Abstract
Betulinic acid, a triterpenoid isolated from the methyl alcohol extract of the leaves of Syzigium claviflorum, was found to have a potent inhibitory activity against human immunodeficiency virus type 1 (HIV-1). Betulinic acid derivatives were synthesized to enhance the anti-HIV activity. Among the derivatives, 3-O-(3′,3′-dimethylsuccinyl) betulinic acid, designated YK-FH312, showed the highest activity against HIV-induced cytopathic effects in HIV-1-infected MT-4 cells. To determine the step(s) of HIV replication affected by YK-FH312, a syncytium formation inhibition assay in MOLT-4/HIV-1IIIB and MOLT-4 coculture, a multinuclear-activation-of-galactosidase-indicator (MAGI) assay in MAGI-CCR5 cells, electron microscopic observation, and a time-of-addition assay were performed. In the syncytium formation inhibition assay or in the MAGI assay for de novo infection, the compound did not show inhibitory effects against HIV replication. Conversely, no virions were detected in HIV-1-infected cell cultures treated with YK-FH312 either by electron microscopic observation or by viral yield in the supernatant. In accordance with a p24 enzyme-linked immunosorbent assay of culture supernatant in the time-of-addition assay, YK-FH312 inhibited virus expression in the supernatant when it was added 18 h postinfection. However, Western blot analysis of the cells in the time-of-addition assay revealed that the production of viral proteins in the cells was not inhibited completely by YK-FH312. These results suggest that YK-FH312 might affect the step(s) of virion assembly and/or budding of virions, and this is a novel mechanism of action of an anti-HIV compound.
The introduction of highly active antiretroviral therapy has improved the quality of life of patients with advanced human immunodeficiency virus (HIV) infection and prevented the development of the disease (13). However, it is impossible to completely eliminate HIV from infected individuals (4), and multidrug-resistant viruses have been isolated from patients receiving long-term medication, even with this therapy (9, 10). In addition, some patients suffered severe side effects with highly active antiretroviral therapy and could not continue the therapy (2, 3). It is important, therefore, to develop more effective anti-HIV reagents targeting different phases of HIV replication from known anti-HIV drugs such as reverse transcriptase (RT) inhibitors and HIV-specific protease inhibitors. In the search for plant-derived natural products as anti-HIV agents, betulinic acid (Fig. 1a), isolated from the methyl alcohol extract of the leaves of Syzigium claviflorum, was found to have potent anti-HIV activity (5). We chemically modified betulinic acid to enhance its activity. Among the derivatives, 3-O-(3′,3′-dimethylsuccinyl) betulinic acid showed the highest anti-HIV activity and did not inhibit the HIV type 1 (HIV-1) RT activity (6). In this study, the mechanism of the anti-HIV activity of this novel compound was examined.
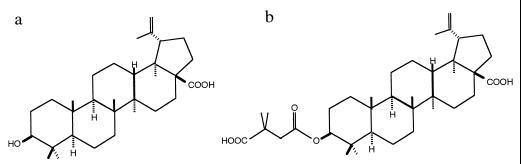
FIG. 1.
Chemical structure of betulinic acid and YK-FH312. (a) Betulinic acid, isolated from the methyl alcohol extract of leaves of S. claviflorum. (b) YK-FH312 has a dimethylsuccinic anhydride at the C-3 hydroxy groups of betulinic acid.
MATERIALS AND METHODS
Compounds.
Betulinic acid was isolated and purified from the methyl alcohol extract of leaves of S. claviflorum. YK-FH312 [3-O-[3′,3′-dimethylsuccinyl] betulinic acid; molecular weight, 584] (Fig. 1b) was synthesized from betulinic acid by chemical modification as described previously (5). The following reagents were used as reference anti-HIV compounds: 3′-azido-2′,3′-dideoxythymidine (AZT) (Sigma Chemical Co., St. Louis, Mo.), a nucleoside RT inhibitor; ritonavir (Abbott Laboratories, Abbott Park, Ill.), a viral protease inhibitor; and curdlan sulfate (molecular mass, 79 kDa; Ajinomoto Co. Ltd., Tokyo, Japan), a virus adsorption inhibitor. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma.
Cells and viruses.
MT-4 cells, a human T-cell line bearing human T-cell leukemia virus type 1, and MOLT-4 cells, a lymphoblastoid T-cell line, were subcultured twice a week at a density of 3 × 105 cells/ml in RPMI 1640 medium (Gibco, Grand Island, N.Y.) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS) (Gibco), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. For the multinuclear-activation-of-galactosidase-indicator (MAGI) assay, MAGI-CCR5 cells were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program and cultured in Dulbecco's modified Eagle medium supplemented with 20% FCS, 0.2 mg of G418 per ml, 50 U of hygromycin B per ml, and 1 μg of puromycin per ml. MAGI-CCR5 cells are clones of MAGI cells which express CCR5, a human chemokine receptor. Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors and isolated by the Ficoll-Hypaque technique. HIV-1IIIB (an X4 HIV-1 strain) was prepared from a culture supernatant of persistently infected MOLT-4/HIV-1IIIB cells and was stored at −80°C.
Anti-HIV-1 assay.
The inhibitory effects of the tested compounds on HIV-1IIIB replication were estimated by the levels of inhibition of virus-induced cytopathic effects (CPE) in MT-4 cells (14). Aliquots of 3 × 105 MT-4 cells per ml were infected with HIV-1IIIB at a multiplicity of infection (MOI) of 0.01. The HIV-infected or mock-infected MT-4 cells were placed in 96-well culture plates (100 μl/well) with 100 μl of various concentrations of the compounds and incubated at 37°C under 5% CO2 at 100% humidity. After 5 days, cell viability was quantified by MTT assay, as described previously (12, 14). The 50% cytotoxic concentration (CC50), 50% effective concentration (EC50), and selectivity index (SI = CC50/EC50) were then calculated from the cell viability for each concentration of the compound. Anti-HIV activity was also investigated in PBMCs infected with HIV-1IIIB and cultured with various concentrations of test compounds. The activity was evaluated by the level of inhibition of p24 core antigen in the culture supernatant, assessed with the HIV-1 p24 core profile enzyme-linked immunosorbent assay (ELISA) kit (Dainabot Co., Tokyo, Japan) (15).
To estimate the anti-HIV-1 activities of the compounds or the infectious titers of compound-treated MOLT-4/HIV-1IIIB culture supernatants, MAGI assays (1, 7) were performed as follows. MAGI-CCR5 cells in Dulbecco's modified Eagle medium containing 10% FCS and antibiotics were cultured in 96-well culture plates at 4 × 104 cells/well for 24 h. The culture medium was then replaced with fresh medium containing a virus solution with or without compounds in duplicate. After 2 days of cultivation, the medium was removed and the cells were fixed with 100 μl of phosphate-buffered saline (PBS) containing 1% formaldehyde and 0.2% glutaraldehyde for 5 min at room temperature. The cells were then washed three times with PBS and incubated for 50 min at 37°C with 100 μl of a staining solution (4 mM potassium ferrocyanide, 4 mM potassium ferricyanide, 2 mM MgCl2, and 400 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside per ml). The reaction was terminated by washing the cells twice with PBS. In cells infected with HIV, the integrated HIV long terminal repeat (LTR) β-gal reporter gene was expressed and the cells turned blue on staining. The blue cells were counted by microscopic observation, and the infectious titer of the virus solution was determined. The anti-HIV-1 activity of the compound was represented as percent inhibition of the blue cell expression and was calculated as follows: [1 − (blue cell counts with a test compound/blue cell counts without a compound)] × 100.
In this MAGI assay, compounds which inhibit viral replication process(es) after provirus integration, such as protease inhibitors, would not cause a decrease in the number of blue cells even if the compound had potential anti-HIV-1 activity.
Syncytium formation assay.
MOLT-4/HIV-1IIIB cells were incubated for 3 days with a test compound (YK-FH312, 5 μg/ml; ritonavir, 5 μg/ml; AZT, 1 μM; or curdlan sulfate, 1 μg/ml). Then, MOLT-4 cells (5 × 105) were cultured with an equal number of the compound-pretreated MOLT-4/HIV-1IIIB cells in a culture plate in the presence of the same compound in duplicate. After 24 h of cocultivation, the number of viable cells was determined by the trypan blue dye exclusion method and the fusion index (FI) was calculated from the mean cell counts of the duplicate culture as follows: 1 − (cell count in test well containing MOLT-4 with MOLT-4/HIV-1IIIB cells/cell count in control well containing MOLT-4 cells only). The fusion inhibition percentage was then estimated from the following: [1 − (FIT/FIC)] × 100, where FIT is the FI of the test sample and FIC is that of the nontreated control sample (16).
Assay for infectious virus release.
To estimate the virus yield from infected cells, MOLT-4/HIV-1IIIB cells were cultured with the test compound (5 μg of YK-FH312 per ml, 5 μg of ritonavir per ml, or 1 μM AZT). After 2 days, the cells were washed to remove viruses already released before addition of the compounds, and the cultures were continued for three more days with fresh media containing the same amounts of the compounds. The infectious titers of compound-treated culture supernatants were estimated by MAGI assay. In addition, the YK-FH312-treated MOLT-4/HIV-1IIIB cells were observed by electron microscopy (8).
Time-of-addition assay.
To clarify the viral replication process inhibited by YK-FH312, a time-of-addition assay was performed as described previously (11). MT-4 cells were exposed to HIV-1IIIB at a high MOI (>1) for 1 h at 37°C to synchronize the virus replicative cycle in the whole cells. After 60 min of virus adsorption, the cells were washed to remove unadsorbed viruses. Parallel cultures were then incubated at 37°C in a CO2 incubator with the addition of 5 μg of YK-FH312 per ml or reference compounds (5 μg of curdlan sulfate per ml, 5 μg of ritonavir per ml, or 1 μM AZT) at 1, 6, 12, 15, 18, and 21 h postinfection. At 0 h, the curdlan sulfate was added to the culture immediately after viral exposure of the cells without virus adsorption. At 24 h postinfection, the cultures were centrifuged and the cells were treated with ISOGEN (Wako Pure Chemical, Osaka, Japan) to isolate viral proteins, and supernatants were stored at −80°C.
The HIV-1 p24 antigen of the culture supernatant was detected and quantified by the HIV-1 p24 core profile ELISA kit (Dainabot) (15), and the viral proteins of the infected cells were analyzed by Western blotting as described previously (11) using 300-fold-diluted human serum from an HIV-infected patient as the primary antibody and 7,000-fold-diluted goat serum anti-human immunoglobulin G (Promega Co., Madison, Wis.) as the secondary antibody.
RESULTS
Anti-HIV activities detected from MTT and MAGI assays.
YK-FH312 inhibited the virus-induced CPE in HIV-1IIIB-infected MT-4 cells and the activity was estimated by MTT assay to have an EC50 of 0.011 μg/ml, a CC50 of 14.03 μg/ml, and an SI of 1,275. Anti-HIV activity was confirmed by inhibition of p24 antigen expression in PBMCs. YK-FH312 also inhibited the p24 expression in the supernatant of PBMC culture detected by an ELISA conducted in parallel with the MTT assay (data not shown). However, YK-FH312 and ritonavir did not prevent blue cell expression in HIV-1IIIB-infected MAGI-CCR5 cells (Table 1). In contrast, AZT and curdlan sulfate treatments showed significant reductions in the numbers of blue cells.
TABLE 1.
Anti-HIV activities determined by MTT and MAGI assaysa
| Compound | MTT assay
|
MAGI assay
|
|||
|---|---|---|---|---|---|
| EC50 (μg/ml) | CC50 (μg/ml) | SI | Concn (μg/ml) | Inhibition (%) | |
| YK-FH312 | 0.011 | 14.03 | 1,275 | 10 | 11.3 |
| 2 | 0 | ||||
| Ritonavir | 1.34 | 441.6 | 330 | 1 | 0 |
| 0.1 | 0 | ||||
| AZT | 0.020b | 280.5b | 13,924 | 0.1b | 91.0 |
| 0.01b | 34.2 | ||||
| Curdlan sulfate | 0.678 | 822.3 | 1,213 | 10 | 77.0 |
| 1 | 31.5 | ||||
Cells were infected with HIV-1IIIB at an MOI of 0.01 for the MTT assay and an MOI of 0.2 for the MAGI assay.
Values are micromolar concentrations.
Effects on syncytium formation.
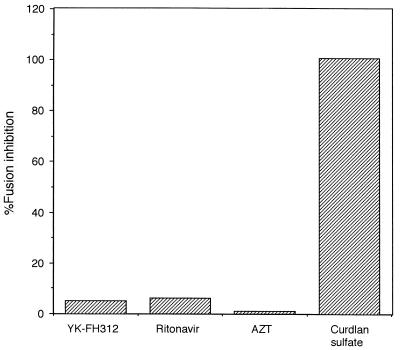
The fusion in the coculture of MOLT-4 cells and YK-FH312-treated MOLT-4/HIV-1IIIB cells was not inhibited, with levels comparable to those of AZT- or ritonavir-treated cells. In contrast, a high percentage of fusion inhibition was observed in the presence of curdlan sulfate (Fig. 2).
FIG. 2.
Inhibitory effects on syncytium formation in coculture of MOLT-4 and MOLT-4/HIV-1IIIB cells pretreated with one of several compounds. MOLT-4/HIV-1IIIB cells were cultured for 3 days with YK-FH312 (5 μg/ml), ritonavir (5 μg/ml), AZT (1 μM), or curdlan sulfate (1 μg/ml) and then cocultured with MOLT-4 for 24 h. The percentage of fusion inhibition was calculated as described in Materials and Methods.
Effects on viral release from MOLT-4/HIV-1IIIB cells.
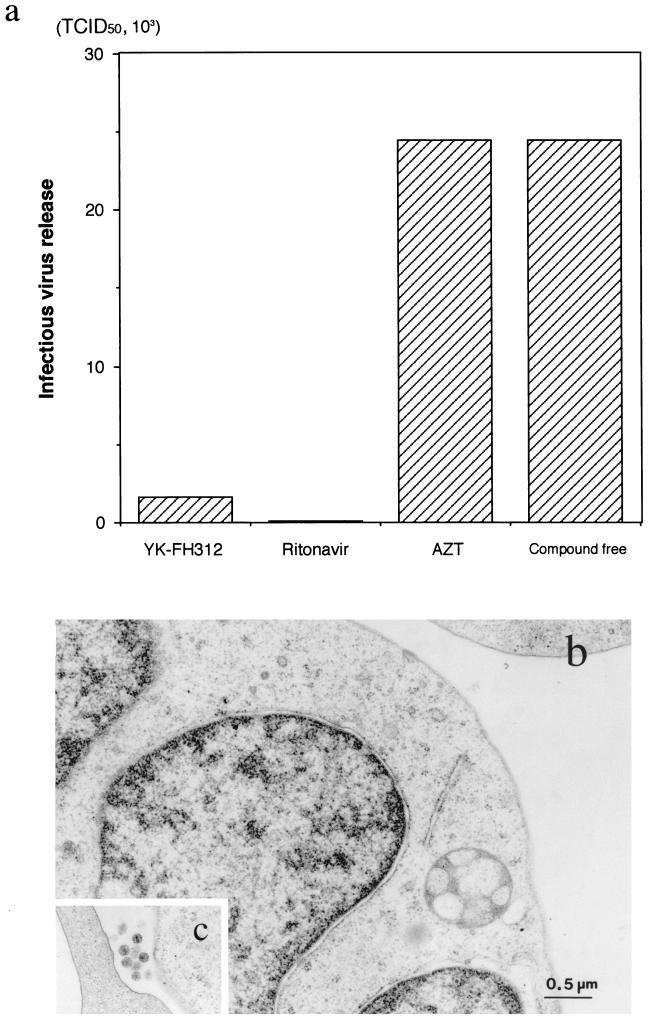
The infectious virus yield in the supernatants of persistently infected MOLT-4/HIV-1IIIB cells cultured in the presence of test compounds was estimated by MAGI assay (Fig. 3a). Ritonavir inhibited the release of infectious HIV-1 from MOLT-4/HIV-1IIIB cells completely, but AZT did not. The infectious titer of the YK-FH312-treated culture supernatant also showed complete suppression when compared with that of the compound-free control. Electron microscopic observation of the YK-FH312-treated and mock-treated cells also suggested that YK-FH312 inhibited virus release from infected cells. No HIV-1 virions were seen around the YK-FH312-treated MOLT-4/HIV-1IIIB cells (Fig. 3b), in contrast to what was observed for the mock-treated cells (Fig. 3c). However, syncytium formation was observed with the coculture of MOLT-4 cells and YK-FH312-pretreated MOLT-4/HIV-1IIIB cells (Fig. 2). Therefore, YK-FH312 does not appear to inhibit viral protein production inside the cell or expression of the viral envelope proteins on the cell surface, but it could prevent release of infectious virions. These findings suggest that YK-FH312 may interfere with the process of viral maturation.
FIG. 3.
Effects of various compounds on infectious HIV production in chronically infected MOLT-4/HIV-1IIIB cells. (a) MOLT-4/HIV-1IIIB cells cultured without compound or with 5 μg of YK-FH312 per ml, 5 μg of ritonavir per ml, or 1 μM AZT. The infectious titers of the culture supernatants were determined by MAGI assay. TCID50, 50% tissue culture infective dose. (b and c) Electron microscopic photographs of YK-FH312-treated MOLT-4/HIV-1IIIB cells (b) and mock-treated cells (c). The magnifications of panels b and c are similar. No HIV virions were found in the YK-FH312-treated culture in contrast to the mock-treated culture.
Viral protein production in HIV-1IIIB-infected MT-4 cells.
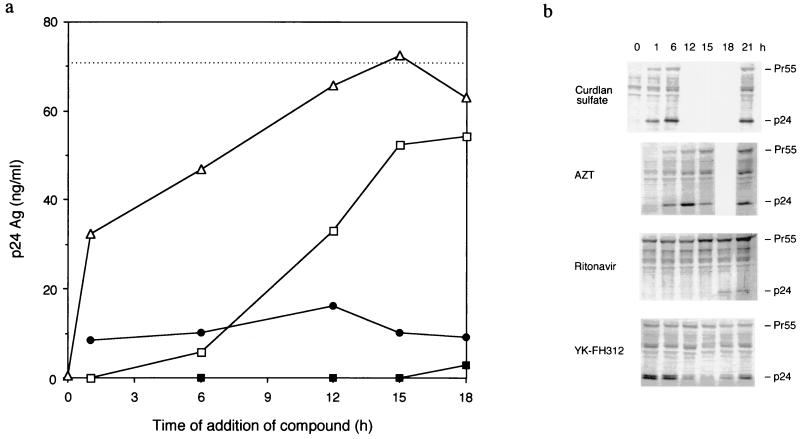
As described above, YK-FH312 appears to inhibit the production of the infectious virion, but it does not inhibit the reverse transcription and production of viral proteins. Therefore, HIV-1-specific p24 antigen levels in the culture supernatant of the time-of-addition assay were estimated by p24 ELISA (Fig. 4a). The levels of p24 antigen in the supernatants of both YK-FH312- and ritonavir-treated cultures were low, even when the compounds were added at 18 h postinfection. In contrast, curdlan sulfate treatment suppressed production of p24 only during the adsorption period, whereas suppression was shown when AZT was added up to 6 h postinfection. To investigate the level of viral protein production, HIV-specific antigens in the cell extract were analyzed by Western blotting (Fig. 4b). The intensity of p24 fragments from cells treated with curdlan sulfate, AZT, and ritonavir changed in the same manner as the level of p24 expression in the supernatants according to the time of addition postinfection. However, the change in the cell-associated p24 antigen in the YK-FH312-treated cells was quite different from that in the supernatant. A high intensity of cell-associated p24 antigen was seen when YK-FH312 was added 0 to 6 h postinfection; the intensity then decreased gradually, reaching its lowest concentration at 15 h and then increasing through 18 to 21 h. Furthermore, double fragments corresponding to p24 antigen were detected only in the YK-FH312-treated cells, whereas a single fragment was detected in the reference compound-treated cells. These findings suggest that YK-FH312 may inhibit virus assembly, as the p24 antigen level inside the cell increased after 15 h postinfection but the p24 titers of the supernatant did not increase even with the addition at 18 h postinfection.
FIG. 4.
Time-of-addition assay. MT-4 cells were infected with HIV-1IIIB at a high MOI. After 60 min of virus adsorption, the cells were washed. Compounds were then added to parallel cultures at different times postinfection or immediately after HIV-1 exposure to the cells without adsorption (at 0 h, only curdlan sulfate was added). At 24 h postinfection, cells and supernatants were collected by centrifugation. (a) Levels of p24 core antigen in culture supernatants treated with YK-FH312 (5 μg/ml) (●), ritonavir (5 μg/ml) (■), AZT (1 μM) (□), or curdlan sulfate (5 μg/ml) (▵). The broken line shows the p24 concentration at 24 h postinfection in the compound-free culture supernatant. Results are the mean concentrations of p24 detected in duplicate experiments. (b) Western blot analysis of cell-associated viral protein.
DISCUSSION
Betulinic acid, a triterpenoid isolated from S. claviflorum (Fig. 1a), exhibited inhibitory activity against HIV-1 replication in vitro (5). Acyl groups were introduced at the C-3 hydroxy groups of betulinic acid, and of the examined derivatives, YK-FH312 (Fig. 1b) was found to have the highest anti-HIV activity (6). However, YK-FH312 did not show inhibition of virus-induced CPE in influenza virus-infected MDCK cells or in herpes simplex virus type 1-infected HeLa cells (data not shown). Western blot analysis revealed that the release of human T-cell leukemia virus type 1 in chronically infected MT-4 cells was not blocked by YK-FH312 (data not shown). These results suggest that the antiviral activity of YK-FH312 may be specific to HIV.
YK-FH312 demonstrated anti-HIV activity in MTT assays but not in our MAGI assay (Table 1). In the MTT assay, inhibition of virus-induced CPE in HIV-infected MT-4 cells was monitored to estimate the anti-HIV activity of the compound. Thus, compounds having anti-HIV activities at any stage of the virus replication cycle can be detected by the MTT assay. In the MAGI assay, on the other hand, the expression of the MAGI-CCR5 integrated LTR β-gal reporter gene was examined (7). Compounds interfering with the process(es) after provirus integration could not prevent the blue cell expression. YK-FH312 showed a potent anti-HIV activity in this assay, suggesting that inhibition of process(es) follows provirus transcription of the virus replication cycle. However, YK-FH312 showed no direct inhibition of RT enzymatic activity in vitro (6). The syncytium formation in the coculture of MOLT-4 cells and YK-FH312-pretreated MOLT-4/HIV-1IIIB cells was not inhibited (Fig. 2), suggesting that YK-FH312 did not inhibit the expression of gp120 on the cell surface. In the time-of-addition assay, the amount of HIV-1-specific p24 core antigen in the supernatant of the YK-FH312-treated culture was low even if the compound was added at 18 h postinfection, and the effect was comparable to that of ritonavir, a viral protease inhibitor (Fig. 4a). However, in a protease inhibition assay, 1 μg of YK-FH312 per ml, about 100 times the EC50, did not produce inhibitory activity (data not shown), suggesting that HIV protease is not a target for YK-FH312. Furthermore, in the time-of-addition assay, the intensity of cell-associated p24 antigen in YK-FH312-treated culture changed in a manner different from that of cultures treated with other compounds and did not correspond to the titer of the cell-free p24 antigen (Fig. 4). When YK-FH312 was added to the HIV-infected MT-4 cells up to 6 h postinfection, a high intensity of cell-associated p24 antigen and a low titer of cell-free p24 antigen were observed. Furthermore, an RT-PCR of the viral LTR U5 domain with YK-FH312-treated MOLT-4/HIV-1IIIB cells revealed that the compound did not inhibit the expression of viral mRNA (data not shown). These findings suggest that the viral proteins were being produced inside the cell but the virion could not be released. The constant intensity of cell-associated Pr55 and two fragments corresponding to p24 in the Western blot analysis of the YK-FH312-treated cultures also supported this hypothesis. The results presented in this study therefore suggest that YK-FH312 interferes with viral maturation and that YK-FH312 is noteworthy as a new anti-HIV reagent with a novel mechanism of action.
REFERENCES
- 1.Arakaki R, Tamamura H, Premanathan M, Kanbara K, Ramanan S, Mochizuki K, Baba M, Fujii N, Nakashima H. T134, a small-molecule CXCR4 inhibitor, has no cross-drug resistance with AMD3100, a CXCR4 antagonist with a different structure. J Virol. 1999;73:1719–1723. doi: 10.1128/jvi.73.2.1719-1723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkman K, ter Hofstede H J, Burger D M, Smeitink J A, Koopmans P P. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Carr A, Samaras K, Thorisdottir A, Kaufmann G R, Chisholm D J, Cooper D A. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 4.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka T, Kashiwada Y, Kilkuskie R E, Cosentino L M, Ballas L M, Jiang J B, Janzen W P, Chen I S, Lee K H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod. 1994;57:243–247. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 6.Kashiwada Y, Hashimoto F, Cosentino L M, Chen C H, Garrett P E, Lee K H. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem. 1996;39:1016–1018. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 7.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohno T, Fujioka Y, Goto T, Morimatsu S, Morita C, Nakano T, Sano K. Contrast-enhancement for the image of human immunodeficiency virus from ultrathin section by immuno electron microscopy. J Virol Methods. 1998;72:137–143. doi: 10.1016/s0166-0934(98)00022-6. [DOI] [PubMed] [Google Scholar]
- 9.Kosalaraksa P, Kavlick M F, Maroun V, Le R, Mitsuya H. Comparative fitness of multi-dideoxynucleoside-resistant human immunodeficiency virus type 1 (HIV-1) in an in vitro competitive HIV-1 replication assay. J Virol. 1999;73:5356–5363. doi: 10.1128/jvi.73.7.5356-5363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molla A, Granneman G R, Sun E, Kempf D J. Recent developments in HIV protease inhibitor therapy. Antivir Res. 1998;39:1–23. doi: 10.1016/s0166-3542(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima H, Ichiyama K, Inazawa K, Ito M, Hayashi H, Nishihara Y, Tsujii E, Kino T. FR901724, a novel anti-human immunodeficiency virus (HIV) peptide produced by Streptomyces, shows synergistic antiviral activities with HIV protease inhibitor and 2′,3′-dideoxynucleosides. Biol Pharm Bull. 1996;19:405–412. doi: 10.1248/bpb.19.405. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima H, Masuda M, Murakami T, Koyanagi Y, Matsumoto A, Fujii N, Yamamoto N. Anti-human immunodeficiency virus activity of a novel synthetic peptide, T22 ([Tyr-5,12, Lys-7]polyphemusin II): a possible inhibitor of virus-cell fusion. Antimicrob Agents Chemother. 1992;36:1249–1255. doi: 10.1128/aac.36.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palella F J, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D the HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 14.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 15.Premanathan M, Nakashima H, Igarashi R, Mizushima Y, Yamada K. Lecithinized superoxide dismutase: an inhibitor of human immunodeficiency virus replication. AIDS Res Hum Retrovir. 1997;13:283–290. doi: 10.1089/aid.1997.13.283. [DOI] [PubMed] [Google Scholar]
- 16.Tochikura T S, Nakashima H, Tanabe A, Yamamoto N. Human immunodeficiency virus (HIV)-induced cell fusion: quantification and its application for the simple and rapid screening of anti-HIV substances in vitro. Virology. 1988;164:542–546. doi: 10.1016/0042-6822(88)90570-3. [DOI] [PubMed] [Google Scholar]