Abstract
REM sleep time in a 12 hr period was found to predict accurately food intake in the subsequent 12 hr period in undisturbed cats fed ad lib. In all but one of the cats, the correlation between REM sleep and subsequent food intake was negative. REM sleep was a better predictor of food intake than either waking, slow wave sleep or previous food intake. Cats were then fed only during the 12 hr day period. It was found that REM sleep at night, during which no food was available, no longer predicted food intake.
SEVERAL studies have suggested that there is a relationship between rapid eye movement (REM) sleep and the regulation of motivated behavior. REM sleep deprivation in the cat facilitates subsequent aggressive and sexual behavior and increases food consumption [ 1 ]. REM sleep deprivation also raises response rates and lowers thresholds for rewarding brain stimulation [ 19].
Conversely, stimulation of brain areas important in the control of motivated behavior has been shown to affect subsequent REM sleep. Rats that are allowed to self-stimulate in the lateral hypothalamus show a depression in subsequent REM sleep and a decreased REM sleep deprivation rebound [18,19]. Cats stimulated in the lateral hypothalamic defense area also show a depression in subsequent REM sleep time [ 14].
However, REM sleep deprivation effects are often confounded by non-specific stress [11,22]. Furthermore, self-stimulation causes an aphysiological activation of brain circuits and produces behaviors that are unlike normal drive behaviors [24]. Therefore, the normal relationship of REM sleep to motivated behavior cannot be determined using these techniques alone.
The present study sought to determine if there was a relationship between spontaneous variations in the quantity of REM sleep and the amount of a motivated behavior (food intake). Undisturbed cats, continuously monitored for sleep and food intake parameters, were used.
METHOD
Animals
Two female (Nos. 23 and 24) and four male (Nos. 25, 28, 32 and 33) cats, all weighing between 2.8 and 3.8 kg. were used. Electrodes for recording EEG and EMG were aseptically implanted while the cats were under pentobarbital (Nembutal) anesthesia. The EMG was recorded from stainless steel wires implanted in the dorsal neck muscles, the EEG from stainless steel screw electrodes. In addition, transcortical, concentric, stainless steel electrodes, which enable visualization of cortical PGO (pontine-geniculate-occipital) spikes, were implanted in Cats 28, 32 and 33, adjacent to the screw electrodes. The EEG electrodes were stereotaxically placed over the suprasylvian cortex at A + 8, 1 ± 9. Recording leads were attached to a 17 pin Amphenol connector, which was then mounted on the skull with dental cement.
The cats were wormed and periodically checked for reinfestation. Drug treatments were discontinued 2 weeks before the collection of EEG data began. All cats were in good health throughout the study.
Procedure
The cats were allowed at least one week to adapt to the recording situation. They were individually caged in 58 × 61 × 48 cm enclosures for recording. Either a Grass Model HI polygraph or a Beckman Model R411 dynograph was used to record data. The cat was separated from the recording machines by a wooden partition. Only one cat at a time was placed in the recording room. White noise from the air conditioning system effectively masked polygraph and other noises. Room temperature was maintained at 23.5 ± 1°C. Lights were on between 0900 and 2100 hr and off between 2100 and 0900 hr. Food and water were weighed, litter and cardboard floor papers changed, and the cage cleaned twice daily, once between 0900 and 0905 hr and once between 2100 and 2105 hr. The food and water were contained in cups attached to the front of the cage. All food spillage from each cat was carefully collected from throughout the cage and weighed along with the food remaining in the food cup. The cats were given enough fresh Purina Cat Chow and water so that no cat ever consumed its entire ration of either. During periods of data collection, the room was not entered except to weigh the food and clean the cage.
REM sleep, slow wave sleep (SWS), and waking (W), were calculated separately for the lights on, day period (0900–2100 hr) and the lights off, night period (2100–0900 hr). The records were scored in consecutive 10 sec epochs. The usual EEG and EMG criteria were used to determine state (see Fig. 1). All scoring was done without any knowledge of food intake values. The total numbers of 10 sec periods of REM sleep, SWS, and W in each 12 hr period were determined for each cat, and were then correlated with the number of grams of food eaten in the preceding, same, and subsequent 12 hr intervals. Depending on the length of the recording period, the number of paired observations ranged from 5 to 11 for each cat. Experiment 1. Continuous recording was carried out for 5 to 9 days and nights while the cats were given ad lib access to food and water. Cats 23, 24, 25 and 28 were used. A second recording session was held for Cat 25, 3 months after its first, and for Cat 28,10 days after its first.
FIG. 1.
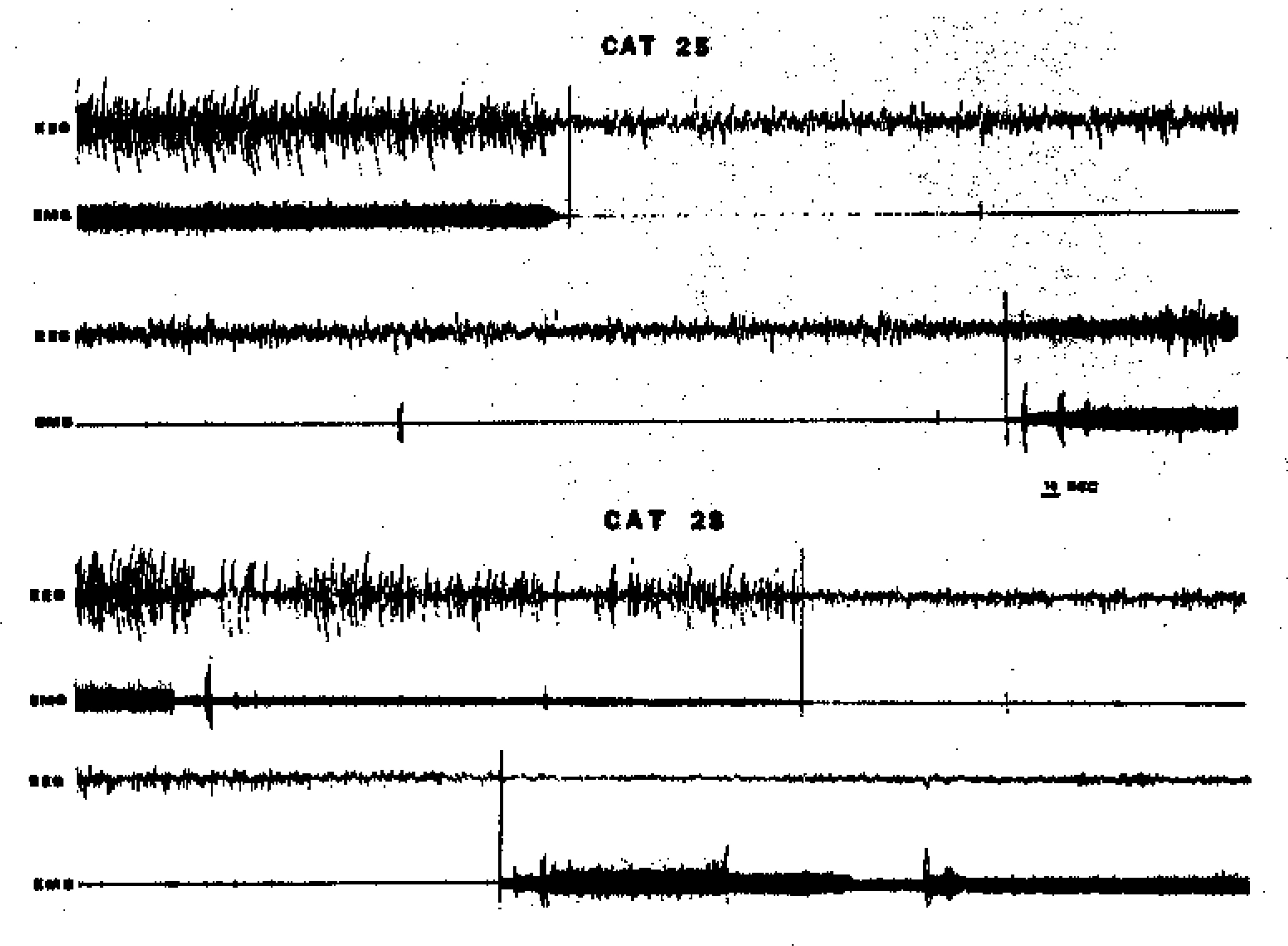
Four samples of the EEG and EMG data used in scoring for sleep state. The top tracings in each pair show a SWS-REM sleep transition. Note the EMG and EEG suppression at REM sleep onset. Cortical PGO spikes are clearly visible as negative monophasic deflections (downward) during REM sleep in Cat 28. The lower tracing in each pair shows a REM sleep offset. Note disappearance of PGO activity and return of muscle tone. Scoring judgments of REM sleep onset and offset are indicated by vertical lines across tracings.
Experiment 2. This experiment was undertaken to see whether food intake during a given 12 hr interval was a necessary condition for REM sleep during that interval to be predictive of subsequent food intake.
Water, but no food, was available during the 12 night hours, viz., 2100 to 0900 hr. Availability of food and water from 0900 to 2100 hr was as in Experiment 1. The cats were allowed one week to adapt to this regimen before recording was begun. Cats 23, 24, 32 and 33 were used.
RESULTS
Experiment 1
The amount of REM sleep in a 12 hr period was found to be correlated with the amount of food intake in the subsequent 12 hr period. An example of the relationship between REM sleep and subsequent food intake is shown in pig. 2. Pearson product-moment correlation coefficients were computed and are presented in Table 1. Separate correlations were calculated between night REM sleep time and subsequent day food intake, and between day REM sleep time and subsequent night food intake. Either day REM sleep or night REM sleep, but never both, showed significant correlations with food intake in the subsequent 12 hr period in all of the cats. All but one of these correlations were negative. Therefore, increased amounts of REM sleep were associated with decreased amounts of food intake in the subsequent 12 hr period. The one exception was the second week’s data for Cat 25, which showed a significant positive correlation. This male cat had produced a significant negative correlation in his first week’s data, collected 3 months previous to obtaining the positive correlation. For individual cats, comparing the day with the night 12 hr period, previous REM sleep predicted food intake better in the period with the greater mean and lower standard deviation of food intake (see Table 1).
TABLE 1.
PEARSON PRODUCT-MOMENT CORRELATIONS BETWEEN FOOD INTAKE IN ONE PERIOD AND REM, SWS, W, AND FOOD INTAKE IN THE PRECEDING OR SAME 12 HR PERIOD IN EXPERIMENT 1. THE NUMBER OF RECORDING PERIODS, AND THE MEANS AND STANDARD DEVIATIONS FOR REM SLEEP PERCENT AND AMOUNT OF FOOD INTAKE IN GRAMS, ARE ALSO SHOWN. REM SLEEP, SWS AND W ARE COMPUTED AS A PERCENTAGE OF THE 12 HR RECORDING TIME
| Day Food Intakes, ‘ Preceding Night Sleep Measures | Night Food Intakes, Preceding Day Sleep Measures | |||||||||||||||
| Cat | REM | W | SWS | Food | N | REM | W | SWS | Food | N | ||||||
|
| ||||||||||||||||
| 23 | −.95† | +.84* | +,73 | +.72 | 6 | +.09 | −.21 | +.40 | +.27 | 6 | ||||||
| 24 | −.34 | +.44 | −41 | −.03 | 8 | −.90† | +.40 | +.31 | −.28 | 7 | ||||||
| 25(1) | −.98† | −.09 | +.38 | −.65 | 5 | . –.19 | +.36 | −.38 | −.67 | 5 | ||||||
| 28(1) | −.84* | +.73 | −.51 | −.54 | 6 | −.38 | −.03 | +.11 | +.68 | 6 | ||||||
| 25(2) | −.04 | −.14 | +.23 | +.27 | 6 | +.80* | −.43 | −.17 | −.40 | 7 | ||||||
| 28(2) | −.74* | +.22 | +.16 | −.30 | 9 | −.24 | +.23 | −.13 | −.20 | 8 | ||||||
|
| ||||||||||||||||
| Night Food Intake, Concurrent Sleep Measures | Day Food Intake, Concurrent Sleep Measures | |||||||||||||||
| Cat | REM | W | SWS | REM Av. S.D. | Food Av. S.D. | N | REM | W | SWS | REM Av. S.D. | Food Av. S.D. | N | ||||
|
| ||||||||||||||||
| 23 | −.49 | +.33 | +.56 | 17 | 4.6 | 36 | 8.4 | 7 | +.72 | −.82* | +.75 | 14 | 4.0 | 27 | 4.4 | 7 |
| 24 | +.48 | −.72 | +.13 | 14 | 1.5 | 48 | 7.7 | 7 | +.45 | +.11 | −.49 | 13 | 1.1 | 40 | 9.5 | 8 |
| 25(1) | +.64 | +.81 | −.95* | 15 | 1.2 | 15 | 4.7 | 5 | +.64 | −.26 | −.06 | 14 | 1.6 | 45 | 3.9 | 5 |
| 28(1) | +.28 | −.14 | +.04 | 11 | 1.4 | 28 | 7.8 | 7 | −.52 | +.04 | +.04 | 17 | 0.8 | 41 | 5.0 | 7 |
| 25(2) | −.87† | +.63 | −.51 | 13 | 2.2 | 21 | 3.7 | 7 | −.35 | −.06 | +.24 | 16 | 3.0 | 33 | 6.0 | 7 |
| 28(2) | −.25 | −.37 | +.50 | 12 | 1.1 | 27 | 7.9 | 9 | +.65 | −.08 | −.15 | 16 | 1.5 | 30 | 10.0 | 8 |
p<0.05, two-tailed probability.
p<0.01, two-tailed probability.
The interval used for prediction did not overlap the subsequent predicted interval. Therefore, the observed correlations could not be merely the result of REM sleep time displacing W time during which eating might have occurred. It might be argued that REM sleep was correlated with some other variable, such as feeding, W, or SWS in the concurrent 12 hr period and that the forward correlation was merely a consequence of this other variable’s relation to subsequent food intake. However, all of the significant correlations between REM and subsequent food intake were larger in magnitude than the corresponding correlations between W, SWS, or food intake and subsequent food intake. The correlations between SWS or food intake and subsequent food intake were not significant for any of the cats. There was only one significant correlation between W (or total sleep time, its reciprocal) and subsequent food intake (Cat 23) and in that case the corresponding correlation between REM sleep and subsequent food intake was somewhat larger than the W-food intake correlation.
It might also be argued that REM sleep was correlated with some other variable in the subsequent interval and that the forward correlation was a consequence of this relationship. However, all of the correlations between REM sleep and subsequent food intake were larger than the correlations between food intake and W or SWS, when all were within the predicted interval.
It appears unlikely, therefore, that the correlation between REM sleep and subsequent food intake was secondary to an underlying change in W or SWS, or to a more direct relationship between food intake in two adjacent 12 hr intervals. These data show that REM sleep is a better predictor of subsequent food intake than previous W, SWS, or food intake.
In no case was the correlation between food intake and subsequent REM sleep significant. Therefore, REM sleep was more strongly related to food intake in the subsequent interval than to intake in the preceding interval.
Experiment 2
Correlations for the data obtained during the 12 hr deprivation condition are listed in Table 2. In no case did REM sleep at night, when food was not available, correlate significantly with subsequent food consumption. However, the means and standard deviations of REM sleep time in both night and day periods were comparable to the values reported in Experiment 1. One of the cats (No. 23) showed a significant relationship between day REM sleep, when food was available, and food intake in the next daytime period 24 hr later. The data from the 12 hr deprivation condition, therefore, indicate that food ingestion is necessary in order to maintain the correlation between REM sleep and subsequent food intake.
TABLE 2.
CORRELATION COEFFICIENTS BETWEEN DAY FOOD INTAKE AND PRECEDING NIGHT REM SLEEP, AND BETWEEN DAY FOOD INTAKE AND PRECEDING DAY REM SLEEP, IN EXPERIMENT 2
| Day Food Intake | Day Food Intake | |||
|---|---|---|---|---|
| Cat | Preceding Night Sleep Measures | N | Preceding Day Sleep Measures | N |
| 23 | −.27 | 11 | −.85† | 10 |
| 24 | +.12 | 5 | −.71 | 5 |
| 32(1) | −.33 | 8 | −.26 | 8 |
| 33(1) | −.07 | 6 | +.35 | 6 |
| 32(2) | −.38 | 5 | +.10 | 5 |
| 33(2) | −.05’ | 5 | −.00 | 5 |
p<0.05, two-tailed probability.
p<0.01, two-tailed probability.
DISCUSSION
These findings demonstrate that there is a relationship between REM sleep and food intake in the undisturbed cat fed ad lib. This correlation is not due to changes in waking or slow-wave sleep nor is it secondary to correlated food intake in adjacent 12 hr periods. Therefore, processes related to REM sleep appear to be tied relatively directly to the regulation of food intake in the undisturbed cat.
Many converging lines of evidence suggest the existence of a direct relationship between REM sleep and food intake. Dement [ 1 ] has shown that REM sleep deprivation produces hyperphagia. Steiner and Ellman [19] have found that REM sleep deprivation changes self-stimulation thresholds in the lateral hypothalamus, an area known to be important in food regulation [2]. Jacobs et al [4] have recorded from neurons in this area after varying amounts of food deprivation. They found that the greatest change in neuronal activity resulting from deprivation occurred during REM sleep. The lateral hypothalamus is also the major area of passage for anteriorly projecting noradren-ergic fibers originating in the locus coeruleus, an area involved in regulating REM sleep [6,12]. Ascending seroto-nergic fibers, important in controlling the phasic events of REM sleep [10], also are concentrated in this area.
Rubinstein and Sonnenschein [I5, 16, 17] have reported evidence of an increase in REM sleep following food ingestion in cats. The relatively short time course of this effect (3 hours) probably accounts for it not being seen in these data, since the present analysis was carried out on 12 hr blocks of data. Rubinstein and Sonnenschein’s results, in concert with the results reported here, suggest a two-way relation between REM sleep and food intake. A decrease in REM sleep would be correlated with an increase in food consumption in the subsequent 12 hr period. This food consumption would then produce an increase in REM sleep in the same period. This feedback relationship would tend to result in stable amounts of both REM sleep and food intake over a 24 hr period.
The typical ultradian REM sleep-waking cycle in the cat is 25 min long [20] and therefore could not be responsible for the high correlations seen between REM sleep in one 12 hr period and food intake in the next 12 hr. However, there are powerful 24 hr cycles of both REM sleep and food intake that could be responsible for the REM sleep-food intake relationship. For example, in the cat, as in other animals, both the occurrence and duration of REM sleep periods are strongly related to the time of day. Sleep that occurs at one point in the circadian cycle will show a predictably different REM sleep percentage than that shown by sleep that occurs at a different point in the 24 hr cycle [20,21]. Similarly in humans, time of day, not duration of prior sleep, appears to be the main determinant of the occurrence and duration of REM sleep periods [23]. A strong circadian modulation is also seen on feeding behavior. Both the duration and the distribution of feeding periods are largely determined by the time of day [8,21]. Thus, both REM sleep and food intake are modulated by strong 24 hr cycles. The data reported here suggest a relationship between the amplitudes of these two circadian cycles.
Control over the diurnal rhythm of blood lipids is one possible mode of REM sleep action. Le Magnen [7] has evidence in the rat of a circadian cycle of 12 hr of lipid synthesis followed by 12 hr of lipid mobilization’. These fluctuations of lipid utilization were found to underlie much of the diurnal variation in food intake. Diurnal distribution of REM sleep has been shown to be related to food intake in the rat [13]. Rubinstein [15] has reported that, in the cat, food intake is effective in modifying REM sleep only during certain periods of the day. Perhaps these periods are linked to specific points in the lipid mobilization cycle. A relationship between these diurnal fluctuations in REM sleep and lipid mobilization could be responsible for the REM sleep-food intake correlation reported here. Similarly, diurnal neurotransmitter rhythms, such as that shown by norepinephrine [9], or other rhythms that interact with both food intake and REM sleep could also be responsible for the correlation.
The REM sleep-food intake correlation makes it possible to predict a substantial proportion of the daily variance in food intake. Thus, fluctuations in food intake between 12 hr periods, which might have been dismissed as resulting from biological variability, appear instead to be predetermined by physiological processes. Since prediction is possible, the daily variation in food intake can be studied as a dependent variable. The processes involved in the consumption of the quota of food set by previous REM sleep periods can therefore be determined.
Previous reports [5,20] have emphasized the stability of REM sleep from day to day in undisturbed cats. The results reported here demonstrate that the small daily variations in amount of REM sleep that do occur have behavioral significance. Other parameters of REM sleep might be usefully correlated with a variety of behavioral measures.
FIG. 2.
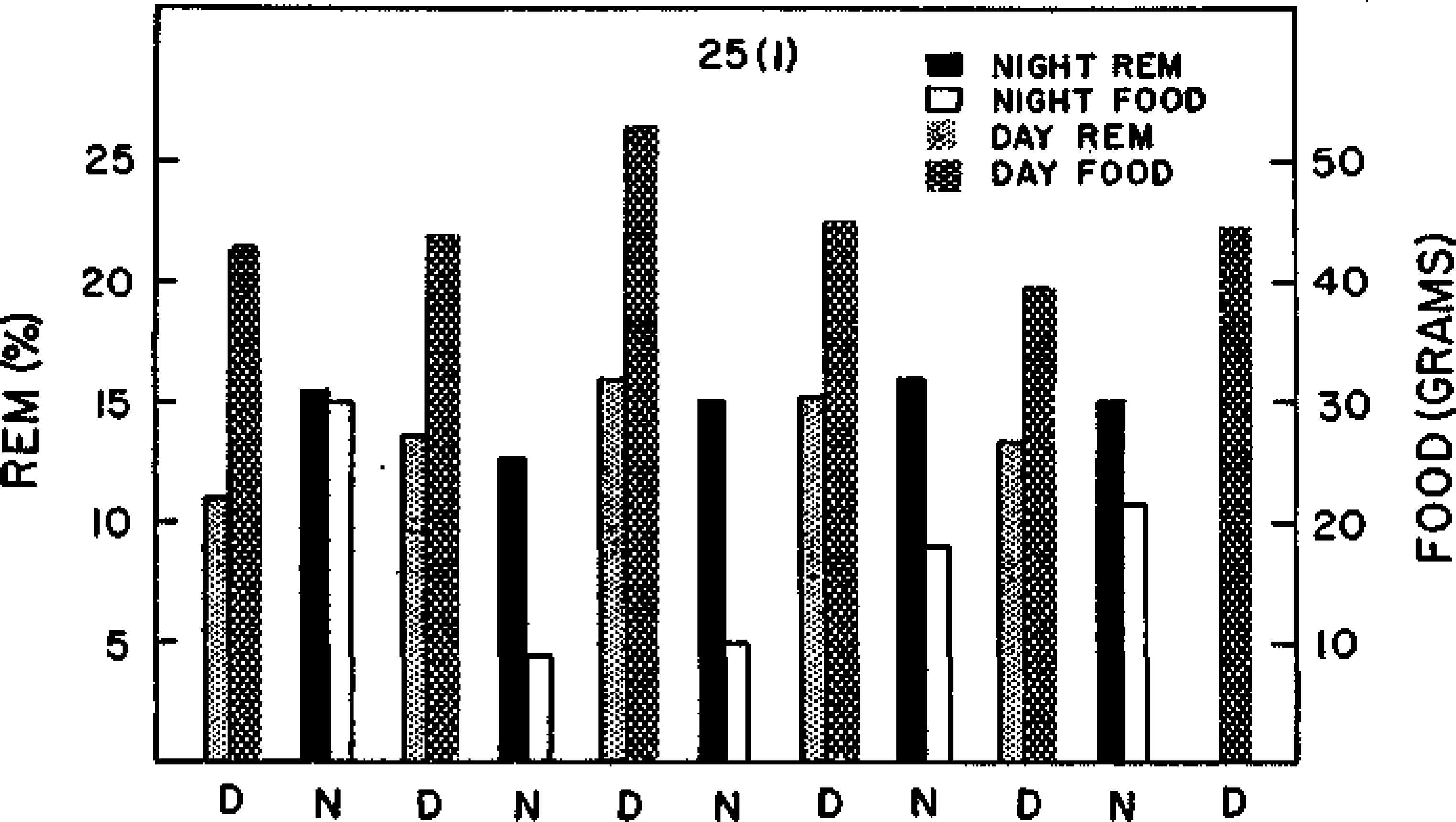
Data from the first recording session for Cat 25 in Experiment 1. Note the significant negative correlation between night REM sleep and food intake on the subsequent day (see Table 1).
ACKNOWLEDGEMENT
This report is partially based on a doctoral dissertation completed at the University of Rochester, The author thanks Drs. Paul Coleman, Jerome Schwartzbaum, Mortimer Rosen and Dennis McGinty.
REFERENCES
- 1.Dement WC The biological role of REM sleep (circa 1968). In: Sleep Physiology and Pathology, edited by Kales A. Philadelphia: J. B. Lippincott, 1969, pp. 245–265. [Google Scholar]
- 2.Epstein AN The lateral hypothalamic syndrome: Its implications for the physiological psychology of hunger and thirst. In: Progress in Physiological Psychology, Vol. 4, edited by Stellar Eand Sprague JM. New York: Academic Press, 1971, pp. 263–311. [Google Scholar]
- 3.Fara JW, Rubinstein EH and Sonnenschein RR. Visceral and behavioral responses to intraduodenal fat. Science 166: 110–111,1969. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs BL, Harper RM and McGinty DJ, Neuronal coding of motivational level during sleep. Physiol Behav. 5: 1139–1143,1970. [DOI] [PubMed] [Google Scholar]
- 5.Jouvet M Neurophysiology of the states of sleep. Physiol. Rev. 47:117–177,1967. [DOI] [PubMed] [Google Scholar]
- 6.Jouvet M The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergebn. Physiol 64: 166–307, 1972. [DOI] [PubMed] [Google Scholar]
- 7.LeMagnen J Advances in studies on the physiological control and regulation of food intake. In: Progress in Physiological Psychology, Vol. 4, edited by Stellar E and Sprague JM. New York: Academic Press, 1971, pp. 204–262. [Google Scholar]
- 8.LeMagnen J and Devos M. Metabolic correlates of the meal onset in the free food intake of rats. Physiol. Behav. 5: 805–814, 1970. [DOI] [PubMed] [Google Scholar]
- 9.Margules DL, Lewis MJ, Dragovich JA and Margutes AS. Hypothalamie norepinephrine; Circadian rhythms and the control of feeding behavior. Science 178: 640–643, 1972. [DOI] [PubMed] [Google Scholar]
- 10.McGinty DJ Neuroehemically defined neurons: Behavioral correlates of unit activity of serotonin-containing cells. In: Brain Unit Activity During Behavior, edited by Phillips MI. Springfield: Charles C. Thomas, 1974, pp. 244–268. [Google Scholar]
- 11.Mendelson W, Guthrie R, Frederick G and Wyatt RJ Should flower pots be used for flowers, pot or rats. In: Sleep Research, Vol. 2, edited by Chase MH, Stern WC and Walter PL. Los Angeles: Brain Information Service, Brain Research Institute, UCLA, 1973, p. 169. [Google Scholar]
- 12.Morgane PJ and Stern WC. Relationship df sleep to neuroanatomical circuits, biochemistry and behavior. Ann. N. Y. Acad. Sci. 193: 95–111,1972. [DOI] [PubMed] [Google Scholar]
- 13.Mouret JR and Bobillier P. Diurnal rhythms of sleep in the rat: Augmentation of paradoxical sleep following alterations of the feeding schedule. Int. J. Neurosci. 2: 265–270,1971. [DOI] [PubMed] [Google Scholar]
- 14.Putkonen PTS and Putkonen AR. Suppression of paradoxical sleep (PS) following hypothalamic defense reactions in cats during normal conditions and recovery from PS deprivation. Brain Res. 26: 333–347,1971. [PubMed] [Google Scholar]
- 15.Rubinstein EH Postprandial drowsiness. J. Am. med. Ass. 222:703,1972. [PubMed] [Google Scholar]
- 16.Rubinstein EH and Sonnenschein RR. Increased REM sleep in the cat after food intake. In: Proc. of the 25th International Congress of Physiological Sciences. 9: 482,1971. [Google Scholar]
- 17.Rubinstein EH and Sonnenschein RR. Sleep cycles and feeding behavior in the cat; role of gastrointestinal hormones. Acta dent venez. 22: 125–128,1971. [Google Scholar]
- 18.Spielman AJ, Mattiace LA, Steiner SS and Ellman SJ. The effects of varying amounts of intracranial self-stimulation on the “normal” sleep cycle of the rat. In: Sleep Research, Vol. 2, edited by Chase MH, Stern WC and Walter PL, UCLA. Los Angeles: Brain Information Service, Brain Research Institute, 1973, p. 38. [Google Scholar]
- 19.Steiner SS and Ellman SJ. Relation between REM sleep and intracranial self-stimulation. Science 177: 1122–1124, 1972. [DOI] [PubMed] [Google Scholar]
- 20.Sterman MB, Knauss T, Lehman D and Clemente CD. Circadian sleep and waking patterns in the laboratory cat. Electroenceph. clin. Neurophysiol. 19: 509–517, 1965. [DOI] [PubMed] [Google Scholar]
- 21.Sterman MB, Lucas EA and Macdonald LR. Periodicity within sleep and operant performance in the cat. Brain Res. 38: 327–341, 1972. [DOI] [PubMed] [Google Scholar]
- 22.Stem W,C,Effects of desynehronized sleep deprivation upon startle response habituation in the rat. Psychon. Sci. 23: 31–32, 1971. [Google Scholar]
- 23.Taub JM and Berger RJ. Sleep stage patterns associated with acute shifts in the sleep-wakefulness cycle. Electroenceph. din. Neurophysiol. 35: 613–619, 1973. [DOI] [PubMed] [Google Scholar]
- 24.Valenstein ES, Cox VC and Kakolewski W. The hypothalamus and motivated behavior. In: Reinforcement and Behavior, edited By Tapp J. New York: Academic Press, 1969, pp. 242–285. [Google Scholar]


