The pinna of the cat and of most other mammals is an exquisitely mobile structure that assists in auditory orientation. The motoneurons innervating the auricular musculature are located in the medial portions of the facial nucleus3,6,8,9,12,20. Sherrington13 first demonstrated that structures caudal to the inferior colliculus were sufficient to control a variety of complex protective reflex movements of the pinna. However, the specific regions in the lower brain stem that might be involved in the regulation of these movements have not been further localized. The present study reports the discovery of a group of neuronal units in the medial pontomedullary reticular formation that discharge in relation to pinna movement.
Units related to pinna movement were recorded in 6 unrestrained, female cats using moveable bundles of 32 μm microwires. Our techniques for recording and for the visual and photographic analysis of behavioral correlates of unit activity have been described previously16 = 19. All units were observed for at least 4 h during spontaneous motor activity and systematic sensory stimulation. Several cells were recorded for 3 or more consecutive days during which time the spike waveform and behavioral correlates of unit discharge were stable. Electrooculograms, splenius electromyograms, cruciate gyrus electroencephalograms, and lateral geniculate activity were polygraphically recorded along with unit activity for sleep stage identification.
Cats were killed with an overdose of Nembutal after passing a 15 μA current for 15 sec through the microwires that had recorded unit activity. After perfusing with saline and 4 % formalin, brains were removed, sectioned in the sagittal or coronal planes, and stained with carbol-fuchsin red Nissl stain. The Prussian blue iron reaction was used to locate the tips of the microwire penetrations. Unit positions were calculated by subtracting microdrive positions at recording locations from the final microdrive position, after allowance was made for brain shrinkage. Units were assigned to appropriate tegmental fields on the basis of cytoarchitectonic patterns. Anatomic landmarks such as the abducens nuclei, genu of the facial nerve, shape of the fourth ventricle, and position of the inferior olive were used to place recording sites with reference to the stereotaxic coordinates on the sagittal plates of the Berman atlas1.
Behavior.
A total of 19 cells that fired in conjunction with ‘spontaneous’ movements of the pinna were observed. All cells were related to movements of the ipsilateral pinna. None were related to movements of the contralateral pinna. Detailed observation of the topography of the pinna movement correlated with unit activity was made in 13 of the cells. Of these, 4 related to caudal, 5 to ventro-caudal and 4 to rostral movements of the pinna. There was no obvious anatomical segregation of these movement subtypes. All but one of the cells related to pinna movement had little or no spontaneous activity when the cat was sitting quietly with the pinna in the normal intermediate position. Rapid active ear rotation in the appropriate direction was accompanied by a burst of unit discharge (Fig. 1). Five cells exhibited tonic discharge if the pinna was maintained by the cat in the displaced position but the remaining cells discharged only during the movement. Vigorous pinna movements were accompanied by bursts up to 30 spikes within a 0.5 sec interval, while slower movements correlated with a single spike or no discharge at all. These cells did not discharge during eye, head, neck or limb movements. In fact, they often were completely silent during very vigorous motor activity such as eating, grooming and a variety of play behaviors as long as the specific ipsilateral pinna movements were not performed. Reflex head shake movements, elicited by placing a small pellet in either concha, or occurring spontaneously, were accompanied by burst discharge in all ear movement cells. This contrasts with the behavior of adjacent cells related to spinal movements, most of which are completely silent during this same head shake movement15.
Fig. 1.
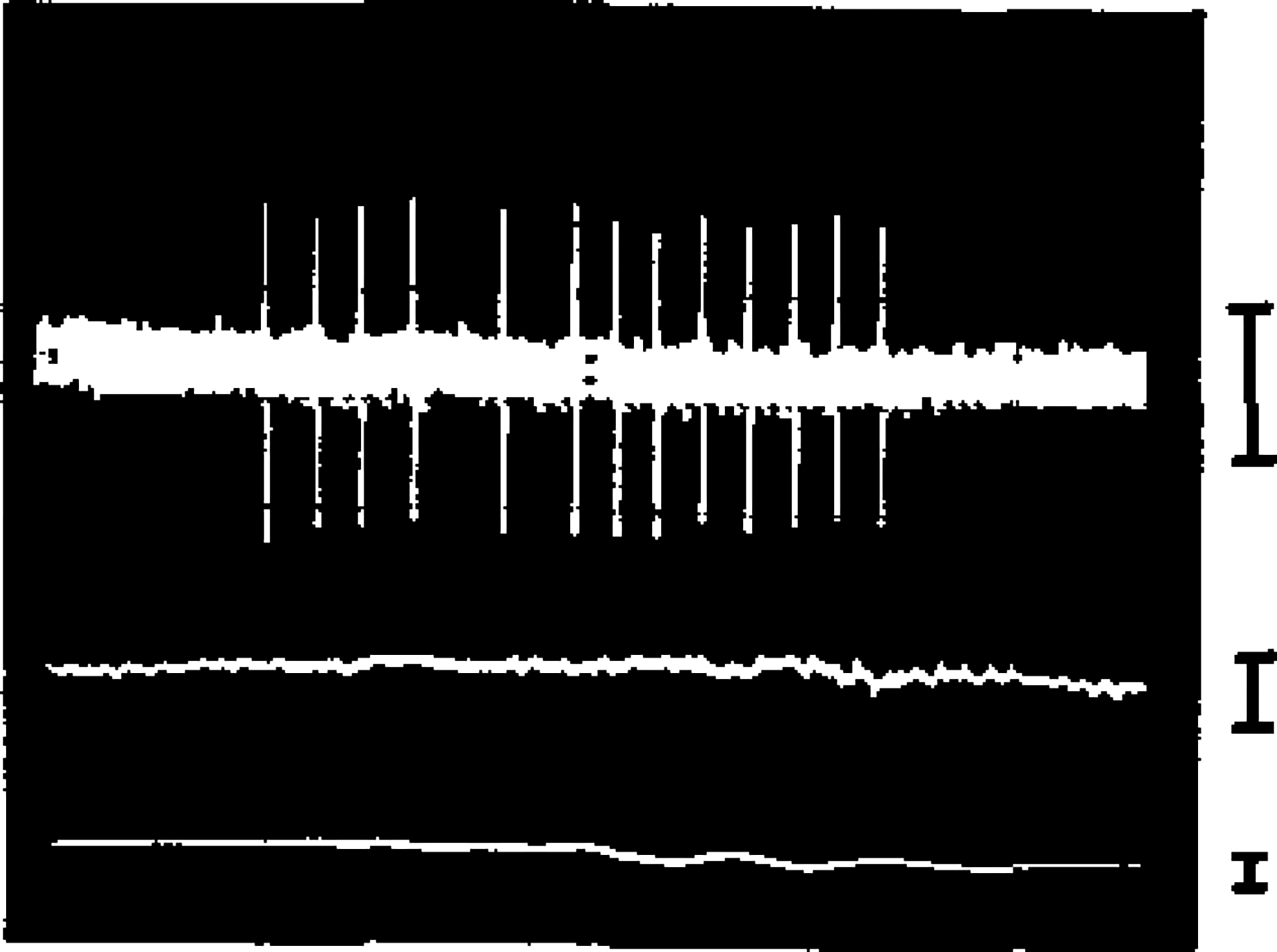
Unit discharge burst during pinna movement. Note lack of correlated activity in simultaneously recorded EOG (lower trace) and splenius EMG (middle trace). Calibrations : EOG, EMG, 100, μV, unit 50, μV; length of sample, 1.0 sec.
Sensory responses.
All cells were tested for response to somatic stimulation. When such stimulation elicited pinna movement, discharge occurred as in the case of spontaneous movements. In the absence of detectable ear movements, 16 of the 19 tested cells had no somatic field. Of the remaining cells, one responded to light manual stroking on any portion of the ipsilateral face. Two cells responded to deep pressure applied to the area from the lower ipsilateral jaw to the region ventral to the pinna. These same 3 cells were totally unresponsive to punctuate stimulation of up to 8 g applied to the same area with an aesthesiometer. Two cells were tested for response to 0.5 msec electric pulses of sufficient intensity to cause a muscle twitch, administered through needle electrodes on the base of the ipsilateral pinna. Neither cell responded. None of the cells showed a response to passive movement of the pinna by the experimenter.
All ear movement cells were tested for response to auditory stimuli. Surprisingly, none responded despite the fact that 40 % of adjacent, non-pinna movement related, pontine reticular cells respond to identical stimuli18. Similarly, none responded to stroboscopic visual stimuli.
Activity during sleep.
All of the units were observed during polygraphically identified slow wave and REM sleep states. All but 3 of the cells (84 %) were silent throughout sleep and quiet waking and therefore fell in the type 1 or ‘NSA’ category previously described17,19. This contrasts with the sleep modulation of activity in adjacent cells only 25 % of which are of this type17
Histology.
We have conducted behavioral analyses on a total of 300 medial reticular formation cells located between stereotaxic coordinates A3 and P 13, LO.8 and 3.0. Those related to pinna movement were all localized to the area between P2 and P8 and especially P4 and P8. Fig. 2 presents the locations of these cells and the proportion of cells at each AP level related to pinna movement. Fig. 3 presents a representative electrode track on which pinna movement cells were recorded.
Fig. 2.
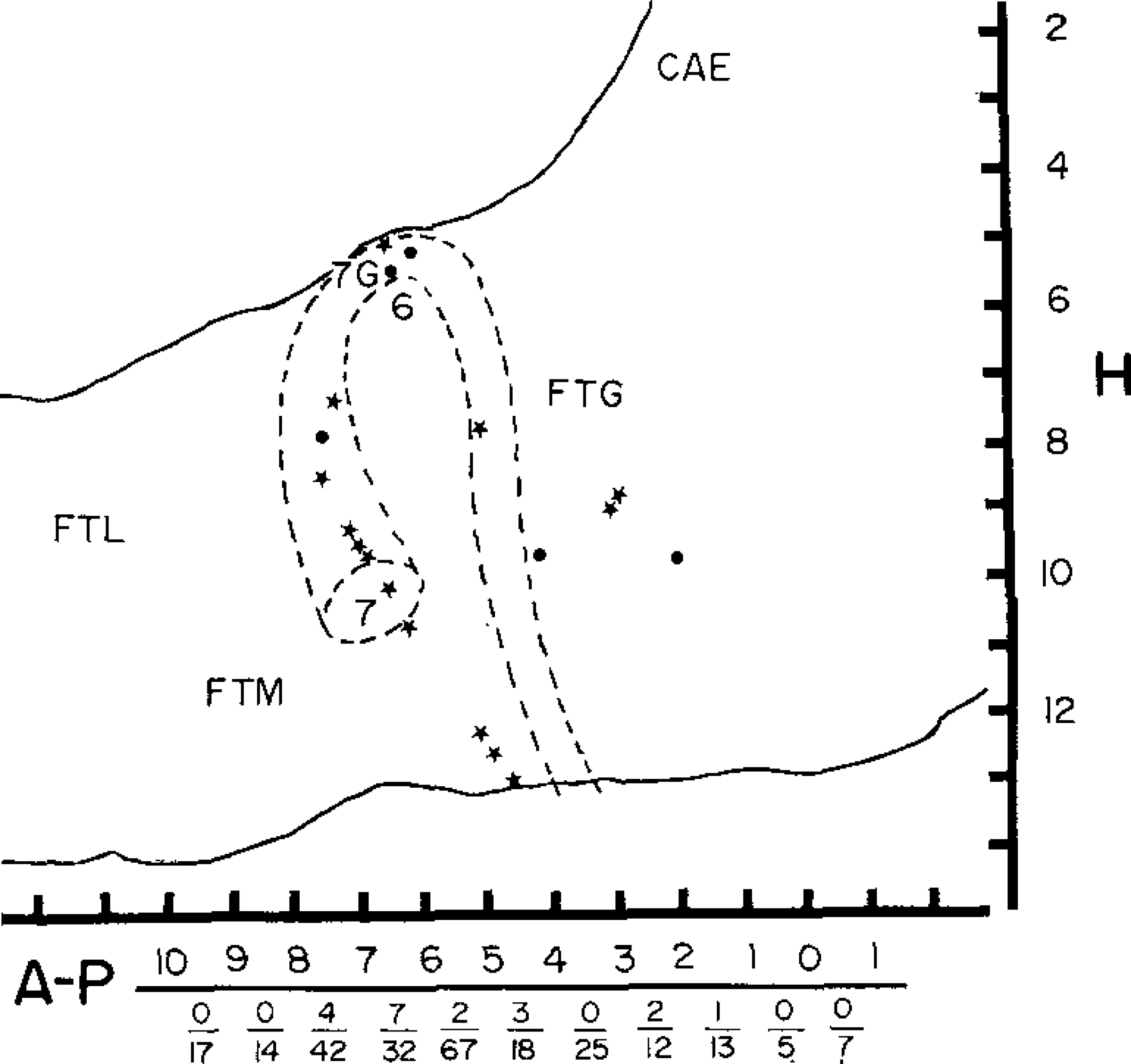
Locations of cells discharging in relation to pinna movement, plotted on sagittal section of the brain stem. Broken lines indicate approximate position of the more laterally located facial nucleus and nerve. Circles indicate units located between 0.8 and 1.7 mm from the midline. Stars indicate units located between 1.8 and 2.5 mm from the midline. Numerators of fractions at bottom of figure indicate number of pinna movement cells, and denominators total number of cells recorded, in each 1 mm segment of the AP axis. FTL, FTM and FTG, lateral, medial and gigantocellular tegmental fields; 6, abducens nucleus; 7G, genu of facial nerve; 7, facial nucleus.
Fig. 3.
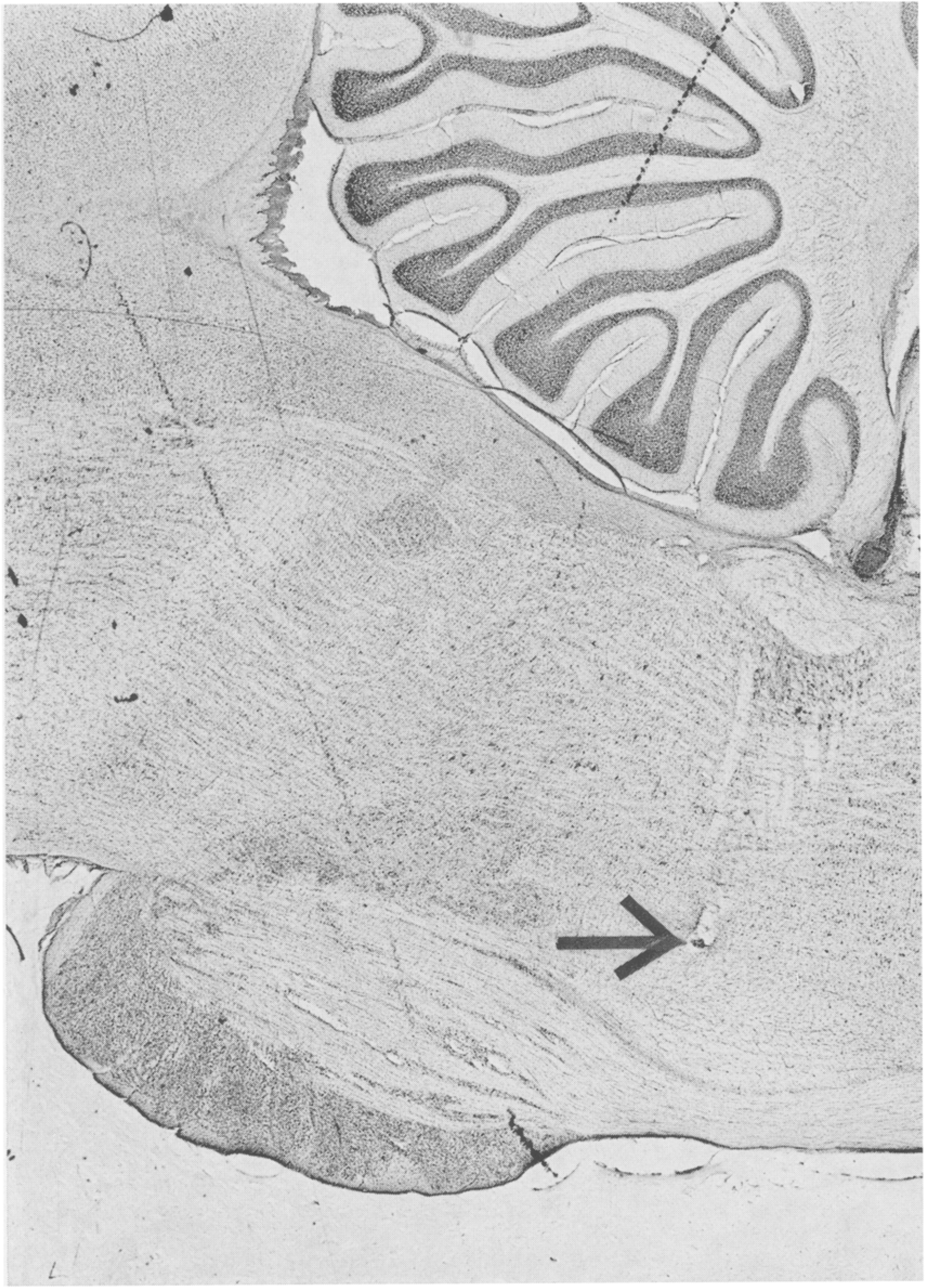
Electrode track whose termination (arrow) is at point at which pinna movement cell was recorded.
Since the facial nucleus and nerve, which innervate the auricular musculature are located at these same AP levels, their approximate position is also sketched. The facial nucleus is located between L3 and L5, i.e. lateral to all of the units presented in Fig. 2. None of our electrode tracks passed through this nucleus. The facial nucleus projects a diffuse spray of axons dorsally and medially, intermingled with somas of reticular formation cells. These fibers converge at the genu of the facial nerve and then bend sharply laterally, exiting the ventral brainstem at L7. None of the pinna movement units were histologically localized to the facial nerve. The 3 units that are mapped at the level of the genu were in the nucleus praepositus hypoglossi on electrode tracts that did not penetrate the genu. However, because a few of the units were near the facial nerve, we analyzed the spike waveform of all cells to see if the waveshapes were consistent with the units being stray facial nerve axons. Spikes were initially negative in 9 cells and initially positive in 10. Signal to noise ratio was generally higher than that recorded in adjacent non-pinna movement cells, ranging from 3/1 to 15/1. Spike amplitude ranged from 70 to 245 μV. Many of these recordings were maintained while moving the microwire bundles up to 200 μm. Spike duration ranged from 0.7 to 1.5 msec with a mean of 1.0 msec. These durations and amplitudes are similar to those reported for reticular formation somas recorded with similar techniques11. The waveshapes were also typical of spikes derived from somas2,7.
The temporal conjunction of unit discharge with specific movements of the pinna would be consistent with these cells triggering the movement or with their receiving sensory or proprioceptive input from the auricle. However, most of these cells do not respond to stimulation of the skin surface of the pinna of surrounding areas. Similarly, we could not activate these units by manipulating pinna position or (in two cases) by electrical stimulation, both of which would produce proprioceptive input. The absence of auditory response in all of these cells is somewhat surprising. However, it is consistent with the relation of unit discharge to active movement of the pinna, since discrete auditory stimuli of the type used here rarely caused pinna movement. Thus, the discharge of reticular pinna movement cells is more closely related to active movement than to sensory input. We have described similar motor relations in units recorded in adjacent medial reticular formation areas14,15,18
Anatomical studies of the auricular portions of the facial nucleus have not shown strong monosynaptic projections from all of the regions in which we found pinna movement cells, although these areas were not the focus of study in these papers6,10,22. Therefore, pinna movement cells may relay to facial nucleus motoneurons in as yet unidentified regions. It is also conceivable that some of these cells may themselves be displaced motoneurons contributing directly to the seventh nerve. The region dorsomedial to the facial nucleus has been described as containing groups of large loosely clumped multipolar cells, labelled by different authors as the retrotrigeminal, accessory facial or accessory abducens nucleus1,4,21, which appear to contribute to the facial nerve20. Several of the more caudal pinna movement cells recorded here may be in these difficult to define and functionally obscure nuclear groups.
Henkel and Edwards6 have described a putative pinna movement control pathway descending from the superior colliculus to paralemniscal areas which in turn relay to the auricular portion of the facial nucleus. This system might interact in the pontine reticular formation with the pinna movement cells described here, or may represent a parallel system.
Pinna movement cells were more common in the medial reticular regions we have investigated than the intensively studied eye movement control cells. This is not surprising when one considers the impressive mobility of the cat’s pinna, which is achieved by at least 20 different muscles5. Like eye movements, pinna movements can be readily quantified and studied in head restrained animals. Unlike eye movements, pinna movements are typically unilateral and the controlling muscles, while numerous, are accessible. Thus, the temporal and synaptic relations between these cells and pinna movement could be fruitfully investigated.
In summary, we have found a localized grouping of brainstem neurons that discharge in conjunction with pinna movement. These cells are situated in the medial reticular formation between the facial nucleus and the region 2 mm rostral to the exit of the facial nerve. They relate to specific active movements of the pinna, most having little or no response to sensory stimuli and low levels of spontaneous and sleep-related activity.
Acknowledgments
Supported by the Medical Research Service of the Veterans Administration and National Institutes of Health Research Grant NS14610.
References
- 1.Berman AL The Brain Stem of the Cat, University of Wisconsin Press, Madison, 1968. [Google Scholar]
- 2.Bishop PO, Burke W and Davis R, The identification of single units in central visual pathways, J. Physiol. (Lond.), 162 (1962) 409–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courville J, The nucleus of the facial nerve; the relation between cellular groups and peripheral branches of the nerve, Brain Research, 1 (1966) 338–354. [DOI] [PubMed] [Google Scholar]
- 4.Crosby EC, Humphrey T and Lauer EW, Correlative Anatomy of the Nervous System, Macmillan, New York, 1962. [Google Scholar]
- 5.Crouch JE, Text-Atlas of Cat Anatomy, Lea and Febiger, Philadelphia, 1969. [Google Scholar]
- 6.Henkel C and Edwards SB, The superior colliculus control of pinna movements in the cat: possible anatomical connections, J. comp. Neurol, 182 (1978) 763–776. [DOI] [PubMed] [Google Scholar]
- 7.Hubel DH, Single unit activity in lateral geniculate body and optic tract of unrestrained cats, J. Physiol. (Lond.), 150 (1960) 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kume M, Uemura M, Matsuda K, Matsushima R and Mizuno N, Topgraphical representation of peripheral branches of the facial nerve within the facial nucleus: a HRP study in the cat, Neurosci. Lett, 8 (1978) 5–8. [DOI] [PubMed] [Google Scholar]
- 9.Martin MR and Lodge D, Morphology of the facial nucleus of the rat, Brain Research, 123 (1977) 1–12. [DOI] [PubMed] [Google Scholar]
- 10.Panneton WM and Martin GF, Midbrain projections to the facial nucleus in the oppossum, Brain Research, 145 (1978) 355–359. [DOI] [PubMed] [Google Scholar]
- 11.Phillips MI, Unit activity recording in freely moving animals; some principles and theory. In Phillips MI (Ed.), Brain Unit Activity During Behavior Vol. 1, Charles C. Thomas, Springfield, Ill., 1973, pp. 5–40. [Google Scholar]
- 12.Radpour S, Organization of the facial nerve nucleus in the cat, Laryngoscope (St. Louis), 87 (1977) 557–574. [DOI] [PubMed] [Google Scholar]
- 13.Sherrington CS, Reflexes elicitable in the cat from pinna vibrissae and jaws, J. Physiol. (Lond.), 51 (1917) 404–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel JM, Behavioral functions of the reticular formation, Brain Res. Rev, 1 (1979) 69–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel JM, Behavioral relations of the medullary reticular formation cells, Exp. Neurol, 65 (1979) 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel JM, Breedlove SM and McGinty DJ, Photographic analysis of relation between unit activity and movement, J. neurosci. Meth. 1 (1979) 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel JM, and McGinty DJ, Brainstem neurons without spontaneous unit discharge, Science, 193 (1976) 240–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel JM and McGinty DJ, Pontine reticular formation neurons: relationship of discharge to motor activity, Science, 196 (1977) 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel JM, McGinty DJ and Breedlove SM, Sleep and waking activity of pontine gigantocellular field neurons, Exp. Neurol, 56 (1977) 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szentagothai J, The representation of facial and scalp muscles in the facial nucleus, J. comp. Neurol, 88 (1948), 207–220. [PubMed] [Google Scholar]
- 21.Taber E, The cytoarchitecture of the brain stem of the cat. I. Brain stem nuclei of the cat, J. comp. Neurol, 116 (1971) 27–70. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi Y; Nakano K, Uemura M, Matsuda K, Matsushima R and Mizuno N, Mesencephalic and pontine afferent fiber systems to the facial nucleus in the cat: a study using the horseradish peroxidase and silver impregnation techniques, Exp. Neurol, 66 (1979) 330–342. [DOI] [PubMed] [Google Scholar]


