Abstract
It has long been known that stimulation of the medial medulla in the decerebrate animal produces bilateral inhibition of muscle tone. In the present study we have found that transection of the brainstem at the ponto-medullary junction attenuates this inhibition. An interaction between medullary and rostal brainstem systems is responsible for the medullary inhibition phenomenon. A similar interaction may produce the inhibition of muscle tone seen in REM sleep.
Keywords: medulla, pons, inhibition, muscle tone, REM sleep
Magoun and Rhines first demonstrated that stimulation of a large portion of the reticular formation of the medial medulla abolished tonic muscle activity and reflex response bilaterally in the response bilaterally in the decerebrate cat 14. It was hypothesized that this effect was due to activation of an intrinsic medullary mechanism with projections descending to the spinal cord. A number of subsequent studies have shown that the medullary inhibition of muscle tone is mediated by hyperpolarization of motoneurons8,11,12
A prolonged bilateral inhibition of muscle tone has been found to occur naturally in REM sleep9. This inhibition is also accompanied by hyperpolarization of motoneurons1–3,5,6,15,16,18 The motor inhibition of REM sleep can be prevented by small lesions placed in dorsolateral pons7,10,19 It has been hypothesized that this pontine region acts by projecting caudally to excite the medullary inhibitory region. Descending projections from the medullary region would then form the final common path for inhibition of motor activity.
If the medullary region responsible for atonia is autonomous, direct stimulation of this area, even after disconnection of the pons, should produce muscle atonia. However, we find that transaction below the level of the pons greatly reduces the number of sites from which medullary inhibition can be produced. Interactions between the medulla and rostral brainstem contribute to the inhibition of muscle tone by medullary stimulation. These results have been reported in preliminary form 20.
Eleven cats served as subjects. Eight were used in acute experiments. Four of the acute preparations were transected at the intercollicular level. The medulla was then stimulated to observe muscle tone inhibition. A second transection was performed at the ponto-medullary level in one of these cats and the stimulation procedures repeated. The 4 other acute preparations were transected only at the ponto-medullary level.
Three cats were used in chronic procedures. They were kept for 28 days after transection at the ponto-medullary level. The procedures for preparing and maintaining these animals are described elsewhere 21. Two of these cats had 4 tripolar electrodes attached to implanted microdrives. These electrodes were used for stimulation between days 7 and 28 after the transection as they were advanced through the medullary inhibitory region. The other chronic cat was stimulated, following the same procedure used in the acute preparations (see below), in a terminal procedure on the 28th day after transection.
Surgical procedures prior to brainstem transection were carried out under halothane/oxygen anesthesia using aseptic technique. Midbrain transection was performed after removing the occipital lobe and hippocampus. A thin wedge of tissue was then aspirated in the coronal plane at the intercollicular or inferior collicular level.
Transection at the ponto-medullary junction was performed after aspirating the medial cerebellum to expose the floor of the fourth ventricle. A spatula was then lowered at 30° off vertical to transect the brainstem.
Blood pressure was monitored through the femoral artery after the more caudal transections, and remained above 100 mm Hg. Expired CO2 was monitored in all cats through an endotrachael tube with a Beckman LB2 CO2 analyzer, and respiration was assisted with a Harvard pump when necessary to maintain CO2 levels below 6%. Core temperature was controlled by a circulating water heating pad triggered by a rectal thermistor. Pairs of stranded stainless steel wires, with 1 cm separation in the sagittal plane, were inserted into the left and right splenius muscles to monitor the activity of the dorsal neck musculature.
Tripolar 30-gauge stainless steel electrodes with 0.5 mm vertical tip separation were used for stimulation. Electrodes were lowered in 0.5 mm steps in the coronal plane. In acute preparations, stimulation was applied at L ± 1.0 and in the midline proceeding in a rostral to caudal direction. Stimulation was performed no less than 2 h after the cessation of halothane anesthesia. Medullary stimulation consisted of 300 or 500 ms trains of 0.1 ms pulses at 60 Hz. Stimulation intensity at each point was varied from 0 to 500 μA. Current levels as high as 500 μA were employed to rule out the possibility that transections had merely elevated the inhibition threshold. Muscle response was displayed on a Grass 78 polygraph and tape recorded along with blood pressure and percent CO2.
Medullary stimulation after intercollicular transection produced a complete bilateral suppression of muscle tone (Fig. 1), as first reported by Magoun13. Current thresholds were below 100 μA. A rebound excitation was often evident immediately after stimulation. Aspiration of the medial cerebellum eliminated this rebound while the inhibitory effect remained (Fig. 1), as has previously been described22.
Fig. 1.
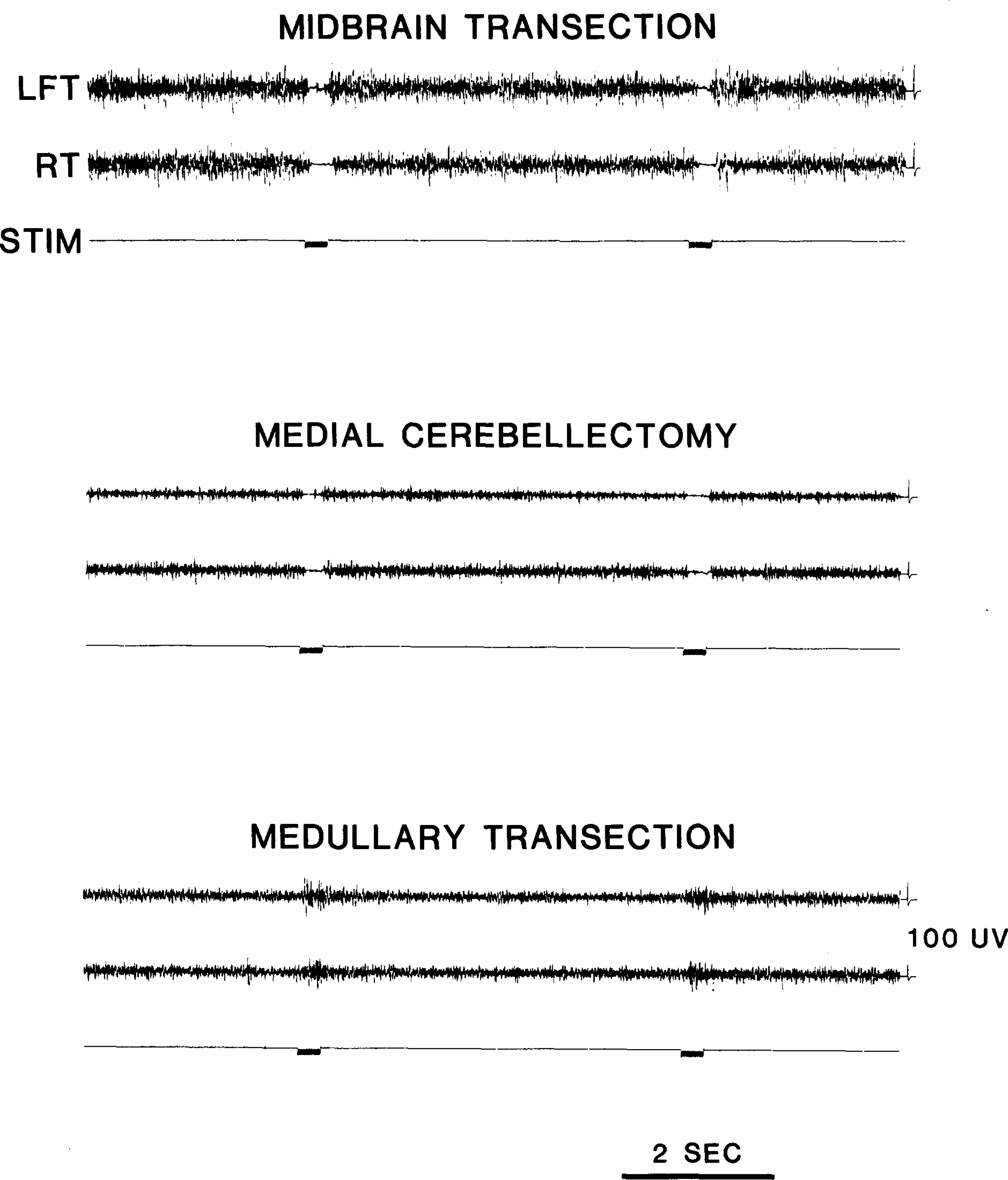
Effect of medullary stimulation on neck muscle tone recorded from electrodes placed in left (LET) and right (RT) splenius muscles. Medullary stimulation produces suppression of muscle tone after decerebration and medial cerebellectomy. Transection at the ponto-medullary junction prevents this suppression. Data are from a single experiment. Stimulation with 0.1 ms, 150 μA pulses at 60Hz for 300 ms was applied in each condition at P9.0, L1.0, H–8.0. Inhibition threshold was 80 μA.
Transection at the ponto-medullary junction (Fig. 2), performed in both acute and chronic preparations, completely eliminated the bilateral suppression of muscle tone in 6 of the 8 transected cats. Stimulation of the region identified by Magoun and Rhines14 produced only excitation of ipsilateral and contralateral neck musculature (Fig. 1) at current thresholds ranging from 80 to 160 μA. Bilateral inhibition was never seen at any current level. In the remaining two cats, bilateral inhibition was found at less than 10% of the sites tested.
Fig. 2.
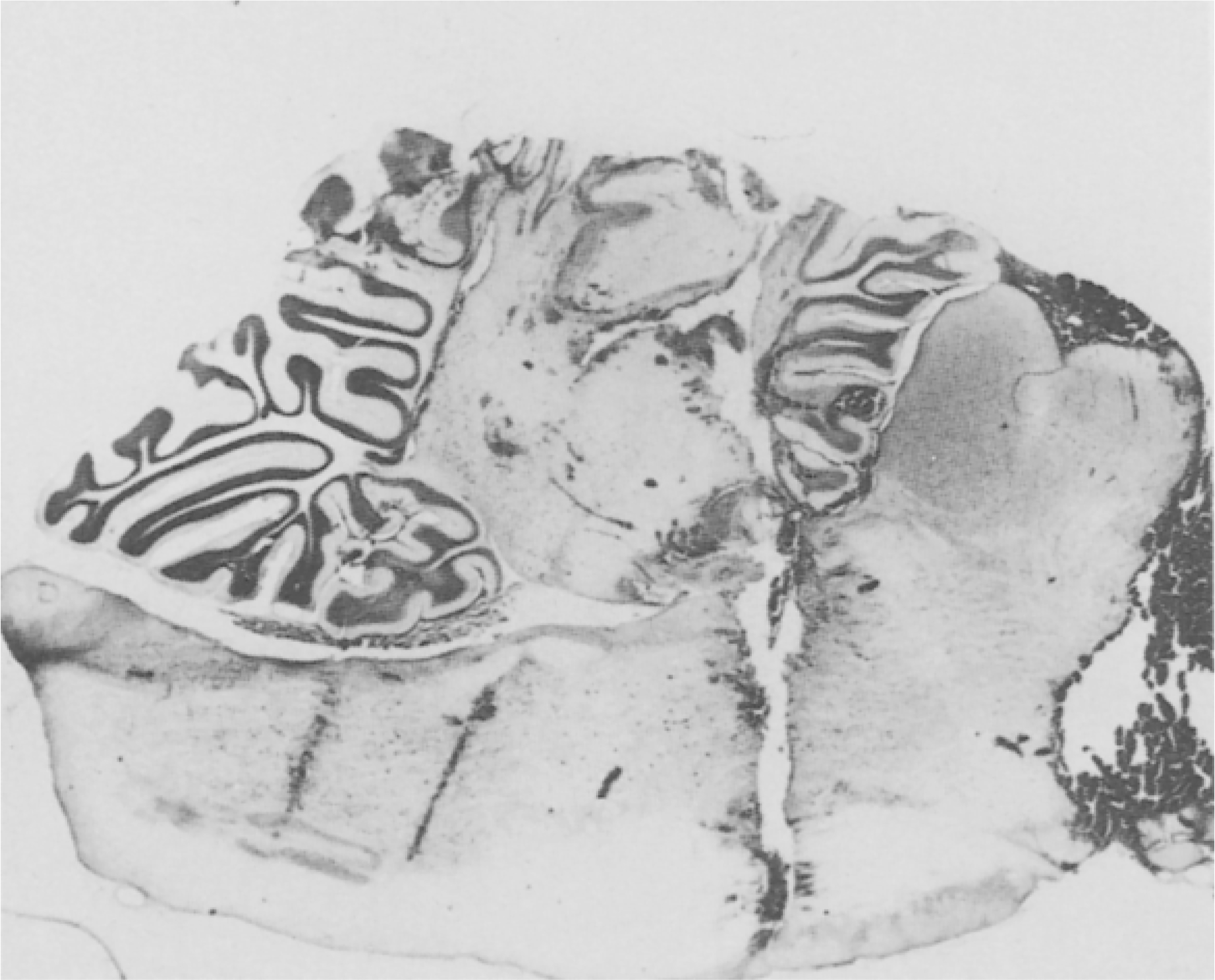
Cat with midbrain and pontine transection. Electrode tracks in medulla pass through inhibitory area. After caudal transection, inhibition could not be evoked by medullary stimulation.
Histological reconstruction of the transection levels and stimulation sites was made for all brains. Transections at both midbrain and ponto-medullary junction were complete except for sparing of less than 0.5 mm of the lateral filaments of the branchium pontis in two cats. In the remaining cats a complete mechanical separation of the brainstem was effected at the transection sites. Results did not differ in these two groups. Ponto-medullary transection levels varied from the most caudal lesion passing through the abducens nucleus (P6.5, H–4) at a 30° angle to vertical, to the most rostral lesions passing at the same angle just caudal to the locus coeruleus complex (P5.4, H–2.8). The region tested for the inhibitory phenomenon extended from P7 to P16, to H–5 to H–10, L0.0 ± 1.0.
In their studies, Niemer and Magoun17 reported that chronic hemisection of the brainstem at the ponto-medullary junction did not prevent the inhibition caused by stimulating either side of the medulla. They interpreted this finding as indicating that systems rostral to the medulla were not required for the inhibitory effect. The present results suggest a different interpretation. Rostral brainstem structures do contribute to medullary inhibition. However, unilateral connection of the pons to medullary structures is apparently sufficient to sustain the effect.
In the early work on medullary inhibition, it was reported that some decerebrate preparations did not produce any motor inhibition when stimulated in the medial medullary region4,22. This was attributed to individual differences between the cats. The present results suggest that the level of transection may have been responsible for this variation. Low decerebrations performed in the anterior fossa will normally destroy a slice of caudal midbrain tissue and, if the damage is extensive, may damage pontine regions. Such damage is less likely with intercollicular transections which will therefore exhibit the inhibitory phenomenon.
In terms of the old controversy over whether the medullary reticular formation exerts non-specific inhibitory effects4,14,22 the present results suggest that non-specific inhibition can be seen if rostral brainstem regions are intact. Loss of these regions leaves an animal in which muscle excitation is produced by medullary stimulation at most medullary sites.
The rostral brainstem contribution to motor inhibition could be mediated by any one of a number of circuits. The dorso-lateral pons and adjacent pontine region contains several cell groups which have direct projections to the spinal cord23. Medullary stimulation could activate these cell groups which could in turn produce spinal motoneuron inhibition. It is also possible that rostral brainstem regions may gate descending medullary inhibition at the spinal or medullary level. Since the full development of inhibition from medullary stimulation requires a minimum of 30–60 ms8,11, there is adequate time for a number of relays or recruitment loops to be involved.
Acknowledgments
Supported by the Medical Research Service of the Veterans Administration, NIH Grant NS14610 and NSF Grant BNS 82-00023.
References
- 1.Chandler SH, Chase MH and Nakamura Y, Intracellular analysis of synaptic mechanisms controlling trigeminal motoneuron activity during sleep and wakefulness, J. Neurophysiol, 44 (1980) 359–371. [DOI] [PubMed] [Google Scholar]
- 2.Chandler SH, Nakamura Y and Chase MH, Intracellular analysis of synaptic potentials induced in trigeminal jaw-closer motoneurons by pontomesencephalic reticular stimulation during sleep and wakefulness, J. Neurophysiol, 44 (1980) 372–382. [DOI] [PubMed] [Google Scholar]
- 3.Chase MH, Chandler SH and Nakamura Y, Intracellular determination of membrane potential of trigeminal motoneurons during sleep and wakefulness, J. Neurophysiol, 44 (1980) 349–358. [DOI] [PubMed] [Google Scholar]
- 4.Gernandt BE and Thulin CA, Reciprocal effects upon spinal motoneurons from stimulation of bulbar reticular formation, J. Neurophysiol, 18 (1955) 113–129. [DOI] [PubMed] [Google Scholar]
- 5.Glenn LL and Dement WC, Membrane potential and input resistance of cat spinal motoneurons in wakefulness and sleep, Behav. Brain Res, 2 (1981) 231–236. [DOI] [PubMed] [Google Scholar]
- 6.Glenn LL, Foutz AS and Dement WC, Membrane potential of spinal motoneurons during natural sleep in cats, Sleep, 1 (1978) 199–204. [PubMed] [Google Scholar]
- 7.Henley K and Morrison AR, A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat, Acta neurobiol. exp, 34 (1974) 215–232. [PubMed] [Google Scholar]
- 8.Jankowska E, Lund S, Lundberg A and Pompeiano O, Inhibitory effects evoked through ventral reticulospinal pathways, Arch. ital. Biol, 106 (1968) 124–140 [PubMed] [Google Scholar]
- 9.Jouvet M, Neurophysiology of the states of sleep, Physiol. Rev, 47 (1967) 117–177. [DOI] [PubMed] [Google Scholar]
- 10.Jouvet M and Delorme F, Locus coeruleus et sommeil paradoxal, C.R. Soc. Biol. (Paris), 159 (1965) 895–899. [Google Scholar]
- 11.Llinas R and Terzuolo CA, Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha-extensor motoneurons, J. Neurophysiol, 27 (1964) 579–591. [DOI] [PubMed] [Google Scholar]
- 12.Llinas R and Terzuolo CA, Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms upon flexor motoneurons, J. Neurophysiol, 28 (1965) 413–422. [DOI] [PubMed] [Google Scholar]
- 13.Magoun HW, Bulbar inhibition and facilitation of motor activity, Science, 100 (1944) 549–550. [DOI] [PubMed] [Google Scholar]
- 14.Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, J. Neurophysiol, 9 (1946) 165–171. [DOI] [PubMed] [Google Scholar]
- 15.Morales F and Chase MH, Postsynaptic control of lumbar motoneuron excitability during active sleep in the chronic cat, Brain Research, 225 (1981) 279–295. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y, Goldberg LJ, Chandler SH and Chase MH, Intracellular analysis of trigeminal motoneuron activity during sleep in the cat, Science, 199 (1978) [DOI] [PubMed] [Google Scholar]
- 17.Niemer WT and Magoun HW, Reticulo-spinal tracts influencing motor activity, J. comp. Neurol, 87 (1947) 367–379. [DOI] [PubMed] [Google Scholar]
- 18.Pompeiano O, Mechanisms responsible for spinal inhibition during desynchronized sleep: experimental study. In Guilleminault C, Dement WC and Passouant P (Eds.), Advances in Sleep Research 3; Narcolepsy, Spectrum, New York, 1976, pp. 411–449. [Google Scholar]
- 19.Sakai K, Sastre JP, Kanamori N and Jouvet M, State-specific neurons in the ponto-medullary reticular formation with special reference to the postural atonia during paradoxical sleep in the cat. In Pompeiano O and Ajmone Marsan C (Eds.), Brain Mechanisms and Perceptual Awareness, Raven Press, New York, 1981. [Google Scholar]
- 20.Siegel JM and Nienhuis R, Pons is required for medullary inhibition of muscle tone, Soc. Neurosci. Abstr, 8 (1982) 957. [Google Scholar]
- 21.Siegel JM,Nienhuis R and Tomaszewski KS, Behavioral states in the chronic medullary and mid-pontine cat, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprague JM and Chambers WW, Control of posture by reticular formation and cerebellum in the intact, anesthetized and unanesthetized and in the decerebrated cat. Amer. J. Physiol, 176 (1954) 52–64. [DOI] [PubMed] [Google Scholar]
- 23.Tohyama M, Sakai K, Salvert D, Touret M and Jouvet M, Spinal projections from the lower brain stem in the cat as demonstrated by the horseradish peroxidase technique. I. Origins of the reticulo-spinal tracts and their funicular trajectories, Brain Research, 173 (1979) [DOI] [PubMed] [Google Scholar]


