Abstract
The phased replacement of oral polio vaccine (OPV) with inactivated polio vaccine (IPV) is expected to significantly complicate mass vaccination campaigns, which are an important component of the global polio eradication endgame strategy. To simplify mass vaccination with IPV, we developed microneedle patches that are easy to administer, have a small package size, generate no sharps waste and are inexpensive to manufacture. When administered to rhesus macaques, neutralizing antibody titers were equivalent among monkeys vaccinated using microneedle patches and conventional intramuscular injection for IPV types 1 and 2. Serologic response to IPV type 3 vaccination was weaker after microneedle patch vaccination compared to intramuscular injection; however, we suspect the administered type 3 dose was lower due to a flawed pre-production IPV type 3 analytical method. IPV vaccination using microneedle patches was well tolerated by the monkeys. We conclude that IPV vaccination using a microneedle patch is immunogenic in rhesus macaques and may offer a simpler method of IPV vaccination of people to facilitate polio eradication.
Introduction
Due largely to the efforts of the Global Polio Eradication Initiative (GPEI), worldwide confirmed polio cases have reached their lowest level in history [1], and the current target for eradication of the disease is fast approaching [2]. This progress has been achieved primarily through mass vaccination using the oral polio vaccine (OPV), which is a live-attenuated vaccine administered orally [3]. Vaccination using OPV offers the advantages of administration by minimally trained personnel in mass campaigns (fixed post or house-to-house); generation of no sharps waste; small package size for simplified storage, transportation and waste disposal; low-cost vaccine; and generation of mucosal immunity.
However, OPV has a major disadvantage: it carries a risk of genetic reversion to a virulent form, which can result in the eme gence and transmission of vaccine-derived polioviruses (VDPVs) [4], which now account for a large fraction of polio cases [5]. To achieve the ultimate goal of eradication, OPV needs to be replaced with inactivated polio vaccine (IPV), which does not carry the risk of paralysis in the recipient or transmission in the community [6].
Plans to switch to IPV are being developed, with the goal of eliminating use of OPV worldwide by 2019 after worldwide introduction of IPV [7]. This is currently underway with the phased withdrawal of OPV type 2 and the transition to bivalent OPV, which will be followed by the complete withdrawal of OPV. However, while IPV overcomes OPV’s major disadvantage of genetic reversion to virulent forms, it also introduces many new disadvantages, such as the need for trained healthcare professionals to administer injections; generation of sharps waste; larger package size of vials, needles and syringes for storage, transport and disposal; multi-dose presentation that leads to vaccine wastage; order of magnitude higher vaccine cost; and poor generation of mucosal immunity on its own [8–10]. Recent studies have found IPV to be a better booster of intestinal immunity in OPV primed persons than an additional dose of OPV, suggesting mass campaigns with IPV could be especially beneficial to the polio endgame [11].
In this study, we propose the use of a microneedle patch to administer IPV by an approach that seeks to capture the safety advantages of IPV without losing the logistical advantages of OPV. Microneedle patches can be applied to the skin in a simple manner, such that microscopic needles painlessly puncture the skin to administer IPV without the need for hypodermic needles [12]. Microneedle patches have previously been used to administer other vaccines in preclinical studies, such as influenza, measles, HPV and others [13–21], but have not yet been studied for IPV vaccination. IPV vaccination using a microneedle patch can eliminate the need for trained healthcare professionals to administer injections, thereby enabling the use of minimally trained personnel to efficiently administer vaccine in house-to-house campaigns in a cost-effective manner. In addition, IPV vaccination using microneedle patches may reduce vaccine cost by possible dose sparing enabled by skin vaccination, as seen for intradermal injection of IPV and other vaccines [22] and generation of improved immunity, as seen for microneedle vaccination using other vaccines [23–25]. Given these motivations, this study developed a dissolving microneedle patch for IPV vaccination and measured the immune response to IPV delivery in the rhesus macaque using the microneedle patch compared to conventional intramuscular injection. This is the first study to assess IPV vaccination using a microneedle patch.
Methods
Concentration of inactivated polio vaccine
Unformulated, monovalent, bulk inactivated polio vaccine was kindly provided by GlaxoSmithKline Biologicals (Rixensart, Belgium). The starting antigen concentration was measured by us as described below to be 2023, 831 and 1081 d-antigen units/mL for IPV types 1, 2 and 3, respectively. The bulk IPV was concentrated using Amicon Ultra centrifuge spin filters with a 100 kDa molecular weight cutoff (EMD Millipore, Billerica, MA). The stock IPV solutions were each concentrated approximately 38-fold by volume and the final antigen concentration was measured to be 56,300, 39,500, and 52,300 d-antigen units/mL for types 1, 2 and 3, respectively. All d-antigen values were determined by ELISA as described below.
Microneedle fabrication: vaccine filling
Molds consisting of a 10×10 array of 300×300×600 μm pyramidal microneedles were fabricated as previously described [26,27]. The concentrated poliovirus vaccine stock was mixed into a casting solution containing 15% w/v sucrose and 300 mM threonine (Sigma–Aldrich, St. Louis, MO) and 20 μL was applied to the microneedle mold, to which vacuum at a pressure of 93.1 kPa was then applied for 20 min. After that, the mold was allowed to further dry in a chemical fume hood for 60 min. Adhesive tape was applied to the dried mold and then quickly peeled away to remove any remaining vaccine present on the mold without removing or damaging the microneedle structures. For the microneedle patch booster dose, the poliovirus vaccine stock was mixed in a casting solution containing 10% w/v maltodextrin (Sigma–Aldrich) instead of sucrose and threonine. The formulation of the booster dose was different from the original vaccination because subsequent in vitro experiments suggested that maltodextrin may be better able to maintain vaccine integrity (unpublished data). The protocol was otherwise identical.
Microneedle fabrication: polymer matrix filling
The matrix solution used to form the microneedle patch backing was composed of 40 wt% gelatin and 15 wt% sucrose (Sigma–Aldrich) mixed in sterile, deionized water. The gelatin solution was mixed for 1 h at 25 °C before use. The matrix solution was cast using a spatula onto the microneedle mold. The molds were placed back into the vacuum system for 90 min and then allowed to dry for 48 h at 25 °C in a chemical fume hood. To remove the dried microneedle patches, a 1.27 cm-diameter disc of polymethyl methacrylate (McMaster-Carr, Atlanta, GA) was covered on one side with double-sided tape (MacTac, Stow, OH) and applied to the back of the mold. The resulting patch was gently peeled away from the mold and stored in a dark, sealed pouch with desiccant (Drierite, Xenia, OH) at 25 °C for less than 24 h until use.
Microneedle patch characterization
Microneedle patches were imaged by brightfield microscopy (Hirox KH-8700, Tokyo, Japan). Their dimensions were quantified using the microscope’s integrated imaging software. To assess microneedle insertion into skin, microneedle patches were pressed by thumb into full-thickness, un-stretched porcine cadaver skin, left in place for 15 min, and then removed. The skin was then exposed to a purple dye, gentian violet (Humco, Texarkana, TX), for 5 min, after which it was wiped off to expose sites of skin puncture stained by the dye. This technique is believed to selectively stain sites of microneedle puncture into the skin [28,29].
Immunization studies
The immune response to IPV vaccination using microneedle patches was tested in the rhesus macaque (Macaca mulatta; Covance Inc, Princeton, NJ). The animals were divided into two groups of four monkeys each. Group 1 received IPV vaccination by intra-muscular injection as a positive control and Group 2 received IPV vaccination by microneedle patch. In this initial study, available resources limited us to four animals per group. Future studies will be needed with a larger statistical sample size.
Female, two-year-old rhesus macaques with a prior history of participation in measles and influenza vaccination studies were enrolled in this study 15 weeks after measles vaccination and 4 weeks after influenza vaccination. To verify that no animals had previous exposure to poliovirus, blood was collected from the femoral vein using Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) and analyzed by micro-neutralization against each of the three poliovirus serotypes, as described below. All animals had initial micro-neutralization titers <2.5 log2 and were considered negative. Animals were anesthetized using ketamine (10 mg/kg) during vaccination and blood collection. For microneedle patch vaccination in Group 2, a section of hair on the upper back of the animal, between the shoulder blades, was removed using electric shears followed by application and removal of depilatory cream (Nair, Church & Dwight Co, Ewing, NJ), according to the manufacturer’s instructions, before the first vaccination (no additional hair removal was needed for the boost dose). After removal of the depilatory cream, the skin was allowed to dry for approximately 20 min before microneedle patch insertion.
The IPV dose in each microneedle patch was characterized by first re-dissolving the patch into sterile saline and then testing for D-antigen content using ELISA. Each lot of four monovalent microneedle patches contained two IPV type 1 patches and one patch each of types 2 and 3 comprising a total of 47.4 ± 5.8, 8.7 ± 1.4, and 38.2 ± 2.6 D-antigen units of IPV types 1, 2 and 3, respectively. In this study, monovalent patches were used to simplify manufacturing, but in on-going studies we have been able to manufacture trivalent patches containing all three IPV serotypes (data not shown). In this study, two patches were needed for IPV type 1 due to the higher dose required and less efficient antigen concentration.
These patches were then pressed onto the hair-free section of the back of each animal in Group 2. This site was chosen to prevent the animals from possibly scratching the site and causing irritation. The patches were left on the skin for 15 min to allow for dissolution of the polymer microneedles in the skin. This time was determined by pressing patches into porcine cadaver skin as described above.
In Group 1, un-concentrated IPV stock solution was diluted using sterile phosphate-buffered saline so that the target dose (i.e., the standard human dose of 40, 8, and 32 d-antigen units of types 1, 2, and 3, respectively) was contained in 500 μL as determined by ELISA. We administered IPV from the same original stock solution used in the creation of the microneedle patches for intramuscular injection in order to have a direct comparison between the two routes of administration using the same antigens. The intramuscular injection was delivered in a single trivalent preparation using a 25-gauge needle into the quadriceps femoris muscle.
Eight weeks after the initial vaccination, all animals were given a second dose of trivalent IPV. This booster dose was delivered using the same route and method as the initial vaccination and consisted of the same dose of IPV types 1, 2, and 3 from the same lot of vaccine. Approximately 10 mL of blood was collected weekly from each animal via the femoral vein for serological analysis, as described below. Following the completion of the study, all animals were transferred to other protocols within CDC. All procedures in this study were approved by the Institutional Animal Care and Use Committees of the Centers for Disease Control and Prevention and the Georgia Institute of Technology.
ELISA assay measurements
Antigen-capture ELISA was used for the detection of d-antigen poliovirus. Poliovirus-specific monoclonal antibodies (mAb) were used as both capture and detection antibodies. Type 1 (NBP1– 05101, Novus Biologicals, St. Louis, MO), type 2 (HYB294–06, Thermo Fisher Scientific, Waltham, MA), or one of two type 3 (HYB300–05 and HYB300–06, Thermo Fisher Scientific) specific antibodies were diluted in 0.05 M carbonate-bicarbonate buffer, pH 9.6. Type 1, 2, and 3 (HYB300–05) capture mAbs were diluted 1:1000 and type 3 (HYB300–06) mAb was diluted 1:500. Detailed methods are available in the Supplemental Materials.
Neutralizing antibody measurements
Samples were tested in triplicate using a standard microneutralization assay for antibodies to poliovirus types 1, 2, and 3 as previously described [30]. Briefly, 80–100 CCID50 of each poliovirus serotype (Sabin strains 1, 2, and 3) and two-fold serial dilutions of serum (starting at 1:4) were combined and preincubated at 35°C for 3 h before addition of HEp-2(C) cells. After incubation for 5 days at 35 °C and 5% CO2, plates were stained with crystal violet and cell viability measured by optical density in a spectrophotometer. Each specimen was run in triplicate, with parallel specimens from one study subject tested in the same assay run; and the neutralization titers estimated by the Spearman-Kärber method [31] and reported as the reciprocal of the calculated 50% endpoint. Each run contained multiple replicates of a reference antiserum pool starting at a 1:32 dilution to monitor performance variation. A serum sample was considered positive if antibodies were present at ≥1:8 dilution [32–34]. The limit of detection for this assay is a 2.5 log2 titer. The precision is ± 0.5 log2 titer (CDC, unpublished data). The limit for seropositivity was defined at ≥3.0 log2 titer.
Statistics
All statistics were calculated using Prism software version 6.02 (Graphpad, La Jolla, CA). Comparisons between individual samples were done using an unpaired t-test with a significance level of p < 0.05. Comparisons between multiple samples were done using a two-way ANOVA with a Tukey post-test.
Results
Microneedle patch design
Microneedle patches were designed to be inexpensive to manufacture, have a small package size, be simple to administer, and generate no sharps waste in order to meet the needs of mass vaccination campaigns in developing countries. The resulting microneedle patches are shown in Fig. 1. They contain a 10 × 10 array of pyramidal microneedles measuring approximately 650 μm in height. With the addition of the supporting backing layer, each microneedle patch has a footprint of 1.27 cm2 and a total volume of 0.19 cm3. Ten patches therefore have a volume of 1.9 cm3. This is considerably smaller than the volume of a ten-dose vial of IPV, ten capped 25-gauge needles and ten 1-mL syringes (i.e., on the order of 50 cm3). Packaging would increase these volumes further.
Fig. 1.
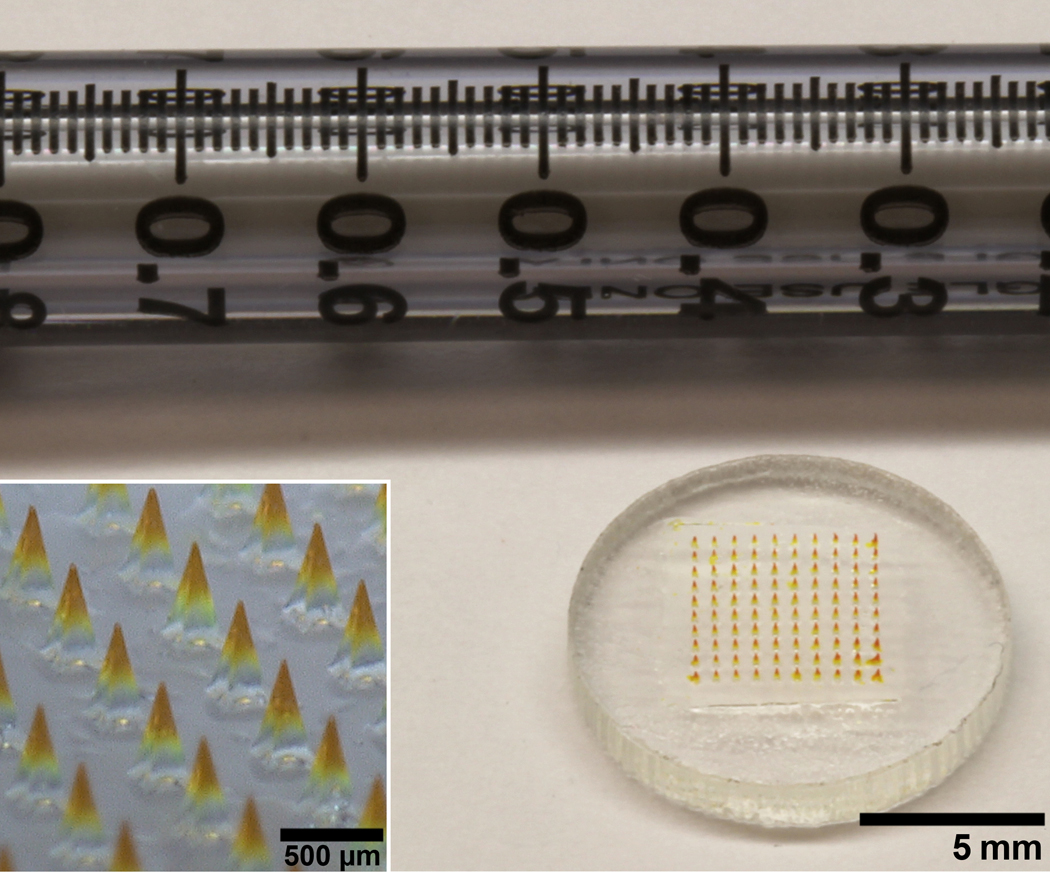
Microneedle patch for IPV vaccination. An array of 100 microneedles made of water-soluble materials encapsulating IPV is mounted on a plastic backing. A 1 mL syringe is included for scale. The microneedles are approximately 650 μm in height and the whole patch is approximately 1.27 cm in diameter. The inset shows a magnified view of the microneedles.
Simple and reliable administration of microneedle patches was facilitated by making microneedles with sharp tips (measured to be 3.6 ± 1.4 μm tip radius). Previous experimental measurements [35] indicate that a 100-needle patch of these microneedles would require a force of less than 40 N to be inserted into skin, which can be easily applied by the thumb of most people [36].
To validate this prediction, microneedle patches were inserted by thumb into full-thickness porcine cadaver skin, left in place for 15 min and then removed. Staining sites of skin penetration showed that at least 99 out of 100 microneedles punctured the skin (Fig. 2). Microscopic imaging of the microneedles after skin insertion showed that the microneedles had substantially dis- solved (Fig. 3). This further confirmed that the microneedle patches were strong enough to pierce the skin and then rapidly dissolve within the skin after insertion. This analysis also demonstrated another important feature of the microneedle patch design, i.e., that microneedle dissolution in the skin produced a sharps-free patch that can be disposed of as non-sharps waste.
Fig. 2.
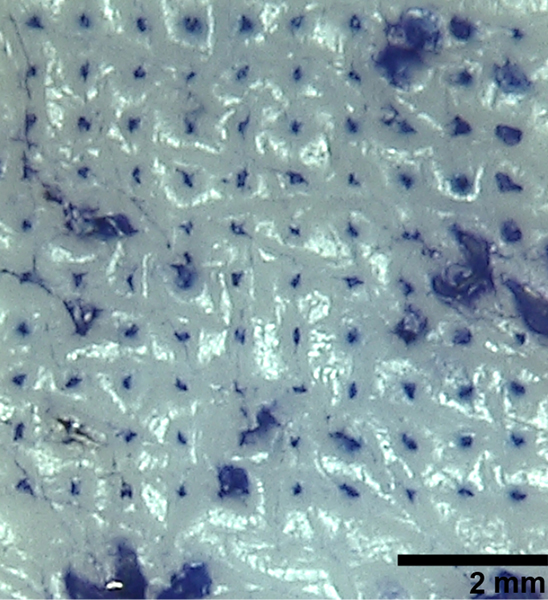
Pig skin stained with dye to reveal sites of skin puncture after microneedle patch insertion. A microneedle patch containing a 10 by 10 array of microneedles was manually applied to shaved porcine cadaver skin and remained in place for 15 min to allow the microneedles to dissolve. The skin was then stained with gentian violet to show the puncture sites.
Fig. 3.
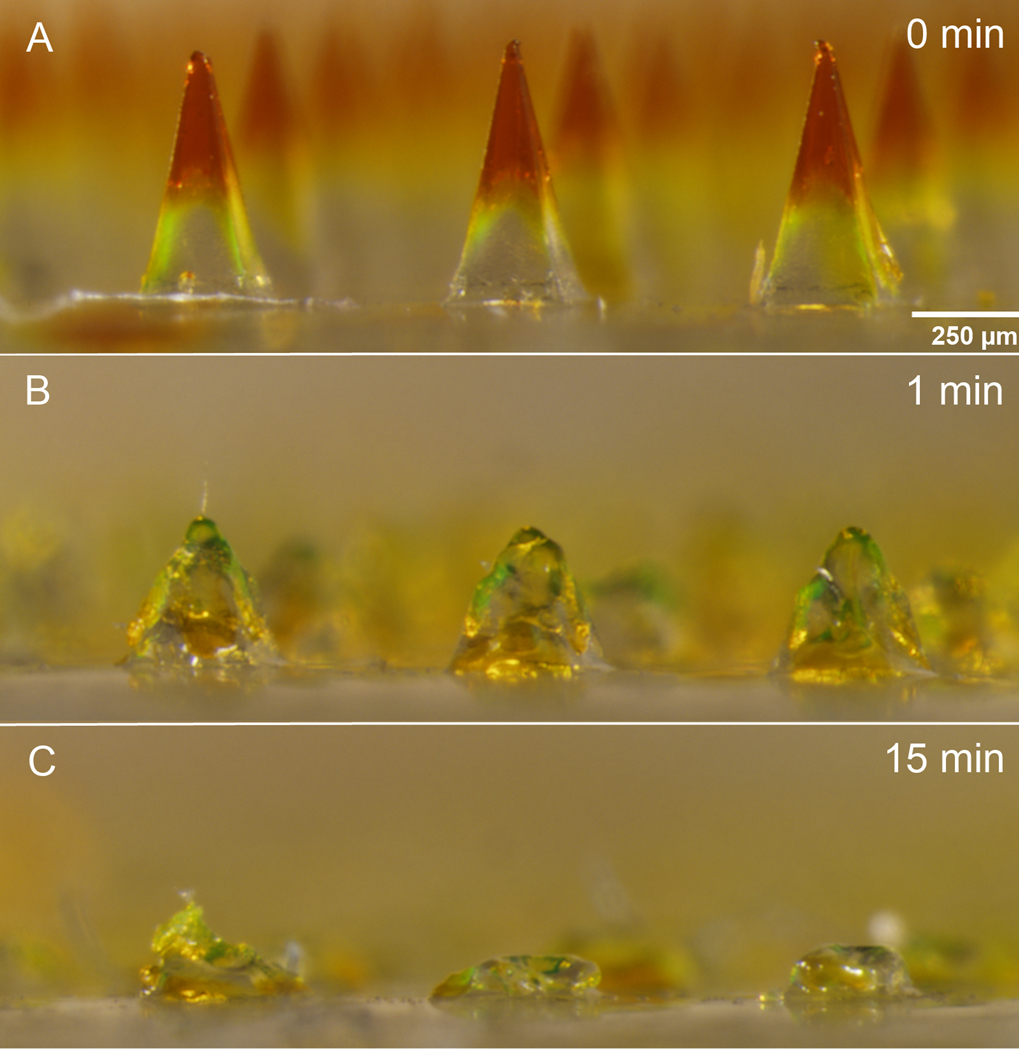
Microneedles before and after insertion into pig skin. Microneedle patches were inserted into shaved porcine cadaver skin and imaged (a) before insertion and (b) 1 and (c) 15 min after insertion to demonstrate the extent of microneedle dissolution in the skin.
Finally, microneedle patches were assayed for their IPV con- tent using a serotype-specific ELISA. Initial testing found that each monovalent patch contained an average of 23.7 ± 4.1, 8.7 ± 1.4, and 38.2 ± 2.6 D-antigen units of IPV type 1, 2, and 3, respectively. However, after completion of the animal study, we found that the commercial monoclonal antibody in the ELISA used to measure the dose of IPV type 3 was not specific for the d-conformation of the antigen (i.e., native capsid conformation). We therefore developed a new ELISA using a different commercial monoclonal antibody specific for the type 3 D-antigen, which indicated that the IPV type 3 dose administered in the animal study using the microneedle patch was at least three-times lower than the dose indicated by the original ELISA. The significance of this difference is discussed below.
Immunization study
The next step in the study was to assess immunogenicity of IPV vaccination using microneedle patches. The rhesus macaque was chosen as the animal model because it has historically been used to test IPV efficacy and viral potency and is arguably the best animal model to simulate a human immune response [37,38]. All animals were seronegative to all three poliovirus serotypes prior to immunization, as measured by neutralization assay. Animals were divided into two groups, one vaccinated using microneedle patches and the other vaccinated by intramuscular injection. All animals received two vaccine doses (by the same route of administration) separated by 8 weeks.
For IPV type 1, neutralizing titers were weak after the first dose by either route of administration, yet at least half of the animals seroconverted (Fig. 4A and D). After the second IPV dose, anti- body titers increased dramatically and 100% seroconversion was achieved. There was no statistically significant difference between antibody responses in the microneedle patch group versus the intramuscular injection group (two-way ANOVA, p > 0.05).
Fig. 4.
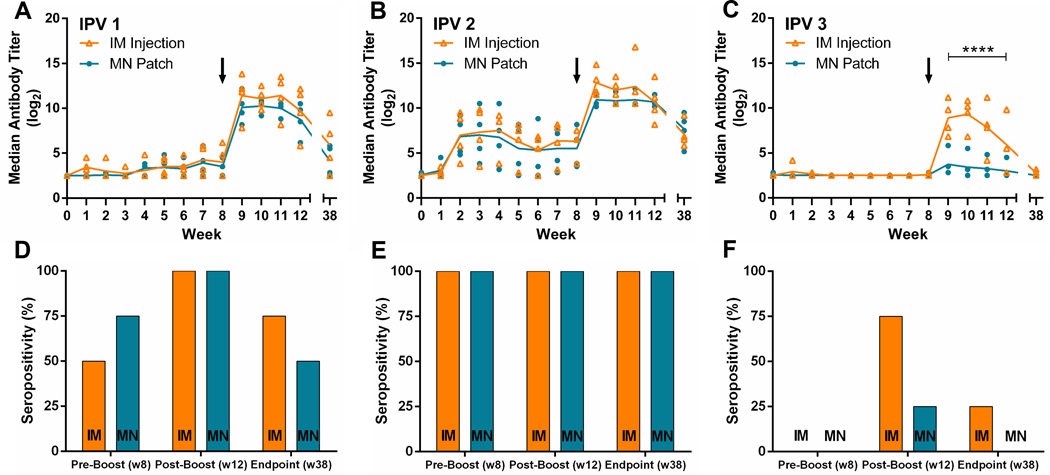
Serologic response and neutralizing antibody titers to poliovirus following vaccination. Rhesus macaques were vaccinated at week 0 and week 8 with IPV given either by microneedle (MN) patch or intramuscular (IM) injection. Serum was collected weekly and analyzed using a serotype-specific micro-neutralization assay, for IPV (A) type 1, (B) type 2 and (C) type 3. Seropositivity is also shown at weeks 8, 12, and 38 (D–F). Each data point represents a single animal while the lines represent the median of each group. The asterisks (****) represent a statistically significant difference (p < 0.0005) between the microneedle patch and intramuscular injection groups as measured by two-way ANOVA. Seropositivity was defined as a titer greater than or equal to 3.0 log2 [32,33].
For IPV type 2, the immune response following the first IPV dose was strong in both groups, exhibiting significantly increased neutralizing titers and 100% seroconversion (Fig. 4B and E). After the second dose, antibody titers increased further. Antibody responses in the microneedle patch group and the intramuscular injection group were statistically indistinguishable (two-way ANOVA, p > 0.05).
For IPV type 3, there were very weak immune responses after the first IPV dose, in both animal groups, such that no animals seroconverted (Fig. 4C and F). After the second dose, antibody responses increased in the intramuscular injection group such that 75% of the animals seroconverted. Antibody responses in the microneedle patch group were weaker and only 25% of the animals seroconverted. Antibody responses in the intramuscular injection group were significantly higher than in the microneedle patch group (two-way ANOVA, p < 0.0005). This difference may be explained by the lower IPV type 3 dose mistakenly given by the microneedle patches (see above).
Sera were also collected 38 weeks after the initial vaccination and tested for neutralizing antibodies in order to examine the longevity of the immune response (Fig. 4). All animals displayed a decrease in titer to each of the three polio serotypes. The median titer for each serotype was not significantly different between the vaccination groups (two-way ANOVA, p > 0.05).
Safety
The vaccination sites were examined daily by animal care staff and no study-related adverse effects were seen in either the microneedle patch or intramuscular injection groups. No swelling, discharge, irritation or other abnormalities were observed at any point during the study. After removal of the microneedle patches, a small grid of puncture sites was faintly visible and minor redness existed, especially where the edges of the patch pressed against the skin (Fig. 5). The grid of puncture sites was no longer visible 2–3 days after insertion (data not shown). No bleeding was observed at any of the sites of microneedle patch vaccination. Microscopic examination of microneedle patches after use revealed almost-complete dissolution of the microneedles, indicating efficient delivery of the encapsulated vaccine (data not shown).
Fig. 5.
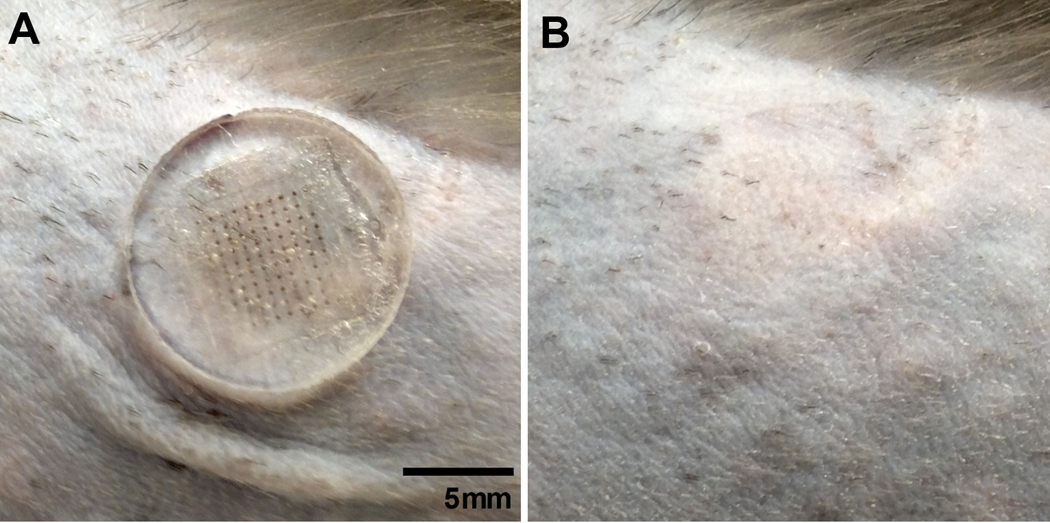
Rhesus macaque skin during and after microneedle patch insertion. A 100-microneedle patch was applied to the skin of a rhesus macaque between the shoulder blades after hair removal and removed after 15 min. The same section of skin was imaged (a) with the patch in place and (b) immediately after patch removal. In (b), a faint grid can be seen where the needles punctured the skin, which disappeared after 2–3 days.
Discussion
The WHO has recommended that member countries begin introducing IPV prior to OPV withdrawal as eradication progresses, to reduce the risk of emergence of vaccine-derived polioviruses [7]. IPV is currently delivered using a needle and syringe, which introduces a number of drawbacks when compared to the oral delivery route utilized by OPV. Hypodermic injection generally requires trained healthcare personnel at fixed-post clinics and increases the risk of disease transmission due to generation of sharps waste. The transition to IPV would be greatly aided by an improved method of delivering IPV that overcomes these logistical barriers [39].
We have developed a microneedle patch using a process that is simple, reproducible and, importantly, scalable to the demands required by the polio eradication program. To enable low-cost manufacturing, the patches were made by a molding process designed to be cost-competitive with vaccine vial filling. In this process, vaccine and excipients (e.g., gelatin and sucrose) were cast onto a micromold, allowed to dry and then packaged (see Methods). In mass production, the costs of excipients and micromolds are expected to be less than that of a needle, syringe and vial, and the cost of the aseptic manufacturing process is expected to be similar to that of filling a sterile vial (unpublished data). Using this process, the cost of microneedle patch manufacturing is expected to be simi- lar to current costs of manufacturing vaccine vials with needles and syringes.
These patches can be inserted into skin without the use of a secondary applicator, simplifying administration and reducing cost. Microneedle patch administration should also not require medically trained personnel. This change could shift the IPV vaccination scheme towards the more efficient strategy currently used with the oral polio vaccine involving house-to-house campaigns con- ducted by minimally trained personnel. Dissolving microneedles in particular have the potential to eliminate the risk of sharps contamination, since the needles disappear after insertion into the skin. Finally, a small, single-dose packaging system could decrease both shipping costs and medical waste, as well as reduce vac- cine wastage associated with multi-dose vials. Our patches were tested in pig cadaver skin and living monkey skin, and found to easily insert with near-complete needle dissolution within 15 min. These are important qualifications for a delivery device intended to overcome many of the hurdles posed by the upcoming transition from OPV to IPV.
The microneedle patches were inserted into the skin of rhesus macaques and delivered at least a full human dose of IPV for types 1 and 2. The vaccine remained immunologically active and induced a potent neutralizing antibody response after two doses, which was statistically indistinguishable from a similar dose delivered by hypodermic injection. The positive response to IPV type 2 is especially important. The final stage of polio eradication calls for the administration of bivalent OPV protecting against polio types 1 and 3, with a supplementary dose of IPV to provide immunity to type 2 [7]. It is expected that this delivery schedule will be carried out throughout the polio endgame strategy until total withdrawal of OPV in 2019 [40].
The immune response to IPV type 3 was inferior when microneedle patch delivery was compared to intramuscular injection. We believe that this was the case at least in part because the ELISA assay used to determine the IPV type 3 dose in the microneedle patches was not specific to the d-antigen conformation of the poliovirus particle, which is thought to be crucial for proper immune recognition of the virus [41]. When comparing the results of our original type 3 ELISA to an improved type 3 ELISA based on a different type 3 d-antigen-specific antibody, tests showed that the original ELISA assay gave readings that were approximately three times higher than the improved ELISA. This suggests that the delivered dose of IPV type 3 was much lower than originally expected. We believe this was a primary factor that contributed to the lower immune response although it is possible that IPV type 3 was less immuno- genic for other reasons as well. Because the reduced IPV type 3 dose administered by microneedle patch was inferior, these data do not provide evidence for dose sparing by skin vaccination as suggested in prior studies [42,43], although this study was not designed to address this question. Further development is in progress to improve the microneedle formulation and manufacturing process, with the goal of minimizing antigen loss for IPV type 3.
This study presents the first assessment of dissolving microneedle patches for polio vaccination as a significant advance over traditional delivery methods. These patches are simple to administer; generate no sharps waste; facilitate storage, transport and disposal due to their small size; and reduce vaccine wastage due to their single-dose presentation. This study showed that microneedle patch vaccination can induce a potent immunologic response by skin delivery of IPV with no significant adverse effects observed. Future work will focus on improving immunogenicity of IPV type 3, conducting human trials, developing low- cost mass-manufacturing methods, and moving quickly toward introducing this new vaccination modality into clinical use. As the endgame nears for the global campaign to eradicate poliomyelitis, microneedles represent a possible solution to many of the final hurdles.
Supplementary Material
Acknowledgements
The authors would like to thank Ryan Johnson and Dr. Robyn Engel for their assistance in bleeding and anesthetizing the animals; William Hendley, Sharla McDonald, Deborah Moore and Yiting Zhang for conducting the serum neutralization assays; Marcus Collins for his help with protocol development and sample collection; and Dr. Paul Rota for acquiring the rhesus macaques and making them available for use in this study. We also thank Dr. James Norman for his help with statistical analyses and Donna Bondy for her administrative assistance.
This work was funded in part by the Bill and Melinda Gates Foundation, the World Health Organization, and the Centers for Disease Control and Prevention’s Global Immunization Division.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest statement: Mark Prausnitz and Chris Edens are inventors of patents that have been or may be licensed to companies developing microneedle-based products and Mark Prausnitz is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products, including Micron Biomedical. The resulting potential conflict of interest has been disclosed and is managed by the Georgia Institute of Technology and Emory University.
References
- 1.Rodriguez-Alvarez M, Jimenez-Corona ME, Cervantes-Rosales R, Ponce de Leon- Rosales S Polio eradication: how long and how much to the end? Arch Med Res 2013, 10.1016/j.arcmed.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Cochi SL, Linkins RW. The final phase of polio eradication: new vaccines and complex choices. J Infect Dis 2012; 205:169–71. [DOI] [PubMed] [Google Scholar]
- 3.Caceres VM, Sutter RW. Sabin monovalent oral polio vaccines: review of past experiences and their potential use after polio eradication. Clin Infect Dis 2001; 33:531–41. [DOI] [PubMed] [Google Scholar]
- 4.Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SG, Cochi SL, Thompson KM. Characterizing poliovirus transmission and evolution: insights from modeling experiences with wild and vaccine-related polioviruses. Risk Anal 2013; 33:703–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duintjer Tebbens RJ, Pallansch MA, Kim JH, Burns CC, Kew OM, Oberste MS, et al. Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Anal 2013; 33:680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinsbroek E, Ruitenberg EJ. The global introduction of inactivated polio vac- cine can circumvent the oral polio vaccine paradox. Vaccine 2010; 28:3778–83. [DOI] [PubMed] [Google Scholar]
- 7.Davis R, Biellik R. Inactivated polio vaccine: its proposed role in the final stages of polio eradication. Pan Afr Med J 2013; 14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog 2012;8: e1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhamodharan A, Proano RA. Determining the optimal vaccine vial size in developing countries: a Monte Carlo simulation approach. Health Care Manage Sci 2012; 15:188–96. [DOI] [PubMed] [Google Scholar]
- 10.Gyawali S, Rathore DS, Shankar PR, Kumar KV. Strategies and challenges for safe injection practice in developing countries. J Pharmacol Pharmacother 2013; 4:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jafari H, Deshpande JM, Sutter RW, Bahl S, Verma H, Ahmad M, et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science 2014; 345:922–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 2012; 64:1547–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding Z, Van Riet E, Romeijn S, Kersten GF, Jiskoot W, Bouwstra JA. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pretreatment. Pharm Res 2009; 26:1635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro- projection array in a mouse model. PLoS ONE 2010;5: e13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, et al. Intra- dermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol 2010; 84:7760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koutsonanos DG, Vassilieva EV, Stavropoulou A, Zarnitsyn VG, Esser ES, Taherb- hai MT, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep 2012;2: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo K, Hirobe S, Yokota Y, Ayabe Y, Seto M, Quan YS, et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J Control Release 2012;160: 495–501. [DOI] [PubMed] [Google Scholar]
- 18.Pattani A, McKay PF, Garland MJ, Curran RM, Migalska K, Cassidy CM, et al. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release 2012; 162:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeMuth PC, Min Y, Huang B, Kramer JA, Miller AD, Barouch DH, et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nat Mater 2013; 12:367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. Measles vaccination using a microneedle patch. Vaccine 2013; 31:3403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kommareddy S, Baudner BC, Bonificio A, Gallorini S, Palladino G, Determan AS, et al. Influenza subunit vaccine coated microneedle patches elicit com- parable immune responses to intramuscular injection in guinea pigs. Vaccine 2013; 31:3435–41. [DOI] [PubMed] [Google Scholar]
- 22.Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ 2011;89:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Zahrani S, Zaric M, McCrudden C, Scott C, Kissenpfennig A, Donnelly RF. Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert Opin Drug Deliv 2012; 9:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang SM, Song JM, Kim YC. Microneedle and mucosal delivery of influenza vaccines. Expert Rev Vaccines 2012; 11:547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol 2013; 785:121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JH, Yoon YK, Choi SO, Prausnitz MR, Allen MG. Tapered conical polymer microneedles fabricated using an integrated lens technique for transdermal drug delivery. IEEE Trans Biomed Eng 2007; 54:903–13. [DOI] [PubMed] [Google Scholar]
- 27.Chu LY, Choi SO, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci 2010; 99:4228–38. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med 2010; 16:915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan S, Murthy N, Prausnitz P. Minimally invasive protein delivery with rapidly dissolving polymer microneedle. Adv Mater 2008; 20:933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. J Infect Dis 1997;175(Suppl. 1): S215–27. [DOI] [PubMed] [Google Scholar]
- 31.Kärber G Beitrag zur kollektiven Behandlung pharmakologischer Reihenver- suche. Arch für Exp Pathol Pharmakol 1931; 162:480–3. [Google Scholar]
- 32.Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J 2001;20:63–75. [DOI] [PubMed] [Google Scholar]
- 33.Bodian D Experimental studies on passive immunization against poliomyelitis in chimpanzees following subclinical infection. Am J Hyg 1953; 58:81–100. [DOI] [PubMed] [Google Scholar]
- 34.Howe HA. Immunization against poliomyelitis: poliomyelitis infection in immunized chimpanzees. Ann N Y Acad Sci 1955; 61:1014–20. [DOI] [PubMed] [Google Scholar]
- 35.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine 2014; 32:1856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li ZM, Goitz RJ. Biomechanical evaluation of the motor function of the thumb. Technol Health Care 2003; 11:233–43. [PubMed] [Google Scholar]
- 37.Dragunsky E, Gardner D, Taffs R, Levenbook I. Transgenic PVR Tg-1 mice for testing of poliovirus type 3 neurovirulence: comparison with monkey test. Biologicals 1993; 21:233–7. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi Y, Misumi S, Muneoka A, Masuyama M, Tokado H, Fukuzaki K, et al. Nonhuman primate intestinal villous M-like cells: an effective poliovirus entry site. Biochem Biophys Res Commun 2008; 368:501–7. [DOI] [PubMed] [Google Scholar]
- 39.Estivariz CF, Pallansch MA, Anand A, Wassilak SG, Sutter RW, Wenger JD, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr Opin Virol 2013; 3:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SG, Kim JH, Cochi SL. Preeradication vaccine policy options for poliovirus infection and disease control. Risk Anal 2013; 33:516–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawyer LA, McInnis J, Albrecht P. Quantitation of D antigen content in inactivated poliovirus vaccine derived from wild-type or sabin strains. Biologicals 1993; 21:169–77. [DOI] [PubMed] [Google Scholar]
- 42.Soonawala D, Verdijk P, Wijmenga-Monsuur AJ, Boog CJ, Koedam P, Visser LG, et al. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine 2013; 31:3688–94. [DOI] [PubMed] [Google Scholar]
- 43.Resik S, Tejeda A, Sutter RW, Diaz M, Sarmiento L, Alemani N, et al. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 2013; 368:416–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


