Abstract
Objective:
Our objective was to compare patterns of dental antibiotic prescribing in Australia, England, and North American (U.S. and British Columbia, Canada).
Design:
Population-level analysis of antibiotic prescription
Setting:
Outpatient prescribing by dentists in 2017
Participants:
Patients receiving an antibiotic dispensed by an outpatient pharmacy
Methods:
Prescription-based rates adjusted by population were compared overall and by antibiotic class. Contingency tables assessed differences in the proportion of antibiotic class by country.
Results:
In 2017, dentists in the United States (US) had the highest antibiotic prescribing rate per 1000 population and Australia had the lowest rate. The penicillin class, in particular amoxicillin, was the most frequently prescribed for all countries. The second most common agents prescribed were clindamycin in the US and British Columbia (Canada) and metronidazole in Australia and England. Broad spectrum agents, amoxicillin-clavulanic acid and azithromycin were the highest in Australia and U.S., respectively.
Conclusion:
There are extreme differences in antibiotics prescribed by dentists in Australia, England, U.S., and British Columbia. The U.S. had twice the antibiotic prescription rate compared with Australia and prescribed the most clindamycin. Significant opportunities exist for the global dental community to update their prescribing behaviour when it comes to use of second-line agents in penicillin allergic patients and contribute to international efforts to tackle antibiotic resistance. Patient safety improvements will result from optimising dental antibiotic prescribing, especially for antibiotics associated with resistance (broad-spectrum agents) or C. difficile (clindamycin). Dental antibiotic stewardship programmes are urgently needed worldwide.
Introduction
Antibiotic resistance is a major public health burden driven by the unnecessary use of antibiotics.1 Dentists contribute significantly to global antibiotic use and are responsible for an estimated 10% of all antibiotics prescribed to humans. FDI World Dental Federation has highlighted the overwhelming case for restricting the use of antibiotics to only when strictly necessary.1
High rates of dental overprescribing in relation to national guidelines have been identified in Australia,2 the United Kingdom (UK)3 and the United States (US).4 Comparing appropriateness between countries is difficult, however, as guidelines for therapeutic and prophylactic uses of antibiotics by dentists differ markedly around the world.1 Unexplained differences in patterns of antibiotic use have been used as a means of assessing unnecessary use of antibiotics; the UK 5-year national action plan identifies priority actions to reduce variation in antibiotic prescribing between healthcare organisations.5
Studies of antibiotic prescribing patterns by general dentists in individual countries have been widely undertaken. In North America, an increasing trend has been identified in British Columbia (BC, Canada)6 and in the United States.7 By contrast, Australia8 and England9 have experienced a reducing trend. The aim of this study was to compare patterns of dental antibiotic prescribing during 2017 in different regions of the world: Australia, England and North America (US and British Columbia, Canada).
Method
This study was a population-level analysis of dental antibiotic prescribing between 1 January and 31 December 2017. Metrics to assess the quantity of antibiotic use were selected from an international consensus of outpatient quantity metrics.10 Prescriptions per defined population counts the number of antibiotic items independently of the prescribed doses (daily dose and duration of the antibiotic course) per 1000 inhabitants.10
Dispensed systemic antibiotic prescriptions from outpatient pharmacies, community and mail service pharmacies prescribed by dentists in Australia, England, the US and BC were included. The drugs were grouped by class: penicillins, cephalosporins, lincosamides (which includes clindamycin), macrolides (which includes erythromycin), nitroimidazoles (which includes metronidazole), tetracyclines and others (which includes trimethoprim with sulfamethoxazole, spiramycin and quinolones).
Data sources
Data for antibiotics dispensed by pharmacists to dental patients in Australia was accessed from the Department of Health relating to the Pharmaceutical Benefits Scheme and the Repatriation Pharmaceutical Benefits Scheme.11 Over 90% of these prescriptions are from the community setting, which is the main source of dental prescriptions.12 The 2017 mid-year population size (24,598,900) was obtained from the Australian Bureau of Statistics.13
Data for antibiotics dispensed by community pharmacists to dental patients in England were accessed from the National Health Service Digital Prescription Cost Analysis.14 These data relate only to antibiotics prescribed to dental patients who receive care through the publicly-funded National Health Service. The 2017 mid-year population size (55,619,430) was obtained from the UK Office for National Statistics.15
The US data sources were obtained from IQVIA LRx relating to all patients, including those who are commercially insured, Medicare, Medicaid and cash pay. These data included 92% of all outpatient prescriptions dispensed in the US. The 2017 population size (325,147,121) was obtained from the US Census Bureau.16
Prescribing data for BC was obtained from the BC Ministry of Health, PharmaNet, a database capturing 99% of outpatient prescriptions in the province.17 The 2017 mid-year population size (4,817,160) was obtained from Statistics Canada.18
No ethics approval was required from the Australian Department of Health as these data were publicly available to facilitate health policy research and analysis. Data from England were available via request to the National Health Service under the UK Freedom of Information Act 2000; use of the National Health Service Business Services Authority datasets is licensed under the terms of the Open Government Licence for Public Sector Information. The University of Illinois at Chicago Investigational Review Board deemed that this study was exempt from review and informed consent. The University of British Columbia IRB approved the protocol used in this study (certificate: HO9–00650).
Outcomes
Three outcomes for each country were described: (1) rate per 1000 population of antibiotic prescription items dispensed, (2) relative proportions of each antibiotic class and (3) rate per 1000 population of each antibiotic type.
Statistical Analysis
Data and statistical analyses were conducted using SAS statistical software version 9.4 (SAS Institute, Inc). Comparison of proportions of prescriptions and each specific antibiotic by country were analysed using chi square or Fisher’s exact tests, with a 2-sided P value of less than or equal to 0.05 being considered significant
Results
In 2017, dentists in the US prescribed 23.6 million antibiotic items, 3.0 million in England, 0.8 million in Australia and 0.3 million in British Columbia (BC). As shown in Figure 1, dentists in the United States (US) had the highest rate (72.6 antibiotic items per 1000 population) and Australia had the lowest rate of antibiotic use (33.2 antibiotic items per 1000 population) during 2017.
Figure 1:
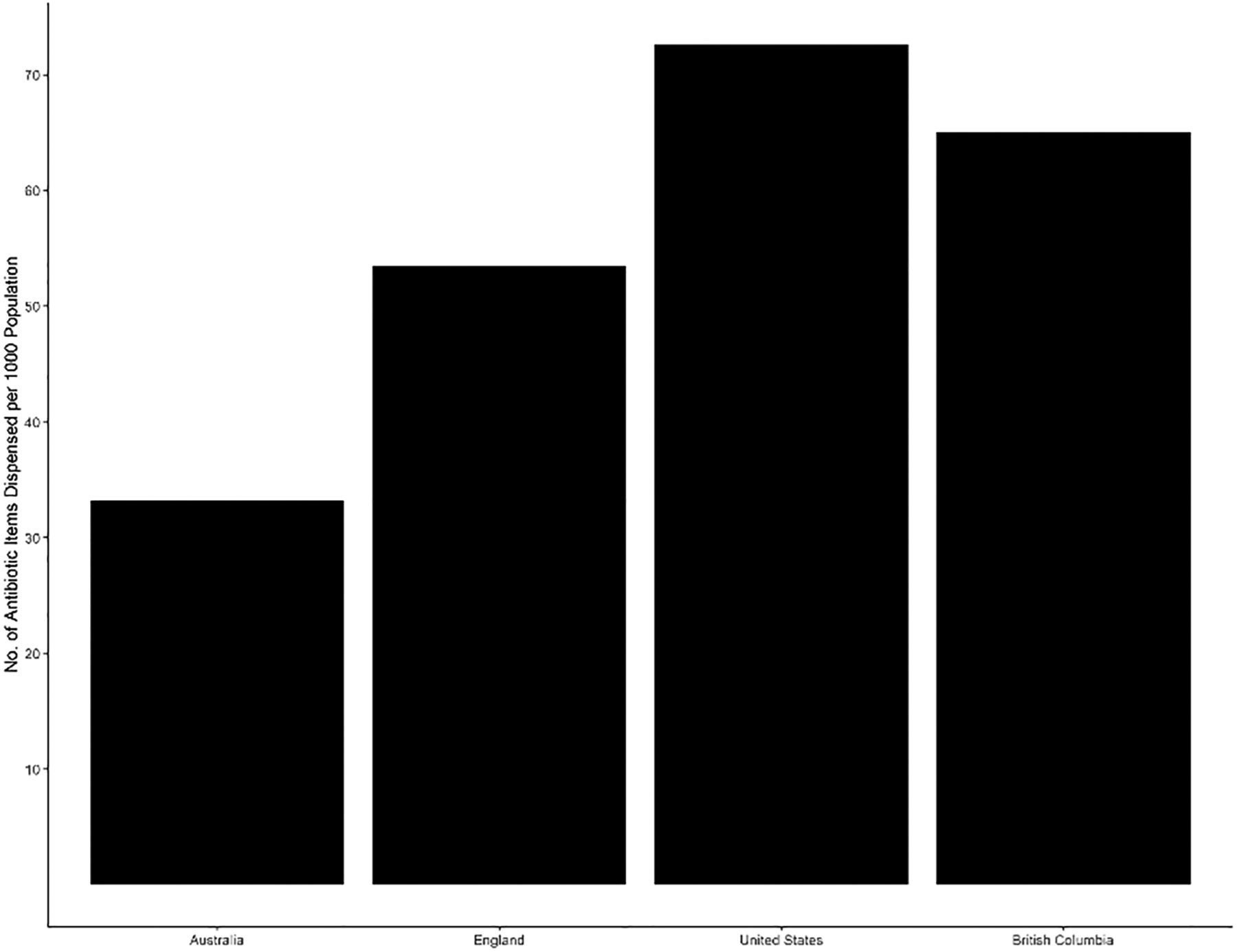
Rate of dental antibiotic prescribing per 1000 population by country
Relative proportions of each antibiotic class:
As shown in Table 1, the penicillin class of antibiotics was the most prescribed in each country (highest: 80.5% in BC and lowest: 66.8% in England). Nitroimidazoles (metronidazole) was the second most frequent class of antibiotic prescribed in England (28.4%) and Australia (13.2%). Lincosamides (clindamycin) was the second most frequent class prescribed in US (14.3%) and BC (12.4%). Macrolides were the third most prescribed class in the US. Cephalosporins were prescribed more often in US (3.9%) and Australia (3.0%) then BC (0.8%) and England (0.2%). Tetracyclines were rarely prescribed in any of the countries.
Table 1 –
Relative proportion of dispensed antibiotic types in 2017 by country.
| Australia | England | United States | British Columbia | |||||
|---|---|---|---|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | |
| Penicillins | ||||||||
| Amoxicillin | 524,166 | 64.2 (64.1–64.3) | 1,959,346 | 65.8 (65.8–65.9) | 14,607,292 | 61.9 (61.9–61.9) | 223,057 | 71.3 (71.1–71.4) |
| Amoxicillin/Clavulanic Acid | 92,903 | 11.4 (11.3–11.5) | 15,578 | 0.5 (0.5–0.5) | 710,856 | 3.0 (3.0–3.0) | 7,954 | 2.5 (2.5–2.6) |
| Phenoxymethylpenicillin | 11,197 | 1.4 (1.3–1.4) | 12,873 | 0.4 (0.4–0.4) | 2,194,226 | 9.3 (9.3–9.3) | 20,999 | 6.7 (6.6–6.8) |
| Other Penicillins | 389 | 0.0 (0.0–0.1) | 0.0 (0.0–0.0) | 23,948 | 0.1 (0.1–0.1) | 43 | 0.0 (0.0–0.0) | |
| Total Penicillins | 628,655 | 77.0 (76.9–77.1) | 1,987,797 | 66.8 (66.7–66.8) | 17,536,322 | 74.3 (74.3–74.3) | 252,053 | 80.5 (80.4–80.7) |
| Cephalosporins | ||||||||
| Cefalexin | 24,056 | 2.9 (2.9–3.0) | 5,593 | 0.2 (0.2–0.2) | 886,761 | 3.8 (3.8–3.8) | 2,515 | 0.8 (0.8–0.8) |
| Other Cephalosporins | 676 | 0.1 (0.1–0.1) | 627 | 0.0 (0.0–0.0) | 32,794 | 0.1 (0.1–0.1) | 102 | 0.0 (0.0–0.0) |
| Total Cephalosporins | 24,732 | 3.0 (3.0–3.1) | 6,220 | 0.2 (0.2–0.2) | 919,555 | 3.9 (3.9–3.9) | 2,617 | 0.8 (0.8–0.9) |
| Lincosamides | ||||||||
| Clindamycin | 41,324 | 5.1 (5.0–5.1) | 14,253 | 0.5 (0.5–0.5) | 3,367,880 | 14.3 (14.3–14.3) | 38,866 | 12.4 (12.3–12.5) |
| Macrolides | ||||||||
| Azithromycin | 0.0 (0.0–0.0) | 2,236 | 0.1 (0.1–0.1) | 1,021,272 | 4.3 (4.3–4.3) | 3,363 | 1.1 (1.0–1.1) | |
| Clarithromycin | 0.0 (0.0–0.0) | 7,654 | 0.3 (0.3–0.3) | 19,432 | 0.1 (0.1–0.1) | 1,602 | 0.5 (0.5–0.5) | |
| Erythromycin | 9,598 | 1.2 (1.2–1.2) | 107,047 | 3.6 (3.6–3.6) | 40,268 | 0.2 (0.2–0.2) | 2,096 | 0.7 (0.6–0.7) |
| Other macrolides | 1,422 | 0.2 (0.2–0.2) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 12 | 0.0 (0.0–0.0) | ||
| Total Macrolides | 11,020 | 1.4 (1.3–1.4) | 116,937 | 3.9 (3.9–3.9) | 1,080,972 | 4.6 (4.6–4.6) | 7,073 | 2.3 (2.2–2.3) |
| Nitroimidazoles | ||||||||
| Metronidazole | 108,041 | 13.2 (13.2–13.3) | 846,207 | 28.4 (28.4–28.5) | 206,682 | 0.9 (0.9–0.9) | 8,651 | 2.8 (2.7–2.8) |
| Tetracyclines | ||||||||
| Doxycycline | 1,719 | 0.2 (0.2–0.2) | 4,611 | 0.2 (0.2–0.2) | 301,508 | 1.3 (1.3–1.3) | 2,008 | 0.6 (0.6–0.7) |
| Tetracycline | 0.0 (0.0–0.0) | 911 | 0.0 (0.0–0.0) | 3,874 | 0.0 (0.0–0.0) | 199 | 0.1 (0.1–0.1) | |
| Other Tetracyclines | 0.0 (0.0–0.0) | 491 | 0.0 (0.0–0.0) | 11,304 | 0.0 (0.0–0.0) | 48 | 0.0 (0.0–0.0) | |
| Total Tetracyclines | 1,719 | 0.2 (0.2–0.2) | 6,013 | 0.2 (0.2–0.2) | 316,686 | 1.3 (1.3–1.3) | 2,255 | 0.7 (0.7–0.8) |
| Other | 642 | 0.1 (0.1–0.1) | 0.0 (0.0–0.0) | 165,440 | 0.7 (0.7–0.7) | 1,432 | 0.5 (0.4–0.5) | |
| Total Antibiotics | 816,133 | 100.0 | 2,977,427 | 100 | 23,593,537 | 100 | 312,947 | 100 |
Rate per 1000 population by antibiotic type
As shown in Figure 2, the highest rate of antibiotic prescribing per 1000 population in each country was amoxicillin, with BC having the highest rate (46.3 prescription items per 1000 population) while the lowest rate was in Australia (21.3/1000) (see Table 2). The rate of prescribing for the broader spectrum antibiotic amoxicillin-clavulanic acid prescribing was highest in Australia (3.8/1000 population) and lowest in England (0.3/1000). The narrowest spectrum antibiotic, phenoxymethylpenicillin, was commonly prescribed in US (6.8/1000) and least in England (0.2/1000).
Figure 2:
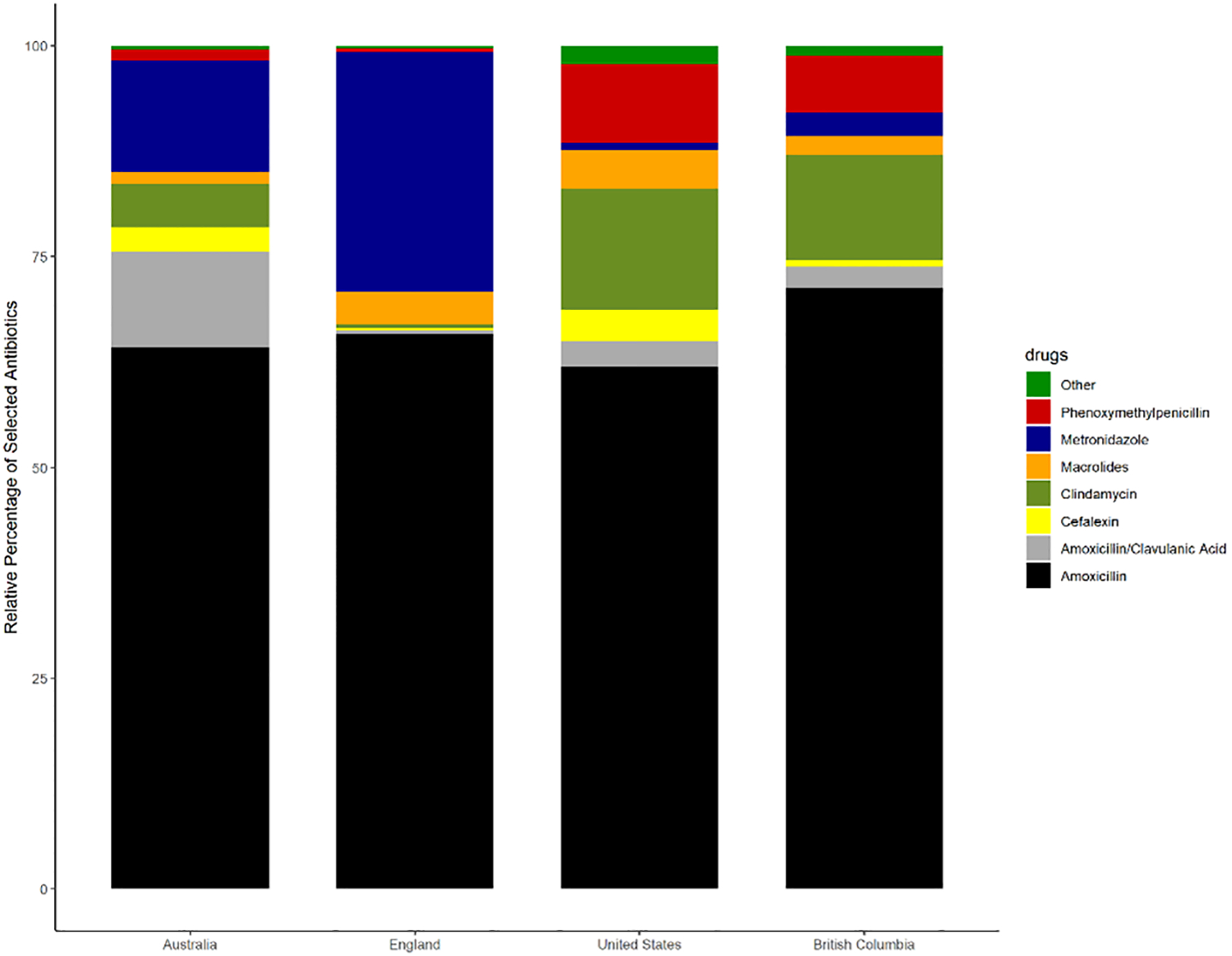
Relative proportions of each antibiotic type by country.
Table 2 –
Rates of dental antibiotic drug class prescribed per 1000 population in 2017 by country.
| Drug class | Australia | England | United States | British Columbia |
|---|---|---|---|---|
| Penicillins | ||||
| Amoxicillin prescription items, Number | 524,166 | 1,959,346 | 14,607,292 | 223,057 |
| Rate per 1000 population (95% CI) | 21.3 (21.3–21.4) | 35.2 (35.2–35.3) | 44.9 (44.9–44.5) | 46.3 (46.1–46.5) |
| Amoxicillin/Clavulanic Acid prescription items, Number | 92,903 | 15,578 | 710,856 | 7,954 |
| Rate per 1000 population (95% CI) | 3.8 (3.8–3.8) | 0.3 (0.3–0.3) | 2.2 (2.2–2.2) | 1.7 (1.6–1.7) |
| Phenoxymethylpenicillin prescription items, Number | 11,197 | 12,873 | 2,194,226 | 20,999 |
| Rate per 1000 population (95% CI) | 0.5 (0.4–0.5) | 0.2 (0.2–0.2) | 6.8 (6.8–6.8) | 4.4 (4.3–4.4) |
| Other Penicillin prescription items, Number | 389 | - | 23,948 | 43 |
| Rate per 1000 population (95% CI) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.1 (0.1–0.1) | 0.0 (0.0–0) |
| Cephalosporins | ||||
| Cefalexin prescription items, Number | 24,056 | 5,593 | 886,761 | 2,515 |
| Rate per 1000 population (95% CI) | 1.0 (1.0–1.0) | 0.1 (0.1–0.1) | 2.7 (2.7–2.7) | 0.5 (0.5–0.5) |
| Other Cephalosporins prescription items, Number | 676 | 627 | 32,794 | 102 |
| Rate per 1000 population (95% CI) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.1 (0.1–0.1) | 0.0 (0.0–0.0) |
| Lincosamides | ||||
| Clindamycin prescription items, Number | 41,324 | 14,253 | 3,367,880 | 38,866 |
| Rate per 1000 population (95% CI) | 1.7 (1.7–.1.7) | 0.3 (0.3–0.3) | 10.4 (10.4–10.4) | 8.1 (8.0–8.1) |
| Macrolides | ||||
| Azithromycin prescription items, Number | - | 2,236 | 1,021,272 | 3,363 |
| Rate per 1000 population (95% CI) | - | 0.0 (0.0–0.0) | 3.1 (3.1–3.1) | 0.7 (0.7–0.7)) |
| Erythromycin prescription items, No. | 9,598 | 107,047 | 40,268 | 2,096 |
| Rate per 1000 population (95% CI) | 0.4 (0.4–0.4) | 1.9 (1.9–1.9) | 0.1 (0.1–0.1) | 0.4 (0.4–0.5) |
| Other Macrolides prescription items, Number | 1,422 | 7,654 | 19,432 | 1,614 |
| Rate per 1000 population (95% CI) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.3 (0.3–0.3) |
| Nitroimidazoles | ||||
| Metronidazole prescription items, Number | 108,041 | 846,207 | 206,682 | 8,651 |
| Rate per 1000 population (95% CI) | 4.4 (4.4–4.4) | 15.2 (15.2–15.3) | 0.6 (0.6–0.6) | 1.8 (1.8–1.8) |
| Tetracyclines | ||||
| Doxycycline prescription items, Number | 1,719 | 4,611 | 301,508 | 2,008 |
| Rate per 1000 population (95% CI) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.9 (0.9–0.9) | 0.4 (0.4–0.4) |
| Tetracycline prescription items, Number | - | 914 | 3,874 | 199 |
| Rate per 1000 population (95% CI) | - | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Other Tetracyclines prescriptions, Number | - | 491 | 11,304 | 48 |
| Rate per 1000 population (95% CI) | - | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Other | ||||
| Other antibiotic prescription items, Number | 642 | - | 165,440 | 1,432 |
| Rate per 1000 population (95% CI) | 0.0 (0.0–0.0) | - | 0.5 (0.5–0.5) | 0.3 (0.3–0.3) |
Metronidazole was the second most frequently used antibiotic in England (15.2 prescription items/1000) and Australia (4.4/1000). Clindamycin was the second most frequent in US (10.4 items/1000) and BC (8.1/1000). The rate of cephalexin use was highest in US (2.7/1000) and lowest in England (0.1/1000). Macrolides were rarely used in Australia or BC; in the US azithromycin was the most prescribed macrolide (3.1/1000) and in England erythromycin (1.9/1000). Doxycycline was the most prescribed tetracycline: the highest rate was in US (0.9 items/1000 population) and lowest in Australia and England (both 0.1/1000).
Discussion
During 2017, wide variation existed in the pattern of antibiotic prescribing by dentists, with the rate per 1000 population in US found to be twice that in Australia which had the lowest prescribing rate. Whilst amoxicillin was the most prescribed dental antibiotic in all four countries, clindamycin was the second most often used in the United States (US) and British Columbia (BC) compared to metronidazole in Australia and UK. Other broad-spectrum agents, such as amoxicillin-clavulanate, and macrolides (such as azithromycin) which are higher resistance potential are also being used by dentists.
Variation in the rate of antibiotic prescribing between the four countries does not seem to be explained by differences in dental health but may be related to differences in the prophylactic use of dental antibiotics aiming to protect people at risk of distance site infections during operative dental procedures. Similar levels of dental health have been previously described using well-established epidemiological outcomes measures of oral health including missing teeth, edentulousness, number of carious teeth and degree of caries experience.19, 20 A wide range of factors are known, however, to influence dental antibiotic prescribing, including access to dental treatment and the existence of (or knowledge about) national guidelines.4, 21–24
National guidelines – indications for dental antibiotics
Dental antibiotic guidelines around the world for therapeutic indications are generally based on the principles of draining infections and removing the cause, with procedures such as dental extraction.25–27 For the prevention of conditions such as infective endocarditis, antibiotic prophylaxis (a single oral dose of amoxicillin 3g or clindamycin 600mg) is indicated by guidance in most countries, including Australia, US and Canada.26, 28 whereas it is not routinely recommended by guidelines used in England.29 The extent to which this fundamental difference in guideline philosophy accounts for differences between England and the other nations in this study is unclear, although it is likely to be relatively small.30, 31 In the US high rates of prophylactic overprescribing (not in accordance with guidelines) are known to occur,4 with suggested explanations including the perioperative use of antibiotics to prevent complications of oral surgery procedures, such as the placement of dental implants and the removal of third molars.6
Ensuring national guidelines are clear about therapeutic, prophylactic and perioperative indications will be important to reducing variation in the rates of dental antibiotic prescribing internationally.30, 31. 27
National guidelines – antibiotic types
The World Health Organization has introduced three classifications of antibiotics as part of its efforts to tackle antibiotic resistance: the AWaRe classification (Access, Watch, Reserve).32 Antibiotics which offer the best therapeutic value while minimising the potential for resistance are included in the ‘Access’ group. Antibiotics which are prone to selecting for resistance are included in the ‘Watch’ group. ‘Last resort’ antibiotics reserved for the treatment of multi-drug resistance infections are included in the ‘Reserve’ group.
Watch group antibiotics include macrolides (such as erythromycin and azithromycin) which should be prioritised in antibiotic stewardship programmes to minimise their use.32 Furthermore, high levels of resistance to erythromycin is associated with bacteria commonly isolated from odontogenic infections and increased incidence of side-effects (such as gastrointestinal adverse effects) and drug interactions in some parts of the world.27 Macrolides were found in this study to have been prescribed by dentists in all four countries, most often in England and the United States. Dental antibiotic stewardship programmes, which focus on reducing the unnecessary use of antibiotics generally and optimising the use of macrolide antibiotics specifically, are required.
Adverse drug reactions to oral antibiotics commonly prescribed by dentists are also important considerations. Common antibiotic-related adverse reactions include nausea, vomiting and diarrhoea. More serious outcomes include allergy, anaphylaxis, bacterial resistance and Clostridoides difficile infection. Clindamycin use in dentistry has been shown to be associated with a significant number of non-fatal and fatal adverse drug reactions30 and community-associated Clostridoides difficile infections have been related to dental use of clindamycin in the US.33 Our finding that clindamycin was prescribed in the US and BC far more often than in England is significant and highlights the importance of a review of dental antibiotic guidelines to ensure they take account of the risks as well as the benefits for patients.
Patient-reported penicillin allergy is the reason dentists in Australia and US prescribe clindamycin.25, 26 In England, macrolides are recommended for patients who are allergic or unable to tolerate penicillin or metronidazole antibiotics.27 This is a significant problem because penicillin allergy is reported in 10–20% of patients, yet a high percentage of penicillin allergy labels on medical records are likely to be erroneous.34 Providing pathways for dentists to refer patients for penicillin-allergy testing and where appropriate de-labelling should further improve patient safety by reducing the use of antibiotics which are known to be associated with increased incidence of Clostridoides difficile, more drug-resistant bacterial infections, longer hospital stays, greater frequency of hospital readmissions, poorer clinical outcomes and increased economic costs.34 In addition, national dental antibiotic guidelines should be updated to take account of the most recent evidence relating to appropriate alternative drugs for people with penicillin allergies, such as cephalosporins.35
Updating and implementing national guidelines
The importance of national dental antibiotic guidelines which take account of the local context including rates of resistance to antibiotics to particular antibiotics, the availability of quality-assured antibiotics and access to dental services has been highlighted by FDI World Dental Federation.1 In particular, it noted that guidelines appropriate in high-income countries may not be appropriate in low and middle-income countries where access to dentistry and high quality antibiotics may differ. As a result, dental antibiotic prescribing guidelines around the world will continue to differ.
Antibiotic stewardship aims to optimise antibiotic prescribing in accordance with national guidelines and has been advocated as one way in which dental teams may contribute to global efforts to tackle antibiotic resistance.1 As factors driving antibiotic prescribing decisions are complex and numerous, there is no ‘one size fits all’ solution: combination of clinical audits, feedback and educational outreach visits have been shown to be particularly effective in primary dental care.36 A systematic review of metrics for evaluating antibiotic stewardship programmes across primary healthcare found that dental studies focused solely on rates of antibiotic prescribing, whereas a wider set of outcomes, such as adverse outcomes and patient satisfaction, were employed in other primary healthcare settings.37 Further research to develop international consensus for a set of dental antibiotic stewardship core outcomes is recommended.
Limitations
It is important to recognise that each nation collects and reports its antibiotic prescribing data in different ways. Data reported in this study for Australia, England and BC are from public health data sources while US data is proprietary. As highlighted in a similar study across Northern Europe,38 the figures for each country are known to be underestimates of the total number of antibiotics prescribed to dental patients and, in particular, the English data does not include prescriptions for patients receiving private treatment. This is also true for the Australian data, although numbers are estimated to be low as all common antibiotics prescribed for dental treatment are listed on the Pharmaceutical Benefits Scheme.11 In contrast, as BC PharmaNet captures all outpatient prescriptions, irrespective of payer, there is reasonable confidence that it captures most dental use.17
It is also important to note that these data include only antibiotics dispensed by pharmacists. Around the world, not all antibiotics are supplied to patients by pharmacists. In England, dentists can supply antibiotics directly to patients and whilst the actual quantity is unknown, it has been estimated that this may represent another 25% of dental antibiotics which are currently uncounted.39 Dentists in Australia may also dispense antibiotics directly, although dental dispensing of medicines is thought to be rare. Regulations in Canada and the US prevent dentists supplying antibiotics. However, in many countries (especially low-middle income countries), more than 60% of antibiotics can be purchased directly without a prescription and not necessarily through a pharmacy.40 The inability to quantify the potentially numerous antibiotics supplied directly is a significant issue, and high priority action is required to ensure systems are in place to monitor all antibiotic use in all countries, irrespective of payer or supplier.
Whilst the routinely collected data included in this study was assessed to be the best available to quantify the amount of antibiotics dispensed to dental patients, it was not possible to assess the indication (such as therapeutic or prophylactic use) for the prescription. Further, it was not possible to assess the appropriateness of the prescribing against national guidelines. This presents a further difficulty for comparing antibiotic prescribing between countries. The necessity for national guidelines which take account of the local context such as patterns of resistant bacteria and access to dental procedures (as advocated by FDI World Dental Federation), present considerable difficulty in drawing conclusions about rates of overprescribing across the international dental community. Further research is recommended to identify how best to present international comparisons for dental antibiotic prescribing to drive quality improvements using the proposed core outcome set for dental antimicrobial stewardship.
In conclusion, concerning differences exist in the pattern of dental antibiotic prescribing around the world. Significant opportunities exist for the global dental community to contribute to international efforts to tackle antibiotic resistance, including by changing from broad spectrum antibiotics (such as amoxicillin/clavulanate) to narrower spectrum antibiotics (such as phenoxymethylpenicillin) and by reducing the use of World Health Organisation Watch antibiotics (such as azithromycin). The dental profession can also contribute to improvements in patient safety by minimising the use of antibiotics associated with increased adverse drug reactions (such as clindamycin), including by reviewing guidelines, auditing compliance, and assisting in efforts to de-label people who identify as penicillin allergic. Dental antibiotic stewardship programmes are urgently required as part of national responses to delivering the WHO global action plan on tackling antimicrobial resistance. Further research to understand locally relevant factors driving unnecessary dental antibiotic prescribing in each country is indicated to support the development of context-appropriate stewardship solutions to the global problem of antibiotic resistance. To enable improvements in the quality of dental antibiotic prescribing around the world, it is vital that governments ensure they have systems to capture all data relating to antibiotic prescribing, irrespective of payer or supplier.
ACKNOWLEDGEMENTS
We are grateful to the BC Ministry of Health for providing the data on antimicrobial consumption for British Columbia, Canada.
Financial support
This work was supported by the National Institute for Health Research integrated academic training programme (Thompson), the Australian Government Research Training Program Scholarship (no. 241616) (Teoh), and the Agency for Healthcare Research and Quality grant R01 HS25177 (Suda and Hubbard).
Role of the funder:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Agency for Healthcare Research and Quality, the Department of Veterans Affairs, the US government, or of IQVIA or any of its affiliated entities. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from IQVIA (source: LRx January 2017 to December 2017, IQVIA Inc). All rights reserved.
Footnotes
Statement of previous presentation: Some of these data were previously presented in an abstract for a poster presented at ID Week, 2020.
Conflicts of interest
W Thompson, L Teoh, C Hubbard, F Marra, D Patrick, A Mamun and K Suda report no conflicts of interest relevant to this article. A Campbell is an employee of IQVIA.
Contributor Information
W Thompson, Division of Dentistry, University of Manchester, Manchester, United Kingdom..
L Teoh, Melbourne Dental School, University of Melbourne, Carlton, Victoria, Australia..
CC Hubbard, Department of Pharmacy Practice, College of Pharmacy, University of Illinois Chicago, Chicago, IL, United States..
F Marra, Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, British Columbia, Canada..
DM Patrick, Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada..
A Mamun, British Columbia Centre for Disease Control, Surrey, British Columbia, Canada..
Campbell Allen, IQVIA, Durham, North Carolina, United States.
K Suda, Department of Medicine, University of Pittsburg, Pittsburg, Pennsylvania, United States..
REFERENCES
- 1.Thompson W, Williams D, Pulcini C et al. The essential role of the dental team in reducing antibiotic resistance. Geneva: FDI World Dental Federation, 2020. [Google Scholar]
- 2.Teoh L, Marino R, Stewart K et al. A survey of prescribing practices by general dentists in Australia. BMC Oral Health 2019; 19: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cope AL, Francis NA, Wood F et al. Antibiotic prescribing in UK general dental practice: a cross-sectional study. Community Dent Oral Epidemiol 2016; 44: 145–53. [DOI] [PubMed] [Google Scholar]
- 4.Suda K, Calip G, Zhou J et al. Assessment of the appropriateness of antibiotic prescriptions for infection prophylaxis before dental procedures, 2011 to 2015. JAMA network open 2019; 2: e193909–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HM Government. Tackling antimicrobial resistance 2019–2024. The UK’s five-year national action plan. London: UK Government, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Marra F, George D, Chong M et al. Antibiotic prescribing by dentists has increased: Why? J Amer Dent Assoc 2016; 147: 320–7. [DOI] [PubMed] [Google Scholar]
- 7.King LM, Bartoces M, Fleming-Dutra KE et al. Changes in US outpatient antibiotic prescriptions from 2011–2016. Clin Infect Dis 2020; 70: 370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teoh L, Stewart K, Marino R et al. Current prescribing trends of antibiotics by dentists in Australia from 2013 to 2016. Part 1. Aus Dent J 2018; 63: 329–37. [DOI] [PubMed] [Google Scholar]
- 9.Thornhill MH, Dayer MJ, Durkin MJ et al. Oral antibiotic prescribing by NHS dentists in England 2010–2017. Br Dent J 2019; 227: 1044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versporten A, Gyssens IC, Pulcini C et al. Metrics to assess the quantity of antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73: vi59–vi66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian Govenment Department of Health. PBS and RPBS Section 85 Date of Supply Data. https://www.pbs.gov.au/info/statistics/dos-and-dop/dos-and-dop.
- 12.Australian Govenment Department ofHealth. Australian Statistics on Medicines 2011. https://www.pbs.gov.au/info/statistics/asm/asm-2011#info_on_asm.
- 13.Australian Government. Australian Bureau of Statistics. https://www.abs.gov.au/.
- 14.NHS Digital. Prescription Cost Analysis, England 2017. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/prescription-cost-analysis-england-2017.
- 15.Office for National Statistics. Population estimates: quality information Mid-2017. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesqualitytools.
- 16.US Census Bureau. Population. https://www.census.gov/topics/population.html.
- 17.Province of British Columbia. Data Stewardship Committee. https://www2.gov.bc.ca/gov/content/health/conducting-health-research-evaluation/data-access-health-data-central/data-stewardship-committee?keyword=data&keyword=stewardship.
- 18.Statistics Canada. Table 17-10-0009-01 Population estimates, quarterly. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901.
- 19.Mejia GC, Elani HW, Harper S et al. Socioeconomic status, oral health and dental disease in Australia, Canada, New Zealand and the United States. BMC Oral Health 2018; 18: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slade G, Nuttall N, Sanders A et al. Impacts of oral disorders in the United Kingdom and Australia. Br Dent J 2005; 198: 489–93. [DOI] [PubMed] [Google Scholar]
- 21.Thompson W, Douglas G, Pavitt S et al. Factors associated with prescribing of systemic antibacterial drugs to adult patients in urgent primary health care, especially dentistry. J Antimicrob Chemother 2019; 74(8): 2139–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canadian Dental Association. A snapshot of oral health in Canada. http://www.cda-adc.ca/stateoforalhealth/snap/.
- 23.Centre for Disease Control and Prevention. Table 78. Dental visits in the past year, by selected characteristics: United States, selected years 1997–2016. 2017. [Google Scholar]
- 24.Manton D, Foley M, Gikas A et al. Australia’s Oral Health Tracker. 2018.
- 25.Lockhart PB, Tampi MP, Abt E et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal-and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J Amer Dent Assoc 2019; 150: 906–21. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oral and Dental Expert Group. Therapeutic guidelines: Oral and dental version 3. Melbourne, Australia: Therapeutic Guidelines Ltd, 2019. [Google Scholar]
- 27.Faculty of General Dental Practitioners. Antimicrobial prescribing for general dental practitioners. In: Palmer N, ed: Faculty of General Dental Practitioners; (UK: ), 2012. [Google Scholar]
- 28.Nishimura RA, Otto CM, Bonow RO et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC 2017; 70: 252–89. [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health & Care Excellence. Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures. CG64 National Institute for Health and Care Excellence; London, 2016. [PubMed] [Google Scholar]
- 30.Thornhill MH, Dayer MJ, Prendergast B et al. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother 2015; 70: 2382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.HSCIC. Prescribing by dentists: 2007, England. https://digital.nhs.uk/data-and-information/publications/statistical/prescribing-by-dentists/prescribing-by-dentists-england-2007.
- 32.WHO. WHO Antibiotic Categorization. https://aware.essentialmeds.org/groups.
- 33.Bye M, Whitten T, Holzbauer S. Antibiotic Prescribing for Dental Procedures in Community-Associated Clostridium difficile cases, Minnesota, 2009–2015. Open Forum Infect Dis 2017; 4: S1. [Google Scholar]
- 34.West R, Smith C, Pavitt S et al. ‘Warning: allergic to penicillin’: association between penicillin allergy status in 2.3 million NHS general practice electronic health records, antibiotic prescribing and health outcomes. J Antimicrob Chemother 2019; 74: 2075–82. [DOI] [PubMed] [Google Scholar]
- 35.Trubiano JA, Stone CA, Grayson ML et al. The 3 Cs of antibiotic allergy—classification, cross-reactivity, and collaboration. J Allergy Clin Immunol 2017; 5: 1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loffler C, Bohmer F. The effect of interventions aiming to optimise the prescription of antibiotics in dental care—A systematic review. PloS one 2017; 12: e0188061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teoh L, Sloan AJ, McCullough MJ et al. Measuring Antibiotic Stewardship Programmes and Initiatives: An Umbrella Review in Primary Care Medicine and a Systematic Review of Dentistry. Antibiotics 2020; 9: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith A, Al-Mahdi R, Malcolm W et al. Comparison of antimicrobial prescribing for dental and oral infections in England and Scotland with Norway and Sweden and their relative contribution to national consumption 2010–2016. BMC Oral Health 2020; 20: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2019 to 2020. London: England, 2020. [Google Scholar]
- 40.Batista AD, A Rodrigues D, Figueiras A et al. Antibiotic Dispensation without a Prescription Worldwide: A Systematic Review. Antibiotics 2020; 9: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]


