Abstract
Background:
The indications, techniques and outcomes for minimally invasive surgical approach for oropharyngeal squamous cell carcinoma (OPSCC) not amenable to transoral resection are not well-described.
Methods:
A retrospective case-series was performed using a prospectively-assembled database of transoral surgery-treated OPSCC patients who underwent a hybrid, transoral, limited pharyngotomy for tumor resection. Disease and functional outcomes were evaluated.
Results:
Twenty patients underwent complete tumor resection using the hybrid approach. Median follow-up was 48 months. No postoperative pharyngocutaneous fistula occurred. One patient (5%) had a local recurrence. Kaplan-Meier estimates for disease-specific survival at 2- and 5-years was 94.4% (95% CI: 84%, 100%) and 87% (95% CI:70%,100%). All but one patient (due to chemoradiotherapy-related chondroradionecrosis) were decannulated, and two required long-term gastrostomy.
Conclusion:
In the absence of favorable transoral, the ‘hybrid’ approach of combined transoral and limited pharyngotomy can accomplish margin-negative primary tumor resection, with a high degree of disease control and functional recovery in selected OPSCC patients.
Keywords: Oropharynx cancer, minimally-invasive surgical technology, p16 positive, pharyngotomy, transoral laser microsurgery
INTRODUCTION
Transoral laser microsurgery (TLM) resection of oropharyngeal squamous cell carcinoma (OPSCC) can achieve excellent oncologic and function outcomes with minimum disruption of uninvolved cervicofacial anatomic structures.1,2 For resectable oropharyngeal tumors, inadequate access is a major contraindication to the transoral approach since it impedes complete oncologic resection, and has been used as justification for consignment of patients to a lip-split mandibulotomy. The technical considerations that can limit transoral access have been categorized by our study group as the 8 ‘T’s of endoscopic access which include: teeth, trismus, transverse dimensions (mandibular), tori (mandibular), tongue, tilt (atlanto-occipital extension, Figure 1), treatment (prior radiotherapy), and tumor.3 However, adoption of a “hybrid” approach, where transoral resection is followed by a transcervical pharyngotomy approach in conjunction with the neck dissection, is able to circumvent some of these limitations and thus, retains the functional benefits of minimally invasive surgery.
Figure 1.
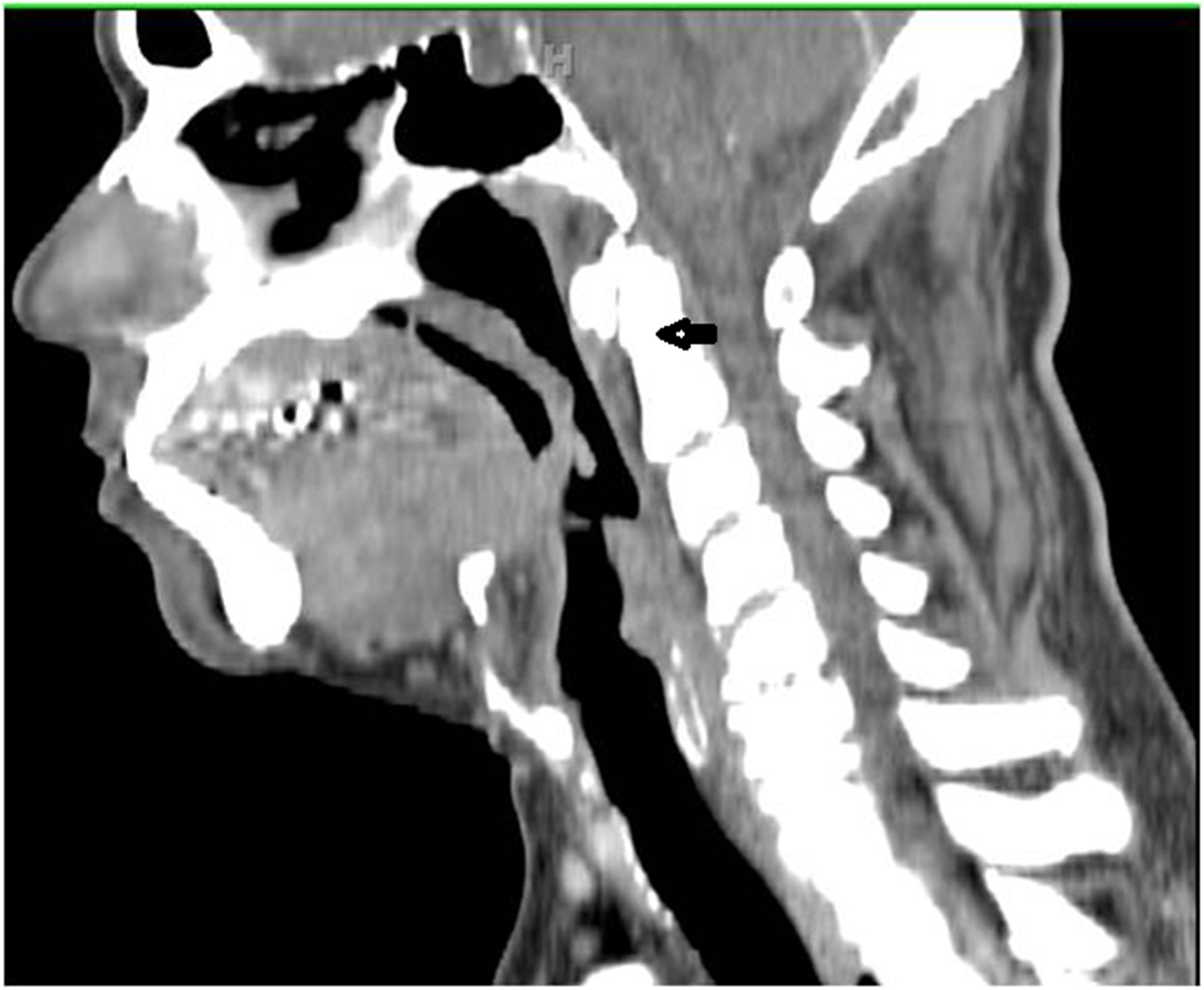
Sagittal computed tomography image showing absence of ‘tilt,’ one of the 8 ‘T’s for unfavorable endoscopic access, due to the stiffening effect of cervical spinal osteoarthritis (arrow).
Our report discusses the indications and techniques of the hybrid transoral, limited pharyngotomy approach as a method to achieve complete resection of oropharyngeal tumors that are not amenable to a transoral approach alone. Oncologic and functional outcomes in a case series of this hybrid approach are also described.
MATERIALS AND METHODS
Data collection:
An electronic data registry of patients with head and neck carcinoma, who had undergone a transoral surgical approach, was searched for patients with TLM-resected OPSCC. All data collection was approved by the Human Research Protection Office. Operative notes were reviewed further to identify a cohort who underwent the hybrid approach as proposed by the senior author for complete tumor resection. The inclusion criteria were (i), histologically-proven primary or recurrent OPSCC (any T, any N, M0 classification) and (ii), tumor resection through hybrid transoral-pharyngotomy approach, where TLM was initially applied for primary resection, followed by a limited pharyngotomy for complete oncologic resection. The reasons for inclusion of patients with T2 disease were poor atlanto-occipital extension, intractable trismus and tumor location relative to endoscopic access. Patients with T4b disease with limited extension to the nasopharynx or infratemporal fossa were considered for the hybrid approach unless preoperatively, tumor was projected to be unresectable due to the high potential for a positive margin, such as skull base extension or tumor surrounding the internal carotid artery.3 Pertinent tumor, pathology and treatment-related data were collected. The indication(s) for performing the hybrid approach, using the 8 ‘T’s classification, was collected from the preoperative examination and intraoperative procedure notes. Data describing treatment-related complications, survival, recurrence, and the presence of tracheostomy and gastrostomy tube (G-tube) at the last follow-up were recorded.
Surgical technique:
Tumor resection was initially performed transorally using TLM techniques as previously described,3 and continued intraoperatively up to the point where further access for oncologically-sound margin-negative resection, could not be achieved due to anatomic or tumor-related constraints. An ipsilateral neck dissection, usually selective, was then performed and transcervical access to the remaining primary tumor achieved through a limited lateral and/or suprahyoid pharyngotomy, for removal of the residual oropharyngeal tumor.
As for technique, the lateral aspect of the hyoid is palpated and the suprahyoid musculature sharply released from the superior aspect of hyoid until the laser-created, oropharyngeal surgical defect in the ipsilateral tongue base or vallecula region is entered. Insertion of a small Deaver retractor via the oral cavity to evaginate soft tissue above the hyoid facilitates external identification of the oropharyngeal laser defect. The hypoglossal nerve, lingual artery and the superior laryngeal nerve are in close proximity, and hence are identified with careful surgical dissection, prior to performing sharp constrictor muscle release around the greater cornu of the hyoid. The ipsilateral lingual artery is resected if invaded by tumor but is also often ligated, along with its branches to the tongue base to decrease the risk of post-operative hemorrhage. The only indication for not ligating the lingual artery was when the primary tumor did not involve the base of tongue. The pharyngotomy may be extended to or across the midline if necessary, and/or down the posterior aspect of thyrohyoid membrane and thyroid lamina, detaching the constrictors to expose the area where remaining resection is required, typically in the deep anterior tongue base or floor of mouth muscle. For lateral pharyngeal wall clearance, the pharyngotomy’s trajectory is aimed away from the persistent disease and seeks to connect to the area of the transoral resection bed that has already been cleared of disease.
The lateral pharyngotomy can also be performed just behind the submandibular gland into the region where deep parapharyngeal space resections have been performed. During this procedure, the facial artery usually needs to be ligated. The pharyngotomy can be enlarged from here down to the level of the hyoid bone, allowing for wider exposure of the tongue base and the supraglottic larynx. The pharyngotomy is held open by the assistant’s retractors, or self-retaining spreader-retractors, and an operating microscope may be re-deployed for an optimal view of the remaining tumor (Figure 2). The remaining tumor is then resected to negative frozen section margins using CO2 laser microsurgery. The midline tongue base as well as the deep anterior geniohyoid region can be accessed using the pharyngotomy approach. For extensive tumors with extension to the supraglottis, a transhyoid pharyngotomy is performed. The ipsilateral hyoid bone may be resected as a safety margin around the tumor. An osteotomy is made through the hyoid bone at approximately the midline using a sagittal saw, and marrow and/or periosteum of the contralateral hyoid bone is sent for frozen section analysis if needed.
Figure 2.
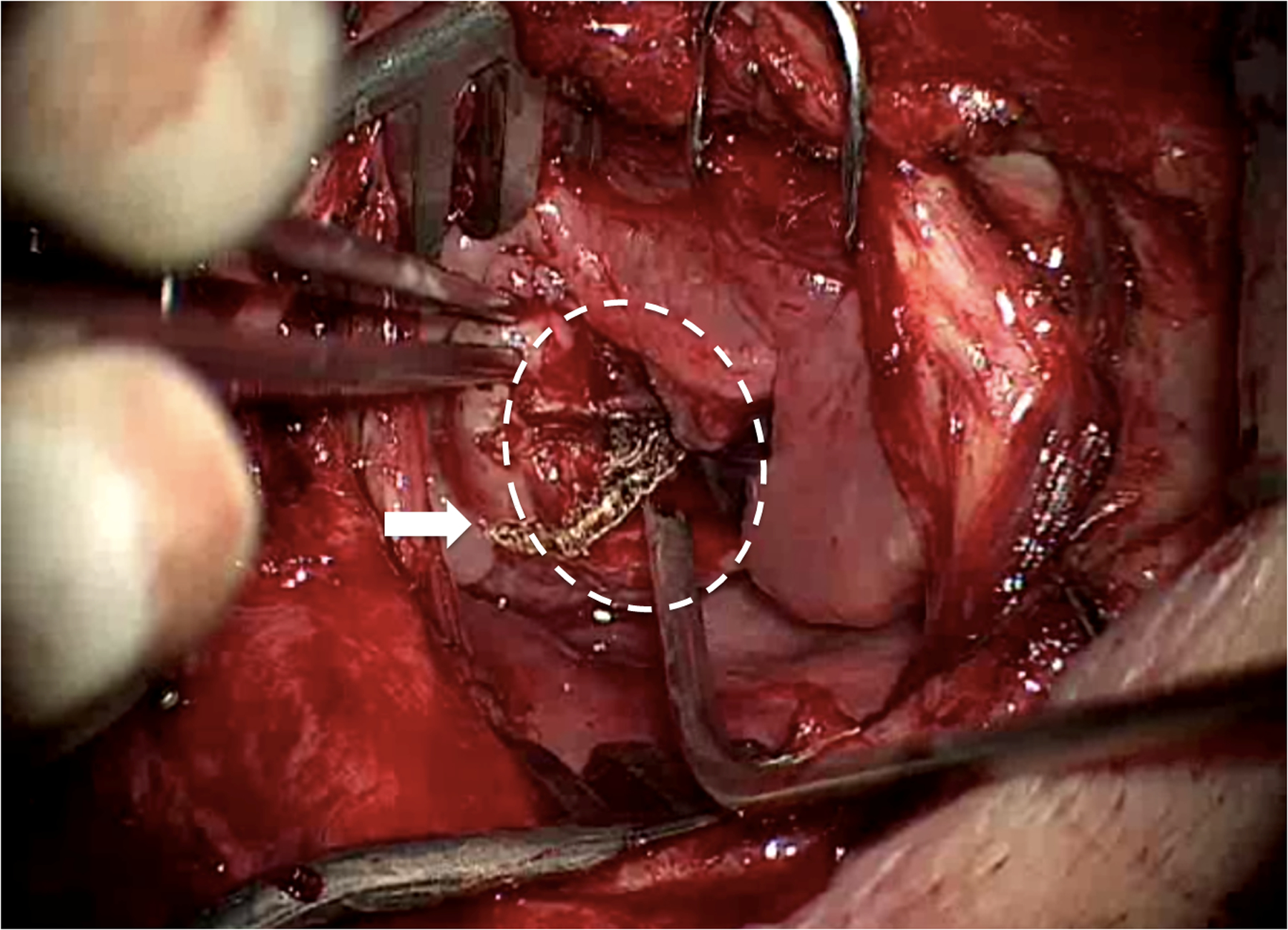
Right lateral limited pharyngotomy (operating microscope view) showing caudal part of the initial TLM defect inside dotted circle, with the previously cut surface of the tongue base seen en face. The trans-pharyngotomy laser cut has been commenced (arrow), traversing both the previous cut tongue surface and starting to extend onto mucosa. This incision was then extended rostrally to complete resection of residual medial tumor, the irregular tissue seen at the forceps tip which could not adequately be accessed transorally, due to patient anatomy.
Reconstruction after pharyngotomy:
The pharyngotomy closure is performed based on the site and size of the opening. The posterolateral margin of the pharyngeal wall is picked up and advanced anteriorly, tension being assessed by haptic feedback. For defects that can be closed primarily, the pharyngeal mucosa and constrictors are closed in an inverting Connell-type stitch using 3–0 Vicryl. This single layer closure is reinforced by anchoring the fascia overlying the strap muscles to the deep fascia of the suprahyoid soft tissue. If needed, 3–0 braided polyglycolic acid suture passed through the hyoid bone and deep tongue musculature to provide additional reinforcement. When needed, an Alloderm implant is quilted onto the base of the repair and is sutured around its perimeter as a patch outside the pharyngeal suture line, on the external aspect. After pharyngotomy repair, the skin flaps are laid down in the usual fashion following a neck dissection, the sternocleidomastoid muscle typically intervening between pharyngeal repair and skin.
For higher pharyngotomy defects, transposition reconstruction is accomplished using local muscles if needed and available, the stylohyoid or digastric. These muscles are released, transposed and sutured into the defect between the submandibular gland, the hyoid bone and the pharyngeal wall. If the resection involves the entire tongue base deeply, and/or > 50% of the pharyngeal wall, reconstruction is accomplished with a fasciocutaneous forearm flap, or if sufficiently thin, an anterolateral thigh flap. Alternatively, the pectoralis muscle or myocutaneous flap can be used. At the end of the procedure, a temporary tracheotomy is performed in large surface area and/or supraglottic larynx resections, to obviate airway decompensation from post-operative pharyngeal mucosal edema and/or hemorrhage.
Perioperative airway and nutrition management:
A tracheostomy is usually performed to secure the airway at the completion of the surgery prior to extubation for patients undergoing large surface area tongue base resection with or without flap reconstruction. Decannulation is performed either prior to discharge or during postoperative follow-up as determined by recovery. Nasogastric feeding is initiated in the initial postoperative period, and rehabilitation measures to improve swallowing are instituted in collaboration with a speech pathologist. Patients with unfavorable progress with swallowing function receive a G-tube which is usually left in place until the completion of adjuvant therapy even if the swallowing improves.
Statistical analysis:
The primary outcome was the local recurrence rate. Secondary outcomes were disease-specific survival (DSS), overall survival (OS), treatment-related complication rate, and permanent and temporary tracheostomy and gastrostomy rates. DSS was estimated from the time of surgery to the date of death from the disease or its treatment. OS was estimated from the time of surgery to the date of death from any cause. In addition to the gastrostomy rate, the swallowing function was also estimated by the Functional Outcome Swallowing Scale (FOSS) at last follow up.4 The FOSS ranks swallowing function from 0 to 5 (0- normal function, 1- normal function with episodic dysphagia, 2-compensated abnormal function, 3-decompensated abnormal function, 4-severely decompensated abnormal function, and 5-nonoral feeding). FOSS scores 0–2 are considered satisfactory for nutritional intake and indicate good swallowing, whereas 3 to 5 indicate poor swallowing, of which 4 to 5 are gastrostomy-dependent. A descriptive analysis was performed along and frequencies were recorded. Survival estimates were computed using Kaplan Meier method. All analyses were performed using SPSS software (IBM SPSS Statistics, Rel 21.0.0, Chicago: IBM Corporation).
RESULTS
A total of 328 patients were treated with TLM for primary or recurrent OPSCC from 1996 to 2013, of which 20 (6%) were identified to have undergone the planned hybrid approach for tumor resection. Inadvertent pharyngotomies were excluded, where no tumor resection occurred through the transcervical approach.5 The decision to perform a hybrid approach was made preoperatively in eight patients, and intraoperatively in the remaining 12. The indications for the hybrid approach are outlined in Table II. About 25% of the 328 patients in the TLM database had T3–T4 tumors, of which 16 required a pharyngotomy as reported in this study. Hence, overall, approxiamtely 20% of the patients with T3–T4 tumors underwent oncologic primary resection through a hybrid approach. Of the four T2 patients that underwent hybrid approach, indications for pharyngotomy were: tumor + tilt (difficulty in neck extension due to cervical arthritis, Figure 1) + trismus in one, tumor + tilt in one, and tumor in two patients (deep glossotonsillar sulcus location in one and infero-lateral pharyngeal wall extension in the other). Demographic and pre-treatment tumor characteristics of the study cohort are illustrated in Table I. Median follow-up duration for the cohort was 48 months (minimum=15, maximum=150 months).
Table II.
Treatment characteristics for the study cohort
| Inferior | 6 |
| Anterior + Inferior | 8 |
| Anterior + Inferior + Lateral | 3 |
| Lateral | 5 |
| Lateral | 10 |
| Transhyoid | 10 |
| Primary | 2 |
| Skin graft | 1 |
| Alloderm | 9 |
| Local flap | 2 |
| Pectoralis major | 3 |
| Radial forearm free flap | 3 |
| None | 4 |
| Yes | 16* |
Table I.
Demographic and tumor characteristics for the study cohort
| Median (minimum-maximum) | 60 (49–75) |
| Male | 17 (85) |
| Female | 3 (15) |
| ACE27 0–1 | 16 |
| ACE27 2–3 | 4 |
| Primary | 17 (85) |
| Recurrent | 3 (15) |
| None | 17 (85) |
| Prior TLM resection | 2 (10) |
| Prior TLM + RT | 1 (5) |
| Tonsil | 6 (30) |
| Tongue base | 14 (70) |
| T2 | 4 (20) |
| T3 | 4 (20) |
| T4a | 10 (50) |
| T4b | 2 (10) |
| N0 | 6 (30) |
| N1 | 1 (5) |
| N2b | 8 (40) |
| N2c | 4 (20) |
| N3 | 1 (5) |
| Tumor | 18 (90) |
| Tumor + Tilt | 1 (5) |
| Tumor + Tilt + Trismus | 1 (5) |
| Positive | 19 (95) |
| Negative | 1 (5) |
ACE 27-Adult Comorbidity Evaluation 27 (0-None, 1-Mild, 2-Moderate, 3-Severe),30 TLM- transoral microsurgery, RT- Radiation
The presence of histologic tumor was confirmed on frozen section analysis of the surgical specimen resected through the pharyngotomy approach in all patients. Tumor resection was performed to negative margins as assessed by frozen sections in all patients. In one patient, the permanent pathology on one of the frozen sections was reported to be microscopically positive, which was addressed with postoperative chemoradiation.
For the purpose of study, the patterns of tumor extension which necessitated a hybrid approach were broadly categorized into anterior, inferior, or lateral extension (Table II). Ventral extension to the midline deep tongue base infiltrating towards or into the oral tongue/floor of mouth musculature was considered “anterior” (Figure 3). Caudad tumor extension to vallecula, pre-epiglottic space, perihyoid tissue, supraglottis and hypopharynx was considered “inferior” (Figure 4). Transverse extension to the posterolateral oropharyngeal or hypopharyngeal wall, parapharyngeal space or infratemporal fossa was considered “lateral” (Figure 5).
Figure 3.
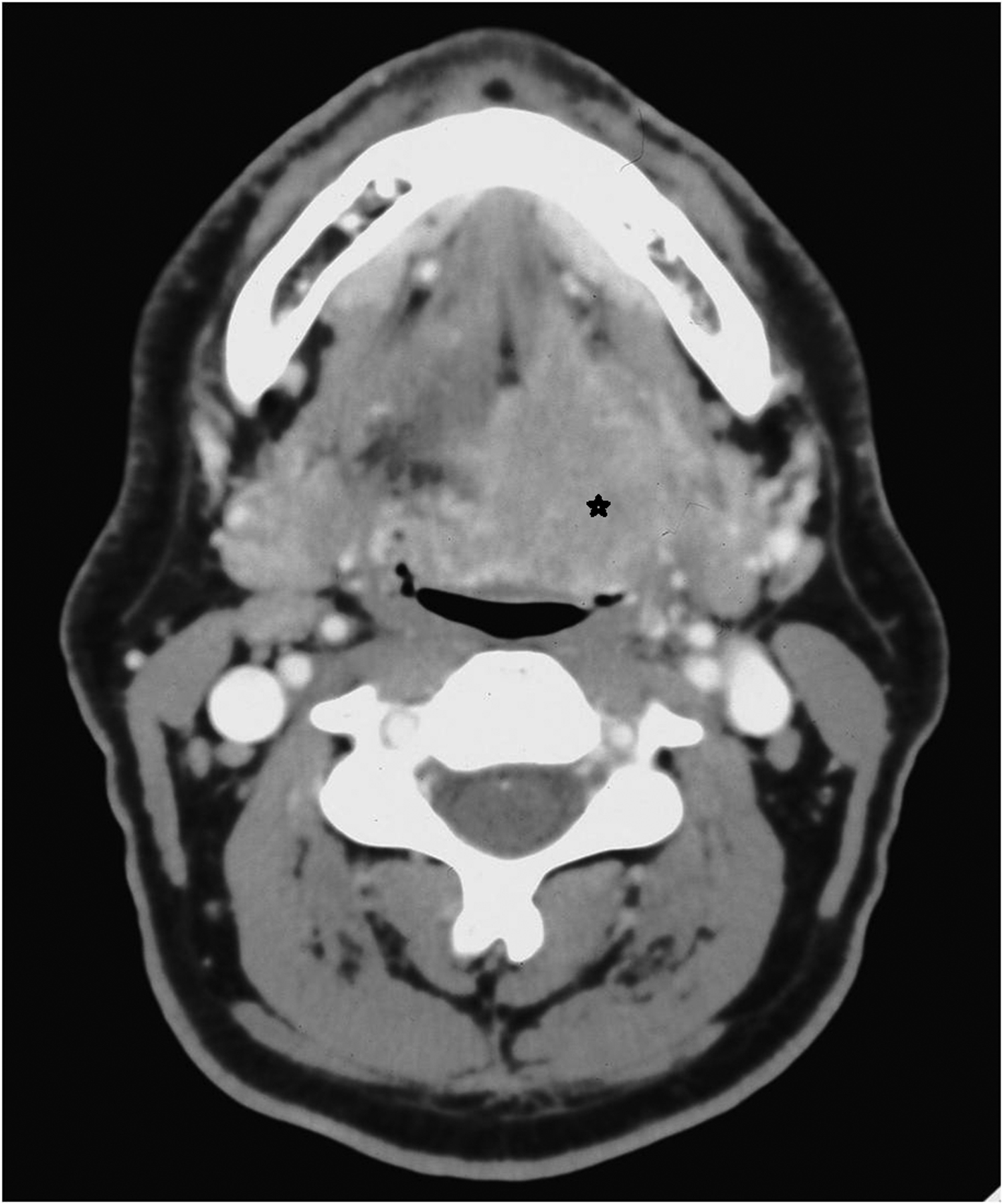
Axial computed tomography image of a left T4 tongue base tumor (asterisk): an example of “anterior” extension.
Figure 4.
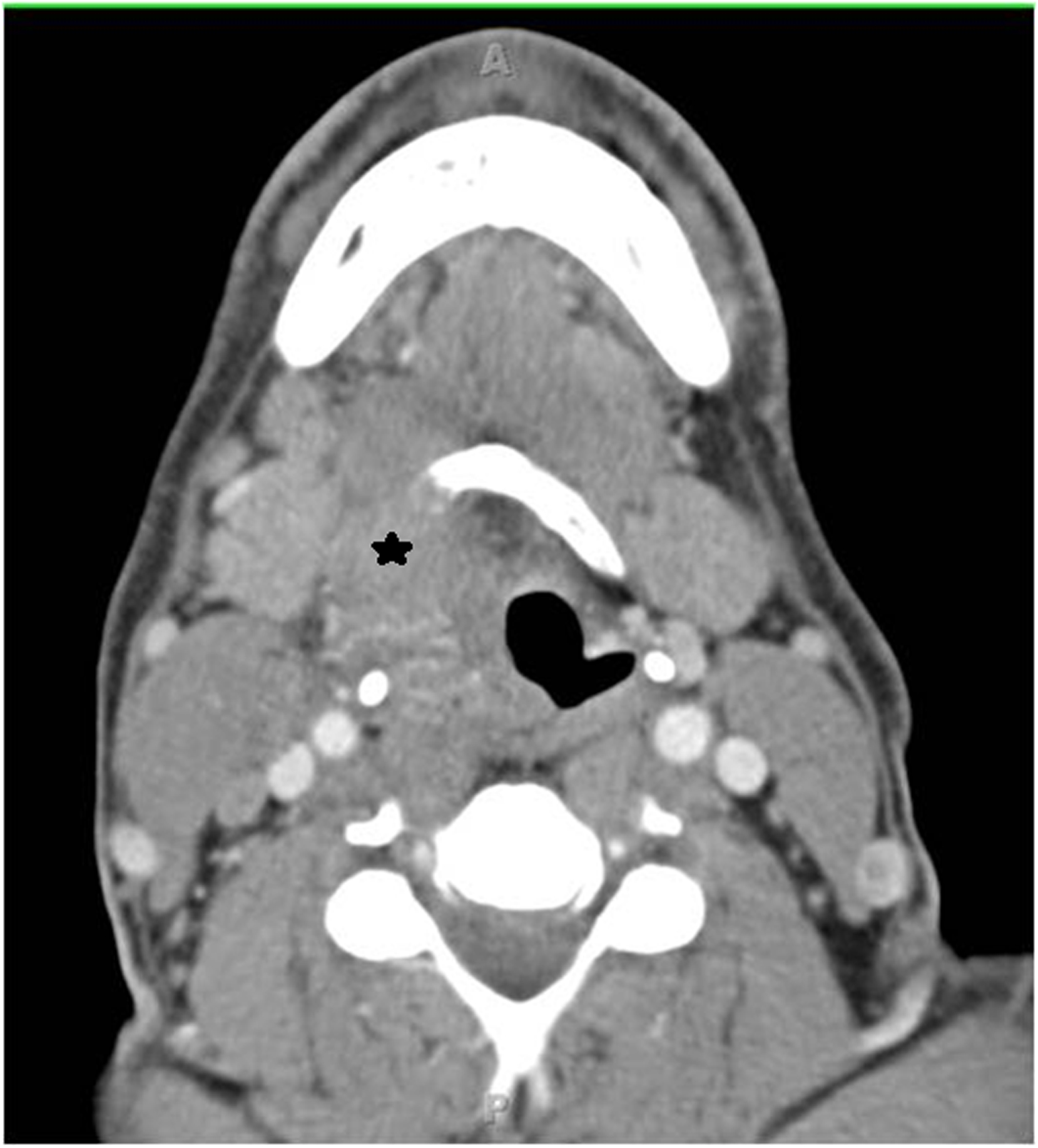
Axial computed tomography image of a right T4 tongue base tumor (asterisk), with erosion of the right hyoid bone and entry into the right pre-epiglottic space: an example of “inferior” extension
Figure 5.
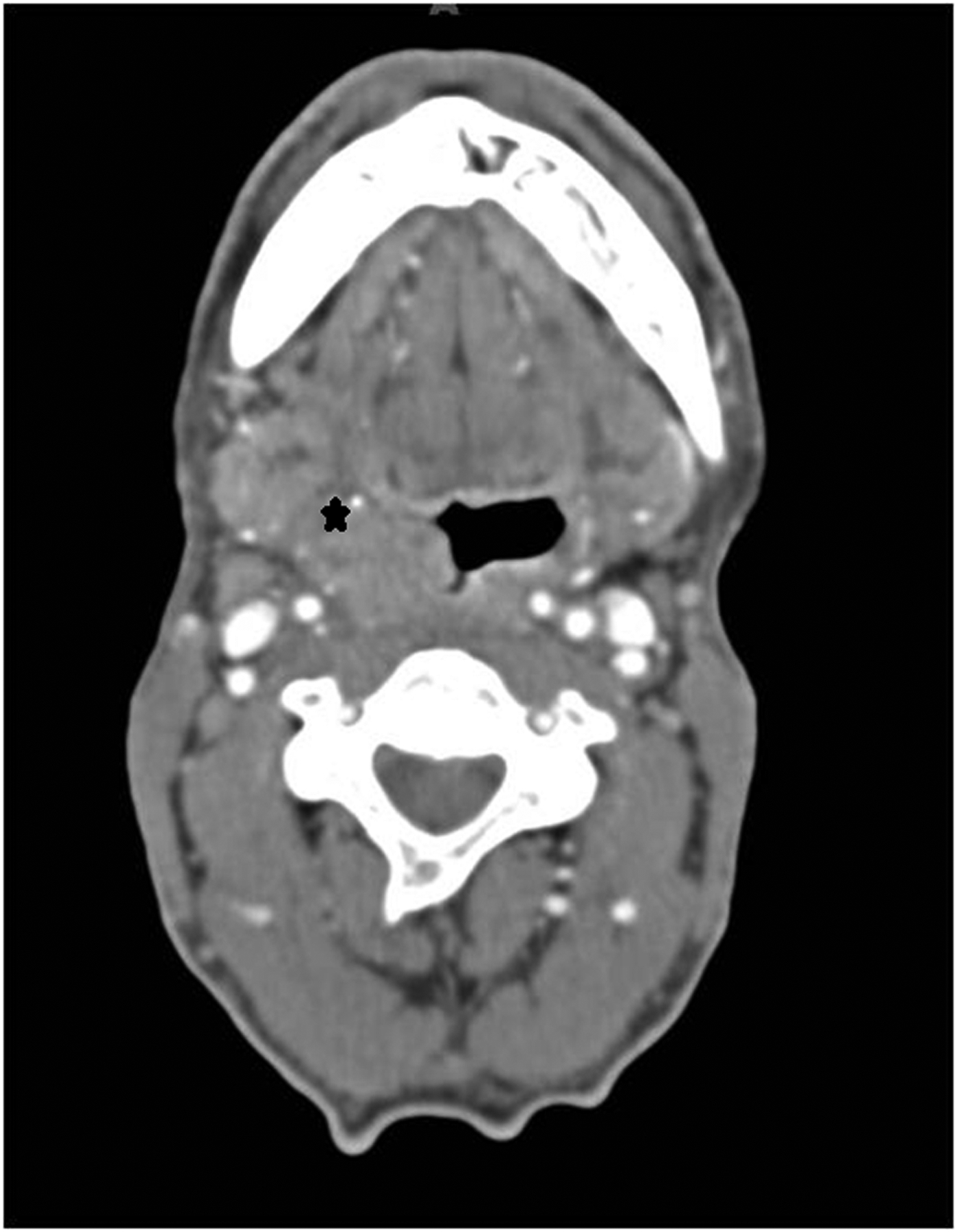
Axial computed tomography image of a right T4 tonsil tumor (asterisk): an example of “lateral” extension.
Reconstruction:
Reconstruction methods for the pharyngotomy defect are presented in Table II. Of the six patients who had free or regional flaps in our series, the primary indication was to reconstruct a large volume surgical defect due to extensive tumor in five, or to close the pharyngotomy along with surgical site reconstruction in one. For the remaining patients, primary closure or closure with local tissue, Alloderm or skin graft was utilized in varying combinations, as described in “Methods.”
Adjuvant therapy:
The indications for adjuvant radiation were advanced pathologic neck disease (≥ N2b-classification) in 15 patients, of which three were recommended, in a multidisciplinary environment, to receive additional chemotherapy due to the presence of extracapsular extension (n=2) and/or positive margins (n=1) on permanent pathology assessment of negative frozen sections.
Complications:
Surgery-related postoperative complications included hematoma on postoperative day 1 requiring surgical evacuation (n=1), wound seroma with spontaneous resolution (n=1), neck swelling on postoperative day 8 concerning for pharyngeal dehiscence with resolution on conservative management (n=1), hemorrhage on postoperative day 19 from friable granulation tissue in vallecula requiring surgical intervention (n=1), and neck wound infection (n=1). One patient had postoperative pneumonia due to aspiration. No clinically confirmed pharyngocutaneous fistulae were recorded. Post-adjuvant treatment complications included radiation ulcer in the radiated primary site of tonsil (n=1), complete esophageal stenosis (n=1), and esophageal stricture requiring dilatation (n=3).
Outcomes (Table III)
Table III.
Oncologic and functional outcomes for the study cohort
| Median (minimum – maximum) | 48 (15 −150) |
| Alive without disease | 15 |
| Alive with disease | 1 |
| Died from disease | 2* |
| Died from other cause | 2 |
| Local | 1 |
| Regional + distant | 1 |
| Distant | 2 |
| Temporary | 15 (preoperative in 1) |
| Permanent | 1 (chondroradionecrosis) |
| Temporary | 15 |
| G-tube at last follow-up | 8** |
died of distant metastasis,
6 out of the 8 had the G-tubes removed but re-inserted due to the following reasons: laryngeal chondroradionecrosis (n=1), disease recurrence (n=3), adjuvant radiation-related esophageal stricture (n=2).
Oncologic outcomes:
At a median follow up of 48 months (min-max: 15–150 months), the local recurrence rate was 5% (n=1/20). The one patient with local recurrence underwent margin-negative TLM resection but recurred again at the primary site and is now alive with disease on supportive therapy. Distant metastasis alone occurred in two patients (one is alive and disease-free status-post pulmonary metastasectomy and one died from disease). Distant metastasis with regional recurrence occurred in one patient (died of disease). The Kaplan-Meier estimates for DSS at 2- and 5- years was 94.4% (95% CI: 84%,100%) and 87% (95% CI: 70%,100%). The OS at 2- and 5- years was 89.5% (95% CI: 76%,100%) and 82.6% (95% CI: 65%,100%). The tumor classification in two patients was T4b due to extension of a recurrent tonsil tumor to the lateral pterygoid muscle in one patient and extension of a primary tonsil tumor in the nasopharynx in the second patient. Both patients were disease-free at their last follow-up of 13.1 and 22 months, respectively from the date of surgery.
Functional outcomes:
The temporary tracheostomy rate was 75% (n=15/20), with one patient receiving a preoperative emergent tracheostomy. All but one, due to chemoradiotherapy-related chondroradionecrosis, were decannulated. At the last follow-up, eight patients were G-tube dependent. Two patients never had their G-tube removed and six had the G-tube removed but reinserted later due to laryngeal chondroradionecrosis in one, disease recurrence in three and radiation-related esophageal stenosis in two. The distribution of FOSS score at the last followup was FOSS 0 in 1, FOSS 1 in 5, FOSS 2 in 3, and FOSS 3 in 3, FOSS 4 in 4 patients, and FOSS 5 in 4 patients.
Oncologic and functional outcomes of patients with regional/free flap reconstruction:
Of the three patients with pectoralis major flap reconstruction of the pharyngotomy defect, all are alive and disease-free. One of these three patients had no G-tube with a FOSS score of 1, one was G-tube dependent with a FOSS score of 5, and one had G-tube removed but it was reinserted due to post-chemoradiation laryngeal chondronecrosis. Of the three patients with free flap reconstruction, two died of distant metastasis, and of these two patients, one did and one did not have a G-tube at the time of death. The third patient is alive without disease and was G-tube dependent at the last follow-up.
DISCUSSION
In our study, access constraints due to tumor extension, trismus, and reduced atlanto-occipital ‘tilt’ were the main indications for a hybrid transoral, limited pharyngotomy resection. We have used the term ‘limited’ to denote the scale of the pharyngotomy, which is considerably smaller than a pharyngotomy approach that is exclusively used for an oropharynx primary resection in the absence of an initial transoral component. A negative margin resection was achieved intraoperatively in all patients. With the exception of one patient, all were free of disease at the primary site, for a local control rate of 95%. There was no recorded postoperative dehiscence of the pharyngotomy repair or fistula. Eighty percent of the patients had clinical T3–T4 classification.
The traditional open-only approach for surgically-managed advanced OPSCC patients has employed a mandibulotomy with or without full-thickness division of the lower lip. Whereas this approach facilitates wide exposure and has advantages for teaching, it also associates with higher complications, morbidity and increased hospital stay.6–12 On this basis, many centers may opt for non-surgical management when the primary tumor is considered ineligible for a transoral approach. However, trends towards better survival outcomes have been reported for primary surgical treatment of advanced OPSCC in both p16-positive and p16-negative series.13–16 TLM is proven to be a safe and effective minimally invasive approach to treat not only early but also selected advanced OPSCC with good oncologic outcomes.13,14,16,17 In a recent series on outcomes of T4a oropharyngeal cancer by Zenga et al, surgically-treated patients had significantly higher survival than non-surgically treated patients, regardless of the p16 status.16 Of the patients treated surgically, two-thirds (66%) underwent TLM resection, some of which were managed with the ‘hybrid’ approach.16 The association of primary surgery for T4 oropharyngeal carcinoma with significantly improved survival provides an evidence-based stimulus for development of minimally invasive approaches, such as the hybrid technique we describe.
The concept of transcervical, mandibulotomy-sparing technique for tumor resection has been described previously. A lateral pharyngotomy or transhyoid pharyngotomy, not combined with TLM, has been proposed for access to advanced oropharyngeal tumors.18–24 Laccourreye et al24,25 reported a series of 91 patients with carcinoma of the lateral oropharynx (80% T1–T2; 20% T3–T4) who underwent resection with a lateral pharyngotomy approach (combined with transoral approach in 12% patients). A positive-margin resection rate of 12%, local failure rate of 18.6%, and a pharyngocutaneous fistula rate of 4.3%, were reported. By contrast, our series had a higher proportion of tongue base tumors (70%) and advanced T-classification of 85% versus 20%.24,25 The tracheostomy rate of 77% reported by Laccoureye et al24,25 was comparable to our temporary tracheostomy rate of 75%.
In another series, Gallet et al compared the oncologic and functional results of 55 patients (73% Tis-T2; 27% T3–T4) treated with a combined transoral-suprahyoid approach versus 19 patients who underwent a mandibulotomy.19 There were no significant differences in the survival or local control between groups, although a lower complication rate was seen in the mandible-sparing group versus the mandibulotomy group (10.9% versus 31.5%). However, by contrast with the mandible-sparing group from Gallet et al, our study cohort had a higher rate of locally advanced T3–T4 tumors (80% versus 27%), and a greater 5-year overall survival (82.6% versus 36.8%). The regional or free flap reconstruction rate was also lower (30% versus 51%) in our series and no postoperative fistulae were noted. In patients requiring a mandibulotomy, postoperative fistula rates as high as 17.4% has been reported in literature.6,26 The T3–T4 primary, we believe, is an emerging relative indication for use of this planned hybrid approach.
Another mandible-sparing technique for resection of oropharyngeal tumors was described by Basterra et al in 1998.26 However, this technique involved a visor and degloving approach with significant soft tissue dissection involving the masseter muscle and the medial pterygoid muscles, and a midline lip-split for creating access to the oropharynx. They described a revised approach with avoidance of the lip-split in 2007 (n=46). A fistula rate of 13% was reported despite the use of local flaps. For oncologic outcomes, a local recurrence rate of 8.7% and an OS of 73%, 48%, and 38% at 1-, 2- and 3- years respectively were reported. In a case series of 21 patients with T3 and T4a oropharynx tumors who underwent resection with a mandible-sparing lateral oropharyngectomy approach, Pelliccia et al27 reported a fistula rate 9.5% and locoregional recurrence rate of 14.3%.27 At a mean follow-up of 23.7 months, Masuda et al28 reported a 100% local control rate with a “mandible preserving pull through oropharyngectomy (MPPO)” technique on 7 patients (5 T3 and 2 T4) technique. In this technique, a modified radical neck dissection was first performed followed by transection of the stylomandibular ligament and detachment of the stylohyoid, styloglossus and stylopharyngeus muscles from the styloid process to gain access to the tumor. Whereas this technique may give increased exposure, in our experience, a modified radical neck dissection for p16-positive oropharyngeal cancer is very infrequent, selective neck dissection being adequate in most cases. Also, the detachment of stylohyoid ligament and stylohyoid muscles from styloid process can be avoided by a simple lateral pharyngotomy. Bozec et al29 presented their experience in 21 patients who underwent oropharyngeal resection without a mandibulotomy. OS, cause-specific survival and disease-free survival were reported as 73%, 76% and 68% respectively. However, all patients in this series underwent reconstruction with a fasciocutaneous radial forearm free flap which, in our experience, was seldom necessary.
There are limitations to this study. It lacks an internal comparison group comprising patients who underwent resection with just a mandibulotomy approach. Lack of p16 testing in historic literature limits comparison, particularly for oncologic outcomes, since 95% of our patients were p16-positive. However, the present report captures the outcomes of the hybrid transoral, limited pharyngotomy approach, a technique which in our experience, provides a feasible way to excise advanced oropharyngeal carcinoma with good oncologic results, acceptable morbidity, and low flap reconstruction requirements.
CONCLUSION
Without access that allows complete transoral resection to negative margins, a combined transoral and pharyngotomy performed at neck dissection, or ‘hybrid’ approach, provides margin-negative primary tumor resection, obviating the need for a lip-splitting incision or mandibulotomy. The soft tissue access for pharyngotomy is already present from the completed neck dissection. This approach provided excellent oncologic control and functional recovery in selected patients across all T-classifications.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Steiner W, Fierek O, Ambrosch P, Hommerich CP, Kron M. Transoral laser microsurgery for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg. 2003;129(1):36–43. [DOI] [PubMed] [Google Scholar]
- 2.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. 2011;33(12):1683–94. [DOI] [PubMed] [Google Scholar]
- 3.Rich JT, Milov S, Lewis JS, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119(9):1709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salassa JR. A functional outcome swallowing scale for staging oropharyngeal dysphagia. Dig Dis. 1999;17(4):230–4. [DOI] [PubMed] [Google Scholar]
- 5.Zenga J, Graboyes EM, Sinha P, Haughey BH. The unplanned intraoperative pharyngotomy: Pull, plug, or patch. Laryngoscope. 2015;125(12):2736–40 [DOI] [PubMed] [Google Scholar]
- 6.Dziegielewski PT, Mlynarek AM, Dimitry J, Harris JR, Seikaly H. The mandibulotomy: friend or foe? Safety outcomes and literature review. Laryngoscope. 2009;119(12):2369–75. [DOI] [PubMed] [Google Scholar]
- 7.Smeele LE, Slotman BJ, Mens JW, Tiwari R. Local radiation dose, fixation, and non-union of mandibulotomies. Head Neck. 1999;21(4):315–8. [DOI] [PubMed] [Google Scholar]
- 8.Genty E, Marandas P, Beautru R, Schwaab G, Luboinski B. [Mandibulotomy for cancer of the oral cavity and oropharynx: functional and carcinologic outcome in 107 cases]. Ann Otolaryngol Chir Cervicofac. 2001;118(1):26–34. [PubMed] [Google Scholar]
- 9.Dai T-S, Hao S-P, Chang K-P, Pan W-L, Yeh H-C, Tsang N-M. Complications of mandibulotomy: midline versus paramidline. Otolaryngol Head Neck Surg. 2003;128(1):137–41. [DOI] [PubMed] [Google Scholar]
- 10.Nam W, Kim H-J, Choi E-C, Kim M-K, Lee E-W, Cha I-H. Contributing factors to mandibulotomy complications: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e65–70. [DOI] [PubMed] [Google Scholar]
- 11.McCann KJ, Irish JC, Gullane PJ, Holmes H, Brown DH, Rotstein L. Complications associated with rigid fixation of mandibulotomies. J Otolaryngol. 1994;23(3):210–5. [PubMed] [Google Scholar]
- 12.Christopoulos E, Carrau R, Segas J, Johnson JT, Myers EN, Wagner RL. Transmandibular approaches to the oral cavity and oropharynx. A functional assessment. Arch Otolaryngol Head Neck Surg. 1992;118(11):1164–7. [DOI] [PubMed] [Google Scholar]
- 13.Seikaly H, Biron VL, Zhang H, et al. Role of primary surgery in the treatment of advanced oropharyngeal cancer. Head Neck. 2016;38 Suppl 1:E571–579. [DOI] [PubMed] [Google Scholar]
- 14.Wang MB, Liu IY, Gornbein JA, Nguyen CT. HPV-Positive Oropharyngeal Carcinoma: A Systematic Review of Treatment and Prognosis. Otolaryngol Head Neck Surg. 2015;153(5):758–69. [DOI] [PubMed] [Google Scholar]
- 15.Psychogios G, Mantsopoulos K, Agaimy A, et al. Outcome and prognostic factors in T4a oropharyngeal carcinoma, including the role of HPV infection. Biomed Res Int. 2014;2014:390825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zenga J, Wilson M, Adkins DR, et al. Treatment Outcomes for T4 Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1118–27. [DOI] [PubMed] [Google Scholar]
- 17.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122 Suppl (September):S13–33. [DOI] [PubMed] [Google Scholar]
- 18.Zeitels SM, Vaughan CW, Ruh S. Suprahyoid pharyngotomy for oropharynx cancer including the tongue base. Arch Otolaryngol Head Neck Surg. 1991;117(7):757–60. [DOI] [PubMed] [Google Scholar]
- 19.Gallet P, Gangloff P, Mastronicola R, et al. Combined transoral and suprahyoid approach for oropharyngeal cancers: an alternative to mandibulotomy. Rev Laryngol Otol Rhinol (Bord). 2011;132(2):95–102. [PubMed] [Google Scholar]
- 20.Civantos F, Wenig BL. Transhyoid resection of tongue base and tonsil tumors. Otolaryngol Head Neck Surg. 1994;111(1):59–62. [DOI] [PubMed] [Google Scholar]
- 21.Metternich FU, Puder C, Brusis T. [Suprahyoid pharyngotomy for surgical therapy of malignant and benign oral and hypopharyngeal tumors]. HNO. 1996;44(5):242–5. [PubMed] [Google Scholar]
- 22.Agrawal A, Wenig BL. Resection of cancer of the tongue base and tonsil via the transhyoid approach. Laryngoscope. 2000;110(11):1802–6. [DOI] [PubMed] [Google Scholar]
- 23.Gopalan KN, Primuharsa Putra SH, Kenali MS. Suprahyoid pharyngotomy for base of tongue carcinoma. Med J Malaysia. 2003;58(4):617–20. [PubMed] [Google Scholar]
- 24.Laccourreye O, Benito J, Menard M, Garcia D, Malinvaud D, Holsinger C. Lateral pharyngotomy for selected invasive squamous cell carcinoma of the lateral oropharynx--part I: how. Laryngoscope. 2013;123(11):2712–7. [DOI] [PubMed] [Google Scholar]
- 25.Laccourreye O, Benito J, Garcia D, Menard M, Bonfils P, Holsinger C. Lateral pharyngotomy for selected invasive squamous cell carcinoma of the lateral oropharynx. Part II: when and why. Laryngoscope. 2013;123(11):2718–22. [DOI] [PubMed] [Google Scholar]
- 26.Basterra J, Bagán JV, Zapater E, Armengot M. Pull-through oropharyngectomy in advanced stage malignant tumours. J Laryngol Otol. 1998;112(4):355–9. [DOI] [PubMed] [Google Scholar]
- 27.Pelliccia P, Martinez Del Pero M, Mercier G, et al. Mini-invasive lateral oropharyngectomy for T3–T4a oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2013;270(4):1419–25. [DOI] [PubMed] [Google Scholar]
- 28.Masuda M, Fukushima J, Kadota H, Kamizono K, Ejima M, Taura M. Mandible preserving pull-through oropharyngectomy for advanced oropharyngeal cancer: a pilot study. Auris Nasus Larynx. 2011;38(3):392–7. [DOI] [PubMed] [Google Scholar]
- 29.Bozec A, Poissonnet G, Chamorey E, et al. [Transoral and cervical approach without mandibulotomy for oropharynx cancer with fasciocutaneous radial forearm free flap reconstruction]. Ann Otolaryngol Chir Cervicofac. 2009;126(4):182–9. [DOI] [PubMed] [Google Scholar]
- 30.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–7. [DOI] [PubMed] [Google Scholar]


