Abstract
Rapid eye movement (REM) sleep is normally accompanied by a complete suppression of tone in the antigravity musculature. Pontine lesions have been shown to block this suppression, producing a syndrome of REM sleep without atonia. We now report that glutamate-induced lesions of the medial medulla, including the nucleus magnocellularis, caudal nucleus gigantocellularis and rostral nucleus paramedianus, produce REM sleep without atonia. These nuclei may function as part of a ponto-medullary system suppressing muscle tone in REM sleep.
Keywords: Rapid eye movement sleep, Muscle tone, Pons, Nucleus magnocellularis, Nucleus gigantocellularis, Nucleus paramedianus, Glutamate
Electrolytic and chemical lesion studies have shown that the dorsolateral pons is required for the loss of muscle tone characteristic of rapid eye movement (REM) sleep [10, 13, 17, 26]. Pontine lesions produce a syndrome of REM sleep without atonia (RSWA), in which extensive motor activity occurs during a state which otherwise resembles REM sleep. Conversely, chemical stimulation of the pons can trigger muscle atonia and other aspects of REM sleep [1, 6, 22, 25]. The descending pathway mediating the action of the pontine atonia mechanism is not clearly understood. Electrical stimulation of the reticular formation of the medial medulla in decerebrated cats, produces a suppression of muscle tone [11, 15]. We have found that chemical stimulation of this area also produces muscle atonia [14]. However, it has not been demonstrated that the medial medulla is required for muscle atonia. Because of its proximity to autonomic and respiratory control regions, electrolytic lesions of this area are frequently lethal. In the present study, we report that chemical lesions of the medial medulla do produce RSWA.
Four cats (3.6–3.9 kg), were implanted, with sleep recording electrodes [24]. In 3 cats (156, 157 and 159), a set of 5 pairs of 19 mm-long 23 gauge stainless-steel guide cannulas were implanted straddling the midline, 1.0 mm dorsal to the cerebellar surface. After recovery from surgery, 0.5 μl microinjections of either acetylcholine (1.1 M) or glutamate (0.2 M), dissolved in Ringer’s solution (pH 7.4), were made in unanesthetized cats (156 and 157) through a 33 gauge cannula. Injections were placed at L ± 1 between P8 and P13, between H – 6 and H – 10 at 0.5 mm increments in depth. A minimum of 24 h elapsed between successive injections through each cannula. A third cat (159) was subjected to control insertions of cannulas as well as subsequent microinjections of Glu (0.4 M/0.5 μl) only into the cerebellum (P9–P12, L1. H-3). As a further control for cerebellar damage, a fourth cat (160) was subjected to an acute lesion procedure. Under Nembutal anesthesia a series of 25 microinjections were made at a 45° angle to the coronal plane to prevent damage to the fastigial nucleus and dorsal vermal region traversed in animals 156 and 157. Microinjections of 0.5 μl of 0.4 M Glu were made into the caudal medullary n. gigantocellularis (MNGC), n. magnocellularis (NMC) and n. paramedianus (NPM).
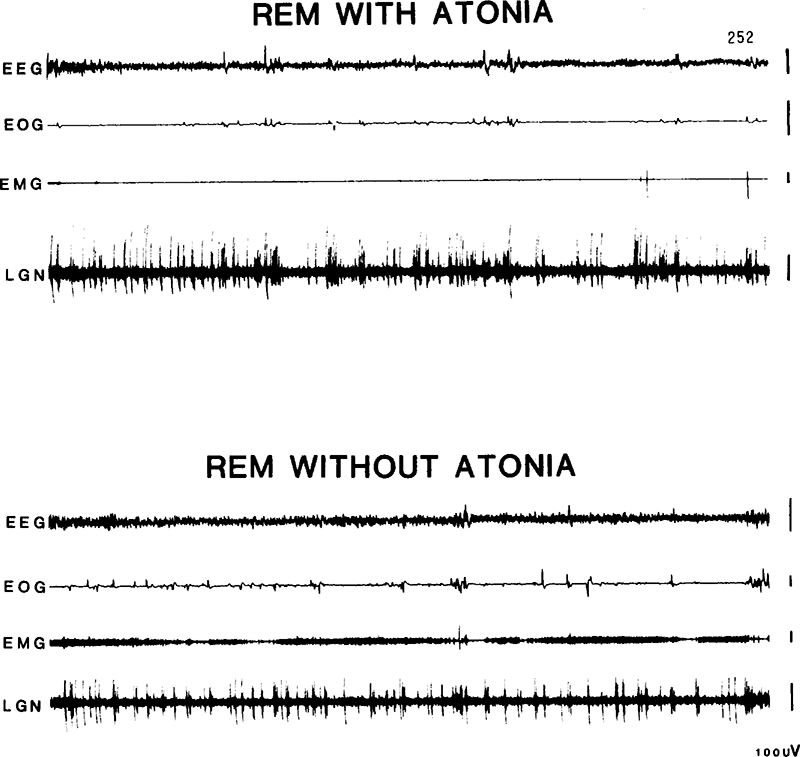
ACh and Glu microinjections produced a variety of behaviors within 5 min of injection, including head and forepaw movements, and emesis. Cats showed normal REM sleep atonia prior to and after the series of medullary ACh injections (Fig. 1, top) but not after the series of medullary Glu injections (n = 14 in 156, n = 15 in 157), when RSWA was observed (Fig. 1, bottom). This sleep stage could be identified by the presence of extreme miosis of the pupil, prolapse of the nictitating membrane, PGO spikes, rapid eye movements, and muscle tone. The cats did not track objects placed in their line of sight and could be awakened from these episodes by tactile or auditory stimulation of the kind sufficient to arouse an intact, sleeping cat. The syndrome appeared after the initial medullary Glu microinjections in cats 156 and 157, but not when Glu was injected in the cerebellum, as observed in Cats 156 (prior to the medullary injections) and 159, who as a control was subject to a series of cerebellar Glu injections. REM sleep periods with sustained muscle tone, were intermixed with REM sleep epochs having some loss of muscle tone, as had been reported in pontine lesioned cats [9]. Video analysis revealed that RSWA was characterized by lifting of the head, slow lateral movements of the head to both sides, chewing-like movements, pawing at the air, extension and alternating movements of all limbs. Quadrupedal locomotion was not seen. RSWA was present in cats 156 and 157 for 15 and 27 days respectively, at which point they were sacrificed. In cat 160, after the acute series of injections, RSWA was seen within 24 h post-injection and persisted throughout a 1-week survival period. Twitching of the kind normally observed during REM sleep was exaggerated in all 3 cats with the syndrome, and was also observable to a lesser extent during non-REM sleep. The vigor of the motor behaviors observed in REM sleep was greater in cat 157 than in cats 156 and 160. The waking behavior of the cats after they manifested RSWA was not obviously different from that observed prior to the lesions.
Fig. 1.
Top – normal REM sleep present prior to glutamate microinjections in cat 157. Bottom – REM sleep without atonia after glutamate induced lesions of the medial medulla (cat 157). EEG, sensorimotor electroencephalogram; EOG, electrooculogram; EMG, electromyogram; LGN, lateral geniculate activity.
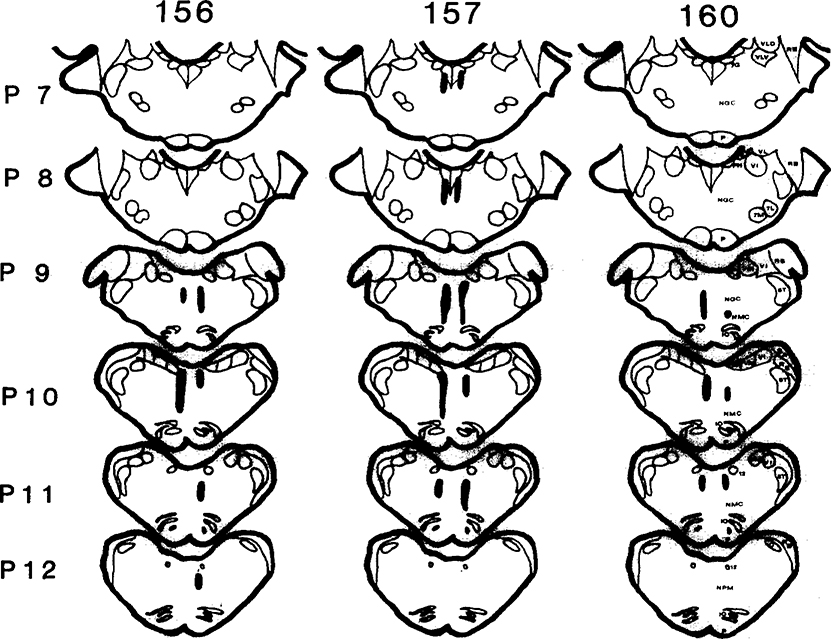
Medullary lesions, characterized by significant neuronal cell loss and fiber damage (156, 157, 160), and extensive gliosis in the animals with longer survival times (156 and 157) were observed at levels corresponding to the caudal MNGC, the NMC, and the rostral portion of NPM [3] (Fig. 2). No lesions were found in any pontine area. Minor damage was found in the fastigial nucleus and the dorsal vermis of the cerebellum in 156 and 157 but not in 160. Total aspiration of the medial cerebellum including the fastigial nucleus has been reported not to affect REM sleep atonia [12, 19], although one study found some disruption of REM sleep atonia after complete ablation of the fastigial nucleus [7].
Fig. 2.
Lesion reconstruction in cats 156, 157 and 160. Damage includes the caudal portions of the medullary nucleus gigantocellularis, nucleus magnocellularis and rostral nucleus paramedianus. Sections are drawn according to the plates of the Berman [3] atlas. 5T, spinal trigeminal tract; 7G, genu of the 7 nerve: 7L, facial n., lateral; 7M, facial n., medial; 12, hypoglossal n.; C, cuneate n.; IO, inferior olive; NGC, n. gigantocellularis; NMC, n. magnocellularis; NPM, n. paramedianus; P, pyramidal tract; PH, n. praepositus hypoglossi; RB, restiform body; VI, inferior vestibular n.; VLD, lateral vestibular n., dorsal; VLV, lateral vestibular n., ventral; VM, medial vestibular n.; SA, stria acustica.
The critical lesion for producing RSWA is smaller than the ‘medullary inhibitory area’ defined by electrical stimulation, but corresponds to the medullary inhibitory areas defined by chemical stimulation [14]. The effective lesions in this study were equal to or smaller in volume than those effective in dorsolateral pontine regions [9, 10, 13]. In contrast, massive electrolytic and kainic acid lesions in the pontine n. gigantocellularis do not disrupt REM sleep atonia [20]. Kainic acid lesions of the lateral pons have recently been shown to produce RSWA [26]. Thus both the dorsolateral pons and medial medulla appear to be required for the suppresion of muscle tone in REM sleep as previously hypothesized [14, 17, 20, 23].
HRP injections in the NMC have demonstrated monosynaptic projections from the dorsolateral pons [20]. Microinjection of Glu, but not ACh, into this nucleus has been shown to produce atonia [14]. Administration of NMDA antagonists in this nucleus blocks the atonia elicited by pontine carbachol injections [14]. Similarly, the administration of an effective neurotransmitter in the NPM, in this case ACh but not Glu, has been shown to produce atonia. Microinjection of ACh antagonists into this nucleus blocks atonia induced by pontine carbachol injection [14]. The NPM is known to have a role in the integration of posture and blood pressure information [5, 16]. Certain medial medullary neurons have high rates of discharge in REM sleep [4, 18, 23]. This convergence of stimulation lesion and recording data supports the hypothesis that these nuclei of the medial medulla contain neurons mediating the suppression of muscle tone during REM sleep.
RSWA has recently been identified in humans [21]. Cataplexy, the sudden loss of muscle tone experienced by narcoleptics, appears to represent a hyperactivity of the mechanism responsible for the suppression of muscle tone in REM sleep [2, 8, 23]. Abnormalities in the pontomedullary inhibitory system could be reponsible for both of these syndromes.
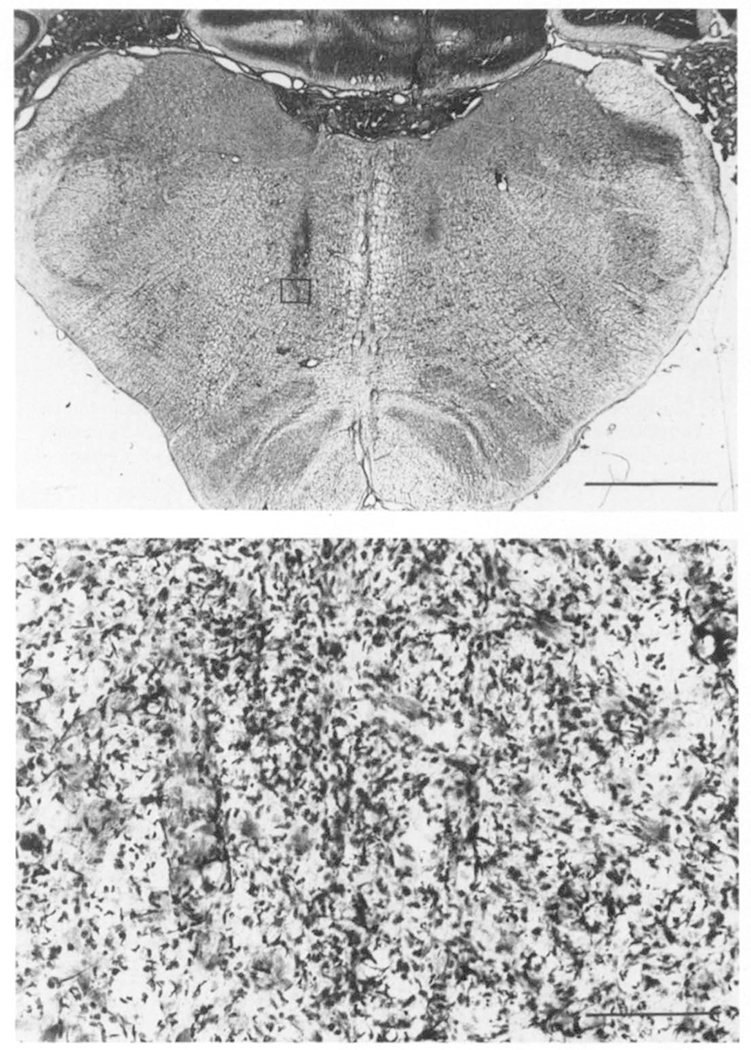
Fig. 3.
Top – photomicrograph showing lesioned areas in cat 157. Bottom – magnification of the area indicated on top section.
Acknowledgments
Supported by the Medical Research Service of the Veterans Administration, USPHS Grants NS14610, MH43811, and HL41370, and the American Narcolepsy Association. We thank Dr. Y. Y. Lai for helpful comments on an earlier version of this manuscript and S. Sampagna for the histological preparations.
Footnotes
Note added in proof. Since submission of this manuscript, an abstract has appeared reporting a similar syndrome of RSWA after quisqualic acid lesions of the medial medulla (Holmes, C.J., Webster, H.H., Zikman, S. and Jones, B.E., Soc. Neurosci. Abstr., 14 (1988) 1308).
References
- 1.Baghdoyan HA, Rodrigo-Angula ML, McCarley RW and Hobson LA., Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions, Brain Res., 306 (1984) 39–52. [DOI] [PubMed] [Google Scholar]
- 2.Baker TL and Dement WC, Canine narcolepsy–cataplexy syndrome: evidence for an inherited monoaminergic-cholinergic imbalance. In McGinty DJ, Drucker-Colin R, Morrison A, and Parmeggiani PL (Eds.), Brain Mechanisms of Sleep, Raven Press, New York, 1985, pp. 199–234. [Google Scholar]
- 3.Berman AL, The Brain Stem of the Cat, University of Wisconsin Press, Madison, 1968. [Google Scholar]
- 4.Chase MH, Enomoto S, Murakami T, Nakamura Y and Taira M, Intracellular potential of medullary reticular neurons during sleep and wakefulness, Exp. Neurol, 71 (1981) 226–233. [DOI] [PubMed] [Google Scholar]
- 5.Elisevich KV, Hrycyshyn AW and Flumerfelt BA, Cerebellar, medullary and spinal afferent connections of the paramedian reticular nucleus in the cat, Brain Res, 332 (1985) 267–282. [DOI] [PubMed] [Google Scholar]
- 6.George R, Haslett WL and Jenden DJ, A cholinergic mechanism in the brainstem reticular formation: Induction of paradoxical sleep, Int. J. Neuropharmacol, 3 (1964) 541–552. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmino S and Strata P, Cerebellum and atonia of the desynchronized phase of sleep, Arch. Ital. Biol, 109 (1971) 210–217. [PubMed] [Google Scholar]
- 8.Guilleminault C, Cataplexy. In Guilleminault C, Dement WC and Passouant P (Eds.), Narcolepsy, Spectrum, New York, 1976, pp. 125–143. [Google Scholar]
- 9.Hendricks JC, Morrison AR and Mann GL, Different behaviors during paradoxical sleep without atonia depend on pontine lesion site, Brain Res, 239 (1982) 81–105 [DOI] [PubMed] [Google Scholar]
- 10.Henley K and Morrison AR, A re-evaluation of the effects of lesions of the positive tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat, Acta. Neurobiol. Exp, 34 (1974) 215–232. [PubMed] [Google Scholar]
- 11.Jankowska E, Lund S, Lundberg A and Pompeiano O, Inhibitory effects evoked through ventral reticulospinal pathways, Arch. Ital. Biol, 106 (1968) 124–140. [PubMed] [Google Scholar]
- 12.Jouvet M, Recherches sur les structures nerveuses et les mechanismes responsables des differentes phases du sommeil physiologique, Arch. Ital. Biol, 100 (1962) 125–206. [PubMed] [Google Scholar]
- 13.Jouvet M and Delorme F, Locus coeruleus et sommeil paradoxal, C.R. Soc. Biol, 159 (1965) 895–899. [Google Scholar]
- 14.Lai YY and Siegel JM, Medullary regions mediating atonia, J. Neurosci, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, J. Neurophysiol, 9 (1946) 165–171. [DOI] [PubMed] [Google Scholar]
- 16.Miura M and Reis DJ, The paramedian reticular nucleus: A site of inhibitory interaction between projections from fastigial nucleus and carotid sinus nerve acting on blood pressure, J. Physiol. (Lond.), 216 (1971) 441–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison AR, A window on the sleeping brain, Sci. Am, 248 (1983) 94–102. [DOI] [PubMed] [Google Scholar]
- 18.Netick A, Orem J and Dement W, Neuronal activity specific to REM sleep and its relationship to breathing, Brain Res, 120 (1977) 197–207. [DOI] [PubMed] [Google Scholar]
- 19.Paz C, Reygadas E and Fernandez-Guardiola A, Sleep alterations following total cerebellectomy in cats, Sleep, 5(3) (1982) 218–226. [DOI] [PubMed] [Google Scholar]
- 20.Sakai K, Some anatomical and physiological properties of pontomesencephalic tegmental neurons with special reference to the PGO waves and postural atonia during paradoxical sleep in the cat. In Hobson JA and Brazier MA (Eds.), The Reticular Formation Revisited, Raven Press, New York, 1980, pp. 427–447. [Google Scholar]
- 21.Schenck CH, Bundlie SR, Patterson AL and Mahowald MW, Rapid eye movement sleep behavior disorder: a treatable parasomnia affecting older adults, J. Am. Med. Assoc, 257 (1987) 1786–1789. [PubMed] [Google Scholar]
- 22.Shiromani PJ, Siegel JM, Tomaszewski KS and McGinty DJ, Alterations in blood pressure and REM sleep after pontine carbachol microinfusion, Exp. Neurol, 91 (1986) 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel JM, Brainstem mechanisms generating REM sleep. In Kryger MK (Ed.), Principals and Practice of Sleep Medicine, Saunders, New York, 1988, in press. [Google Scholar]
- 24.Siegel JM, Wheeler RL and McGinty DJ, Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep, Brain Res, 179 (1979) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Dongen PAM, Broekkamp CLE and Cools AR, Atonia after carbachol microinjections near the locus coeruleus in cats, Pharmacol. Biochem. Behav, 8 (1978) 527–532. [DOI] [PubMed] [Google Scholar]
- 26.Webster HH and Jones BE, Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat II. Effects upon sleep–waking states, Brain Res, 458 (1988) 285–302. [DOI] [PubMed] [Google Scholar]