Abstract
Background
During the last two decades, researchers have suggested that the changes of malaria cases in African highlands were driven by climate change. Recently, a study claimed that the malaria cases (Plasmodium falciparum) in Oromia (Ethiopia) were related to minimum temperature. Critics highlighted that other variables could be involved in the dynamics of the malaria. The literature mentions that beyond climate change, trends in malaria cases could be involved with HIV, human population size, poverty, investments in health control programmes, among others.
Methods
Population ecologists have developed a simple framework, which helps to explore the contributions of endogenous (density-dependent) and exogenous processes on population dynamics. Both processes may operate to determine the dynamic behaviour of a particular population through time. Briefly, density-dependent (endogenous process) occurs when the per capita population growth rate (R) is determined by the previous population size. An exogenous process occurs when some variable affects another but is not affected by the changes it causes. This study explores the dynamics of malaria cases (Plasmodium falciparum and Plasmodium vivax) in Oromia region in Ethiopia and explores the interaction between minimum temperature, HIV, poverty, human population size and social instability.
Results
The results support that malaria dynamics showed signs of a negative endogenous process between R and malaria infectious class, and a weak evidence to support the climate change hypothesis.
Conclusion
Poverty, HIV, population size could interact to force malaria models parameters explaining the dynamics malaria observed at Ethiopia from 1985 to 2007.
Keywords: Malaria dynamics, Climate, HIV, Poverty, Social instability, Population size
Background
During the last two decades, researchers have suggested that the increase of the malaria burden was driven by climate change [1–8]. These studies lead to extensive debates about the importance of climate in the malaria burden in these locations [9–19]. Despite the evident relationship between malaria and climate, principally between seasons, other variables could be involved in the increase of the malaria burden. Globally, the literature suggests that the positive trends in malaria could also be affected by the spread of the Human Immunodeficiency Virus (HIV) [20–24], poverty level [25–27], health campaigns [27–30] and human population size [31, 32]. Alonso et al. [6] have suggested that although temperature was the main driver of malaria dynamics in Kericho tea plantations, temperature could interact with rural population size and HIV prevalence. A recent study [19] found that the HIV incidence together with rural population size could also influenced malaria dynamics in Kericho district in Kenya suggesting that climate had a negligeable effect on malaria dynamics.
In anoher recent study [16], authors have claimed that the dynamics of the monthly numbers of malaria (Plasmodium falciparum) cases were closely related to changes in minimum temperature levels in the region Oromia in Ethiopia, which increased until 1998, them, stabilized and declined from 2000 to 2005 (Fig. 1). In 2005, Ethiopia joined in a global campaign to eradicate malaria [16]. Hence, the authors suggested that the reduction of both malaria cases and minimum temperature (2000–2005) were linked and climate could act synergically with malaria interventions from 2005 on. In resume, climate was the main driver of malaria dynamics in Ethiopia prior the introduction of control programmes. In this study, the data was re-analysed from 1985 to 2007 from the study [16] testing the temperature effects and other variables using inter-annual (annual averages of monthly minimum temperatures and malaria cases) data for malaria dynamics in Ethiopia.
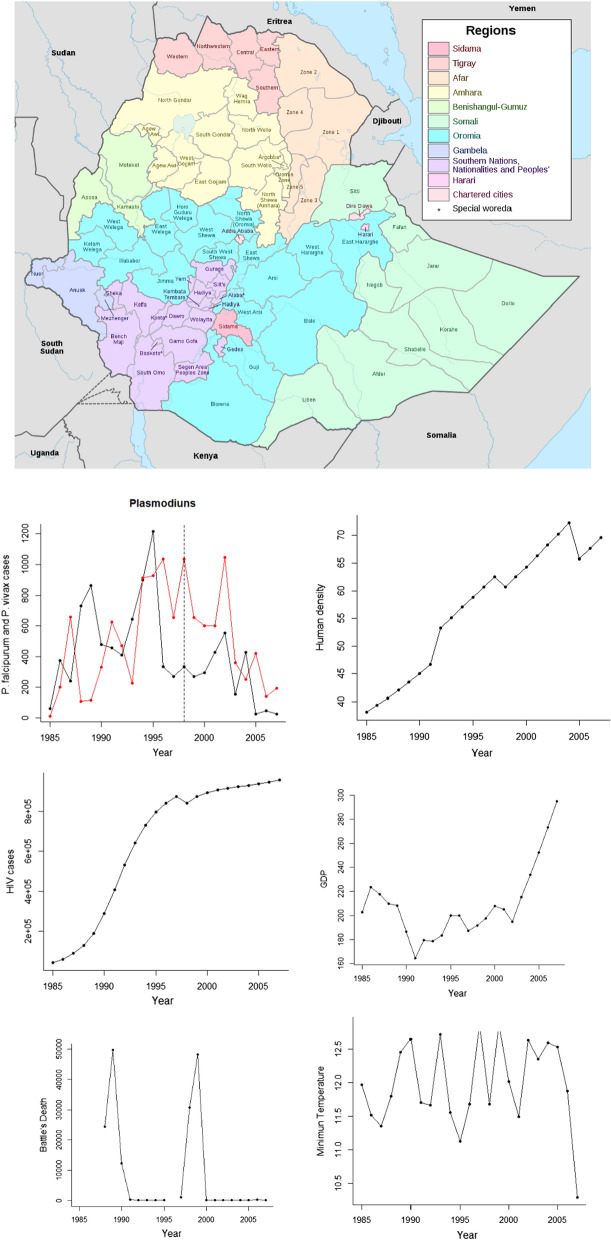
Fig. 1.
Location of Oromia zone (in blue inside Ethiopia). The site was the Debre Zeit are near of Addis Abada, with meteorological stations ranging from 1600 to 2500 m above sea level. Annual data for Plasmodiums annual cases (P. vivax in red, vertical dash line separates two chronological periods claimed in the study 2020), HIV new cases, Per capita GDP, Human density, annual Minimum temperature and death related to civil conflicts as a proxy of social instability. Map was taken from (Sarah Vaughan, Archived August 13, 2011), at the Wayback Machine (University of Edinburgh: Ph.D. Thesis, 2003), p. 240 n. 259)
After three decades of decline at global level, around 1980, malaria started to rise and once again became a major health problem, principally in Africa. During the same period (the 1980s decade), HIV started to spread globally [19, 25, 26]. HIV may increase malaria contagion, accelerate progression rates from latent to infectious stage and may delay the recovery of infected individuals, which increases the period in which an infected individual may transmit malaria. In addition, individuals only acquire malaria immunological defenses following several episodes. By affecting the immunological system, HIV removes the acquired defenses (i.e. herd immunity) increasing the susceptible pool (the population at risk of infection) [33, 34].
The increase of human population density (rural and urban) may affect malaria dynamics through reducing the distance between infected and susceptible individuals. Also, the increase of population size expands agriculture and urban frontiers over natural rural areas [19, 35, 36]. This expansion requires deforestation. Deforestation changes the Anopheles spp. aquatic food web structure, usually freeing larvae from predators [35]. Deforestation may also increase the number of mosquito breading sites and accelerates the mosquito life cycle by means of an increase in local temperature, resulting in high mosquito population biomass and malaria transmission in rural areas and cities [31, 32, 35, 36], like in the Oromia district in Ethiopia.
Ethiopia is a low-income country composed of ethno-regions (districts). Poverty and political instability, including civil war (between districts) could also affect diseases dynamics and/or epidemic episodes. Malaria, HIV and many others diseases are related to poverty. Difficult access, low-quality health system and inefficient control programmes results in high diseases’ burdens. Social and political instabilities (like civil conflicts) may also impact diseases by concentrating refugees in low sanitary conditions. Social conflicts (e.g. civil wars) impact food production and drain resources from potential disease control programmes, increasing undernourishment and individual’s susceptibility to diseases [37, 38].
Ethiopan malaria scenario may be more influenced by other variables than minimum temperature. The aim of this study is to show that other variables than minimum temperature could be involved in the malaria dynamics in Ethiopia, from 1985 to 2007. Additionally, the dynamics of Plasmodium vivax were considered, which were lacking from the [16] study despite of available data.
Methods
Population ecologists have developed a simple framework, which helps to explore the contributions of endogenous and exogenous processes on population dynamics. Briefly, density-dependent (endogenous process) occurs when the per capita population growth rate (R) is determined by population's previous size. An exogenous process occurs when some variable affects the population but is not affected by the changes it causes. Climate variables and governmental disease control policies are known examples of exogenous pulse/press perturbations with relation to diseases. Both, endogenous and exogenous processes may operate to determine the dynamic behavior of a particular population through time [39–41]. Therefore, a more complete understanding of the dynamics of a population is achieved when both endogenous and exogenous processes are considered. Using this framework, recent studies have captured the trends of measles, tuberculosis, HIV, malaria and pertussis at city, country and global scales [19, 42–45].
The ecological principles mentioned above have analogies in epidemiological processes. Following the introduction of an infected individual in a naïve population, the infected class (It) is expected to grow exponentially driven by R0, the basic reproductive number, since there are almost unlimited susceptible individuals (resources). Nevertheless, as the infected class grows, the susceptible pool is depleted and declines the per infected transmission rate. R0 becomes RE, the realized reproductive number, a process known as self-limiting, analogous to the principle of intra-specific competition [19, 42, 45]. Higher infected class may increase contact rate enhancing transmission rate, analogous to the intra-specific cooperation principle. The per capita population rate of change (R) is the corner stone of the framework adopted in this study [39–41]. R can be estimated by the natural log differences between actual and past numbers of infected individuals, which are adopted in this work.
The recent study of 2020 [16] suggested that the malaria dynamics in Oramia region (Ethiopia) showed distinct chronological domains, from 1985 to 1998 and from1998 to 2007. Hence, the time series from 1985–1998 and from 1998 to 2007 were re-analysed. In advance, the malaria dynamics showed signs of a negative correlation between RIt and It-1 (infected population size class) for both periods and Plasmodium spp. (see Table 1), which can be captured by the following Ricker model:
| 1 |
where RI is the per capita growth rate of the infected class, Rmax is maximum population rate of change (analogous to R0), It − 1 is the infected class (annual averages of malaria cases) in the previous year, K is the stable malaria incidence carrying capacity. Q is the pendent when RI is zero at the carrying capacity of infectious class and measures the degree intraspecific competition, self-limiting, around K.
Table 1.
The logistic model’s results for P. falciparum and P. vivax from 1985 to 1998 and from 1998 to 2007 without and with the lateral effects of per capita GDP (GDP), as a proxy of poverty, human density (Density), minimum temperature (Temperature), New HIV cases (HIV) and deaths related to civil conflicts (Battle)
| Pathogen | Period | Model | Variables | Parameters | R2 | AIC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rmax | K | Q | a | |||||||
| P. falciparum | 1985–1998 | 1 | 1.82 | 549.87 | 0.467 | 0.499 | 25.011 | |||
| P. falciparum | 1985–1998 | 2 | GDP | 1.94 | 1503.45 | 0.44 | − 4.87 | 0.501 | 24.147 | |
| P. falciparum | 1985–1998 | 3 | Density | 1.94 | 856.05 | 0.44 | − 5.934 | 0.483 | 24.605 | |
| P. falciparum | 1985–1998 | 4 | HIV | 1.94 | 633.6 | 0.44 | − 0.00016 | 0.438 | 24.58 | |
| P. falciparum | 1985–1998 | 5 | Temperature | 1.94 | 125.07 | 0.44 | 35.63 | 0.477 | 24.824 | |
| P. falciparum | 1985–1998 | 6 | Battle | 1.94 | 601.411 | 0.44 | − 0.0017 | 0.5766 | 15.728 | |
| P. falciparum | 1985–1998 | 7 | Temperature | GDP | 0.51 | 1588.33 | 0.44 | − 0.3799 | 0.65 | 28.262 |
| P. falciparum | 1985–1998 | 8 | Temperature | HIV | 0.51 | 92.63 | 2.27 | − 0.00019 | 0.734 | 14.662 |
| P. falciparum | 1985–1998 | 9 | Temperature | Density | 0.51 | 219.912 | 2.27 | 0.795 | 0.638 | 28.854 |
| P. falciparum | 1985–1998 | 10 | Temperature | Battle | 0.51 | 751.7 | 2.27 | − 0.00021 | 0.478 | 10.865 |
| P. falciparum | 1985–1998 | 11 | GDP | Density | 0.51 | 519.81 | 2.27 | 0.0182 | 0.624 | 29.206 |
| P. falciparum | 1985–1998 | 12 | GDP | HIV | 0.51 | 628.9 | 2.27 | 7.12E–06 | 0.631 | 29.059 |
| P. falciparum | 1985–1998 | 13 | GDP | Battle | 0.51 | 751.3 | 2.27 | − 1.2E–05 | 0.722 | 10.918 |
| P. falciparum | 1985–1998 | 14 | HIV | Density | 0.51 | 794.7 | 2.27 | 0.000291 | 0.421 | 13.449 |
| P. falciparum | 1985–1998 | 15 | HIV | Battle | 0.51 | 743.5 | 2.27 | − 2.2E–08 | 0.747 | 10.763 |
| P. falciparum | 1985–1998 | 16 | Density | Battle | 0.51 | 751.9 | 2.27 | − 0.00005 | 0.723 | 10.859 |
| P. vivax | 1985–1998 | 17 | 3.01 | 524 | 0.292 | 0.352 | 35.32 | |||
| P. vivax | 1985–1998 | 18 | GDP | 3.01 | 2143.56 | 0.319 | − 8.27 | 0.784 | 33.971 | |
| P. vivax | 1985–1998 | 19 | Density | 3.01 | − 498.96 | 0.319 | 19.82 | 0.849 | 31.683 | |
| P. vivax | 1985–1998 | 20 | HIV | 3.01 | 226.5 | 0.319 | 0.00059 | 0.867 | 30.662 | |
| P. vivax | 1985–1998 | 21 | Temperature | 3.01 | − 672.8 | 0.319 | 101.5 | 0.791 | 35.11 | |
| P. vivax | 1985–1998 | 22 | Battle | 3.01 | 629.06 | 0.319 | − 0.0054 | 0.507 | 23.015 | |
| P. vivax | 1985–1998 | 23 | Temperature | GDP | 3.01 | 1784.74 | 0.319 | − 0.546 | 0.79 | 34.762 |
| P. vivax | 1985–1998 | 24 | Temperature | Density | 3.01 | − 517.495 | 0.319 | 1.715 | 0.845 | 31.21 |
| P. vivax | 1985–1998 | 25 | Temperature | HIV | 3.01 | 224.9 | 0.319 | 5.05E–05 | 0.865 | 30.635 |
| P. vivax | 1985–1998 | 26 | Temperature | Battle | 3.01 | 627.2 | 0.319 | − 4.3E–05 | 0.548 | 23.054 |
| P. vivax | 1985–1998 | 27 | GDP | Density | 3.01 | − 249.573 | 0.319 | 0.077 | 0.805 | 33.489 |
| P. vivax | 1985–1998 | 28 | GDP | HIV | 3.01 | 225.3 | 0.319 | 0.000031 | 0.868 | 30.743 |
| P. vivax | 1985–1998 | 29 | GDP | Battle | 3.01 | 629.6 | 0.319 | − 2.7E–05 | 0.584 | 22.943 |
| P. vivax | 1985–1998 | 30 | HIV | Density | 3.01 | 186.2 | 0.319 | − 0.00022 | 0.313 | 14.311 |
| P. vivax | 1985–1998 | 31 | HIV | Battle | 3.01 | 657.7 | 0.319 | − 3.5E–07 | 0.343 | 17.232 |
| P. vivax | 1985–1998 | 32 | Density | Battle | 3.01 | 616.9 | 0.319 | − 0.00008 | 0.596 | 23.321 |
| P. falciparum | 1998–2007 | 33 | 18.01 | 72.46 | 0.094 | 0.369 | 31.429 | |||
| P. falciparum | 1998–2007 | 34 | GDP | 0.47 | − 614.852 | 2.061 | 4.38 | 0.611 | 29.549 | |
| P. falciparum | 1998–2007 | 36 | Density | 0.47 | 2710.63 | 2.061 | − 35.26 | 0.887 | 19.969 | |
| P. falciparum | 1998–2007 | 37 | HIV | 0.47 | 8708 | 2.061 | − 0.0091 | 0.942 | 14.247 | |
| P. falciparum | 1998–2007 | 38 | Temperature | 0.47 | 1743.2 | 2.061 | − 118.7 | 0.653 | 28.761 | |
| P. falciparum | 1998–2007 | 39 | Battle | 0.47 | 237.395 | 2.061 | 0.0101 | 0.383 | 26.384 | |
| P. falciparum | 1998–2007 | 40 | Temperature | GDP | 0.47 | 421.205 | 2.061 | − 0.062 | 0.575 | 30.109 |
| P. falciparum | 1998–2007 | 41 | Temperature | HIV | 0.47 | − 568.6 | 2.061 | 1.328 | 0.930 | 15.878 |
| P. falciparum | 1998–2007 | 42 | Temperature | Density | 0.47 | 2567.31 | 2.061 | − 2641 | 0.901 | 19.122 |
| P. falciparum | 1998–2007 | 43 | Temperature | Battle | 0.47 | 2373 | 2.061 | 0.00087 | 0.383 | 26.381 |
| P. falciparum | 1998–2007 | 44 | GDP | HIV | 0.47 | − 371.2 | 2.061 | 0.000062 | 0.882 | 20.291 |
| P. falciparum | 1998–2007 | 45 | GDP | Density | 0.47 | 2023.22 | 2.061 | − 0.125 | 0.705 | 27.531 |
| P. falciparum | 1998–2007 | 46 | GDP | Battle | 0.47 | 237.4 | 2.061 | 0.000052 | 0.383 | 26.384 |
| P. falciparum | 1998–2007 | 47 | HIV | Density | 0.47 | − 580.3 | 2.061 | 0.00023 | 0.941 | 15.345 |
| P. falciparum | 1998–2007 | 48 | HIV | Battle | 0.47 | 237.3 | 2.061 | 1.7E – 06 | 0.382 | 26.372 |
| P. falciparum | 1998–2007 | 49 | Density | Battle | 0.47 | 237.4 | 2.061 | 0.000152 | 0.384 | 26.388 |
| P. vivax | 1998–2007 | 50 | 0.5 | 185 | 0.094 | 0.401 | 20.519 | |||
| P. vivax | 1998–2007 | 51 | GDP | 0.5 | 1833.35 | 0.784 | − 7.306 | 0.661 | 17.219 | |
| P. vivax | 1998–2007 | 52 | HIV | 0.5 | − 357.1 | 0.784 | 0.0016 | 0.789 | 16.364 | |
| P. vivax | 1998–2007 | 53 | Density | 0.5 | 1379.7 | 0.784 | − 15.47 | 0.527 | 18.912 | |
| P. vivax | 1998–2007 | 54 | Temperature | 0.5 | 2822.9 | 0.784 | − 199.8 | 0.602 | 17.76 | |
| P. vivax | 1998–2007 | 55 | Battle | 0.5 | 203.054 | 0.784 | 0.247 | 0.406 | 13.341 | |
| P. vivax | 1998–2007 | 56 | Temperature | GDP | 0.5 | 2627.7 | 0.784 | − 0.908 | 0.822 | 13.245 |
| P. vivax | 1998–2007 | 57 | Temperature | Density | 0.5 | 1535.2 | 0.784 | − 1.451 | 0.590 | 18.046 |
| P. vivax | 1998–2007 | 58 | Temperature | HIV | 0.5 | 6059 | 0.784 | − 0.0005 | 0.757 | 14.159 |
| P. vivax | 1998–2007 | 59 | Temperature | Battle | 0.5 | 203.429 | 0.784 | 0.019 | 0.407 | 13.341 |
| P. vivax | 1998–2007 | 60 | GDP | Density | 0.5 | 1544.97 | 0.784 | − 0.089 | 0.660 | 17.324 |
| P. vivax | 1998–2007 | 61 | GDP | HIV | 0.5 | 1455 | 0.784 | − 6E–06 | 0.707 | 16.831 |
| P. vivax | 1998–2007 | 62 | GDP | Battle | 0.5 | 202.4 | 0.784 | 0.00129 | 0.406 | 13.368 |
| P. vivax | 1998–2007 | 63 | HIV | Density | 0.5 | − 452.9 | 0.784 | 0.00217 | 0.837 | 12.56 |
| P. vivax | 1998–2007 | 64 | HIV | Battle | 0.5 | 205.9 | 0.784 | 3.5E–0 6 | 0.407 | 13.283 |
| P. vivax | 1998–2007 | 65 | Density | Battle | 0.5 | 201.2 | 0.784 | 0.0041 | 0.407 | 13.323 |
The results suggest a weak effect of minimum temperature to malaria dynamics for all periods and species. Models were compared and selected for each period and for each species based on high R2 and lower AIC. From 1985 to 1998 for P. falciparum, the models 11, 13, 16 delivered the best results. For the same period and for P. vivax the models 25 and 28 delivered the best results. From 1998 to 2007 (P. falciparum) the models 37, 41 and 47 delivered the best results. From 1998 to 2007 (P. vivax) the models 56 and 63 delivered the best results. The logistic model without exogenous effects can be interpreted as a null model and models with exogenous effects as alternative models
The effect of exogenous variables on malaria dynamics will be assessed using Royama methodology [39], whom classified three basic exogenous effects on the logistic model (Eq. 1): vertical, lateral and non-linear effects. A vertical effect on R changes the relative position of the R function by changing proportionally both Rmax (y-scale) and K (x-scale) intercepts but not he non-linear parameter Q. Lateral effects occur when only the K is affected (Eq. 2), and the non-linear effects when the variable affects both Rmax and K, but disproportionally. This study uses inter-annual data instead of monthly data, mainly because there are no monthly data for human population size, nor HIV indices among other variables. This leaves us with less degree of freedom (annual data), thus the study explores the lateral effects of the variables. The lateral exogenous effects can be tested as follows [39–41]:
| 2 |
where a is the linear coefficient that measures the effect of any exogenous variable (Vt) on the self-limiting model parameters. Other parameters are as above.
The HIV national new cases per year, population density and death related to civil conflicts, as a proxy of social instability (Battle in Table 1), were obtained from the World Bank (https://www.worldbank.org/en/home) and World Health Organization (https://www.who.int/) websites. The national trends were interpreted as a proxy for what occurs at a smaller scale (Oromia region) [19, 45]. Falciparum and vivax malaria cases and the minimum Temperature (°C) data was obtained from the study [16], where only the minimum Temperature (°C) was considered. Equation 1 measures the endogenous contribution to malaria dynamics. Equation 2 measures the effects of the mentioned exogenous variables. This study presents the results of the effects of each of the variables, but,mainly focusses on the interaction between the exogenous variables.
The best model was selected based on the R2 (Coefficient of determination) and on the Akaike information criteria (AIC) [46] for each species and period. The predictability, the goodness of fit (R2) of a model usually increase by adding more variables and AIC penalizes the model in regard to the number of variables included, meaning that less variables needed to explain an observed pattern more parsimonious is the model. Equations 1 and 2 were fitted using the nls library in R through non-linear regression analysis [47].
Results
The malaria increase in numbers could be captured by the logistic growth model (Table 1, model 1, 17, 33 and 50) for the distinct species and periods. The logistic model alone explained more than 40% of the variance of the R levels, the self-limiting process between the infectious class and susceptible pool. Table 1 also shows the effects of the mentioned variables on carrying capacity (K) of the infectious class.
The logistic models with the interaction between GDP and human population size (model 11), GDP and social instability (model 13). HIV new cases and social instability (Battle in Table 1, model 14) and human population size and social instability (model 16) delivered similar and the bests results (higher R2 and lower AIC) for P. falciparum, from 1985 to 1998.
For P. vivax, the interaction between the effects of HIV new cases and GDP (model 28) delivered the best result (Table 1. Higher R2 and lower AIC). The interaction between the effects of the minimum temperature with HIV new cases (model 25) gave similar results, from 1985 to 1998.
From 1998 to 2007, the model for P. falciparum with HIV new cases as exogenous lateral effect without interactions delivered the best results (model 37) followed by the modes with interactions between HIV new cases and population size (model 47) and minimum temperature and HIV new cases (model 41), respectively. For P. vivax, the interaction between HIV new cases and human density (model 63) gave the best model (Higher R2 and lower AIC values) followed by the interaction between minimum temperature and GDP (model 56, Table 1).
Discussion
The results suggest that the interactions between HIV new cases, human density and GDP gave better results than models without exogenous variables and models with exogenous variables, but without interactions suggesting that climate change had an almost negligeable effect in terms of P. falciparum and P. vivax dynamics in Ethiopia. In this study, the manner in which the effects of these variables could interact to explain the changes in malaria dynamics will be discussed.
Both malaria and HIV are related to poverty [24–27], which can be exacerbated by civil conflicts [37, 38]. Poverty may force individuals to engage in risk behaviours (to avoid extreme poverty) and be more susceptible to contract HIV (sex workers), and hence, malaria [19–21].The spread of HIV could remove the herd immunity effect. The interaction between malaria and HIV is suspected to be synergetic [20], because malaria-infected individuals show an increase of the HIV host cells in the immunological system. This could produce a longer period of the acute phase of HIV, increasing infectivity between individuals and susceptibility to future malaria infections. Besides the synergy, the effects of HIV seem to be greater than the effects of malaria on the spread of HIV, because the infection period and AIDS stage may last for years reducing the immune defense efficiency [21–24]. The spread of HIV could expand the population at risk of contracting malaria in the growing population of Ethiopia, which may increase the contact rate and create mosquitoes breeding sites trough deforestation. Civil conflicts affect crops, favouring undernourishment (susceptibility), reducing health care investments and intensify human movement (refugees), which can amplify malaria distribution and burden [48, 49].
All these variables may also explain the malaria decline period and serve as alternative hypothesis to the claimed effect of minimum temperature. From 1998 to 2007, GDP levels increased, HIV levels increased but at lower rates (almost reaching an equilibrium), civil conflicts diminished and population density rose, but, as for HIV, at lower rates. GDP levels started to increase since 1990 and accelerated at the end of time series (1998 to 2007). Higher income may allow individuals to invest out-of-pocket for treating malaria. From 1998 to 2000, social instability peaked and dropped at low levels from 2000 onwards. The increase in income, the reduction of civil instability, the increase of HIV and human population size at low rates, could interact to explain the malaria decrease burden from 1998 to 2007.
It is important not to rule out climate change. Minimum temperature also delivered good results as exogenous force on the logistic model, but only when other variables were added (the models with interactions). This could be simply because of the stronger effects of others variables, and/or the fact that interannual data was used instead of monthly data, which could capture more precisely the malaria cases peaks. Climate variables could always provide an initial set of potential variables, which could influence the dynamics of malaria (not only minimum temperature). Temperature can accelerate larval development rate (including more life-cycles per season) and reduce the differences between seasons. Local rises in temperature could interact with increase in human density and HIV to explain the increase of malaria cases during the first period (1985–1998).
A recent study revised the climate effect on P. falciparum in Kericho tea plantations [19] and the results from this study showed that temperature (maximum, mean and minimum) and rain (average) had an almost negligeable role on malaria dynamics. The interaction of human population size and HIV levels had more contribution in explaining malaria dynamics than climate change, which reinforces the results from this study for Ethiopia, where others variables could have more influence on malaria burden than climate change [19]. In 2000, several countries joined in a global effort to halt and begin to reverse the incidence of malaria by 2015 (Millennium Development Goals 6- Target 6C) [26, 27]. African countries received international funding and developed efficient national strategies to achieve the Millennium Development Goals [50–54]. In many African countries, this occurred in 2005, one year after USA made a massive investment to increase ART (Antiretroviral Therapy) coverage in sub-Saharan Africa [27, 28].
In Ethiopia, the interaction between the effects of human density, HIV, GDP, and in some cases civil disorder could explain the decline of cases for P. falciparum (and similarly for P. vivax) from 1998 to 2005 in Ethiopia. This study also highlights that efforts to control malaria and HIV could interact in order to achieve the malaria Millennium Development Goals 6- Target 6C [54–58].
Conclusion
Here, the Population Ecology Theory was adopted to re-explore malaria cases dynamics in Ethiopia. This study presents a distinct scenario to explain that others large-scale phenomena (HIV, population size, among others) could have influenced malaria dynamics at a higher level than climate change.
The framework employed is based on the per capita population rate of change (RI), which is surrounded by plausible ecological principles and is hence an advantageous starting point to explore disease dynamics. Any government may disentangle RI in its components (new per capita infections and per capita mortality), explore which of them are most important for RI trends and explore the contributions of endogenous and exogenous processes. Hence, this approach, based on simple principles based on population ecology theory, could be included as a supplement to WHO reports with minimal cost- and time-demanding efforts, which could provide insights and hypotheses and may facilitate the testing and estimation of the drivers of disease dynamics.
Funding
FAMK receives a postdoctoral scholarship from FONDECYT (Folio: 319,001). TPM receives a productivity scholarship from CNPq (PQ 302121/2019-6). FMJ and ML receive receives funding: ANID PIA/BASAL FB0002.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patz JA, Hulme M, Rosenzweig C, Mitchell TD, Goldberg RA, Githeko AK, et al. Regional warming and malaria resurgence. Nature. 2002;420:627–628. doi: 10.1038/420627a. [DOI] [PubMed] [Google Scholar]
- 2.Gagnon AS, Smoyer-Tomic KE, Bush ABG. The El Niño Southern Oscillation and malaria epidemics in South America. Int J Biometeor. 2002;46:81. doi: 10.1007/s00484-001-0119-6. [DOI] [PubMed] [Google Scholar]
- 3.Kovats RS, Bouma MJ, Hajat S, Worrall E, Haines A. El Niño and health. Lancet. 2003;362:1481–1489. doi: 10.1016/S0140-6736(03)14695-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhou G, Minakawa N, Githeko AK, Yan G. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci USA. 2003;101:2375–2380. doi: 10.1073/pnas.0308714100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso D, Bouma MJ, Pascual M. Epidemic malaria and warmer temperatures in recent decades in an East African highland. Proc R Soc B. 2011;278:1661–1669. doi: 10.1098/rspb.2010.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual M, Ahumada JA, Chaves LF, Rodo X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proc Natl Acad Sci USA. 2006;103:5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay SI, Rogers DJ, Shanks GD, Myers MF, Snow RW. Malaria early warning in Kenya. Trends Parasitol. 2001;17:95–99. doi: 10.1016/S1471-4922(00)01763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascual M, Cazelles B, Bouma MJ, Chaves LF, Koelle K. Shifting patterns: malaria dynamics and rainfall variability in an African highland. Proc R Soc B. 2008;275:123–32. doi: 10.1098/rspb.2007.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pascual M, Dobsonb AP, Boumac MJ. Underestimating malaria risk under variable temperatures. Proc Natl Acad Sci USA. 2009;106:13645–13656. doi: 10.1073/pnas.0906909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanks GD, Hay SI, Omumbo JA, Snow RW. Malaria in Kenya’s Western Highlands. Emerg Infect Dis. 2005;11:1425–1432. doi: 10.3201/eid1109.041131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haftu A, Haftom H, Emiru B, Meseret H, Girma M. Spatio-temporal dynamics of malaria expansion under climate change in semi-arid areas of Ethiopia. Environ Hazar. 2019;18:400–413. doi: 10.1080/17477891.2019.1609405. [DOI] [Google Scholar]
- 13.Esayas E, Tufa A, Massebo F, Ahemed A, Ibrahim I, Dillu D, et al. Malaria epidemiology and stratification of incidence in the malaria elimination setting in Harari Region. Eastern Ethiopia Infect Dis Poverty. 2020;9:160. doi: 10.1186/s40249-020-00773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabaro D, Birhanu Z, Yewhalaw D. Analysis of trends of malaria from 2010 to 2017 in Boricha District. Southern Ethiopia Malar J. 2020;19:88. doi: 10.1186/s12936-020-03169-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalil FS, Hasen BM, Kabeta WS. Trends of malaria morbidity and mortality from 2010 to 2017 in Bale Zone, Ethiopia: analysis of surveillance data. Infect Drug Resist. 2020;13:4379–4387. doi: 10.2147/IDR.S284281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodó X, Martinez PP, Siraj A, Mercedes P. Malaria trends in Ethiopian highlands track the 2000 ‘slowdown’ in global warming. Nat Commun. 2021;12:1555. doi: 10.1038/s41467-021-21815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern DI, Gething PW, Kabaria CW, Temperley WH, Noor AM, Okiro EA, et al. Temperature and malaria trends in highland East Africa. PLoS ONE. 2011;6:e24524. doi: 10.1371/journal.pone.0024524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omumbo JA, Lyon B, Waweru SM, Connor SJ, Thomson MC. Raised temperatures over the Kericho tea estates: revisiting the climate in the East African highlands malaria debate. Malar J. 2011;10:12. doi: 10.1186/1475-2875-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krsulovic FAM, Moulton TP, Lima M, Jacksic F. Epidemic malaria dynamics in Eastern Africa Highlands: the role of climate change and human population growth. Ecol Evolut Bio. 2021;6:23–30. doi: 10.11648/j.eeb.20210601.15. [DOI] [Google Scholar]
- 20.Cuadros DF, Branscum AJ, Crowley PH. HIV–malaria co-infection: effects of malaria on the prevalence of HIV in East sub-Saharan Africa. Int J Epidemiol. 2011;40:931–939. doi: 10.1093/ije/dyq256. [DOI] [PubMed] [Google Scholar]
- 21.Bates I, Fenton C, Gruber J, Lalloo D, Lara AM, Squire SB, et al. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease .Part 1: determinants operating at individual and household level. Lancet Infect Dis. 2004;4:267–77. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- 22.Foglia G, Sateren WB, Renzullo PO, Bautista CT, Langat L, Wasunna MK, et al. High prevalence of HIV infection among rural tea plantation residents in Kericho Kenya. Epidemiol Infect. 2008;136:694–702. doi: 10.1017/S0950268807009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beyene HB, Tadesse M, Disassa H, Beyene MB. Concurrent Plasmodium infection, anemia and their correlates among newly diagnosed people living with HIV/AIDS in Northern Ethiopia. Acta Trop. 2017;169:8–13. doi: 10.1016/j.actatropica.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Korenromp EL, Brian G, Williams BG, de Vlas SJ, Gouws E, Gilks CF, et al. Malaria attributable to the HIV-1 epidemic Sub-Saharan Africa. Emerg Infect Dis. 2005;11:1410–1419. doi: 10.3201/eid1109.050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic. Geneva; 2013.
- 26.UNAIDS. Global AIDS update. Geneva; 2016.
- 27.WHO. World Malaria Report 2019. Geneva; World Health Organization, 2019.
- 28.Trape JF, Pison G, Spiegel A, Enel C, Rogier C. Combating malaria in Africa. Trends Parasitol. 2002;18:224–230. doi: 10.1016/S1471-4922(02)02249-3. [DOI] [PubMed] [Google Scholar]
- 29.Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee MC, Kazura J, et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty. 2018;7:103. doi: 10.1186/s40249-018-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martens P, Hall L. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis. 2000;6:103–109. doi: 10.3201/eid0602.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali A, Berhane Y, Deressa W. Review of the interplay between population dynamics and malaria transmission in Ethiopia. Ethiop J Health Dev. 2006;20:136–205. [Google Scholar]
- 33.Doolan DL, Doban C, Baird K. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu-Raddad L, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 35.Loiola CCP, da Silva CJM, Tauil PL. Controle da malária no Brasil: 1965 a 2001. Pan Am J Public Health. 2002;11:235–44. doi: 10.1590/S1020-49892002000400005. [DOI] [PubMed] [Google Scholar]
- 36.Amaral S, Câmara G, Monteiro AMV. Análise Espacial do Processo de Urbanização da Amazônia. INPE: Brasil. 2001.
- 37.WHO. Bridging the gaps. Geneva, World Health Organization, Geneva. 1995.
- 38.Croissant A, Trinn C. Culture identity and conflict in Asia and Southeast Asia. ASIEN. 2009;110:13–43. [Google Scholar]
- 39.Royama T. Analytical population dynamics. London: Chapman and Hall; 1992. [Google Scholar]
- 40.Berryman AA. Principles of population dynamics and their application. London: Garland Science; 1999. [Google Scholar]
- 41.Berryman AA, Turchin P. Identifying the density-dependent structure underlying ecological time series. Oikos. 2001;92:265–270. doi: 10.1034/j.1600-0706.2001.920208.x. [DOI] [Google Scholar]
- 42.Lima M. A link between the North Atlantic Oscillation and measles dynamics during the vaccination period in England and Wales. Ecol Letters. 2002;12:302–314. doi: 10.1111/j.1461-0248.2009.01289.x. [DOI] [PubMed] [Google Scholar]
- 43.Lima M, Estay SA, Fuentes R, Rubilar P, Broutin H, Chowell-Puente G. Whooping cough dynamics in Chile (1932–2010): disease temporal fluctuations across a north-south gradient. BMC Infect Dis. 2005;15:590. doi: 10.1186/s12879-015-1292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krsulovic FAM, Lima M. Tuberculosis epidemiology at the country scale: self-limiting process and the HIV effects. PLoS ONE. 2016;11:e0153710. doi: 10.1371/journal.pone.0153710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krsulovic FAM, Lima M, Moulton TP, Jaksic F. Different patterns of HIV expansion between high-income and non-rich countries and the potential drivers during the 1980s. IJSBAR. 2022;62:127–146. [Google Scholar]
- 46.Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer; 2002. [Google Scholar]
- 47.R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing: Vienna. 2016.
- 48.Acemoglu D, Robinson JA. Why is Africa poor? Econ Hist Devel Reg. 2010;25:21–50. [Google Scholar]
- 49.Anerjee A, Iyer L. History, institutions, and economic performance: the legacy of colonial land tenure systems in India. Am Econ Rev. 2005;95:1190–1213. doi: 10.1257/0002828054825574. [DOI] [Google Scholar]
- 50.Ministry of Health. The epidemiology and control profile of malaria in Kenya: reviewing the evidence to guide the future vector control. Ministry of Health: Nairobi. 2016.
- 51.Solomon T, Loha E, Deressa W, Gari T, Lindtjørn B. Spatiotemporal clustering of malaria in southern-central Ethiopia: a community-based cohort study. PLoS ONE. 2019;14:e0222986. doi: 10.1371/journal.pone.0222986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghebreyesus TA, Alemayehu T, Bosman A, Witten KH, Teklehaimanot A. Community participation in malaria control in Tigray region Ethiopia. Acta Trop. 1996;61:145–156. doi: 10.1016/0001-706X(95)00107-P. [DOI] [PubMed] [Google Scholar]
- 53.White MT, Griffin JT, Churcher TS, Ferguson NM, Basáñez MG. Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasit Vectors. 2011;4:153. doi: 10.1186/1756-3305-4-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jima D, Getachew A, Bilak H, Steketee RW, Emerson PM, Graves PM, et al. Ethiopia Malaria Indicator Survey Working Group. Malaria indicator survey 2007, Ethiopia: coverage and use of major malaria prevention and control interventions. Malar J. 2010;9:58. doi: 10.1186/1475-2875-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiner RC, Jr, Guerra C, Donnelly MJ, Bousema T, Drakeley C, Smith DL. Estimating malaria transmission from humans to mosquitoes in a noisy landscape. J R Soc Interface. 2015;12:20150478. doi: 10.1098/rsif.2015.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.African Centre for Technology Studies . Climate change and development consultation on key researchable issues. London: International Institute for Environment and Development; 2005. [Google Scholar]
- 57.Yousif EH, Kweka EJ. Malaria in East African highlands during the past 30 years: impact of environmental changes. Front Physiol. 2012;3:315. doi: 10.3389/fphys.2012.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhatt S, Weiss DJ, Mappin B, Dalrymple U, Cameron E, Bisanzio D, et al. Coverage and system efficiencies of insecticide-treated nets in Africa from 2000 to 2017. eLife. 2015;4:e09672. doi: 10.7554/eLife.09672. [DOI] [PMC free article] [PubMed] [Google Scholar]