Summary:
Blood pressure and heart rate were monitored in narcoleptic dogs by means of a chronically implanted catheter placed in the descending aorta. Changes in these variables were recorded during spontaneously occurring cataplectic episodes. We found no reliable change in blood pressure associated with cataplexy onset. However, heart rate showed a marked increase prior to the onset of cataplexy, with peak heart rates being reached at or shortly after the disappearance of muscle tone. Autonomic events correlated with increased heart rate may contribute to the triggering of cataplexy in narcoleptics.
Keywords: Narcolepsy, Cataplexy, Heart rate, Blood pressure, Dog
It is well known that cataplectic attacks in humans are often triggered by emotions such as laughter, anger, surprise, excitement, fear, and embarrassment and by sexual intercourse and athletic activity (1). Since such situations tend to increase heart rate, it has long been suspected that cardiovascular changes may be a triggering event in cataplexy. A series of studies by Puizzilout and colleagues (3–5) provided some support for this hypothesis. These workers found that in chronic encéphale isolé cats stimulation of the baroreceptors produced a loss of muscle tone resembling cataplexy. This was followed by other signs of REM sleep. Scrima (6) has hypothesized that a similar activation of the baroreceptors may trigger cataplexy in human narcolepsy. Sachs and Kaijser (7,8) have reported that narcoleptic patients have reduced reactivity in the cardiovascular system. Therefore, it is of considerable interest to determine if cardiovascular changes accompany cataplectic attacks. In this study we have examined such changes in genetically narcoleptic dogs (1).
METHODS
One male (#142) and two female (#139 and 141) puppies aged from 3 to 4 months served as subjects. The dogs were Doberman and Labrador crossbreeds and were transported from the Stanford Narcoleptic Dog Colony to Los Angeles, where the studies were conducted. Using Halothane anesthesia, the dogs were implanted with screw electrodes over sensorimotor and posterior lateral cortex for recording electroencephalograms (EEGs). Screw electrodes were also placed in the orbit or frontal bone for recording the electro-oculogram (EOG). Stranded stainless steel wires were inserted into the dorsal neck musculature for recording the electromyogram (EMG). A Swan-Ganz 5F catheter was inserted through the femoral artery and guided into the descending aorta. It was led subcutaneously to terminate on the dog’s head plug. The catheter was flushed with heparinized saline every 12 h and a continuous flow of 4 cc/h of heparinized saline was maintained during recording sessions using a Sorenson valve. Blood pressure was recorded with a Statham pressure transducer and was displayed on a polygraph along with EEG, EOG, and EMG. A cardiotachometer was employed to display heart interbeat intervals derived from the blood pressure pulse wave. In dog #142 respiration was recorded with a thoracic strain gauge.
The subjects were trained to stand quietly in a sling (Chatham Medical Arts) that supported most of their weight. The experiments were performed between 0800 and 1900 h in a quiet room with a low level of natural light. The dogs could see only the wall of the laboratory, ~ 3 feet away. No attempt was made to trigger cataplexy by presenting food, water, or other stimuli. Spontaneous episodes of sleep and cataplexy occurred as the dogs rested undisturbed in the sling. Systolic and diastolic blood pressure, heart rate, and EMG were measured prior to and during 10 consecutive artifact-free cataplectic episodes and eight consecutive REM sleep bouts.
RESULTS
Figure 1 presents data from representative cataplectic attacks in each of the experimental subjects. Blood pressure changes prior to cataplexy onset were inconsistent. However, heart rate showed a substantial and consistent increase prior to cataplexy onset. Apnea frequently accompanied cataplexy, as can be seen in dog #142.
FIG. 1.
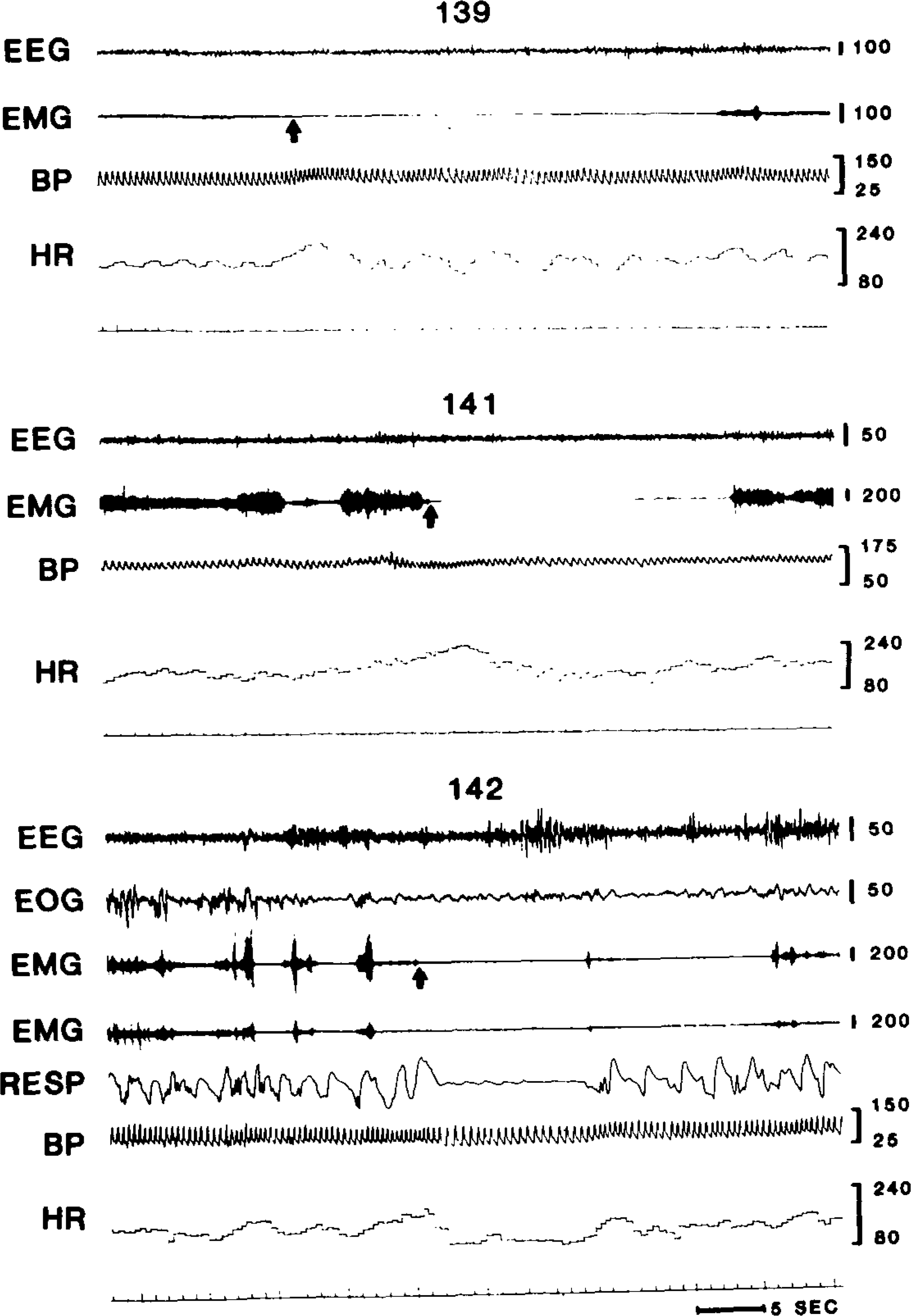
Polygraph tracings of cataplexy onset in dogs #139, 141, and 142. Arrows indicate points scored as cataplexy onset. EEG, sensorimotor electroencephalogram (calibration in (μV); EMG, dorsal neck electromyogram (left and right neck derivations shown in dog 142); EOG, orbital electro-oculogram; RESP, respiration; BP, blood pressure in mm Hg; HR, heart rate in beats/min.
Figure 2 presents data averaged from consecutive, artifact-free, spontaneous cataplectic attacks. EMG levels began to fall 3–4 s prior to cataplexy onset, defined as the point at which muscle tone diminished to zero. Systolic and diastolic blood pressure showed no significant change in the 10 s preceding cataplexy onset. However, heart rate declined consistent change beginning at least 10 s prior to the onset of cataplexy. Heart rate declined rapidly after cataplexy onset. Heart rate at cataplexy onset was significantly greater than heart rate 10 s prior to cataplexy onset (p < 0.05, F test). These changes are of very large magnitude, with heart rate increasing by a mean of 18% in the 10 s preceding cataplexy onset and falling by a mean of 10% in the 10 s after cataplexy onset. The heart rate changes were highly reliable. Nine out of the 10 consecutive cataplectic episodes recorded in each dog were associated with an increase in heart rate in the 10 s preceding cataplexy (p < 0.0001, Sign test). In dog #139 the tenth episode was associated with no heart rate change, while a small heart rate decrease was observed in the tenth cataplectic episode in dogs #141 and 142.
FIG. 2.
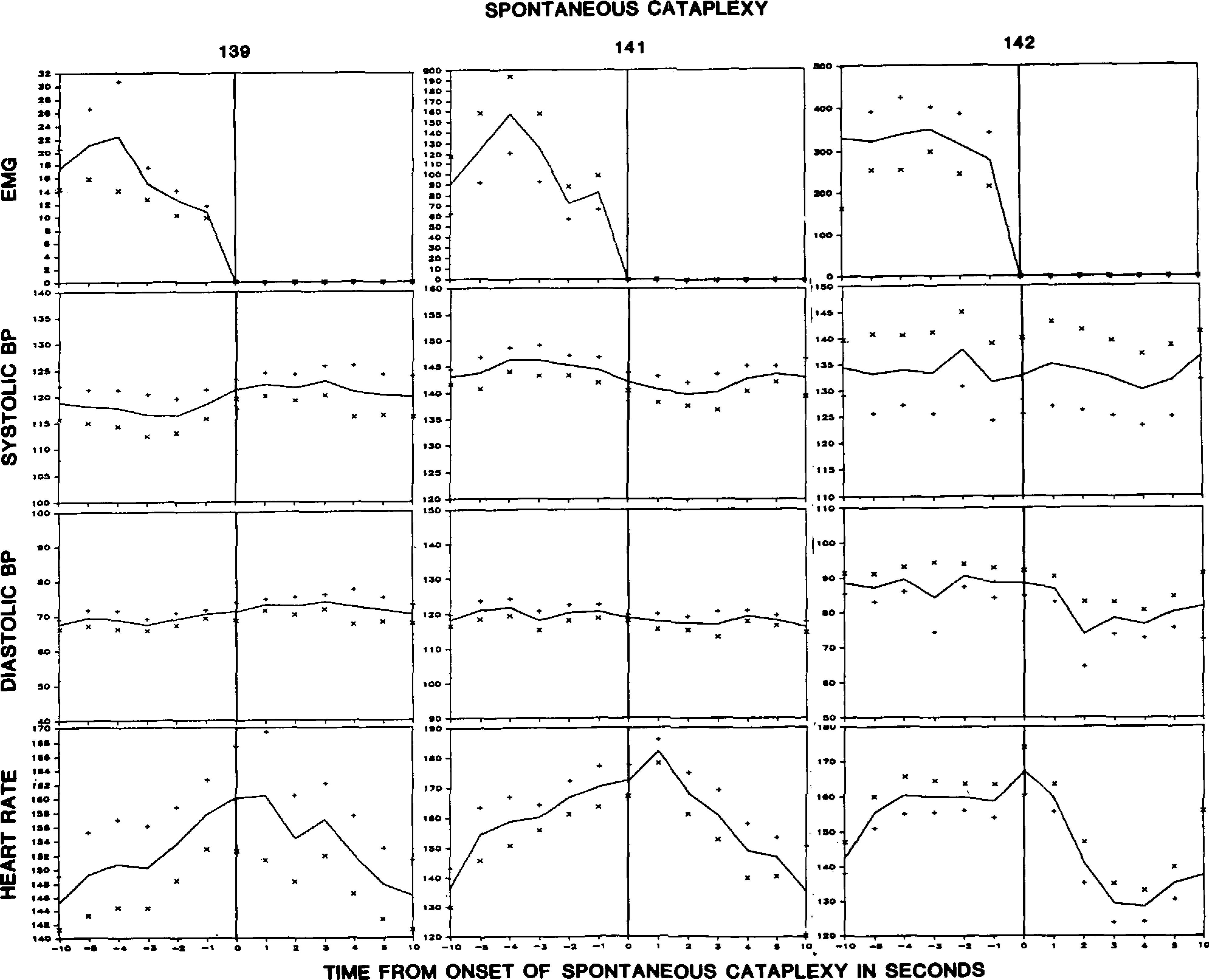
Averaged electromyogram (EMG), blood pressure (BP), and heart rate changes during the period beginning 10 s prior to cataplexy onset and ending 10 s after cataplexy onset. Graphs are based on 10 cataplectic episodes in dogs #141 and 142 and on 7 episodes in dog #139. + and × signs indicate standard error.
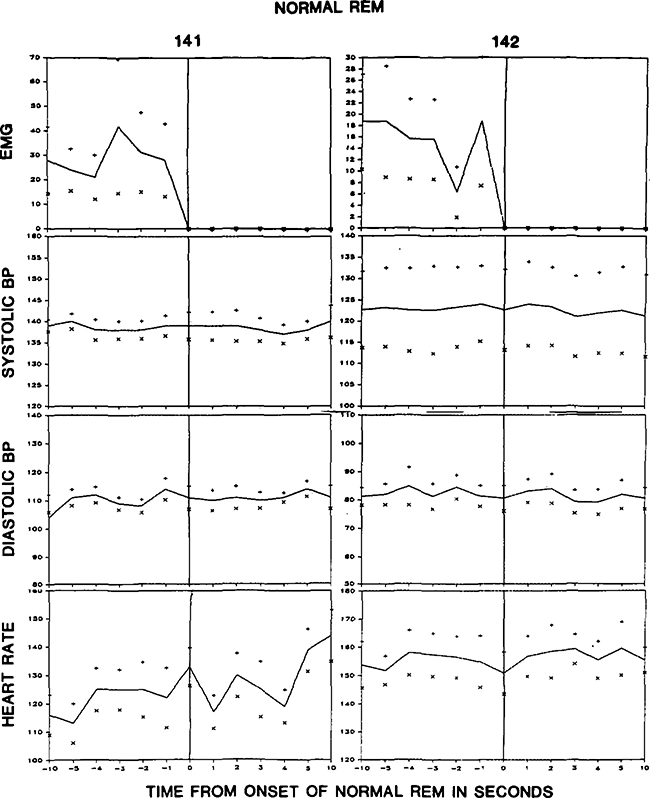
Heart rate changes at cataplexy onset could be seen as relating primarily to EMG reduction or to the change from waking to the cataplectic state. Therefore, we conducted a similar analysis on cardiovascular changes during REM sleep onset in two of the dogs (Fig. 3). Heart rate did not increase at REM sleep onset. While heart rates 10 s prior to REM sleep onset were not significantly different from those 10 s prior to cataplexy onset, heart rates at cataplexy onset were significantly greater than those at REM sleep onset (p < 0.0002, F test).
FIG. 3.
Averaged electromyogram (EMG), blood pressure (BP), and heart rate during the period beginning 10 s prior to transition from NREM to REM sleep and ending 10 s after REM sleep onset. Averages are based on eight REM sleep onsets in each dog.
DISCUSSION
We found a consistent and large magnitude increase in heart rate prior to cataplexy onset. This increase presumably reflects a change in the sympathetic-parasympathetic balance, which precedes the EMG reduction defining cataplexy. The heart rate increase does not seem to be a simple correlate of changes in the EMG level because it precedes the EMG reduction and does not occur with the EMG offset seen in REM sleep episodes in the same animals.
Despite the increase in heart rate preceding cataplexy, we found no consistent change in blood pressure during the transition to the cataplectic state. However, it remains possible that blood pressure change could trigger or block cataplexy. Therefore, further study and manipulation of cardiovascular variables in narcoleptic subjects would be useful.
Acknowledgment:
This research was supported by the Medical Research Services of the Veterans Administration, PHS grant NS14610, and NSF grant BNS00023.
REFERENCES
- 1.Delashaw JB Jr, Foutz AS, Guilleminault C, Dement WC. Cholinergic mechanisms and cataplexy in dogs. Exp Neurol 1979;66:745–57. [DOI] [PubMed] [Google Scholar]
- 2.Guilleminault C Cataplexy. In Guilleminault C, Dement WC, Passouant P, eds. Narcolepsy, New York: Spectrum, 1976:125–43. [Google Scholar]
- 3.Puizillout JJ, Foutz AS. Vago-aortic nerves stimulation and REM sleep: evidence for a REM-triggering and a REM-maintenance factor. Brain Res 1976;111:181–4. [DOI] [PubMed] [Google Scholar]
- 4.Puizillout JJ, Foutz AS. Characteristics of the experimental reflex sleep induced by vago-aortic nerve stimulation. Electroencephalogr Clin Neurophysiol 1977;42:552–63. [DOI] [PubMed] [Google Scholar]
- 5.Puizillout JJ, Temaux JP, Foutz AS, Fernandez G. Les stades de sommeil de la preparation “encephale isole”: I Declenchement des pointes ponto-geniculo-occipitales et du sommeil phasique a ondes lentes. Role des noyaux du raphe. Electroencephalogr Clin Neurophysiol 1974;37:561–76. [DOI] [PubMed] [Google Scholar]
- 6.Scrima L An etiology of narcolepsy-cataplexy and a proposed cataplexy neuromechanism. Int J Neuroscience 1981;15:69–86. [DOI] [PubMed] [Google Scholar]
- 7.Sachs C, Kaijser L. Autonomic control of reflexes in narcolepsy. J Neurol Neurosurg Psychiatr 1980;43:535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachs C, Kaijser L. Autonomic regulation of cardiopulmonary functions in sleep apnea syndrome and narcolepsy. Sleep 1982;5:227–38. [DOI] [PubMed] [Google Scholar]