Abstract
Trypanosoma cruzi is the protozoan agent that causes Chagas' disease, a major health problem in Latin America. Better drugs are needed to treat infected individuals. The sterol biosynthesis pathway is a potentially excellent target for drug therapy against T. cruzi. In this study, we investigated the antitrypanosomal activities of a series of compounds designed to inhibit a key enzyme in sterol biosynthesis, oxidosqualene cyclase. This enzyme converts 2,3-oxidosqualene to the tetracyclic product, lanosterol. The lead compound, N-(4E,8E)-5,9, 13-trimethyl-4,8, 12-tetradecatrien-1-ylpyridinium, is an electron-poor aromatic mimic of a monocyclized transition state or high-energy intermediate formed from oxidosqualene. This compound and 27 related compounds were tested against mammalian-stage T. cruzi, and 12 inhibited growth by 50% at concentrations below 25 nM. The lead compound was shown to cause an accumulation of oxidosqualene and decreased production of lanosterol and ergosterol, consistent with specific inhibition of the oxidosqualene cyclase. The data demonstrate potent anti-T. cruzi activity associated with inhibition of oxidosqualene cyclase.
Trypanosoma cruzi is transmitted to humans by a bite from the insect vector (triatomines), by blood transfusion, or by transmission from mother to fetus. An estimated 16 to 18 million people in South and Central America (30) and 50,000 to 100,000 people in the United States (16) are infected with T. cruzi. The chronic phase typically occurs 10 to 20 years after contracting the parasite and affects 10 to 30% of those infected. Cardiac and gastrointestinal pathology are the most common manifestations of chronic disease. Recent clinical evidence showed that aggressive antiparasitic therapy (using benznidazole) had a beneficial effect on cardiomyopathic progression (28), suggesting an important role for etiologic treatment in the management of patients infected with T. cruzi. Unfortunately, the two compounds which have served as the principal antiparasitic drugs for Chagas' disease, benznidazole and nifurtimox, are highly toxic and fail to cure most patients with chronic disease (8). Better drugs are urgently needed to treat patients with Chagas' disease.
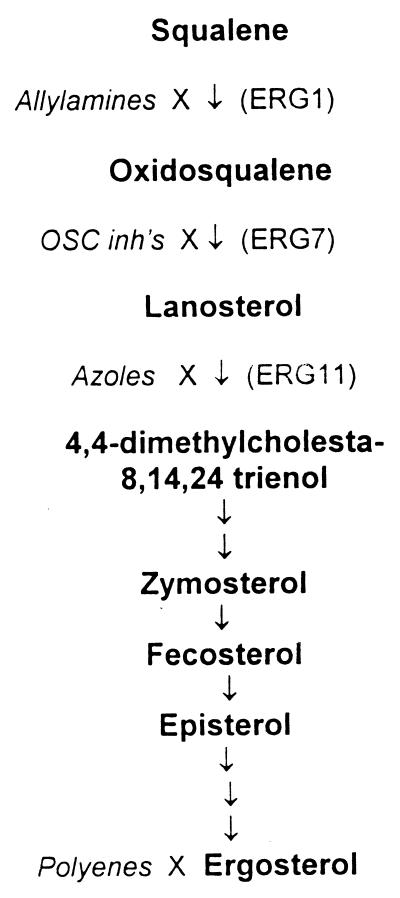
Sterol biosynthesis is a proven target for antimicrobial chemotherapy. For example, most effective antifungal agents act on sterol biosynthesis enzymes or their products. Some of these drugs, including azole antifungals, have been shown to have potent effects against T. cruzi (4, 9–11, 19, 23, 25). Itraconazole has entered the clinical arena for treating patients with Chagas' disease, showing parasitologic cure rates of 53% (3). The azole drugs (itraconazole, ketoconazole, etc.) target the lanosterol C14-demethylase enzyme in the ergosterol biosynthesis pathway (Fig. 1). Azole drugs cause the accumulation of 14α-methylsterols and decreased production of ergosterol (25). The allylamine antifungal drug terbinafine inhibits squalene epoxidase in the sterol biosynthesis pathway (Fig. 1). Terbinafine was shown to be synergistic with ketoconazole against cultures of T. cruzi (17, 24). The polyene antifungal drug amphotericin B works by directly associating with ergosterol to disrupt the integrity of the cell membrane. Amphotericin B and its liposomal preparations have potent anti-T. cruzi activities (14, 31).
FIG. 1.
Biosynthesis of ergosterol as characterized in yeast. The pathway shows the major intermediates (bold characters) and enzymes by their yeast designations (ERG1, squalene epoxidase; ERG7, oxidosqualene cyclase; ERG11, C14-demethylase). Drug classes that act on selected sites in the pathway are shown in italics.
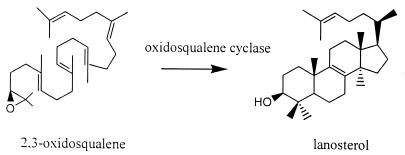
The synthesis of lanosterol is an essential step in the production of mature sterols. In yeast and higher eukaryotes (including humans), oxidosqualene cyclase (OSC) directly catalyzes the synthesis of lanosterol from 2,3-oxidosqualene (Fig. 2). This is a remarkably complex cyclization-rearrangement reaction involving the formation of a total of six new carbon-carbon bonds by a single enzyme (2). Inhibitors of OSC are under investigation as potential antifungal drugs (12) and cholesterol-lowering drugs (1, 6). One series of OSC inhibitors was designed as electron-poor aromatic mimics of a monocyclized transition state or high-energy intermediate formed from oxidosqualene (21). In this paper, we report that these compounds have potent activities against T. cruzi and inhibit sterol biosynthesis in these organisms.
FIG. 2.
Conversion of 2,3-oxidosqualene to lanosterol.
MATERIALS AND METHODS
Test compounds.
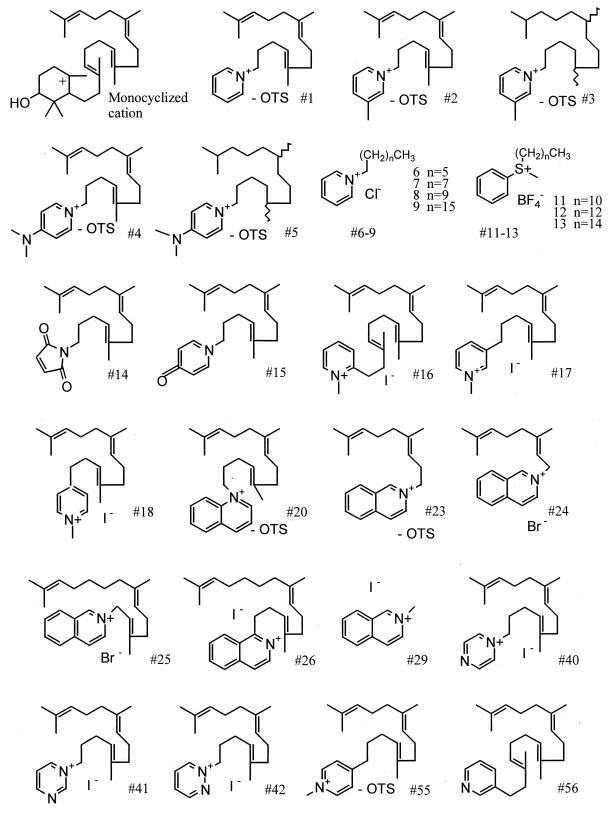
Benznidazole (Rochagan; Roche Pharmaceuticals, Rio de Janeiro, Brazil) was extracted from tablets with methanol-CHCl3 (1:1) and further purified via flash silica gel chromatography. OSC inhibitor no. 1 (N-[4E, 8E]-5,9, 13-trimethyl-4,8, 12-tetradecatrien-1-ylpyridinium) and no. 2 to 9 were synthesized as previously reported (21). The synthesis methods of other compounds are available from J. Griffin. The structures of selected compounds are shown in Fig. 3. All compounds were dissolved in dimethyl sulfoxide. The final concentration of dimethyl sulfoxide did not exceed 0.2%, a concentration which did not alter cell growth.
FIG. 3.
Structures of OSC inhibitors and the monocyclized cationic intermediate formed in the cyclization of oxidosqualene to lanosterol. OTS, tosylate.
Parasites and culture procedures.
The Tulahuen strain of T. cruzi (22) that expresses the Escherichia coli β-galactosidase gene was described previously (5). Peru and Sonya strains of T. cruzi (20) were kindly provided by S. Croft (London School of Hygiene and Tropical Medicine, London, United Kingdom). The VL2067 strain (from Minas Gerais, Brazil) was kindly provided by J. Peralta (Federal University, Rio de Janeiro, Brazil). Epimastigotes were grown in liver infusion tryptone broth with 10% fetal bovine serum, penicillin, and streptomycin as previously described (27). Amastigotes were grown at 37°C in monolayers of murine 3T3 fibroblasts in RPMI 1640 (Biowhittaker Inc., Walkersville, Md.) with 10% fetal bovine serum, penicillin, streptomycin, and glutamine as previously described (27).
Transfection and cloning of parasites.
In order to perform drug screening assays using a colorimetric method (5), we transfected the Peru, Sonya, and VL2067 strains with the E. coli lacZ gene. The plasmid (pBS:CL-Neo-01-BC-LacZ-10) was first linearized for integrative transformation, and 5 μg of DNA was electroporated into epimastigotes as previously described (7). Transfectants were selected by growth in G418 (Gibco BRL, Rockville, Md.) at 500 μg/ml. The drug-resistant population of epimastigotes was cloned by limiting dilution. Clones were tested for the ability to catalyze the colorimetric reaction with the substrate, chlorophenolred-β-d-galactopyranoside (CPRG; Boehringer-Mannheim, Indianapolis, Ind.) (5). The clones were transformed into mammalian-stage parasites by inoculation of the culture onto monolayers of 3T3 fibroblasts at 37°C. After approximately one week, intracellular amastigotes were visible microscopically, and shortly thereafter the host cells spontaneously lysed and released trypomastigotes. These trypomastigotes were used to infect subsequent monolayers of fibroblasts. β-Galactosidase expressing T. cruzi clones that were observed to have essentially the same growth rate in fibroblasts as untransfected parasites were used for subsequent experiments.
Growth inhibition assay of mammalian stages of T. cruzi.
Drug screening against β-galactosidase expressing strains of T. cruzi was performed as described previously (5). The assays were performed in 96-well tissue culture plates (Costar, Cambridge, Mass.). 3T3 fibroblasts were inoculated at 103/well using RPMI 1640 without phenol red (Biowhittaker Inc.) plus 10% fetal bovine serum and glutamine. The next day, the plates were seeded with 3T3-derived trypomastigotes at 104/well. After 4 h, drugs were added in serial dilutions to give a final volume of 200 μl/well. The plates were incubated at 37°C in 5% CO2 atmosphere for 7 days. At this time, CPRG (100 μM final) and Nonidet P-40 (0.1% final) (Sigma Chemical Co., St. Louis, Mo.) were added and the plates were incubated at 37°C for approximately 4 h. Wells with β-galactosidase activity turned the media from yellow to red, and this was quantified on an enzyme-linked immunosorbent assay reader at A570. To learn whether drugs inhibited the growth of mammalian host cells, control wells containing 3T3 fibroblasts and drug were incubated for 3 days and then developed with AlamarBlue (Alamar Biosciences Inc., Sacramento, Calif.) (5).
Analysis of sterol products.
T. cruzi epimastigotes (1 × 107 in 1 ml of culture medium) were incubated for 24 h with 100 μCi of RS[5-3H]mevalonolactone (American Radiolabel Chemicals, St. Louis, Mo.) with or without inhibitors. The cells were centrifuged and washed once in phosphate-buffered saline. The pellets were resuspended in 1 ml of chloroform-methanol (2:1) and agitated for 3 h at room temperature. The sample was reduced to approximately 50 μl under a stream of N2 gas. The remaining solution was extracted twice with petroleum ether (1 ml per extraction), and the sample was dried down under N2 gas in glass vials. The sample was then resuspended in 50 μl of chloroform-methanol and spotted on silica gel thin-layer chromatography (TLC) plates. The plates were developed with toluene-dethyl ether (9:1), dried, sprayed with EN3HANCE (NEN, Boston, Mass.), and subjected to autoradiography. The standard, 2,3,22,23-dioxidosqualene, was kindly provided by S. Matsuda (Rice University, Houston, Tex.). This compound was visualized on the TLC plate with iodine vapor.
Statistical analyses.
Effective concentrations causing 50% growth inhibition (EC50) were calculated by nonlinear regression analysis using the statistical software program Prism (GraphPad Software Inc., San Diego, Calif.).
RESULTS
Anti-T. cruzi activities of inhibitors.
Twenty-eight new compounds, ketoconazole, and benznidazole were tested against mammalian stages (amastigotes and trypomastigotes) of T. cruzi grown in coculture with murine 3T3 fibroblasts. All compounds were tested against the Tulahuen strain, and selected compounds were tested against mammalian-stage parasites of the Sonya, Peru, and VL2067 strains (Table 1.) Twelve compounds had EC50s of ≤25 nM against the Tulahuen strain (compounds no. 1, 2, 3, 4, 5, 13, 16, 17, 18, 20, 25, and 55). By comparison, the EC50 for benznidazole against Tulahuen T. cruzi was 300 nM. (Benznidazole kills T. cruzi by a mechanism other than affecting sterol biosynthesis.) The sterol biosynthesis inhibitor ketoconazole was about 10-fold more potent against T. cruzi than the best OSC inhibitors that were tested. There was a general correlation between the observed EC50 values and toxicity towards murine 3T3 fibroblasts; however, the concentration of OSC inhibitors that was toxic to fibroblasts was generally more than 100 times the concentration that inhibited the mammalian-stage parasites. The epimastigote forms of Tulahuen T. cruzi were found to be relatively resistant to sterol biosynthesis inhibitors, with EC50 values (after 5 days in culture) of 2 μM for OSC inhibitor no. 1 and 1 μM for ketoconazole, respectively (data not shown). When free T. cruzi trypomastigotes were suspended in RPMI plus 10% fetal bovine serum at 37°C in the presence of compound no. 1, the slender shape of the parasites changed to rounded morphologies after 4 to 6 h and by 24 h, most of the cells had disintegrated. In contrast, trypomastigotes treated with ketoconazole were normal appearing for the first 24 h and slowly died after 3 to 4 days.
TABLE 1.
EC50 values for OSC inhibitors against four strains of T. cruzi and mammalian host cells.a
| OSC inhibitor (no.) | EC50 (nM) against:
|
||||
|---|---|---|---|---|---|
|
T. cruzi strains
|
Murine 3T3 fibroblasts | ||||
| Tulahuen | Sonya | Peru | VL2067 | ||
| 1 | 20 | 46 | 30 | 130 | 2,000 |
| 2 | 9 | 1,500 | |||
| 3 | 14 | 16 | 9.8 | 45 | 1,500 |
| 4 | 12 | 1,300 | |||
| 5 | 2 | 4 | 1 | 6 | 880 |
| 6 | 21,500 | 50,000 | |||
| 7 | 591 | 15,000 | |||
| 8 | 97 | 1,800 | |||
| 9 | 24 | 84 | 37 | 158 | 600 |
| 11 | 256 | 4,100 | |||
| 12 | 40 | 109 | 33 | 343 | 1,400 |
| 13 | 25 | 95 | 17 | 127 | 670 |
| 14 | 1,830 | 100,000 | |||
| 15 | 2,190 | 25,000 | |||
| 16 | 11 | 520 | |||
| 17 | 10 | 22 | 25 | 29 | 3,000 |
| 18 | 12 | 700 | |||
| 20 | 2 | 10 | 2.9 | 15 | 500 |
| 23 | 714 | 6,400 | |||
| 24 | 56 | 5,500 | |||
| 25 | 3 | 9.6 | 4.9 | 9 | 600 |
| 26 | 16,000 | 18,000 | |||
| 29 | 100,000 | >100,000 | |||
| 40 | 714 | 10,400 | |||
| 41 | 1,915 | 23,400 | |||
| 42 | 104 | 4,100 | |||
| 55 | 25 | 30 | 17 | 44 | 1,000 |
| 56 | 1,750 | 45,000 | |||
| Ketoconazole | 0.2 | 0.3 | 0.2 | 0.8 | 40,000 |
| Benznidazole | 300 | 660 | 110 | 1,050 | 200,000 |
Experiments were repeated at least once for all compounds against the Tulahuen strain with very similar results.
Analysis of sterol products.
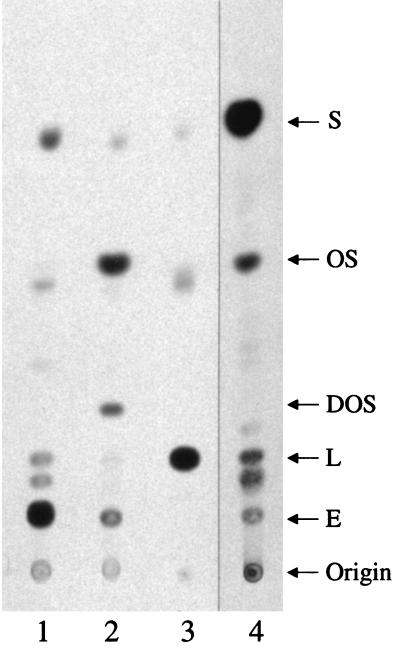
In order to determine whether the test compounds inhibited the target enzyme in T. cruzi, OSC, we analyzed the endogenous sterols of parasites grown with or without drug. The analysis depended on the incorporation of the radiolabeled substrate, [3H]mevalonolactone, into the sterols of live cells. It was necessary to analyze free-growing epimastigotes since the substrate is utilized by the mammalian host cells in which the mammalian-stage parasites grow. After 24 h, growth of the parasites as compared with the untreated control was as follows: OSC inhibitor no. 1 (5 μM), 47%; ketoconazole (25 μM), 80%. TLC analysis shows that OSC inhibitor no. 1 led to the accumulation of oxidosqualene and a dramatic reduction in lanosterol and ergosterol when compared to untreated epimastigotes (Fig. 4). In addition, a new product appeared between the oxidosqualene spot and the lanosterol spot. This product is superimposed on standard dioxidosqualene when cospotted on the same plate, indicating its probable identity as 2,3,22,23-dioxidosqualene (Rf = .28). Epimastigotes treated with ketoconazole (which blocks lanosterol C14-demethylase) led to accumulation of product comigrating with lanosterol and a complete depletion of ergosterol.
FIG. 4.
TLC of cellular sterols from T. cruzi epimastigotes grown without drugs (lane 1) or with 5 μM OSC inhibitor no. 1 (lane 2) or with 25 μM ketoconazole (lane 3). The parasites were grown for 24 h in the presence of [3H]mevalonalactone and the indicated drugs. Sterols from a Candida albicans extract and their identification are shown for comparison (lane 4). (Lane 4 was separated from lane 3 by three intervening lanes that were removed from the figure for simpler display.) Note the reduction in ergosterol in parasites treated with the OSC inhibitor or ketoconazole. S, squalene; OS, 2,3-oxidosqualene; DOS, dioxidosqualene; L, lanosterol; E, ergosterol. The digital image was produced with Adobe Photoshop 5.0.
Synergy studies with OSC inhibitor and ketoconazole.
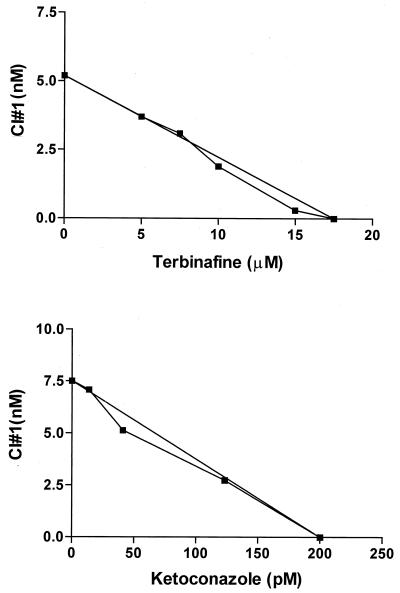
Growth inhibition assays with various concentrations of OSC inhibitor no. 1 and ketoconazole or terbinafine (checkerboard setup) were performed with mammalian-stage Tulahuen T. cruzi. Since these compounds act on separate enzymes in sterol biosynthesis, it was hypothesized they might act synergistically against cultured parasites. The results show that the drugs had additive but not synergistic effects on the parasites (Fig. 5).
FIG. 5.
Isobologram of OSC inhibitor no. 1 and ketoconazole or terbinafine against mammalian-stage T. cruzi. CI, cyclase inhibitor.
DISCUSSION
A series of 28 compounds designed to inhibit oxidosqualene cyclases (21) were tested for in vitro activity against four strains of T. cruzi (Tulahuen, Sonya, Peru, and VL2067). Twelve of these compounds had an EC50 of ≤25 nM against the mammalian stages of the parasites, which is more than 10-fold more potent in vitro than the established antitrypanosomal drug, benznidazole. The most potent oxidosqualene mimics were approximately 10-fold less potent than the azole drug, ketoconazole (Table 1). Most of the compounds demonstrated selective activity against parasites over mammalian host cells; e.g., compound no. 1 was toxic to the parasites at a concentration >100 times lower than the concentration at which it inhibited 3T3 fibroblasts (Table 1). The greater sensitivity of parasite cells suggests either greater inhibition of the parasite enzyme than the mammalian enzyme or greater susceptibility of T. cruzi to the consequences of OSC inhibition. The drugs were active against four diverse strains of T. cruzi, indicating the effectiveness was not strain specific.
Compound no. 1 contains a pyridinium ion with an alkyl side chain resembling squalene. This structure mimics a monocyclized cationic intermediate or transition state formed in the cyclization of oxidosqualene to lanosterol (Fig. 3) (21). Compounds containing a pyridinium ion were typically potent against the live parasites. Similarly, the isoquinoline derivatives (no. 24 and 25) and the thioether derivatives (no. 11 to 13) had excellent activity. Structures with aromatic rings with keto groups (e.g., no. 14 and 15) were considerably less potent, as were compounds with additional nitrogen atoms in the aromatic ring (no. 40 to 42). The length of the alkyl side chain was also important for potency against the live parasites. For example, compound no. 6 with a 6-carbon side chain was about 1/1,000 as potent as the comparable structure (no. 9) with a 16-carbon side chain. Also, compound no. 29 (isoquinoline with a one-carbon side group) had very little activity compared to other isoquinoline derivatives (no. 23 to 25) with longer side chains. These observations fit with the hypothesis that these compounds mimic oxidosqualene and block the activity of OSC in live T. cruzi.
Further support for the target of action of the compounds was provided by the analysis of sterols produced in live epimastigotes. The analysis demonstrated that compound no. 1 inhibited the production of lanosterol and further downstream sterols, as would be predicted if OSC was blocked. In addition, there was an accumulation of the substrate oxidosqualene as well as a new product that migrated with an Rf value similar to that of 2,3,22,23-dioxidosqualene. This metabolite has been shown to accumulate when OSC activity is inhibited (12, 15). This metabolite probably is formed by the action of squalene epoxidase on the accumulating 2,3-oxidosqualene. It was not possible to test the effects of OSC inhibitors on growing mammalian-stage parasites because the radiolabeled substrate gets incorporated into the host cells, complicating the analysis. However, since sterol biosynthesis occurs in mammalian-stage T. cruzi (18), it seems probable that the test compounds inhibit OSC in the mammalian stage as well. The greater sensitivity of the mammalian-stage than insect-stage (epimastigote) parasites was a consistent finding for three different classes of drugs tested (OSC inhibitors, ketoconazole, and benznidazole) and may reflect their decreased ability to penetrate epimastigotes.
Epimastigotes treated with OSC inhibitor no. 1 had less growth (47% of control) than did parasites treated with ketoconazole (80% of control) at 24 h even though ergosterol synthesis was more completely inhibited by ketoconazole (see Fig. 4). This suggests the possibility that the cause of parasite cell death with the OSC inhibitors may not be due solely to ergosterol depletion but rather may include another mechanism, such as the accumulation of a toxic byproduct (e.g., 2,3,22,23-dioxidosqualene) or concomitant membrane disruption. Membrane effects are supported by the observation that trypomastigotes cultured in the presence of OSC inhibitor developed altered morphology and died more rapidly than did trypomastigotes cultured with ketoconazole.
Experiments to detect synergy between OSC inhibitor no. 1 and ketoconazole or terbinafine were done because other drugs acting in combination on sterol biosynthesis have been shown to synergistically inhibit growth of trypanosomatid parasites (26, 13). The lack of synergy between OSC inhibitor no. 1 and ketoconazole or terbinafine (Fig. 5) raises interesting questions. In yeast there is evidence that azole drugs act, at least in part, by causing the accumulation of abnormal sterols that disrupt cell membrane integrity (29). It is possible that the action of OSC inhibitor to deplete lanosterol (the substrate of ketoconazole) may lead to reduced accumulation of the abnormal sterols that mediate the effects of ketoconazole. Thus the OSC inhibitor might partly undermine the mechanism of action of azoles. Similarly, terbinafine did not act synergistically with OSC inhibitor no. 1. If the OSC inhibitor's antiparasitic effects occur by the generation of a toxic byproduct, then one would predict that a compound acting upstream in the pathway (i.e., terbinafine) would reduce the substrate which gets converted into the toxic byproduct and thus detract from the activity of the OSC inhibitor. The fact that the action of the OSC inhibitors and the other sterol biosynthesis inhibitors was additive (and not antagonistic) allows for the possibility to use such compounds in combination. It also remains possible that even though the study compounds were shown to inhibit oxidosqualene cyclase in T. cruzi, the compounds might also act on the parasites by another unknown mode of action.
In summary, this paper describes potent in vitro activities of a series of oxidosqualene cyclase inhibitors against four strains of T. cruzi. The findings further validate sterol biosynthesis as a target for anti-T. cruzi chemotherapy (23). Additional studies will determine the effectiveness of these compounds in animal models of Chagas' disease. This paper also reports the creation of three new strains of T. cruzi (in addition to the Tulahuen strain previously reported) that carry the recombinant gene for expression of β-galactosidase.
ACKNOWLEDGMENTS
We acknowledge Constanze Brocke, Clemens Dertelt, Iris Escher, Roger Lee, YonQi Mu, Ed Olhava, Ingo Rose, Bradley Sharpe, Joyce Siregar, and Nuria Tamayo for synthesizing the test compounds. We thank Jerry Bressi for purifying benznidazole from tablets. We thank Seiichi Matsuda for providing dioxidosqualene. We are grateful for excellent technical assistance from Lisa Nguyen and Lynn Barrett.
This research was supported by National Institutes of Health grant AI01258 to F.S.B. and National Institutes of Health grant AI01023 and American Heart Association grant-in-aid to A.J.W. and W.C.V.V. Support to J.G. came from the National Science Foundation (CHE-9018241), the Arnold and Mabel Beckman Foundation, the California affiliate of the American Heart Association, and the Alfred P. Sloan Foundation.
REFERENCES
- 1.Abe I, Prestwich G D. Squalene epoxidase and oxidosqualene: lanosterol cyclase—key enzymes in cholesterol biosynthesis. In: Cane D E, editor. Comprehensive natural products chemistry. New York, N.Y: Elsevier; 1999. pp. 267–298. [Google Scholar]
- 2.Abe I, Rohmer M, Prestwich G D. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem Rev. 1993;93:2189–2206. [Google Scholar]
- 3.Apt W, Aguilera X, Arribada A, Perez C, Miranda C, Sanchez G, Zulantay I, Cortes P, Rodriguez J, Juri D. Treatment of chronic Chagas' disease with itraconazole and allopurinol. Am J Trop Med Hyg. 1998;59:133–138. doi: 10.4269/ajtmh.1998.59.133. [DOI] [PubMed] [Google Scholar]
- 4.Brener Z. An experimental and clinical assay with ketoconazole in the treatment of Chagas disease. Mem Inst Oswaldo Cruz. 1993;88:149–153. doi: 10.1590/s0074-02761993000100023. [DOI] [PubMed] [Google Scholar]
- 5.Buckner F S, Verlinde C L M J, La Flamme A C, Van Voorhis W C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattel L, Ceruti M, Viola F, Delprino L, Balliano G, Duriatti A, Bouvier-Nave P. The squalene-2,3-epoxide cyclase as a model for the development of new drugs. Lipids. 1986;21:31–38. doi: 10.1007/BF02534300. [DOI] [PubMed] [Google Scholar]
- 7.Chung S H, Gillespie R D, Swindle J. Analyzing expression of the calmodulin and ubiquitin-fusion genes of Trypanosoma cruzi using simultaneous, independent dual gene replacements. Mol Biochem Parasitol. 1994;63:95–107. doi: 10.1016/0166-6851(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 8.Croft S L. The current status of antiparasitic chemotherapy. Parasitology. 1997;114:S3–S15. [PubMed] [Google Scholar]
- 9.Docampo R. Biochemical and ultrastructural alterations produced by miconazole and econazole in Trypanosoma cruzi. Mol Biochem Parasitol. 1981;3:169–180. doi: 10.1016/0166-6851(81)90047-5. [DOI] [PubMed] [Google Scholar]
- 10.Docampo R, Schmunis G A. Sterol biosynthesis inhibitors: potential chemotherapeutics against Chagas disease. Parasitol Today. 1997;13:129–130. doi: 10.1016/s0169-4758(97)01021-1. [DOI] [PubMed] [Google Scholar]
- 11.Goad L J, Berens R L, Marr J J, Beach D H, Holz G G. The activity of ketoconazole and other azoles against Trypanosoma cruzi: biochemistry and chemotherapeutic action in vitro. Mol Biochem Parasitol. 1989;32:179–190. doi: 10.1016/0166-6851(89)90069-8. [DOI] [PubMed] [Google Scholar]
- 12.Goldman R C, Zakula D, Capobianco J O, Sharpe B A, Griffin J H. Inhibition of 2,3-oxidosqualene-lanosterol cyclase in Candida albicans by pyridinium ion-based inhibitors. Antimicrob Agents Chemother. 1996;40:1044–1047. doi: 10.1128/aac.40.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haughan P A, Chance M L, Goad L J. Synergism in vitro of lovastatin and miconazole as anti-leishmanial agents. Biochem Pharm. 1992;44:2199–2206. doi: 10.1016/0006-2952(92)90347-l. [DOI] [PubMed] [Google Scholar]
- 14.Horvath A E, Zierdt C H. The effect of amphotericin B on Trypanosoma cruzi in vitro and in vivo. J Trop Med Hyg. 1974;77:144–149. [PubMed] [Google Scholar]
- 15.Kelly R, Miller S M, Lai M H, Kirsch D R. Cloning and characterization of the 2,3-oxidosqualene cyclase-coding gene of Candida albicans. Gene. 1990;87:177–183. doi: 10.1016/0378-1119(90)90299-7. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhoff L V. American trypanosomiasis (Chagas' disease)—a tropical disease now in the United States. N Engl J Med. 1993;329:639–644. doi: 10.1056/NEJM199308263290909. [DOI] [PubMed] [Google Scholar]
- 17.Lazardi K, Urbina J A, de Souza W. Ultrastructural alterations induced by two ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1990;34:2097–2105. doi: 10.1128/aac.34.11.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liendo A, Visbal G, Piras M M, Piras R, Urbina J A. Sterol composition and biosynthesis in Trypanosoma cruzi amastigotes. Mol Biochem Parasitol. 1999;104:81–91. doi: 10.1016/s0166-6851(99)00129-2. [DOI] [PubMed] [Google Scholar]
- 19.McCabe R E, Remmington J S, Araujo F G. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. Am J Trop Med Hyg. 1986;35:280–284. doi: 10.4269/ajtmh.1986.35.280. [DOI] [PubMed] [Google Scholar]
- 20.Neal R A, Van Bueren J. Comparative studies of drug susceptibility of five strains of Trypanosoma cruzi in vivo and in vitro. Trans R Soc Trop Med Hyg. 1988;82:709–714. doi: 10.1016/0035-9203(88)90208-8. [DOI] [PubMed] [Google Scholar]
- 21.Rose I C, Sharpe B A, Lee R C, Griffin J H, Capobianco J O, Zakula D, Goldman R C. Design, synthesis and in vitro evaluation of pyridinium ion based cyclase inhibitors and antifungal agents. Bioorg Med Chem. 1996;4:97–103. doi: 10.1016/0968-0896(95)00177-8. [DOI] [PubMed] [Google Scholar]
- 22.Taliaferro W H, Pizzi T. Connective tissue reaction in normal and immunized mice to a reticulotropic strain of Trypanosoma cruzi. J Infect Dis. 1954;96:199–207. doi: 10.1093/infdis/96.3.199. [DOI] [PubMed] [Google Scholar]
- 23.Urbina J A. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology. 1997;114:S91–S99. [PubMed] [Google Scholar]
- 24.Urbina J A, Lazardi K, Aguirre T, Piras M M, Piras R. Antiproliferative synergism of the allylamine SF 86–327 and ketoconazole on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1988;32:1237–1242. doi: 10.1128/aac.32.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbina J A, Vivas J, Ramos H, Larralde G, Aguilar Z, Avilan L. Alteration of lipid order profile and permeability of plasma membranes from Trypanosoma cruzi epimastigotes grown in the presence of ketoconazole. Mol Biochem Parasitol. 1988;30:185–196. doi: 10.1016/0166-6851(88)90111-9. [DOI] [PubMed] [Google Scholar]
- 26.Urbina J A, Vivas J, Visbal G, Contreras L M. Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes by delta 24(25)-sterol methyl transferase inhibitors and their combinations with ketoconazole. Mol Biochem Parasitol. 1995;73:199–210. doi: 10.1016/0166-6851(95)00117-j. [DOI] [PubMed] [Google Scholar]
- 27.Van Voorhis W C, Eisen H. FL-160: a surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J Exp Med. 1989;169:641–652. doi: 10.1084/jem.169.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas' disease with benznidazole: clinical and serological evolution of patients with long-term follow-up. Am Heart J. 1994;127:151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 29.Watson P F, Rose M E, Ellis S W, England H, Kelly S L. Defective sterol C5–6 desaturation and azole resistance: a new hypothesis for the mode of action of azole antifungals. Biochem Biophys Res Commun. 1989;164:1170–1175. doi: 10.1016/0006-291x(89)91792-0. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Control of Chagas' disease. WHO Tech Rep Ser. 1991;811:1–93. [PubMed] [Google Scholar]
- 31.Yardley V, Croft S L. In vitro and in vivo activity of amphotericin B-lipid formulations against experimental Trypanosoma cruzi infections. Am J Trop Med Hyg. 1999;61:193–197. doi: 10.4269/ajtmh.1999.61.193. [DOI] [PubMed] [Google Scholar]