assessment. Thus, a narrowing of the QOL treatment effect size over time is not due to any evident lack of durability of INV outcomes. A similar pattern was seen in the COURAGE trial QOL outcomes for all the component SAQ subscales.5 One possible explanation for this pattern relates to the previously reported progressive increase in spontaneous MIs for the CON arm. A common occurrence in cardiology is that a CCD patient with stable angina who experiences an MI often has relief of anginal symptoms, possibly because the culprit myocardial region is no longer viable or fed by a stenotic coronary artery. In more recent times, an additional factor is the substantially increased likelihood of associated invasive management at the time of acute MI hospitalization. Thus, it is possible that some of the continued modest improvement in QOL scores for the CON arm could reflect an unexpected consequence of the higher late MI rate and attendant revascularization.7 Additional analyses are planned to examine whether the incremental narrowing of the INV:CON treatment difference is associated with more use of revascularization and/or with intensification of anti-anginal medical therapies in more symptomatic patients randomized to the CON strategy.
Limitations
Several caveats should be considered in the interpretation of our data. First, for administrative (particularly site enrollment burden) and financial reasons, our initial plans to collect a QOL battery at baseline and during follow-up from all enrolled patients in ISCHEMIA proved infeasible. The plan was modified so that the SAQ-7 was collected on the parent ISCHEMIA enrollment, and the battery of additional instruments were collected on a subgroup. While nonrandom selection into the substudy could bias our findings in complex ways, concordance of the larger trial QOL results with the present findings provides important reassurance that our findings are relevant for the trial overall. Second, ISCHEMIA was not a masked trial and knowledge of the treatment assignments could have distorted some of our measurements. However, the presence of larger treatment effects where expected based on previous research and no treatment effects in other domains, together with the durability of these effects over time, reduces the likelihood that the treatment benefits we observed for INV are due to either ascertainment bias or a nonspecific placebo-type effect. Third, some missing data are inevitable in a large, complex, international trial involving repeated patient interviews. The analysis method we used to accommodate the repeated measures nature of our measurements tolerates even a moderate amount of missingness without the need for imputation. Finally, in our intention-to-treat analysis, mean QOL scores in the INV arm were quite stable between 12 months and the last assessment, while scores in CON tended to show additional modest improvement. To the extent that this narrowing of mean treatment differences reflects increased late use of revascularization in the CON arm, our results may underestimate the true treatment-related (rather than strategy-related) differences.
Conclusions
In patients enrolled in ISCHEMIA with chronic coronary disease and moderate or severe ischemia on functional testing, an initial invasive strategy provided clinically important QOL benefits but only when patients had more frequent anginal episodes at the time of evaluation for treatment. Measures of depression, perceived stress, and optimism showed no treatment differences irrespective of baseline angina status. Invasive treatment of moderate or severe ischemia with rare or absent baseline angina was not associated with a meaningful improvement in QOL.
Supplementary Material
CLINICAL PERSPECTIVES.
What is new?
Patients with more frequent baseline angina reported greater improvements in the symptom, physical functioning, and psychological well-being dimensions of QOL when treated with an invasive strategy.
Patients who had rare/absent angina at baseline reported no consistent treatment-related QOL differences.
Measures of depression, perceived stress, and optimism showed no treatment differences irrespective of baseline angina status.
What are the clinical implications?
Clinicians can provide patients with expectations of quality of life benefits to be gained with an early invasive strategy when discussing risks and benefits of treatment options for chronic coronary disease.
ACKNOWLEDGEMENTS
We are particularly indebted to the coordinators at the ISCHEMIA sites who collected study data and to the patients who agreed to provide their quality of life data for the trial.
Drs. Mark and Anstrom had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data analysis was conducted by Kevin J. Anstrom, PhD, Robert Bigelow, PhD, and Sophia Anderson, MB of the Duke Clinical Research Institute.
SOURCES OF FUNDING:
Supported by grants U01HL105907, U01HL105462, U01HL105565, and U01HL105561 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the United States Department of Health and Human Services.
The main ISCHEMIA trial was also supported by grants (U01HL105907, U01HL105462, and U01HL105561) from the National Heart, Lung, and Blood Institute, by Arbor Pharmaceuticals and AstraZeneca Pharmaceuticals, and by a Clinical and Translational Science Award (11UL1 TR001445) from the National Center for Advancing Translational Sciences. Devices or medications used in the trial were provided by Abbott Vascular, Medtronic, St. Jude Medical, Volcano, Amgen, Arbor Pharmaceuticals, AstraZeneca Pharmaceuticals, Espero Pharmaceuticals, Merck Sharp & Dohme, Omron Healthcare, and Sunovion Pharmaceuticals
DISCLOSURES
Dr. Daniel B. Mark reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from HeartFlow, grants from Merck, outside the submitted work.
Dr. John A. Spertus reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; personal fees from Bayer, personal fees from Novartis, personal fees from Myokardia, personal fees from Merck, personal fees from Janssen, personal fees from United Healthcare, grants from American College of Cardiology, personal fees from Blue Cross Blue Shield of Kansas City, outside the submitted work; In addition, Dr. Spertus has a patent Copyright to Seattle Angina Questionnaire with royalties paid.
Dr. Robert Bigelow reports no funding.
Ms. Sophia Anderson reports no funding.
Ms. Melanie R. Daniels reports no funding.
Dr. Kevin J. Anstrom reports grants from funding from the National Institutes of Health, Merck, Bayer, Pfizer, and the Patient-Centered Outcomes Research Institute.
Ms. Khaula Baloch reports no funding.
Dr. David J. Cohen reports grant support from Abbott Vascular, Boston Scientific, Medtronic, Edwards Lifesciences, Corvia, CSI, Svelte and consulting income from Abbott Vascular, Boston Scientific, Medtronic, Edwards Lifesciences.
Dr. Claes Held reports grants from National Heart, Lung, and Blood Institute.
Dr. Shaun Goodman reports grants and personal fees from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants and personal fees from Bayer, personal fees from Boehringer Ingelheim/Eli Lilly, grants and personal fees from Bristol Myers Squibb/Pfizer, grants and personal fees from CSL Behring/PERFUSE, grants and personal fees from Daiichi-Sankyo/American Regent/DCRI, personal fees from Esperion/C5, grants and personal fees from Ferring, personal fees from GlaxoSmithKline, personal fees from HLS Therapeutics, personal fees from Merck, grants and personal fees from Novartis, personal fees from NovoNordisk, grants and personal fees from Regeneron/Sanofi, personal fees from Servier, outside the submitted work.
Dr. Sripal Bangalore reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Abbott Vascular, personal fees from Biotronik, Pfizer, Amgen, and Reata outside the submitted work.
Dr. Derek Cyr reports grants from National Heart, Lung and Blood Institute during the conduct of the study.
Dr. Harmony R. Reynolds reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Abbott Vascular, non-financial support from Siemens, non-financial support from BioTelemetry, outside the submitted work.
Dr. Karen P. Alexander reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Yves D. Rosenberg reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Gregg W. Stone reports grants and personal fees from the National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from Terumo, Amaranth, and Shockwave; personal fees and other from Valfix; personal fees from TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Claret, and Sirtex; personal fees and other from Ancora and Qool Therapeutics; other from Cagent, Applied Therapeutics, Biostar family of funds, and MedFocus family of funds; personal fees and other from SpectraWave; personal fees from MAIA Pharmaceuticals; personal fees and other from Orchestra Biomed; other from Aria; personal fees from Vectorious; and other from Cardiac Success, outside the submitted work.
Dr. David J. Maron reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Judith S. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular; Medtronic, Inc.; Abbott Laboratories (formerly St. Jude Medical, Inc); Royal Philips NV (formerly Volcano Corporation); Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc, Sunovion Pharmaceuticals, Inc. Espero BioPharma; and Amgen, Inc; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP.
ABBREVIATIONS
- CCD
Chronic coronary disease
- CCS
Canadian Cardiovascular Society
- CON
Initial conservative strategy
- DASI
Duke Activity Status Index
- DCRI
Duke Clinical Research Institute
- INV
Initial invasive strategy
- ISCHEMIA
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches trial
- LOT-R
Life Orientation Test-Revised
- NHLBI
National Heart, Lung, and Blood Institute
- NIH
National Institutes of Health
- PHQ-8
Patient Health Questionnaier-8
- PSS-4
Perceived Stress Scale-4
- QOL
Quality of life
- SAQ-7
7-item Seattle Angina Questionnaire
- SAQ
19-item Seattle Angina Questionnaire
- VAS
Visual analog scale
Footnotes
Supplemental Materials
Quality of Life Instruments Collected in the ISCHEMIA Trial
Clinical Trial Registration: clinicaltrials.gov identifier: NCT01471522, https://clinicaltrials.gov/ct2/show/NCT01471522
REFERENCES
- 1.Abdallah MS, Wang K, Magnuson EA, Spertus JA, Farkouh ME, Fuster V, Cohen DJ and Freedom Trial Investigators. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310:1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen DJ, Van Hout B, Serruys PW, Mohr FW, Macaya C, den Heijer P, Vrakking MM, Wang K, Mahoney EM, Audi S, et al. Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med. 2011;364:1016–1026. [DOI] [PubMed] [Google Scholar]
- 3.Hlatky MA, Rogers WJ, Johnstone I, Boothroyd D, Brooks MM, Pitt B, Reeder G, Ryan T, Smith H, Whitlow P, et al. Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1997;336:92–99. [DOI] [PubMed] [Google Scholar]
- 4.Neill WA, Branch LG, De Jong G, Smith NE, Hogan CA, Corcoran PJ, Jette AM, Balasco EM and Osberg S. Cardiac disability. The impact of coronary heart disease on patients’ daily activities. Arch Intern Med. 1985;145:1642–1647. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. [DOI] [PubMed] [Google Scholar]
- 6.Mark DB. Assessing quality-of-life outcomes in cardiovascular clinical research. Nat Rev Cardiol. 2016;13:286–308. [DOI] [PubMed] [Google Scholar]
- 7.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, Lopez-Sendon J, Alexander KP, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE Jr., Boden WE, Weintraub WS, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ISCHEMIA Trial Research Group, Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Alexander KP, Senior R, Boden WE, Stone GW, Goodman SG, Lopes RD, et al. Baseline characteristics and risk profiles of participants in the ISCHEMIA randomized clinical trial. JAMA Cardiol. 2019;4:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M and Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 12.EuroQol G EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 13.Rose GA and Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 14.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT and Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. [DOI] [PubMed] [Google Scholar]
- 15.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR and Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–654. [DOI] [PubMed] [Google Scholar]
- 16.Hays RD, Sherbourne CD and Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217–227. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Kamarck T and Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 18.Cohen S and Williamson GM. Perceived stress in a probability sample of the United States. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 19.Scheier MF, Carver CS and Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. [DOI] [PubMed] [Google Scholar]
- 20.Spertus J and Mark D. ISCHEMIA trial update. Am Heart J. 2019;218:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark DB, Lee KL and Harrell FE Jr. Understanding the role of P values and hypothesis tests in clinical research. JAMA Cardiol. 2016;1:1048–1054. [DOI] [PubMed] [Google Scholar]
- 22.Thomas M, Jones PG, Arnold SV and Spertus JA. Interpretation of the Seattle Angina Questionnaire as an outcome measure in clinical trials and clinical care: A review. JAMA Cardiol. 2021;6:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Lamee R, Howard JP, Shun-Shin MJ, Thompson D, Dehbi HM, Sen S, Nijjer S, Petraco R, Davies J, Keeble T, et al. Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease. Circulation. 2018;138:1780–1792. [DOI] [PubMed] [Google Scholar]
- 24.Al-Lamee RK, Shun-Shin MJ, Howard JP, Nowbar AN, Rajkumar C, Thompson D, Sen S, Nijjer S, Petraco R, Davies J, et al. Dobutamine stress echocardiography ischemia as a predictor of the placebo-controlled efficacy of percutaneous coronary intervention in stable coronary artery disease: the stress echocardiography-stratified analysis of ORBITA. Circulation. 2019;140:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Juniper EF, Walter SD, Griffith LE and Goldstein RS. Interpreting treatment effects in randomised trials. BMJ. 1998;316:690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks MM, Chung SC, Helmy T, Hillegass WB, Escobedo J, Melsop KA, Massaro EM, McBane RD, Hyde P, Hlatky M, et al. Health status after treatment for coronary artery disease and type 2 diabetes mellitus in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial. Circulation. 2010;122:1690–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mark DB, Pan W, Clapp-Channing NE, Anstrom KJ, Ross JR, Fox RS, Devlin GP, Martin CE, Adlbrecht C, Cowper PA, et al. Quality of life after late invasive therapy for occluded arteries. N Engl J Med. 2009;360:774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold SV, Kosiborod M, Li Y, Jones PG, Yue P, Belardinelli L and Spertus JA. Comparison of the Seattle Angina Questionnaire with daily angina diary in the TERISA clinical trial. Circ Cardiovasc Qual Outcomes. 2014;7:844–850. [DOI] [PubMed] [Google Scholar]
- 29.Mark DB, Anstrom KJ, Sheng S, Baloch KN, Daniels MR, Hoffmann U, Patel MR, Cooper LS, Lee KL, Douglas PS, et al. Quality-of-life outcomes with anatomic versus functional diagnostic testing strategies in symptomatic patients with suspected coronary artery disease: Results from the PROMISE randomized trial. Circulation. 2016;133:1995–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


