Abstract
Purpose
To investigate clinical efficacy and safety of 3D printing coplanar template-assisted iodine-125 (125I) seed implantation as a palliative treatment for inoperable pancreatic cancer.
Material and methods
Consecutive 28 patients (16 males and 12 females, median age of 64 years) with histologically diagnosed pancreatic cancer who underwent 3D printing coplanar template-assisted 125I seed implantation between June 2016 and May 2019 were analyzed. Among these 28 patients, 9 (32.1%) and 19 (67.9%) patients were presenting with tumor node metastasis (TNM) stage IIB and stage III cancer, respectively. Seed implantation was conducted for pain palliation intent in 25 patients and recurrent cancer after radiotherapy in 3 patients.
Results
No significant differences were found between pre-planned and post-operative dosimetric parameters, involving D90, D100, V90, V100, V150, conformity index, external index, and homogeneity index (all p > 0.05). Two months after implantation, pain relief rate was 76% (19/25) for pain patients. Overall tumor response rate (complete response + partial response) was 60.7% (complete response 0 patients, partial response 17 patients, stable disease 8 patients, and progressive disease 3 patients). Median survival was 10.5 months and estimated 1-year survival rate was 26.7%. Only one patient presented with a slightly upper gastro-intestinal hemorrhage, and another patient suffered from incomplete intestinal obstruction soon after implantation, both recovered after conservative medical treatment without a prolonged hospital stay. No major complication was observed.
Conclusions
3D printing coplanar template-assisted 125I seed implantation appears to be safe and effective palliative treatment for inoperable pancreatic cancer with favorable clinical outcomes.
Keywords: 3D printing, coplanar template, iodine-125, seed implantation, pancreatic cancer
Purpose
Pancreatic cancer is one of the leading lethal malignancies [1]. In the past few decades, substantial treatment advance was absent in patients with pancreatic cancer, though recent adoption of precision medicine may have a potential improvement on survival [2]. Unfortunately, the 5-year overall survival is only 9% for patients with pancreatic cancer, which is the lowest rate among all cancers, and has changed a little over the past few decades [1]. Currently, surgery remains the only chance for cure of pancreatic cancer, while treatment option is limited to chemo-radiotherapy or chemotherapy for majority of patients with locally advanced pancreatic cancer, yielding a poor prognosis [1, 3]. As a form of radiotherapy, radioactive iodine-125 (125I) seed implantation demonstrated favorable efficacy for local tumor control, survival, and pain palliation in pancreatic cancer [4-13]. High quality of seed implantation is critical for improving patients’ outcomes [9, 14, 15].
Computed tomography (CT) guidance was commonly used to improve the outcome of radioactive 125I seed implantation, which is not a real-time image, and 125I seeds implanted into the tumors by free-hand. Therefore, a 3D printing template is used to assist radioactive 125I seed implantation for cancers in the head and neck, thorax, retroperitoneal region, and pelvic cavity, which appears to be a safe and effective method for high-quality seed implantation [16-19]. Post-operative dosimetry was well consistent with that of pre-plan, with high accuracy of seed implantation for retroperitoneal lymphatic metastasis and head and neck cancers [17, 20].
Preliminary application of 3D printing coplanar template for radioactive 125I seed implantation in 25 patients with advanced pancreatic cancer appeared to be safe and effective to improve implantation accuracy and optimize dosimetric distribution [12]. Since clinical outcomes involving tumor response in patients’ survival has not yet been reported, the present study aimed to investigate dosimetric outcomes, clinical efficacy involving tumor response, patients’ survival, pain palliation, and safety of 3D printing coplanar template-assisted 125I seed implantation as palliative treatment for inoperable pancreatic cancer.
Material and methods
Study design
Clinical data of consecutive patients with histological diagnosis of pancreatic adenocarcinoma treated with 3D printing coplanar template-assisted 125I seed implantation from June 2016 to May 2019 in our institute were retrospectively reviewed. Twenty-eight patients (16 males and 12 females; median age, 64 years, range, 45-72 years) were identified and included in this study. Nine and 19 patients were presenting with tumor node metastasis (TNM) stage IIB and stage III cancer, respectively (Table 1).
Table 1.
Clinical characteristics of patients (n = 28)
| Item | n (%) | ||
|---|---|---|---|
| Age (years) | 64 (45-72) | ||
| Sex | |||
| Male | 16 (57.1) | ||
| Female | 12 (42.9) | ||
| Karnofsky performance scale | |||
| 70 | 15 (53.6) | ||
| 80 | 11 (39.3) | ||
| 90 | 2 (7.1) | ||
| Diagnosis | |||
| Inoperable pancreatic adenocarcinoma | 25 (89.3) | ||
| Recurrent pancreatic adenocarcinoma | 3 (10.7) | ||
| Tumor location | |||
| Head of the pancreas | 18 (64.3) | ||
| Body and tail of the pancreas | 10 (35.7) | ||
| Size of the lesions (cm) | 3.2-5.2 | ||
| TNM stage | |||
| Stage IIB | 9 (32.1) | ||
| T2N1M0 | 2 (7.1) | ||
| T3N1M0 | 7 (25.0) | ||
| Stage III | 19 (67.9) | ||
| T4N0M0 | 6 (21.4) | ||
| T4N1M0 | 13 (46.0) | ||
| Pain intensity | 25 (89.3) | ||
| Severe | 8 (28.6) | ||
| Moderate | 11 (39.3) | ||
| Mild | 6 (21.4) | ||
| Previous treatment | |||
| Radiotherapy | 3 (10.7) | ||
| Percutaneous trans-hepatic cholangial drainage/biliary stenting | 2 (7.2) | ||
| None | 23 (82.1) | ||
TNM – tumor/node/metastasis
All patients were discussed and enrolled after a multidisciplinary consultation. Indications for seed implantation were as follows: 1. Pain palliation intent for inoperable patient due to advanced pancreatic cancer or poor condition; 2. Recurrent/residual/progressive pancreatic adenocarcinoma after surgery/external beam radiation therapy (EBRT)/chemotherapy. Among the 28 patients, seed implantation was conducted for pain palliation intent in 25 patients and recurrent cancer after radiotherapy in 3 patients. Among the 25 patients presenting with pain after receiving oral analgesics, 8 patients suffered from severe pain, 11 from moderate pain, and 6 from mild pain. Three levels of pain intensity referred to an algorithm, including mild pain (1-3), moderate pain (4-7), and severe pain (8-10) (using a numerical rating scale with 0 – no pain to 10 – worst pain) [21]. Contraindications for seed implantation were as follows: 1. Infection; 2. Any active concomitant distant cancer/metastasis; 3. Estimated life expectancy < 1 month; 4. No suitable needle pathway for necessary seed implantation during pre-planing, i.e., impossibility to avoid adjacent vital organs at risk or hard to fulfill dose requirement; 5. Severe coagulation disorders and uncontrolled jaundice; and 6. Pregnancy or any comorbidities of clinical concern. The study was approved by the Hospital Medical Science Research Ethics Committee. All patients had signed an informed consent form for seed implantation. As this was a retrospective study without releasing personal information, the requirement to obtain written informed consent for participation in the study was waived.
Dosimetric outcomes, pain relief, tumor response, survival, and complications were evaluated. Primary endpoints included pain relief and tumor response, and the remaining was defined as secondary endpoints. Tumor response was determined according to tumor response standards suggested by the World Health Organization. Definitions of complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and overall response rate are listed in Table 2. Overall survival was defined as a duration from seed implantation until time of death from any cause or last follow-up. Complications were determined using common terminology criteria for adverse events (CTCAE) version 4.0 (CTCA) [22].
Table 2.
Response rate definition according to the World Health Organization
| Response | Definition |
|---|---|
| Complete response (CR) | Complete disappearance of the lesion lasting for more than 4 weeks |
| Partial response (PR) | Size of the lesion (maximum diameter × vertical diameter) decreased by more than 50%, and then maintained for 4 weeks |
| Stable disease (SD) | Size of the tumor decreased by less than 50% or increased by less than 25% |
| Progressive disease (PD) | Size of the tumor increased by more than 25% |
| Overall response rate | Rate of CR + PR |
Pre-plan
All patients were fixed by vacuum negative pressure pad in a supine position according to location of the lesion, and underwent a CT scan with intravenous contrast at 5-mm slice thicknesses, 1-3 days before seed implantation. Position lines were marked. Obtained images were sent to brachytherapy treatment planning system (BT-TPS, Beijing Tianhang Kelin Science and Technology Development Co. Ltd, China). Then, gross tumor volume (GTV) and organ at risk (OAR) were delineated. Pre-plan was designed according to determined prescription dose (90-110 Gy) and radioactivity of seeds (0.4-0.6 mCi).
Needle pathway was designed based on location of the lesion and OAR adjacent to the tumor. Critical organs, such as the vena cava, aorta abdominalis and its’ primary branches, and main portal vein were all avoided. However, the liver, stomach, small intestine, and colon could be penetrated during seed implantation if necessary. Dose distribution on GTV and OAR was designed. Data, such as needle pathway and entrance, were obtained from BT-TPS for simulation of a coplanar template (2 cm thickness with specifications of 8 cm × 8 cm/10 cm × 10 cm) using 3D printing software. A coplanar template was printed by a 3D printer according to the simulation data, which was used to secure the needle pathway and entrance (Figure 1). The coplanar template was 2 cm in thickness with a 20 × 20 pinhole square array for an 18-gauge needle, with each pinhole separated 0.5 cm from the other.
Fig. 1.
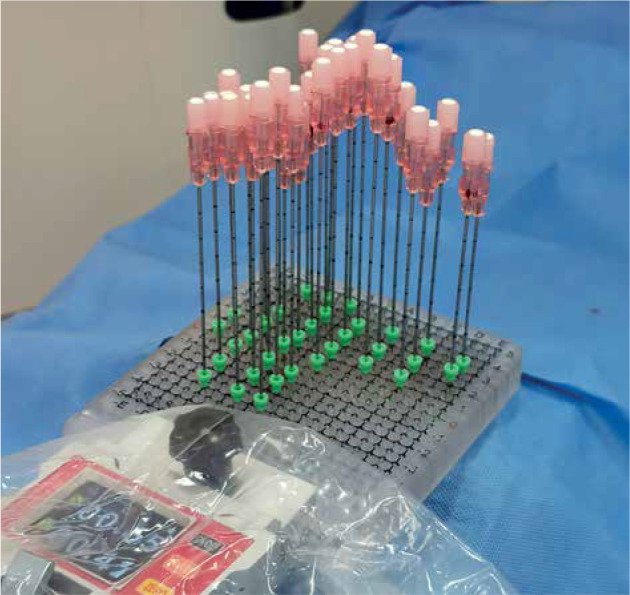
3D printing coplanar template was placed on the body surface to secure needle pathway and entrance. Coplanar template was 2 cm in thickness with a 20 × 20 pinhole square array for 18-gauge needle, with each pinhole separated 0.5 cm from the other
Iodine-125 seed implantation
All patients were fixed by a vacuum negative pressure pad per pre-plan and were breath-hold trained. Supporting device for 3D printing coplanar template was connected to the bed (Figure 2A). The needle entrance was marked on the skin. After skin disinfection and local anesthesia with 2% lidocaine (combined with intravenous enhanced anesthesia using remifentanil), the 3D-printed coplanar template was placed according to pre-plan. All 18-gauge needles were inserted step-by-step into the lesions through the holes on the coplanar template (Figure 2B, C). During the insertion, needles’ direction was adjusted under CT guidance. When all the needles were deemed in place, 125I seeds were then implanted in a retracement manner according to the pre-plan (Figure 2D). Prescription dose of 90 Gy in 6 patients, 100 Gy in 10 cases, and 110 Gy in 12 patients were prescribed with 125I seeds (0.4 mCi in 6 cases, 0.5 mCi in 18 cases, and 0.6 mCi in 4 cases; 0.8 mm × 4.5 mm, 6711-99 type, Beijing Atomic Technology Co. Ltd, China).
Fig. 2.
Procedure of 3D printing coplanar template-assisted iodine-125 (125I) seed implantation. A) Pre-operative enhanced CT were scanned at 5-mm slice thicknesses for the patient with pancreatic carcinoma. B-D) All 18-gauge needles were inserted into the lesions through the holes on coplanar template, and 125I seeds were implanted in a retracement manner according to pre-plan. E) Post-operative validation and dose curve. F) CT scan for tumor response 2 months after seed implantation (partial response in this case)
Post-operative dosimetric verification
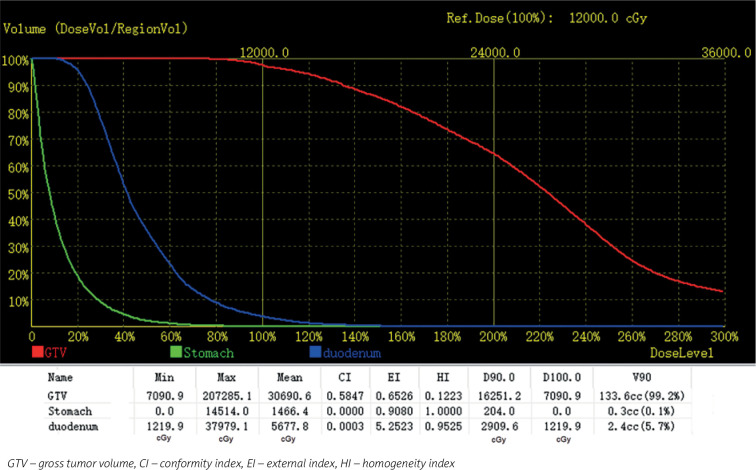
Dosimetric verification was performed after seed implantation, and post-operative CT data set was transferred to the planning station. Dosimetric parameters of GTV included D90 (minimum prescribed dose of 90% target volume), D100 (minimum prescribed dose of 100% target volume), V90 (volume covered by 90% prescription dose as a percentage of target volume), V100 (volume covered by 100% prescription dose as a percentage of target volume), and V150 (volume covered by 150% prescription dose as a percentage of target volume). Moreover, conformity index (CI) was used to evaluate conformity of dose distribution, external index (EI) was applied to describe percentage of volume received beyond prescription dose to target volume, and homogeneity index (HI) is used to evaluate dose distribution uniformity (Figures 2E, F and 3). All patients were requested to stay in bed for 8 hours after seed implantation.
Fig. 3.
Dose-volume histogram of the patient
Follow-up
Gemcitabine-based chemotherapy was applied 1-2 weeks after implantation according to temporal National Comprehensive Cancer Network (NCCN) guidelines. CT with intravenous contrast/magnetic resonance imaging (MRI) of the abdomen, chest X-ray/CT were routinely performed every 2-3 months during follow-up. No patients were lost to follow-up. Evaluation of tumor response was examined according to CT images at the initial 2-3 months’ follow-up after seed implantation.
Statistical analysis
Data were expressed as mean ± standard deviation. A paired t-test was used to compare continuous variable of pre-planned and post-operative dosimetric verification. Survival analysis was evaluated using Kaplan-Meier methods. A p-value of less than 0.05 was defined as statistically significant. SPSS statistical software version 26.0 was used for statistical analysis.
Results
Dosimetric outcomes
Iodine-125 seeds were successfully implanted for all the 28 patients, with procedure lasting for 1-2 hours. The median number of needles and iodine seeds were 8 (range, 4-33) and 53.5 (range, 15-122) per patient, respectively. The difference between pre-planned and post-operative dosimetric data was not statistically significant (all p > 0.05) (Table 3).
Table 3.
Comparisons of pre-plan and post-operative parameters of coplanar template-assisted seed implantation for pancreatic cancer (n = 28)
| Index | Pre-planned | Post-operative | T-value | P-value |
|---|---|---|---|---|
| D90 (Gy) | 128.80 ±15.51 | 123.30 ±18.02 | 2.104 | 0.057 |
| D100 (Gy) | 60.29 ±11.33 | 63.88 ±20.81 | –0.620 | 0.547 |
| V90 (%) | 97.13 ±2.26 | 95.53 ±2.57 | 1.098 | 0.322 |
| V100 (%) | 91.30 ±8.05 | 91.17 ±2.93 | 0.520 | 0.96 |
| V150 (%) | 66.85 ±9.51 | 66.76 ±6.45 | 0.034 | 0.973 |
| GTV volume (cm3) | 48.48 ±16.90 | 52.42 ±30.98 | –1.843 | 0.090 |
| Iodine-125 seeds | 47.15 ±16.90 | 53.61 ±20.71 | –1.924 | 0.078 |
| CI | 0.76 ±0.14 | 0.74 ±0.16 | 1.300 | 0.218 |
| EI (%) | 27.21 ±21.31 | 26.57 ±23.86 | 0.264 | 0.796 |
| HI (%) | 24.63 ±17.99 | 24.05 ±15.57 | 0.327 | 0.749 |
GTV – tumor target volume, CI – conformability index, EI – external index, HI – homogeneity index
Efficacy
Pain relief
At the first follow-up 2-3 months after seed implantation, the pain was statistically significantly relieved compared with that before seed implantation (visual analog scale pain score, 5.80 ±2.50 vs. 2.68 ±2.17, p < 0.001), and the pain relief rate was 76% (n = 19/25). Moreover, among the eight patients with severe pain before implantation, three downgrades to moderate pain, four downgrades to mild pain, and one with no change after implantation were noted. Of the eleven patients with moderate pain before implantation, seven downgrades to mild pain and four with no change after the procedure were observed. In these six patients who suffered from mild pain before implantation, five patients were completely relieved from pain, and the remaining one patient showed no change after implantation (Table 4).
Table 4.
Pain level of 25 patients
| Pain level | Before seed implantation n (%) | After seed implantation n (%) |
|---|---|---|
| Severe pain | 8 (32.0) | 1 (4.0) |
| Moderate pain | 11 (44.0) | 7 (28.0) |
| Mild pain | 6 (24.0) | 12 (48.0) |
| Completely relieved | 0 (0.0) | 5 (20.0) |
Tumor response and patient survival
CR was not found, and PR was observed in 17 cases, SD was noted in 8 patients, and PD was observed in 3 cases. The overall response rate was 60.7% at the first follow-up 2-3 months after seed implantation. The relationship between tumor response and pain relief rate for patients is presented in Figure 4.
Fig. 4.
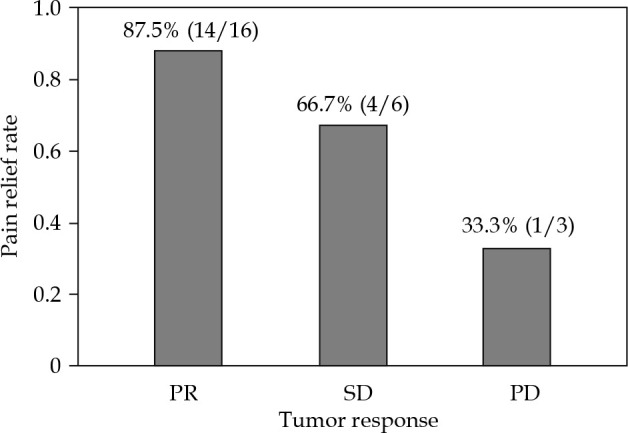
Relationship between tumor response and pain relief rate of 28 patients
During a median follow-up period of 13 months (range, 3-23 months), 23 patients died because of local tumor progression, and 5 patients died because of distant metastases. The median overall survival was 10.5 months, and the estimated 1-year survival was 26.77%.
Safety
Only one patient presented with a slightly upper gastro-intestinal hemorrhage, and another patient suffered from incomplete intestinal obstruction soon after seed implantation; both recovered after conservative medical treatment without a prolonged hospital stay. No severe complications, including pancreatitis, pancreatic leakage, and gastro-intestinal perforation were observed.
Discussion
The present study investigated the dosimetric outcomes, clinical efficacy, and safety of 3D printing coplanar template-assisted 125I seed implantation under CT guidance for inoperable pancreatic cancer, and obtained a high-rate of local control without major complication. The post-operative dosimetry was well consistent with that of the pre-plan.
Iodine-125 seed kills pancreatic cancer cells and induces apoptosis [23]. The effectiveness of 125I seed implantation depends on radiation dose delivered to target area, which is influenced by seed distribution involving the spacing interval and parallelism as well as the needle’s depth and angle during implantation. The pancreas is a retroperitoneal organ that is adjacent to the digestive tract and mesenteric vessels. Traversing the gastro-intestinal tract is inevitable in 125I seed implantation for pancreatic cancer, which is unamiable for precise needle puncture by free-hand, and seed distribution may lead to radiation cold or hot spots, especially in large volume lesions. The reasons for imprecise seed implantation might include the following: 1. Deviated needle pathways caused by the peristalsis in either gastro-intestinal tract or gastro-intestinum, muscle contraction, and long-distance of the pathway; 2. The presence of intra-tumor necrosis; 125I seeds might migrate due to gravity.
Various image guidance was reported for seed implantation of pancreatic cancer. Recently, Jin et al. [24] prospectively evaluated the clinical efficacy and safety of endoscopic ultrasonography-guided interstitial implantation of radioactive 125I seeds in 22 patients with advanced pancreatic cancer. Estimated median survival time was 9.0 months. Partial remission was achieved in three cases (13.6%) for 4 weeks, and in 10 patients (45.5%), the disease remained stable. Visual analog scale pain score dropped from 5.07 to 1.73 (p-value < 0.01) 1 week after brachytherapy, but increased again to 3.53 1 month later. There were no obvious complications following therapy. In addition, the combination of percutaneous stenting with 125I seed implantation and chemotherapy may bring remission of obstructive jaundice combined with an increased survival for obstructive jaundice treatment caused by unresectable pancreatic head cancer [25]. However, long-term efficacy of this treatment needs to be confirmed with further prospective studies [25]. CT-guided radioactive 125I seeds implantation was the most reported. Zhongmin et al. [10] examined the clinical efficacy of CT-guided radioactive 125I seeds implantation treatment in 31 patients with unresectable pancreatic cancer. Tumor response, which was demonstrated on repeated CT 2 months post-treatment, revealed CR in 3 cases, PR in 16 patients, SD in 9 cases, and PD in 3 patients, and the median survival time for all patients was 10.31 months, which is comparable to our results.
For obtaining a favorable dosimetric distribution, the 3D printing template appears to be feasible for CT-guided 125I seed implantation of pancreatic cancer. Huang et al. [12] evaluated 3D printing coplanar template for iodine-125 seed implantation therapy in 12 patients with pancreatic cancer and compared with 13 patients who received free-hand seed implantation. In this study, a better dosimetric parameter was observed for the 3D printing coplanar template group, and the difference was statistically significant (V100: 91.05% ±4.06% vs. 72.91% ±13.78%, p-value < 0.05). Mild hemorrhage was observed in one patient with a peritoneal local hematoma due to mesenteric vein damage from 125I seed implantation needle. No major procedure-related complications were observed. In the present study, the D90, V90, and V100 were slightly lower than that of pre-plan, but not statistically significant; the difference in pre-planned and post-operative CI and EI were also not statistically significant, which indicated a favorable dosimetric distribution.
External beam radiotherapy (EBRT) is a non-invasive method and may be useful for pain relief in pancreatic cancer patients. In a systematic review published by Buwenge et al. [26], of the 14 included studies (479 patients) reporting the effect of stereotactic radiotherapy on pain relief, the overall response rate (CR and PR) of 84.9% (95% CI: 75.8-91.5%) with high heterogeneity was reported, which seems superior to that reported in the present study; while SBRT resulted in acute and late toxicity (grade ≥ 3) rates of 3.3-18.0% and 6.0-8.2%, respectively, including duodenal obstruction/ulcer, small bowel obstruction, duodenal bleeding, hemorrhage, and gastric perforation. Furthermore, seed implantation may be used in recurrent pain after EBRT. More recently, high-dose-rate brachytherapy was tested for pancreatic cancer, however, the number of related studies is limited [27-29], which might provide a well-tolerated additional therapeutic option in the future, when high-quality evidence is available [28]. Even though there were no serious complications in this small cohort, the risk of lethal bleeding or infection should certainly not be underestimated in seed implantation. For safety concern, penetration of stomach, small intestine, or colon indeed should be maximally avoided, but is inevitable in some situations. If this was the case, we would suggest the patient for gastro-intestinal preparation using laxative one day before the procedure, with additional prophylactic antibiotics on the day of the procedure, and fasting for 24-48 h after the procedure. When abdominal pain is relieved without abdominal tenderness, patient may be discharged.
The present study has several limitations. First, the retrospective nature of the study is prone to having potential selection bias. Second, the single cohort study without a control group limits comparison of outcomes of seed implantation with and without 3D printing coplanar template guidance. Third, a relatively small group of patients was included, and sub-group analysis was not available due to insufficient statistical power.
Conclusions
3D printing coplanar template-assisted 125I seed implantation appears to be an effective and safe palliative treatment for inoperable pancreatic cancer, demonstrating favorable clinical outcomes.
Funding
Projects of Medical and Health Technology Development Program in Shandong Province (Grant No. 202009030304) to LL W and National Key Research and Development Plan of China (Grant No. 2019YFB1311300) to JJ W supports the implementation (e.g., labor cost and data collection) and publication of the project.
Ethics approval
The study was approved by the Tengzhou Central People’s Hospital Medical Science Research Ethics Committee. All patients had signed an informed consent form for seed implantation. As this is a retrospective study without releasing personal information, the requirement to obtain written informed consent to participants of the study was waived.
Disclosure
The authors report no conflict of interest.
References
- 1.Neoptolemos JP, Kleeff J, Michl Pet al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol 2018; 15: 333-348. [DOI] [PubMed] [Google Scholar]
- 2.Pishvaian MJ, Blais EM, Brody JRet al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol 2020; 21: 508-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleeff J, Korc M, Apte Met al. Pancreatic cancer. Nat Rev Dis Primers 2016; 2: 16022. [DOI] [PubMed] [Google Scholar]
- 4.Mohiuddin M, Rosato F, Barbot Det al. Long-term results of combined modality treatment with I-125 implantation for carcinoma of the pancreas. Int J Radiat Oncol Biol Phys 1992; 23: 305-311. [DOI] [PubMed] [Google Scholar]
- 5.Peretz T, Nori D, Hilaris Bet al. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys 1989; 17: 931-935. [DOI] [PubMed] [Google Scholar]
- 6.Joyce F, Burcharth F, Holm HH, Stroyer I. Ultrasonically guided percutaneous implantation of iodine-125 seeds in pancreatic carcinoma. Int J Radiat Oncol Biol Phys 1990; 19: 1049-1052. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Qi E, Liu Fet al. The application of a three-dimensional visualized seed planning and navigation system in (125)I seed implantation for pancreatic cancer. Onco Targets Ther 2018; 11: 619-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Jiang Y, Li Jet al. Intraoperative ultrasound-guided iodine-125 seed implantation for unresectable pancreatic carcinoma. J Exp Clin Cancer Res 2009; 28: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia SN, Wen FX, Gong TTet al. A review on the efficacy and safety of iodine-125 seed implantation in unresectable pancreatic cancers. Int J Radiat Biol 2020; 96: 383-389. [DOI] [PubMed] [Google Scholar]
- 10.Zhongmin W, Yu L, Fenju Let al. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol 2010; 20: 1786-1791. [DOI] [PubMed] [Google Scholar]
- 11.Han Q, Deng M, Lv Y, Dai G. Survival of patients with advanced pancreatic cancer after iodine125 seeds implantation brachytherapy: A meta-analysis. Medicine (Baltimore) 2017; 96: e5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Lu J, Chen KMet al. Preliminary application of 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. World J Gastroenterol 2018; 24: 5280-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Angio G, Hilaris BS, Arthur Ket al. Iodine 125 implantation for unresectable cancer of the pancreas. Postgrad Med 1970; 47: 226-230. [DOI] [PubMed] [Google Scholar]
- 14.Chargari C, Deutsch E, Blanchard Pet al. Brachytherapy: An overview for clinicians. CA Cancer J Clin 2019; 69: 386-401. [DOI] [PubMed] [Google Scholar]
- 15.Butler WM, Merrick GS. Clinical practice and quality assurance challenges in modern brachytherapy sources and dosimetry. Int J Radiat Oncol Biol Phys 2008; 71: S142-146. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Jiang Y, Ji Zet al. Efficacy and safety of CT-guided (125)I seed implantation as a salvage treatment for locally recurrent head and neck soft tissue sarcoma after surgery and external beam radiotherapy: A 12-year study at a single institution. Brachytherapy 2020; 19: 81-89. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Jiang Y, Ji Zet al. Dosimetry, efficacy, and safety of three-dimensional printing noncoplanar template-assisted and CT-guided (125)I seed implantation for recurrent retroperitoneal lymphatic metastasis after external beam radiotherapy. Brachytherapy 2020; 19: 380-388. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Wang H, Jiang Yet al. The efficacy and dosimetry analysis of CT-guided (125)I seed implantation assisted with 3D-printing non-co-planar template in locally recurrent rectal cancer. Radiat Oncol 2020; 15: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu B, Jiang P, Ji Zet al. Brachytherapy for lung cancer. Brachytherapy 2021; 20: 454-466. [DOI] [PubMed] [Google Scholar]
- 20.Qiu B, Jiang Y, Ji Zet al. The accuracy of individualized 3D-printing template-assisted I(125) radioactive seed implantation for recurrent/metastatic head and neck cancer. Front Oncol 2021; 11: 664996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swarm RA, Paice JA, Anghelescu DLet al. Adult Cancer Pain, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019; 17: 977-1007. [DOI] [PubMed] [Google Scholar]
- 22.Chen AP, Setser A, Anadkat MJet al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 2012; 67: 1025-1039. [DOI] [PubMed] [Google Scholar]
- 23.Ma JX, Jin ZD, Si PRet al. Continuous and low-energy 125I seed irradiation changes DNA methyltransferases expression patterns and inhibits pancreatic cancer tumor growth. J Exp Clin Cancer Res 2011; 30: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin Z, Du Y, Li Zet al. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy 2008; 40: 314-320. [DOI] [PubMed] [Google Scholar]
- 25.Chi Z, Chen L, Huang Jet al. A novel combination of percutaneous stenting with iodine-125 seed implantation and chemotherapy for the treatment of pancreatic head cancer with obstructive jaundice. Brachytherapy 2021; 20: 218-225. [DOI] [PubMed] [Google Scholar]
- 26.Buwenge M, Macchia G, Arcelli Aet al. Stereotactic radiotherapy of pancreatic cancer: a systematic review on pain relief. J Pain Res 2018; 11: 2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milickovic N, Tselis N, Karagiannis Eet al. Iridium-Knife: Another knife in radiation oncology. Brachytherapy 2017; 16: 884-892. [DOI] [PubMed] [Google Scholar]
- 28.Omari J, Heinze C, Wilck Aet al. Efficacy and safety of CT-guided high-dose-rate interstitial brachytherapy in primary and secondary malignancies of the pancreas. Eur J Radiol 2019; 112: 22-27. [DOI] [PubMed] [Google Scholar]
- 29.Franck C, Hass P, Malfertheiner Pet al. Combined systemic chemotherapy and CT-guided high-dose-rate brachytherapy for isolated local manifestation of pancreatic cancer after surgical resection. Digestion 2018; 98: 69-74. [DOI] [PubMed] [Google Scholar]