SUMMARY
Single units, recorded in the medial medullary reticular formation (RF) in unrestrained, behaving cats, discharged in conjunction with specific movements and postures. Most cells were also active during REM sleep. Discharge rates in active waking and REM sleep were positively correlated and discharge patterns in these states were similar. We conclude that activity in these cells is related to the motor activation occurring in both active waking and REM sleep. We found no cells whose discharge was related in a non-specific way to motor tone or to REM sleep atonia. We discuss mechanisms by which medullary units with specific motor relations may give rise when stimulated to the relatively non-specific motor effects previously reported.
Keywords: medulla, reticular formation, unit activity, sleep, cat
INTRODUCTION
Electrical stimulation of the medial medullary reticular formation (RF) melts the motor tone of decerebrate rigidity and exerts an inhibitory effect on a variety of reflexes2,8,10–12,14. The similarity of the atonia induced by stimulation of this area to the loss of motor tone in REM sleep suggests that the medullary RF may have a role in mediating REM sleep atonia, or even an ‘executive’ role in precipitating the REM sleep state7,16. The role of cells in this region in the behaving, waking animal and in natural sleep states is unclear, since investigations of these cells have generally been restricted to immobilized or paralyzed preparations. We have therefore undertaken a systematic exploration of the medial medullary RF in unrestrained cats to determine the discharge patterns of these cells during natural waking and sleep states. Portions of these data have been reported in preliminary form23,26.
METHODS
Four female and two male cats were implanted with electrodes for recording sensorimotor electroencephalogram (EEG), electro-oculogram (EOG), lateral geniculate nucleus (LGN) ponto-geniculo-occipital spikes, and neck electromyogram (EMG). Units were recorded in freely moving, unrestrained cats using moveable bundles of 32 μm microwires. Electrophysiological, surgical, and histological techniques have been previously described27. Most of the recording sites in the present study were located near or caudal to the border of the bony tentorium22 and therefore it was possible to make vertical penetrations for most unit recordings. Two penetrations off vertical were made in the coronal plane, and one in the sagittal plane. All cells were recorded for at least one complete sleep cycle (waking, slow wave sleep (SWS), REM sleep, waking) and most were studied for more than 8 h. Behavioral observations were systematically conducted24,25. Extended periods of study, lasting for 4 or more hours were often required to adequately observe and characterize the behavioral correlates of unit discharge.
Unit discharge rates were determined ‘on line’ by an automated counting system that printed out unit rates and time code readings for consecutive 10 sec epochs. The time code was also printed on the polygraph record, thereby allowing us to correlate unit data with electroencephalographic variables to determine discharge rates during different states. Tape recordings of the unit signal were checked when polygraphic EMG tracings suggested the presence of movement artifact that might cause spurious counts.
RESULTS
Cell types
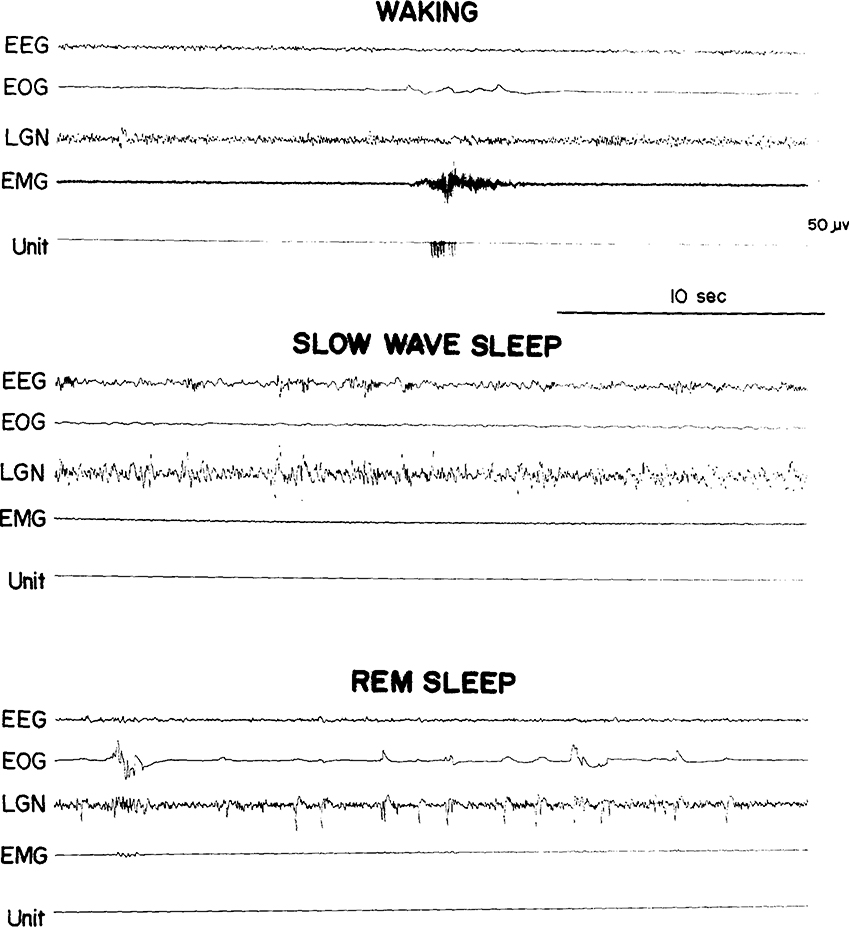
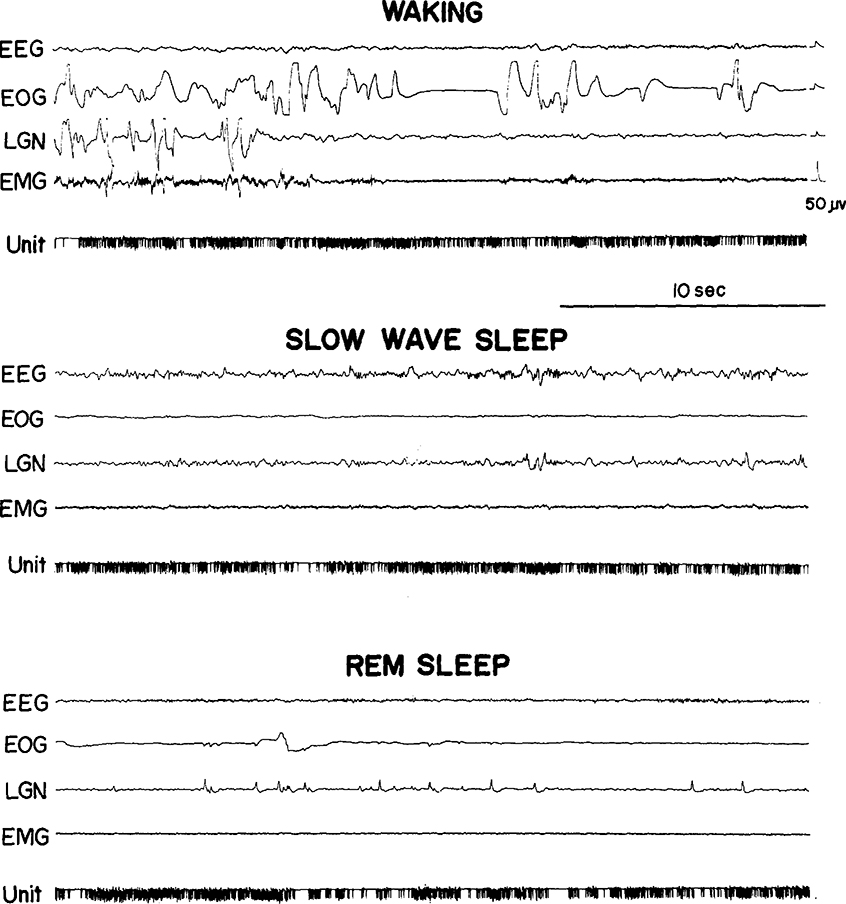
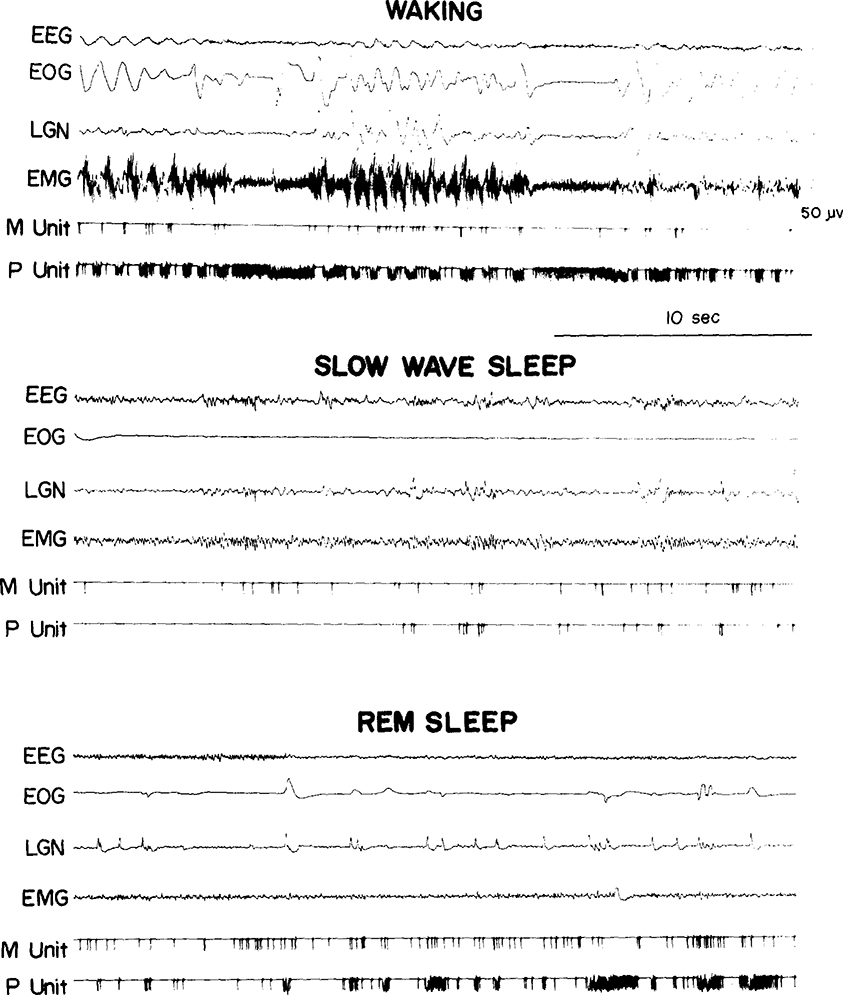
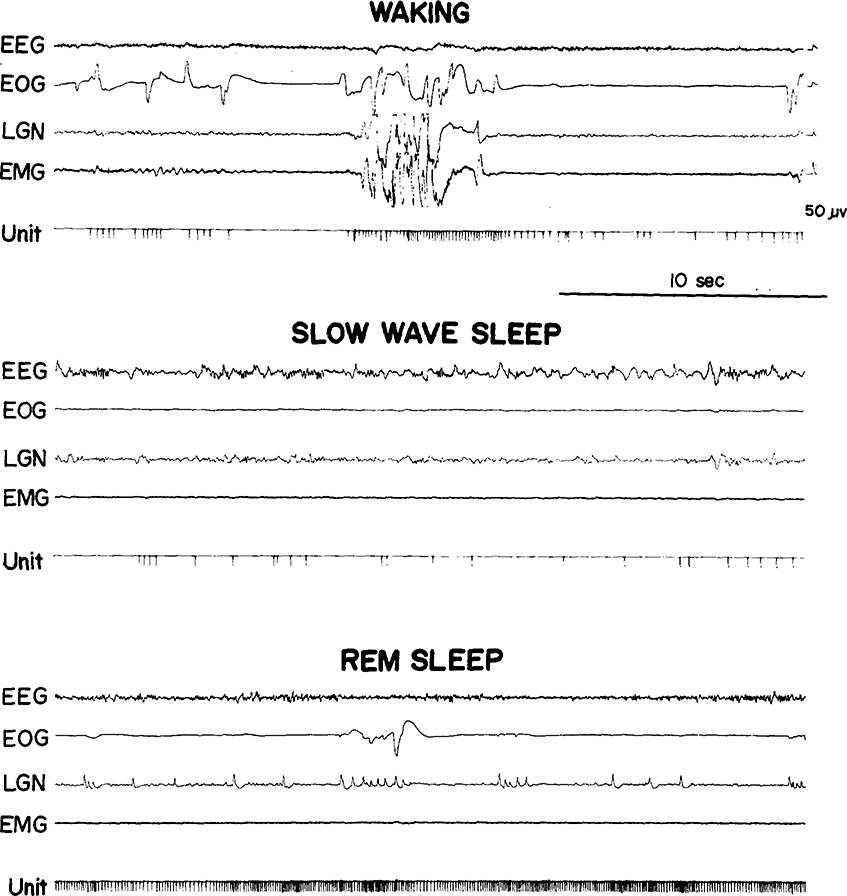
We saw the same 3 cell types in the medullary RF that we previously reported in pontine RF regions27. Type 1 cells (Fig. 1) showed no spontaneous activity in quiet waking and sleep and have previously been labeled ‘NSA’ cells 24. They discharged only during specific waking movements or in response to particular stimuli. Type 2 cells (Fig. 2) were tonically active at high rates in quiet waking and SWS and increased discharge during waking movements and in REM sleep periods. Type 3 cells (Fig. 3) were spontaneously active at low rates in quiet waking and SWS and, like type 2 cells, increased their discharge during both active waking and REM sleep. Our criteria for deciding cell type are: type 1 cells must show a minimum of 60 sec of silence in both SWS and REM sleep and no spontaneous activity in quiet waking. Type 2 cells must have SWS discharge rates in excess of 4/sec. Type 3 cells must have SWS rates equal to or less than 4/sec. Of the 48 cells recorded, 15 (31 %) were type 1, 19 (40 %) were type 2, and 14 (29 %) were type 3. Thus, in the medullary area, the proportion of type 2 cells is higher and the proportion of type 3 cells lower than in the pontine RF.
Fig. 1.
A medullary type 1 cell. EEG, sensorimotor electroencephalogram; EOG, electrooculogram; LGN, lateral geniculate nucleus activity; EMG, electromyogram; Unit, the pulse output of a window discriminator monitoring the amplified microwire signal.
Fig. 2.
A medullary type 2 cell.
Fig. 3.
A medullary type 3 cell (M Unit) recorded simultaneously with a pontine type 3 cell (P Unit). Note that the medullary cell shows less intense bursts of activity than the pontine cell in waking and REM sleep.
Discharge rates
Table I lists the waking and sleep discharge rate for 20 type 2 and 3 cells. All rates are given in discharges/sec. Maximum and minimum rates are based on 10 sec samples. Type 1 cells were silent in SWS, REM sleep and quiet waking and had arithmetic mean maximum waking discharge rates of 34.9/sec (standard deviation = 30.5) and geometric mean maximum rates of 25.2/sec (n = 7) based on 10 sec samples. All medullary RF cells showed considerable rate variation in waking. Maximum rates of discharge were invariably associated with movement or the adoption of specific postures. In all but one case (see below) discharge rates during movement exceeded mean REM sleep rates. All type 3 and all but one of the type 2 cells had higher mean discharge rates during REM sleep than during SWS. The one exceptional type 2 cell had approximately equal rates in both states (21.4/sec in SWS and 21.1/sec in REM sleep).
TABLE I.
Discharge rates during waking and sleep
| Waking |
REM sleep |
SWS mean rate | ||||
|---|---|---|---|---|---|---|
| 10-sec maximum | 10-sec minimum | mean rate | 10-sec maximum | 10-sec minimum | ||
|
| ||||||
| Overall (n = 31) | ||||||
| Arithmetic mean | 40.44 | 3.58 | 16.49 | 36.32 | 4.82 | 7.89 |
| Standard deviation | 26.49 | 5.21 | 15.12 | 27.95 | 5.79 | 8.51 |
| Geometric mean | 29.51 | 1.18 | 9.60 | 23.94 | 1.45 | 3.83 |
| Type 2 (n = 9) | ||||||
| Arithmetic mean | 56.15 | 5.56 | 24.98 | 50.87 | 7.76 | 12.11 |
| Standard deviation | 20.96 | 5.99 | 13.92 | 25.68 | 5.84 | 8.66 |
| Geometric mean | 49.80 | 2.80 | 21.82 | 44.90 | 4.63 | 10.20 |
| Type 3 (n = 22) | ||||||
| Arithmetic mean | 16.86 | 0.60 | 3.74 | 14.50 | 0.41 | 1.56 |
| Standard deviation | 12.45 | 0.78 | 2.58 | 13.30 | 0.69 | 1.49 |
| Geometric mean | 13.47 | 0.25 | 2.81 | 9.32 | 0.17 | 0.88 |
Medullary RF cells had higher rates of spontaneous activity in quiet waking than pontine RF cells, but showed smaller phasic rate variations during both waking movement and active REM sleep periods (i.e. REM sleep with eye movements and phasic muscle activity). The latter result is illustrated by the simultaneous recording of a pontine and a medullary RF cell shown in Fig. 3. The overall ratio of maximum waking rate to minimum waking rate in medullary cells was 11.3 and the ratio of maximum REM sleep rate to minimum REM sleep rate was 7.5. Both values were lower than those seen previously in pontine regions where the corresponding ratios were 60.2 and 83.9 (ref. 27). The absence of phasic rate variations was most obvious in those medullary cells that were related to postural attitudes.
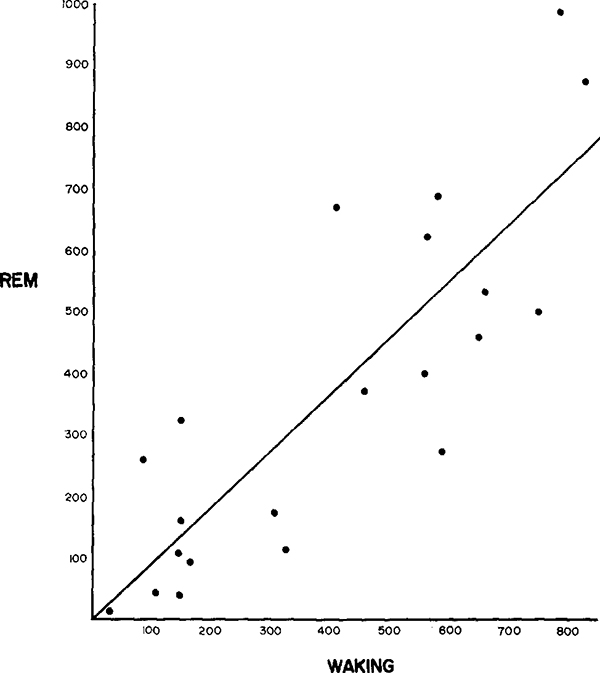
As can be seen in Figs. 1, 2 and 3, the discharge patterns in active waking tended to be quite similar to those seen in active REM sleep; i.e. cells with phasic discharge patterns in W tended to discharge phasically in REM sleep and cells with high rates in active waking had high rates in active REM sleep. This relation is documented by the high correlations between maximum waking rates and maximum REM sleep rates (r = +0.83, n = 21, P < 0.002 two-tailed). Fig. 4 shows a scatter plot of the relationship between waking and REM sleep rates.
Fig. 4.
A scatter plot showing the positive correlation of waking and REM sleep rates in medullary cells, and the least squares best fit line. Maximum 10 sec REM sleep and waking counts are plotted.
Relation to REM sleep
We saw no cells that discharged specifically in relation to the atonia of REM sleep. All but one of the recorded cells showed discharge rates that exceeded their mean REM sleep rate during active waking movements. The lone exception is shown in Fig. 5. This cell was studied for more than 24 h. It had a maximum waking rate of 8.6/sec, minimum waking rate of 0.2/sec, maximum REM sleep rate of 26.2/sec, minimum REM sleep rate of 7.7/sec, and mean REM sleep rate of 15.8/sec. Its discharge was extremely regular with few discharge bursts or pauses. During waking, this cell’s discharge was related to postures requiring a maintained neck twist to the ipsilateral side. Discharge onset with such a movement is illustrated in the middle of the waking period of Fig. 5. Several cells with similar specific postural relations were seen. In other postures the cells were often completely silent.
Fig. 5.
Posture related cell with regular discharge in waking and REM sleep.
Several techniques were employed in an attempt to find cells that might be predominantly or exclusively active in REM sleep. (1) On the assumption that cells discharging predominantly in REM sleep might show some low rate of activity in other states, microwires were routinely scanned for unit activity for at least 15 and often as long as 45 min before commencing recordings. During this period the cat was typically awake or in SWS. Using this procedure we have in the past detected cells with little or no spontaneous activity including cells that showed silent periods of over 40 min24. (2) Whenever a cell was recorded, at least one additional pair of functioning microwires was oscilloscopically monitored to determine if any new units appeared during REM sleep. (3) After recording sessions, magnetic tapes of REM sleep unit activity were replayed, photographed, and compared with waking unit records to see if there were any amplitude or waveshape changes that might indicate the appearance of new units in REM sleep. None of these techniques revealed cells with discharge restricted to REM sleep. Clearly, most medullary RF cells do not discharge specifically in relation to the atonia of REM sleep. If REM selective cells exist in this area they must have virtually no waking discharge and be relatively rare. Such cells might also be localized to regions of the medullary RF not explored in the present study.
Behavior
During waking, cells discharged in relation to specific movements. The most common cell types were those related to back and lateral head movements (75 % of encountered cells) and facial movements (10 %). Medullary RF cells were more likely to relate to axial musculature and less likely to relate to lateral head movements than pontine RF cells25. Medullary cells were usually related to movement in a particular direction, i.e. a cell might discharge consistently when a cat turned to its right but would not discharge when it made the same movement to its left. The behavioral correlates of discharge in these cells will be presented in detail elsewhere.
Histology
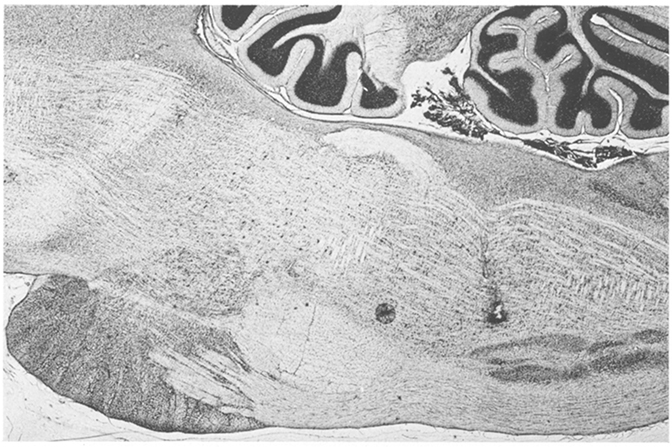
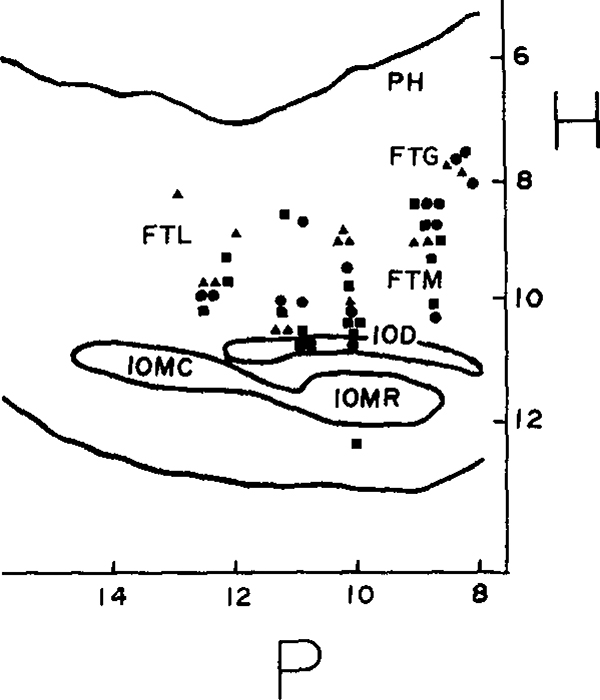
Cells were located in areas that Berman1 labels lateral, magnocellular and gigantocellular tegmental field, and three cells were localized to the inferior olive. A representative electrode tract is shown in Fig. 6. Fig. 7 shows the locations of these units in the sagittal plane. Units were located between 0.8 and 1.6 mm lateral to the midline except for those between P10.5 and P11.5. These were recorded during penetrations in the coronal plane: the ventral group were 1.8 to 2.1 mm from the midline and the dorsal pair of units were 2.5 and 2.7 mm from the midline. Type 1 cells tended to be more dorsally located, although there was some intermingling of cell types.
Fig. 6.
Representative electrode tract in medullary RF.
Fig. 7.
Locations on units plotted on sagittal section of Berman’s atlas1 at L1.2. Type 1 cells are represented by triangles, type 2 by squares and type 3 by circles. P, mm posterior to stereotaxic zero; H, mm ventral to stereotaxic zero; FTG, gigantocellular tegmental field; FTL, lateral tegmental field; FTM, magnocellular tegrnental field; IOD, dorsal accessory nucleus of the inferior olive, caudal division; IOMR, medial accessory nucleus of the inferior olive, rostral division; PH, nucleus praepositus hypoglossi.
DISCUSSION
Medullary RF cells, like pontine units 25,27, discharge in relation to specific movements or to maintained postures. Even high levels of activity in these cells during waking is not accompanied by general reductions in motor tone. Their low discharge rates in quiet waking and SWS, and elevated discharge rate both during active waking and REM sleep is also similar to that seen in adjoining pontine RF regions. In both regions discharge rates in REM sleep and in active waking movements are positively correlated. Thus, neither pontine nor medullary units show a pattern of activity that can readily explain the motor tone suppression of REM sleep. Both the ‘facilitatory’ pontine RF region and the ‘inhibitory’ medullary RF region show similar rate changes during the sleep–waking cycle.
While both pontine and medullary RF cells relate to specific movements in waking, the synaptic mechanisms underlying these effects need not be the same. It is conceivable, for example, that the behavioral relations of medullary cells reflect largely inhibitory postsynaptic processes at the spinal cord motoneuronal level while similar behavioral relations seen in pontine RF neurons involve mostly facilitatory processes. It has been shown that stimulation of medullary RF areas in acute preparations evokes mono- and polysynaptic IPSPs in spinal cord motoneurons, while stimulation of pontine RF regions produces EPSPs5,8,10,11,19. One can explain the effects of supraliminal medullary RF stimulation as being due to the synchronous activation of many units each having different specific motor correlates, but most having similar inhibitory synaptic effects. The summation of these effects in the spinal cord would produce a nonspecific inhibition of motor tone. A similar explanation may help reconcile reports that liminal electrical stimulation of the RF produces discrete movements involving reciprocal changes in muscle activity4,28 with earlier findings that more intense electrical stimulation produces nonspecific excitation or inhibition 12,17,21. Stimulation at threshold level may recruit a few units mediating specific movements and therefore result in reciprocal changes in spinal motoneuronal pools. More intense stimulation, by recruiting large numbers of units affecting different ventral horn areas, would produce ‘nonspecific’ effects.
While we find that most cells in the medial RF do not relate to motor tone in a nonspecific way, we cannot rule out the possibility that some small number of cells in this area have this relation. A recent study by Netick et al. 16 reported finding 4 cells in the medullary RF that discharged at very low rates in waking and SWS but showed high levels of tonic discharge in REM sleep. We can confirm the existence of cells that show little activity in quiet waking and SWS and show tonic activity in REM sleep. However, we found that these cells also showed tonic activity during specific postures in waking. Since Netick et al. were recording in cats immobilized by head and body restraints they would probably not have observed this waking activity. Therefore the cells they report as REM sleep selective may be similar to the posture related cells we see. The Netick et al. study did not determine the proportion of encountered medullary RF cells that discharged selectively in REM sleep. Therefore the possibility that such cells are rare and were missed in our study cannot be rejected.
We found that the discharge rates and patterns of medullary RF units are similar in active waking and REM sleep. Similar results were found in pontine RF areas13,27,29. At a physiological level this similarity can be explained as reflecting similar patterns of synaptic input and similar cell membrane characteristics of RF cells in these two states. At a behavioral level it can be noted that, for the motor system, REM sleep is a time of intense activity, similar to active waking3,6,9,18. This activity can be observed in the twitching and rapid eye movements that are the defining characteristics of REM sleep, although hyperpolarization of motoneurons prevents the somatic expression of most of the descending motor signals 15,20. The similarity of discharge in brainstem motor systems in waking and REM sleep may reflect a similar central programming of movements in these two states.
ACKNOWLEDGEMENTS
Supported by the Medical Research Service of the Veterans Administration and N.I.H. Research Grant NS14610.
REFERENCES
- 1.Berman AL, The Brain Stem of the Cat, University of Wisconsin Press, Madison, 1968, 175 pp. [Google Scholar]
- 2.Engberg I, Lundberg A and Ryall RW, Reticulospinal inhibition of transmission in reflex pathways, J. Physiol. (Lond.), 194 (1968) 201–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evarts EV, Temporal patterns of discharge of pyramidal tract neurons during sleep and waking in the monkey, J. Neurophysiol, 27 (1964) 152–171. [DOI] [PubMed] [Google Scholar]
- 4.Gernandt BE and Thulin CA, Reciprocal effects upon spinal motoneurons from stimulation of bulbar reticular formation, J. Neurophysiol, 18 (1955) 113–129. [DOI] [PubMed] [Google Scholar]
- 5.Grillner S and Lund S, The origin of a descending pathway with monosynaptic action on flexor motoneurons, Acta. physiol. scand, 74 (1968) 274–284. [DOI] [PubMed] [Google Scholar]
- 6.Harper RM and Jacobs BL, Red nucleus neuronal activity during sleep and wakefulness, Sleep Res, 1 (1972) 20. [Google Scholar]
- 7.Hoshino K and Pompeiano O, Selective discharge of pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cat, Arch. ital. Biol, 114 (1976) 244–277. [PubMed] [Google Scholar]
- 8.Jankowska E, Lund S, Lundberg A and Pompeiano O, Inhibitory effects evoked through ventral reticulospinal pathways, Arch. ital. Biol, 106 (1968) 124–140. [PubMed] [Google Scholar]
- 9.LaMarre Y, Filion M and Cordeau JP, Neuronal discharges of the ventrolateral nucleus of the thalamus during sleep and wakefulness in the cat. I. Spontaneous activity, Exp. Brain Res, 12 (1971) 480–498. [DOI] [PubMed] [Google Scholar]
- 10.Llinas R and Terzuolo CA, Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha-extensor motoneurons, J. Neurophysiol, 27 (1964) 579–591. [DOI] [PubMed] [Google Scholar]
- 11.Llinas R and Terzuolo CA, Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms upon flexor motoneurons, J. Neurophysiol, 28 (1965) 413–422. [DOI] [PubMed] [Google Scholar]
- 12.Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, J. Neurophysiol, 9 (1946) 165–171. [DOI] [PubMed] [Google Scholar]
- 13.McGinty DJ and Siegel JM, Neuronal activity patterns during rapid-eye-movement sleep: relation to waking patterns. In Drucker-Colin RR and McGaugh JL (Eds.), Neurobiology of Sleep and Memory, Academic Press, 1977, pp. 135–158. [Google Scholar]
- 14.Nakamura Y, Nozaki S, Takatori M and Kikucki M, Possible inhibitory neurons in the bulbar reticular formation involved in the cortically evoked inhibition of the masseteric motoneuron of the cat, Brain Research, 115 (1976) 512–517. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Goldberg LJ, Chandler SH and Chase MH, Intracellular analysis of trigeminal motoneuron activity during sleep in the cat, Science, 199 (1978) 204–207. [DOI] [PubMed] [Google Scholar]
- 16.Netick A, Orem J and Dement W, Neuronal activity specific to REM sleep and its relationship to breathing, Brain Research, 120 (1977) 197–207. [DOI] [PubMed] [Google Scholar]
- 17.Niemer WT and Magoun HW, Reticulo-spinal tracts influencing motor activity, J. comp. Neurol, 87 (1947) 367–379. [DOI] [PubMed] [Google Scholar]
- 18.Pellet J, Tardy MF, Harlay F, Dubrocard S et Gilhodes JC, Activité spontanée des cellules de Purkinje chez la chat chronique: étude statistique des spikes complexes, Brain Research, 81 (1974) 75–96. [DOI] [PubMed] [Google Scholar]
- 19.Peterson BW, Pitts NG, Fukushima K and Mackel R, Reticulospinal excitation and inhibition of neck motoneurons, Exp. Brain Res, 32 (1978) 471–489. [DOI] [PubMed] [Google Scholar]
- 20.Pompeiano O, Mechanisms of sensorimotor integration during sleep, Progr. Physiol. Psychol, 3 (1970) 1–179. [Google Scholar]
- 21.Rhines R and Magoun HW, Brain stem facilitation of cortical motor response, J. Neurophysiol, 9 (1946) 219–229. [DOI] [PubMed] [Google Scholar]
- 22.Siegel JM, A stereotaxic map of the bony tentorium of the cat, Physiol. Behav, 13 (1974) 715–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel JM, Unit activity in the reticular formation and REM sleep. In Conf. Functions of Sleep and its Clinical Implications, Mexico City, 1977. [Google Scholar]
- 24.Siegel JM and McGinty DJ, Brainstem neurons without spontaneous unit discharge, Science, 193 (1976) 240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel JM and McGinty DJ, Pontine reticular formation neurons: relationship of discharge to motor activity, Science, 196 (1977) 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel JM and McGinty DJ, Sleep and waking activity of medullary gigantocellular nucleus cells Sleep Res, 7 (1978) 50. [Google Scholar]
- 27.Siegel JM, McGinty DJ and Breedlove SM, Sleep and waking activity of pontine gigantocellular field neurons, Exp. Neurol, 56 (1977) 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprague JM and Chambers WW, Control of posture by reticular formation and cerebellum in the intact, anesthetized and unanesthetized and in the decerebrated cat, Amer. J. Physiol, 176 (1954) 52–64. [DOI] [PubMed] [Google Scholar]
- 29.Vertes RP, Selective firing of rat pontine gigantocellular neurons during movement and REM sleep, Brain Research, 128 (1977) 146–152. [DOI] [PubMed] [Google Scholar]