Abstract
Background
Hypertension remains the major risk factor for cardiovascular diseases (CVDs) worldwide with a prevalence and mortality in low- and middle-income countries (LMICs) among the highest. The early detection of hypertension risk factors is a crucial pillar for CVD prevention.
Design and method
This cross-sectional study included 4284 subjects, mean age 46 ± 16SD, 56.4% females and mean BMI 26.6 ± 3.7 SD. Data were collected through a screening campaign in rural area of Kirehe District, Eastern of Rwanda, with the objective to characterize and examine the prevalence of elevated blood pressure (BP) and other CVD risk factors. An adapted tool from the World Health Organization STEPwise Approach was used for data collection. Elevated BP was defined as ≥ 140/90 mm/Hg and elevated blood glucose as blood glucose ≥ 100 mg/dL after a 6-h fast.
Results
Of the sampled population, 21.2% (n = 910) had an elevated BP at screening; BP was elevated among individuals not previously known to have HTN in 18.7% (n = 752). Among individuals with a prior diagnosis of HTN, 62.2% (n = 158 of 254) BP was uncontrolled. Age, weight, smoking, alcohol history and waist circumference were associated with BP in both univariate analyses and multivariate analysis.
Conclusion
High rates of elevated BP identified through a health screening campaign in this Rwandan district were surprising given the rural characteristics of the district and relatively low population age. These data highlight the need to implement an adequate strategy for the prevention, diagnosis, and control of HTN that includes rural areas of Rwanda as part of a multicomponent strategy for CVD prevention.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-022-02606-9.
Keywords: High blood pressure, Hypertension, Screening, Rwanda
Background
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality worldwide [1, 2] and approximately 80% of all cardiovascular deaths occur in low- and middle-income countries [1, 3]. By 2030, cardiovascular deaths are projected to increase to 23 million globally [4] and double in Sub-Saharan Africa (SSA) from 1 million deaths in 2013 [5].
Hypertension (HTN) is the most prevalent risk factor for CVD mortality worldwide [3]. Although hypertension is a well-document public health threat in developing countries, population-based data on HTN prevalence is scarce from SSA. Based on one report, the estimated prevalence of HTN in SSA is 16.2%, ranging from 10.6% in Ethiopia to 29.6% in Ghana. In SSA, the prevalence of HTN is also higher in urban (20.7%) compared with rural (13.7%) areas [6], a difference generally attributed to lifestyle changes commonly associated increasing urbanization (physical inactivity, obesity, smoking, and alcohol consumption) [7]. Similar to other LMICs, Rwanda is experiencing epidemiological transition in urban areas with changes in lifestyle behaviors which portends increased healthcare burden, morbidity, and mortality from CVDs.
Early detection and management remain a key cost-effective strategy for the prevention of chronic CVDs [6, 8, 9]. However, there is a lack of data on the prevalence of CVD risk factors among adults living in remote rural areas which are less geographically accessible. This fundamental data gap hampers efforts to characterize the CVD risk profile in mainly rural regions, further impeding efforts to establish appropriate preventive measures targeting these underserved populations [1, 10]. To bridge this gap, this study aimed to measure the rates of and risk factors for HTN in people living in a rural area of Rwanda through a screening campaign the Kirehe District, an eastern province of Rwanda.
Methods
Study design and procedures
This was a cross-sectional study that included adult (≥ 18 years) Rwandan people who participated in campaign on voluntary basis for five days from 23rd to 29th September, 2018. The study design followed the other reported screening studies design [11]. In addition, it has integrated elements from Health Beliefs Model (HBM) [12] to explore the rates and predictors of HTN in the community. The screening was conducted by trained volunteers (medical students and nurses) from health centers (HCs) in the Kirehe district hospital catchment area. The core role of the Kirehe district leadership was to arrange with local authorities to sensitize and invite people for screening by using local and public communications and radio publicity spots. The screening site was designed by HCs leadership and staff of Rwanda Biomedical Centre (RBC), a government institution under the Ministry of Health responsible for implementing health polices and health services in the community.
Study setting
The study was conducted in 8 sectors in the Kirehe District that were systematically selected. The Kirehe District, one of the rural area districts situated in Eastern province of Rwanda bordering Tanzania, covers a total area of 1118.5 Km2 with a population of 340,983 (52% female) according to National Institute of Statistics of Rwanda (NISR). Ninety percent (90%) of the economic activity in the district depends on agriculture and livestock [13].
Data collection
Data collection was done after verbal consent of participants by using an adapted World Health Organization STEPwise Approach to NCD Risk Factor Surveillance tool [14]. Data collected included demographics, social history of alcohol intake and smoking, prior history of hypertension, and time since last meal. Measurements included anthropometrics, blood pressure and blood glucose. After a resting in a seated position for 5 min, blood pressure (BP) was taken three times separated by 1 min following a standardized protocol with a calibrated and automated BP machine (Omron M2, Kyoto, Japan). The mean of the 2nd and 3rd readings was recorded. Waist circumference (WC) was measured in triplicate at the level of the midpoint between the lower margin of the last palpable rib and the top of the iliac crest after expiration using a 203 cm Seca® measuring tape (all Seca GmbH & Co. KG., Hamburg, Germany); the mean of the three WC measures was used for analysis [15]. Weight was measured using a digital Seca® 813 scale and height using a Seca® 213 stadiometer. As the BP measures were taken casually and do not necessarily reflect a well-established hypertension diagnosis [16], we used the term of “Elevated BP” to reflect a systolic BP greater than or equal to 140 mmHg and/or a diastolic BP greater than or equal to 90 mmHg [17, 18]. This definition of elevated BP has nothing do with the definition of hypertension staging, used in some guidelines [16]. Data on the use of anti-hypertensive medications was not collected; a separate question surveyed whether the subject had ever been diagnosed with HTN.
A screening blood glucose (BG) was measured using a portable-battery driven Accu-Check® Aviva (Roche Diagnostics GmbH, Mannheim, Germany); the number of fasting hours as recorded and categorized form 0–6 h and 6 h and above (6 h corresponding to the night period from midnight to 6.00 a.m. where no food intake is usually expected). This was decided after the pilot where we found most of participant not remembering the exact time of their last food intake (not only food or meal but also including all kinds of mouth intake, a common situation in rural families).
As the BG measures were taken casually and do not necessarily reflect a well-established diabetes diagnosis [19], we used the term “elevated blood glucose” to reflect glycaemia greater than or equal to 100 mg/dL [19–21]. Participants who had elevated BP or self-reported use of HTN medications or had elevated BG were referred to the nearest health center for regular follow-up. Those individuals with normal BP were counselled on NCD risk reduction for primary prevention. The blood glucose and blood pressure results were communicated to participants immediately. The study was conducted in accordance with the principles of the Declaration of Helsinki and local Rwandan regulations.
Statistical methods and data analysis
Data was captured by a study team person using spreadsheet software program (Microsoft Excel, Microsoft Corporation, 2018), then de-identified and transferred to STATA 15.5 Version (Stata Corporation, College Station, TX) for analysis. Data are presented as mean ± standard deviation (SD) or percentage (number). Comparisons were performed by unpaired t-tests, one-way ANOVA and χ2-test as appropriate. Results with p values < 0.05 were considered statistically significant. Univariate and multivariate regression analyses were used to assess relationships between different risk factors and the presence of elevated BP in the total population and in subgroups defined by the absence or presence of a prior diagnosis of HTN.
We inspect the correlations between the predictors, using a correlation map to assess the collinearity, and highly correlated variables were entered one by one to test the best model. All variables in bivariate analyses with p value < 0.05 were considered for inclusion in multivariate regression model.
Results
Participant characteristics by blood pressure category
A total of 4284 participants in Kirehe district completed a 5-day screening program as part of routine monthly physical activity national program. The participants were largely middle-aged (mean age: 46 ± 16 years, range: 18–98 years), female (56, 4%), mildly overweight, and the majority had a history of alcohol intake (Table 1). Females were younger than males (mean age: 45 ± 16 vs. 47 ± 16 years; p < 0.001). More than one-third of participants (34%) reported history of smoking; 14.8% (n = 636) were current smokers and 19.2% (n = 821) were former smokers.
Table 1.
Participants’ characteristics and elevated blood pressure (BP) outcome
| Participant characteristics | Total screened population N = 4284 |
Comparisons by elevated blood pressurea | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal BP n = 3374 |
All elevated BP n = 910 (21.2%) |
Elevated BP in known hypertensive subjects n = 158 (62.2%) of 254 |
Elevated BP in non-known hypertensive subjects n = 752 (18.7%) of 4030 |
|||||
| Mean ± SD or % (n) | p valueb | Mean ± SD or % (n) | p valueb | Mean ± SD or % (n) | p valueb | |||
| Female (%) (n) | 56.4 (2417) | 57.0 (1922) | 54.4 (495) | 0.165 | 54.4 (86) | 0.530 | 54.4 (409) | 0.197 |
| Age (years) | 46 ± 16 | 44 ± 15 | 55 ± 15 | < 0.001 | 56 ± 14 | < 0.001 | 55 ± 15 | < 0.001 |
| Height (cm) | 166 ± 4 | 166 ± 4 | 166 ± 4 | 0.486 | 166 ± 4 | 0.193 | 166 ± 4 | 0.180 |
| Weight (kg) | 73.6 ± 9.3 | 72.8 ± 8.2 | 76.7 ± 12 | < 0.001 | 76.2 ± 12.1 | < 0.001 | 76.7 ± 12 | < 0.001 |
| BMI (kg/m2) | 26.6 ± 3.7 | 26.4 ± 3.3 | 27.7 ± 4.6 | < 0.001 | 27.8 ± 4.7 | < 0.001 | 27.7 ± 4.7 | < 0.001 |
| Waist circumference (cm) | 74.4 ± 16.6 | 72.7 ± 15.6 | 80.4 ± 18.6 | < 0.001 | 80.0 ± 18.2 | < 0.001 | 80.5 ± 18.6 | < 0.001 |
| Smoking history (%) (n) | 34.0 (1457) | 26.6 (896) | 61.6 (561) | < 0.001 | 61.4 (97) | < 0.001 | 61.7 (464) | < 0.001 |
| Alcohol history (%) (n) | 63.8 (2732) | 58.1 (1959) | 84.9 (773) | < 0.001 | 82.9 (131) | < 0.001 | 85.4 (642) | < 0.001 |
| Systolic BP (mmHg) | 127 ± 18 | 119 ± 12 | 153 ± 14 | < 0.001 | 153 ± 16 | < 0.001 | 153 ± 13 | < 0.001 |
| Diastolic BP (mmHg) | 76 ± 11 | 72 ± 8 | 88 ± 9 | < 0.001 | 89 ± 9 | < 0.001 | 88 ± 9 | < 0.001 |
| Blood glucose (mg/dL) | 101 ± 22 | 100 ± 21 | 105 ± 25 | < 0.001 | 103 ± 30 | 0.051 | 105 ± 24 | < 0.001 |
| Elevated blood glucose (%) (n)c | 18.2 (779) | 16.5 (558) | 24.3 (221) | < 0.001 | 7.6 (12) | 0.003 | 27.8 (208) | < 0.001 |
Table combine both descriptive data on the total screened population (first column) and univariate participants characteristics comparison between each time the normal BP group versus elevated BP, versus poorly controlled BP and versus new discovered cases BP groups
aElevated blood pressure was defined as a systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg
bComparison by unpaired t-test or χ2, Normal BP as comparison group of all p values
cElevated blood glucose ≥ 100 mg/dL after minimum 6-h fast
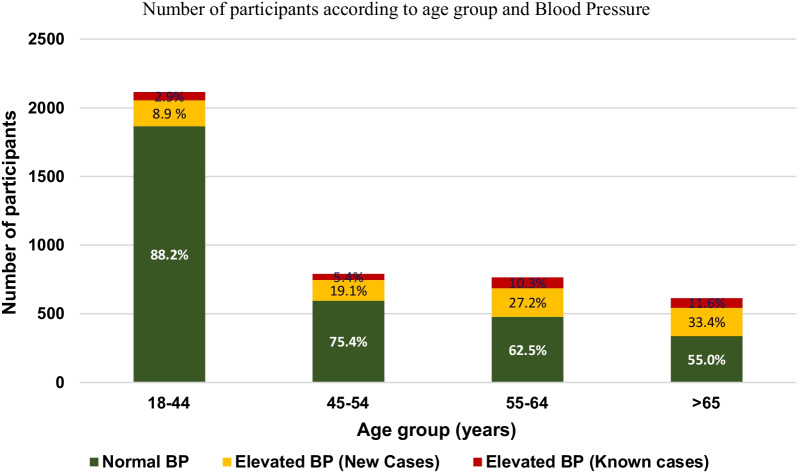
Of the 4284 individuals in the sampled cohort, 21.2% (n = 910) had elevated BP. Also 158 of 910 (17.4%) with elevated BP had a prior diagnosis of HTN and represent 62.2% of all individuals with a prior diagnosis of HTN (158/254). 752 of 910 (82.6%) individuals detected with elevated BP were without a prior diagnosis of HTN and represent 18.7% of all participants without history of HTN. A separate analysis which included known cases with controlled hypertension showed a normal BP with mean systolic blood pressure (SBP) of 127 ± 19 mmHg, and mean diastolic pressure (DBP) of 77 ± 11 mmHg (Additional file 1). The proportion of new discovery of elevated BP progressively increased with advancing age: 19.1% in 45–54 year old, 27.2% in 55–64 year old, and 33.4% among those with age > 65 years (Fig. 1).
Fig. 1.
Number of participants according to age group and blood pressure
Gender was similar between Normal BP and Elevated BP categories (Table 1). Age, weight, body mass index (BMI), and waist circumference were all higher in the Elevated BP categories compared with the Normal BP (all p < 0.001). Fasting blood glucose concentration was significantly higher in the all elevated BP and Elevated BP among Non-Known Hypertensive subjects. The prevalence of smoking history, alcohol consumption, and elevated blood glucose are all higher in the Elevated BP categories compared with the Normal BP category (all p < 0.005).
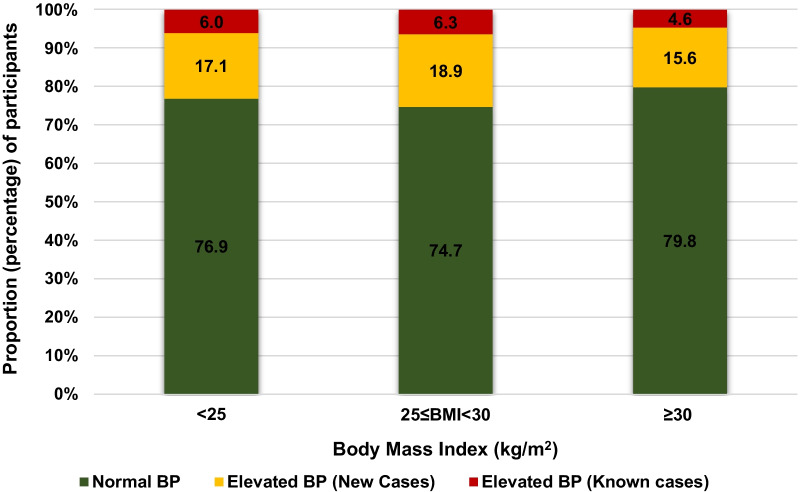
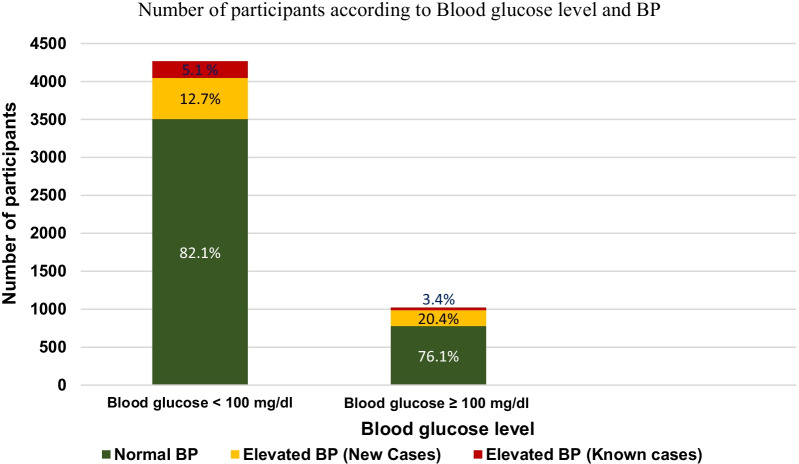
The distribution of BP category was similar across BMI categories (Fig. 2). An elevated BG was found in 18.2% (n = 779) of participants, with a greater proportion of elevated BP among those participants with BG ≥ 100 mg/dL (Fig. 3). Among the 713 participants (16.6%) with a reported diagnosis of diabetes, 616 (79.1%) had a BG ≥ 100 mg/dL; BG was elevated in 163 participants without a reported diagnosis of diabetes (3.8% of the total screened population, 4.6% among participants without a reported diagnosis of diabetes) (Additional file 1).
Fig. 2.
Number of participants by BMI and BP class
Fig. 3.
Number of participants according to blood glucose level and BP
Independent risk factors of elevated BP in the 3 groups: general screened population, known hypertensive population and the newly discovered elevated BP sub-groups
Table 2 presents a multivariate analysis results of risk factors in the Normal BP group versus each of the three Elevated BP categories: All Elevated BP, Elevated BP in Known Hypertensive subjects, and Elevated BP in Non-Known Hypertensive subjects. The findings revealed that advanced age was independently associated with progressively increased risk of having elevated BP in all three comparisons (all p < 0.001). The relationship between Elevated BP category with overweight (25 ≤ BMI < 30 kg/m2) and obesity (BMI ≥ 30 kg/m2), weight, waist circumference, and fasting blood glucose were less consistent. Current smoking, alcohol consumption, and fasting blood glucose are significant predictors of Elevated BP in multivariate models (Table 2).
Table 2.
Participants characteristics associated elevated BP in multivariate analysis
| Normal BP versus all elevated BPa | Normal BP versus elevated BP in known hypertensive subjects | Normal BP versus elevated BP in non-known hypertensive subjects | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years) | ||||||
| 18–44 | Refd | Refd | Refd | |||
| 45–54 | 1.8X [1.46–2.36] | < 0.001* | 1.87 [1.10–3.18] | 0.021* | 1.76 [1.35–2.88] | < 0.001* |
| 55–64 | 3.05 [2.41–3.85] | < 0.001* | 3.81 [2.35–6.19] | < 0.001* | 2.81 [2.17–3.63] | < 0.001* |
| ≥ 65 | 3.91 [3.03–5.03] | < 0.001* | 4.43 [2.62–7.49] | < 0.001* | 3.63 [2.75–4.78] | < 0.001* |
| Weight (Kg) | 1.02 [1.00–1.04] | 0.024* | 1.02 [0.99–1.06] | 0.187 | 1.03 [1.01–1.05] | 0.009* |
| BMI (kg/m2) | ||||||
| < 25 kg/m2 | Refd | Refd | Refd | |||
| 25 ≤ BMI < 30 kg/m2 | 0.55 [0.44–0.71] | < 0.001* | 0.80 [0.49–1.30] | 0.368 | 0.49 [0.38–0.64] | < 0.001* |
| ≥ 30 kg/m2 | 0.73 [0.48–1.10] | 0.134 | 0.93 [0.42–2.11] | 0.875 | 0.63 [0.40–1.00] | < 0.050* |
| Waist circumference (Cm) | 1.01 [1.00–1.02] | < 0.001* | 1.01 [1.00–1.02] | 0.012* | 1.01 [1.00–1.02] | 0.001* |
| Smoking history (%) | ||||||
| Never smoked | Refd | Refd | Refd | |||
| Ex-smoker | 1.04 [0.83–1.30] | 0.753 | 1.35 [0.86–2.14] | 0.189 | 1.09 [0.85–1.39] | 0.493 |
| Current smoker | 5.06 [4.05–6.31] | < 0.001* | 5.71 [3.68–8.86] | < 0.001* | 5.7X [4.50–7.25] | < 0.001* |
| Alcohol history (%) | ||||||
| Never | Refd | Refd | Refd | |||
| 0–5 years | 2.48 [1.85–3.32] | < 0.001* | 2.50 [1.37–4.56] | 0.003* | 2.48 [1.80–3.51] | < 0.001* |
| 6–10 years | 2.76 [1.85–3.32] | < 0.001* | 3.38 [1.72–6.28] | < 0.001* | 2.73 [1.90–3.94] | < 0.001* |
| 11–15 years | 2.79 [1.92–4.06] | < 0.001* | 3.21 [1.55–6.61] | 0.002* | 3.04 [2.03–4.54] | < 0.001* |
| > 15 years | 1.97 [1.57–2.48] | < 0.001* | 1.52 [0.93–2.47] | 0.092* | 2.11 [1.64–2.71] | < 0.001* |
| Fasting blood glucose (mg/dL) | 1.00 [1.00–1.01] | 0.034* | 1.01 [1.00–1.02] | 0.007 | 1.00 [1.00–1.01] | 0.055 |
| Elevated blood glucose (%)b | 0.91 [0.73–1.14] | 0.426 | 0.16 [0.08–0.33] | < 0.001 | 1.15 [0.91–1.46] | 0.235 |
aElevated Blood pressure was defined as a systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg
bComparison by unpaired t-test or χ2 versus Normal BP
cElevated blood glucose ≥ 100 mg/dL after minimum 6-h fast
dRef: reference category
Table shows results from the multivariate analysis assessing independent participants’ characteristics associated to the elevated BP, comparison between each time the normal BP group versus elevated BP, versus poorly controlled BP and versus new discovered cases BP groups
Discussion
This report revealed two main unexpected findings: A high rate of Elevated BP among individuals not known to have hypertension (18.7%), a higher rate of elevated BP in individuals with a known diagnosis of hypertension (62.2%), and high rates of elevated blood glucose (79.1%). High rates of elevated BP and elevated BG identified through a health screening campaign in this Rwandan district were surprising given the rural characteristics of the district and relatively low population age (46.4 ± 15.8 years).
Awareness and hypertension control in this study
This report identified a high prevalence of elevated BP in individuals not previously known to have hypertension. This finding confirms both a high prevalence and a poor awareness of HTN typical of [17] especially in Sub-Sahara Africa [6, 22]. However, it is lower compared to the pooled weighted awareness rate of 16.9% in 1990, 29.2% in 2000 and 33.7% in 2010 reported by Adeloye et al. [23] from population-based studies on hypertension in Africa. The availability of data on awareness rates of hypertension in sub-Saharan Africa (SSA) in general, and from rural areas in particular, are scattered and generated by a wide range of studies differing in methodology thus limiting the opportunity for reliable comparisons [7, 24, 25]. However, the recent study by Chow et al. [26] reports that only one in three individual is aware of their hypertension status (i.e. higher than in our study), and about only 8% have their blood pressure controlled (i.e. lower than in our study). The poor treatment control in African settings has been largely reported by other authors, however the 37.8% reported rate by this study is higher than the reported average of 23.4% [26]. The high proportion of rural dwellers with uncontrolled hypertension in our study can be partially explained by geographically inaccessibility to health facilities for treatment monitoring.
High prevalence found in this study
This study identified an overall prevalence of Elevated BP of 21.2% (95% CI = 20.0–22.4%). A prior population-based study in Rwanda estimated the prevalence of HTN in people between 15 and 64 years old at 15.3% (16.4% for males and 14.4% for females) [22], indicating that ~ 1 million people are living with HTN in Rwanda. The reported rate of elevated BP in this study was also lower than the 27.7% reported by a workplace-screening program conducted in an urban Ethiopian setting [27]. Other studies from rural areas of Sub-Saharan Africa have reported variable rates of HTN prevalence that ranges from 5 to 52% [8, 28, 29]. Adeloye et al. [23] had estimated hypertension pooled prevalence pooled in Africa at 26.1% (95% CI: 23.6–33.6). In line with those other pooled data, the results presented in the current study confirm a serious concern of the rising prevalence of hypertension in rural Rwanda. This threat is further exacerbated by an under-resourced healthcare system; the nationwide network of healthcare clinics available to treat NCDs in Rwanda only serve approximately 80,000 patients, representing a coverage of < 10% [30]. These data indicate that the majority of Rwandan adults with HTN are not only untreated, but undiagnosed.
Participants’ characteristics in this study
The participant characteristics associated with elevated BP in this study are similar to the preliminary data reported by Muggli et al. [28] from another screening event held in the rural area of the District of Nyaruguru (Southern Rwanda) which found a much low prevalence of hypertension at 8.8%. However, that screening event reported a median age of 32 years, much younger than 46 ± 16 years reported in this study. This lower age likely contributes to the low prevalence of hypertension found in their report. As reported in other studies [7, 10, 17, 29], gender was not significantly associated to elevated BP in our study. However, consistent with other studies [25, 31, 32], advancing age was a significant predictor of elevated BP in this study.
The association between overweight and elevated blood pressure has been long-established, including in African populations [15, 33–35]. This study revealed a high prevalence of overweight and obesity as noted in other prior studies conducted in rural Rwandan areas [22]. The relationship between BP and anthropometric indices such as BMI, WC, waist to hip ratio (WHpR), and waist to height ratio (WHtR) have been described in others studies [33]. Our study was not powered to assess this comparison between overweight indices, and some indices were not available for analysis. Therefore, we added all raw data on weight, height, BMI and WC into analysis both univariate and multivariate after assess collinearity with correlation matrix between variables. As the distribution of BP category was similar across BMI categories, it was not conclusive to clearly draw a conclusion on the association between the BMI categories and the elevated BP in our study. This was probably due to the small sample size in cells.
Nevertheless, this finding is unexpected and further work is needed to validate the association identified in this study since these are in contrast with other reports from rural India [34] and Nigerian [35] which identify a higher BMI as a major risk factor for hypertension [18, 36, 37].
Other risk factors identified in multivariable analysis as independently associated to elevated BP are in line with previous Rwanda WHO STEPs study by Nahimana et al. [22]. In their nationwide study, a logistic regression model revealed that age, alcohol consumption, blood glucose levels, and raised BMI were significantly associated with hypertension, a finding confirmed in this study.
Implication for strategy for the prevention, diagnosis and treatment of hypertension in Rwanda
This study brings additional evidence to support tailored measures for the prevention, diagnosis and treatment of hypertension in rural populations in Rwanda. Recent reviews analyzing root causes for poor blood pressure control in Rwanda [38] and in Eastern Sub-Saharan Africa [39] highlight this unmet healthcare need. On a global scale, the World Health Organization (WHO) has created a target to reduce heart attacks and stroke by 25% by 2025 [17], and the World Heart Federation (WHF) launched a roadmap focusing on raised BP awareness, treatment, and control during the 2015 World Health Assembly in Geneva [40]. Monitoring through systematically organized periodic screening campaigns for HTN, diabetes, and other NCD risk factors in rural population remains a key strategy for optimizing treatment and control [24]. Early detection with opportunistic screening campaigns can also mitigate multiple barriers like poor health education literacy [38] and low socio-economic status [39]. However, given the need to balance competing healthcare priorities including infectious diseases, nutritional deficiencies, and maternal and perinatal morbidity and mortality, a reallocation of healthcare resources towards continuous monitoring of NCDs in LMICs is necessary [6].
Study limitations
This study fills in a scientific data gap and its large sample size is adequate for subgroup comparisons. This is also one of the first studies to characterize the blood pressure and blood glucose profiles in a remote rural area in Rwanda through a community screening. However, it has also some limitations. First, given the current study is not a systematic population-based screening, bias from participant self-selection likely influenced the results of this study. Second, given the cross-sectional design of this screening study, we are unable to determine temporality and causality in the study; therefore, causation can only be inferred. Third, like other opportunistic screening studies wherein the BP measurement are taken at a single visit, this is indeed a limitation. However, the BP measurements were taken through a rigorous and standardized protocol (3 times, with 1-min intervals, after rest period). Additionally, all subjects with elevated BP were referred to nearest health facilities -as usual procedure, where hypertension diagnosis was confirmed for all the detected elevated BP cases, and followed an appropriate management. The follow up data are not part of this manuscript. The study reports only elevated BP and not prevalence of hypertension because the three blood pressure measurements were performed only on one occasion. Fourth, we were unable to explore the contribution of other risk factors such as unhealthy diet, salt intake, physical activity and psychosocial stress, the lack of information on those risk factors that significantly affects the prevalence of hypertension and limits the strength of the conclusions. Such parameters were not easy to legitimate and scientifically evaluate through an opportunistic screening campaign, so the related information were not available during casual data collection.” Additionally, the quantification of alcohol intake and smoking where rather empirical in the region where traditional alcoholic drinks [41] and traditional pipe smoking were hard to define according to international standards [42]. There might be also social desirability biases to underestimating some of the lifestyle and behavioral questions, such as smoking and alcohol consumptions. Despite these limitations, the study makes significant contribution and fills a substantial gap in the current Rwanda and regional context. The use of standardized and robust methodologies, tools and high response rate observed in the study increased its representativeness and strengthens its value in informing the CVDs prevention by enabling tailored preventive measures and optimize the treatment control.
Conclusion
This opportunistic screening findings confirm a high rate of newly discovered elevated BP and poor control among rural population in Rwanda. This study identified age, current smoking, and alcohol history and waist circumference to be the risks associated with elevated BP. Having such high rate of newly discovered abnormal BP from that rural population would imply an epidemiological risk profile transition for hypertension, that needs further studies. Nevertheless, these data support the need to strengthen also in rural areas of Rwanda an adequate strategy for the prevention, early diagnosis and treatment of hypertension. Future longitudinal studies to analyse in more details the specific CVDs risk in that population are needed.
Supplementary Information
Additional file 1. Additional descriptive analysis on participants’ characteristics and diabetes cases (old versus new cases).
Acknowledgements
We would like to thank the staff of Partners on Health/Inshuti mu Buzima for their support and contributions towards the successful completion of the study.
Abbreviations
- BP
Blood pressure
- BG
Blood glucose
- BMI
Body Mass Index
- CVDs
Cardiovascular diseases
- DBP
Diastolic blood pressure
- HC
Health center
- HBC
Health belief models
- HTN
Hypertension
- LMICs
Low middle income countries
- NCDs
Non-communicable diseases center
- NISR
National Institute of Statistics of Rwanda
- RBC
Rwanda Biomedical Center
- SBP
Systolic blood pressure
- WC
Waist circumference
- WHpR
Waist to hip ratio
- WHtR
Waist to height ratio
- WHO
World Health Organization
- WHF
World Heart Federation
Author contributions
Conceptualization, methodology, data collection: EN, EH, GN, SD, and FU; Data curation and data analysis: RM, MT and JNU; Original draft preparation: RM, MT, EM, JNU, LT, AB, TC, MB, DR, LDF and MT; Review and Editing: EN, EM, RM, AN, VDR, KS, LT, AB, TWC, MB, DR, LDF and MT. All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Partners in Health/Inshuti Mu Buzima (PHI/IMB) and Government of Rwanda through Rwanda Biomedical Center (Ministry of Health). PHI/IMB is an international non-profit organization that supports the Ministry of health in implementation of health services in three districts namely Burera, Kirehe and Kayonza. The funding parties have no involvement in the design of the study, analysis, interpretation of data or writing this publication.
Availability of data and materials
The dataset used and/or analyzed during this study is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Routinely collected program data analyzed for this study are maintained by the Rwanda Biomedical Centre (RBC), Division of Non-communicable Diseases. The ethical procedures for the collection of these data were governed by the Medical Research Council of Rwanda, and site authorizations were obtained from the Ministry of Health for hosting sites. Secondary analyses of routinely collected and de-identified data are exempted for additional ethical clearance by RBC. Approval (No. 3653/RBC/2021) for utilization of the data was obtained by RBC. Verbal consent was obtained from all eligible participants to the screening before blood sample and interview-based data collection. The study was conducted in accordance with the principles of the Declaration of Helsinki and local Rwandan regulations.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumann AA, Mutabazi V, Brown AL, Hooley C, Reeds D, Ingabire C, Ndahindwa V, Nishimwe A, Cade WT, de Las Fuentes L, Proctor EK, Karengera S, Schecthman KB, Goss CW, Yarasheski K, Newsome B, Mutimura E, Davila-Roman VG. Dissemination and implementation program in hypertension in Rwanda: report on initial training and evaluation. Glob Heart. 2019;14:135–141. doi: 10.1016/j.gheart.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A. Hypertension International Society of Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Kotwani P, Kwarisiima D, Clark TD, Kabami J, Geng EH, Jain V, Chamie G, Petersen ML, Thirumurthy H, Kamya MR, Charlebois ED, Havlir DV, Search Collaboration Epidemiology and awareness of hypertension in a rural Ugandan community: a cross-sectional study. BMC Public Health. 2013;13:1151. doi: 10.1186/1471-2458-13-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathers Loncar DCD. Updated projections of global mortality and burden of disease, 2002–2030: data sources, methods and results. Geneva: World Health Organization; 2005. pp. 1–130. [Google Scholar]
- 5.Amegah AK. Tackling the growing burden of cardiovascular diseases in Sub-Saharan Africa. Circulation. 2018;138:2449–2451. doi: 10.1161/CIRCULATIONAHA.118.037367. [DOI] [PubMed] [Google Scholar]
- 6.Twagirumukiza M, De Bacquer D, Kips JG, de Backer G, Stichele RV, Van Bortel LM. Current and projected prevalence of arterial hypertension in sub-Saharan Africa by sex, age and habitat: an estimate from population studies. J Hypertens. 2011;29:1243–1252. doi: 10.1097/HJH.0b013e328346995d. [DOI] [PubMed] [Google Scholar]
- 7.Schutte AE, Srinivasapura Venkateshmurthy N, Mohan S, Prabhakaran D. Hypertension in low- and middle-income countries. Circ Res. 2021;128:808–826. doi: 10.1161/CIRCRESAHA.120.318729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agyemang C. Rural and urban differences in blood pressure and hypertension in Ghana, West Africa. Public Health. 2006;120:525–533. doi: 10.1016/j.puhe.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 9.John O, Campbell NRC, Brady TM, Farrell M, Varghese C, Velazquez Berumen A, Velez Ruiz Gaitan LA, Toffelmire N, Ameel M, Mideksa M, Jaffe MG, Schutte AE, Khan T, Lopez Meneses LP. The 2020 "WHO technical specifications for automated non-invasive blood pressure measuring devices with cuff". Hypertension. 2021;77:806–812. doi: 10.1161/HYPERTENSIONAHA.120.16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banyangiriki J, Phillips J. Prevalence of hypertension among working adults in Rwanda. Iran J Public Health. 2013;42:925–926. [PMC free article] [PubMed] [Google Scholar]
- 11.Kavishe B, Biraro S, Baisley K, Vanobberghen F, Kapiga S, Munderi P, Smeeth L, Peck R, Mghamba J, Mutungi G, Ikoona E, Levin J, Bou Monclus MA, Katende D, Kisanga E, Hayes R, Grosskurth H. High prevalence of hypertension and of risk factors for non-communicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med. 2015;13:126. doi: 10.1186/s12916-015-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones CL, Jensen JD, Scherr CL, Brown NR, Christy K, Weaver J. The Health Belief Model as an explanatory framework in communication research: exploring parallel, serial, and moderated mediation. Health Commun. 2015;30:566–576. doi: 10.1080/10410236.2013.873363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute of Statistics Rwanda . EICV 3 thematic report-Rwanda. Kigali: NISR; 2012. [Google Scholar]
- 14.World Health Organization . WHO STEPS surveillance manual: the WHO STEPwise approach to chronic disease risk factor surveillance. Geneva: WHO; 2005. [Google Scholar]
- 15.W.H.O Expert Consultation. Waist circumference and waist-hip ratio report of a WHO Expert Consultation, Geneva, 8–11 December 2008. 2011.
- 16.Xie Y, Ma M, Li Z, Guo X, Sun G, Sun Z, Zheng J, Sun Y, Zheng L. Elevated blood pressure level based on 2017 ACC/AHA guideline in relation to stroke risk in rural areas of Liaoning province. BMC Cardiovasc Disord. 2019;19:258. doi: 10.1186/s12872-019-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dzudie A, Ojji D, Anisiuba BC, Abdou BA, Cornick R, Damasceno A, Kane AL, Mocumbi AO, Mohamed A, Nel G, Ogola E, Onwubere B, Otieno H, Rainer B, Schutte A, Ali IT, Twagirumukiza M, Poulter N, Mayosi B, Pascar Hypertension Task Force members Development of the roadmap and guidelines for the prevention and management of high blood pressure in Africa: proceedings of the PASCAR hypertension task force meeting: Nairobi, Kenya, 27 October 2014. Cardiovasc J Afr. 2015;26:82–85. [PubMed] [Google Scholar]
- 18.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Sr, Williamson JD, Wright JT., Jr ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;2018(138):e484–e594. doi: 10.1161/CIR.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 19.Solomon S, Mulugeta W. Disease burden and associated risk factors for metabolic syndrome among adults in Ethiopia. BMC Cardiovasc Disord. 2019;19:236. doi: 10.1186/s12872-019-1201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heianza Y, Arase Y, Fujihara K, Tsuji H, Saito K, Hsieh SD, Kodama S, Shimano H, Yamada N, Hara S, Sone H. Screening for pre-diabetes to predict future diabetes using various cut-off points for HbA(1c) and impaired fasting glucose: the Toranomon Hospital Health Management Center Study 4 (TOPICS 4) Diabet Med. 2012;29:e279–e285. doi: 10.1111/j.1464-5491.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- 22.Nahimana MR, Nyandwi A, Muhimpundu MA, Olu O, Condo JU, Rusanganwa A, Koama JB, Ngoc CT, Gasherebuka JB, Ota MO, Okeibunor JC. A population-based national estimate of the prevalence and risk factors associated with hypertension in Rwanda: implications for prevention and control. BMC Public Health. 2017;18:2. doi: 10.1186/s12889-017-4536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adeloye D, Basquill C. Estimating the prevalence and awareness rates of hypertension in Africa: a systematic analysis. PLoS ONE. 2014;9:e104300. doi: 10.1371/journal.pone.0104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J. Screening for hypertension in adults. JAMA. 2021;325:1688. doi: 10.1001/jama.2021.5288. [DOI] [PubMed] [Google Scholar]
- 25.Yonga G, Okello FO, Herr JL, Mulvaney A, Ogola EN. Healthy Heart Africa: a prospective evaluation of programme outcomes on individuals' hypertension awareness, screening, diagnosis and treatment in rural Kenya at 12 months. Cardiovasc J Afr. 2020;31:9–15. doi: 10.5830/CVJA-2019-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, Kazmi K, Lanas F, Wei L, Lopez-Jaramillo P, Fanghong L, Ismail NH, Puoane T, Rosengren A, Szuba A, Temizhan A, Wielgosz A, Yusuf R, Yusufali A, McKee M, Liu L, Mony P, Yusuf S, Pure Study investigators Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 27.Angaw K, Dadi AF, Alene KA. Prevalence of hypertension among federal ministry civil servants in Addis Ababa, Ethiopia: a call for a workplace-screening program. BMC Cardiovasc Disord. 2015;15:76. doi: 10.1186/s12872-015-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muggli F, Parati G, Suter P, Bianchetti M, Radovanovic D, Umulise A, Muvunyi B, Ntaganda E. Blood pressure in a population of a rural area of Rwanda: preliminary data. J Hypertens. 2021;39:e400. doi: 10.1097/01.hjh.0000749228.89530.93. [DOI] [Google Scholar]
- 29.Katchunga PB, Twagirumukiza M, Kluyskens Y, Kaishusha D, Baguma M, Bapolisi A, Cikomola J, Ntabure T, Callens S, M'Buyamba-Kabangu JR, Van Bortel L. Blood pressure in the Congolese adult population of South Kivu, Democratic Republic of Congo: preliminary results from the Bukavu Observ Cohort Study. Rev Epidemiol Sante Publique. 2015;63:339–345. doi: 10.1016/j.respe.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 30.Rwanda Biomedical Center. Rwandans encouraged to go for early screening to prevent NCDs. 2021;2021.
- 31.Everett B, Zajacova A. Gender differences in hypertension and hypertension awareness among young adults. Biodemogr Soc Biol. 2015;61:1–17. doi: 10.1080/19485565.2014.929488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drain PK, Hong T, Hajat A, Krows M, Govere S, Thulare H, Moosa MYS, Bassett I, Celum C. Integrating hypertension screening at the time of voluntary HIV testing among adults in South Africa. PLoS ONE. 2019;14:e0210161. doi: 10.1371/journal.pone.0210161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutema BT, Chuka A, Ayele G, Megersa ND, Bekele M, Baharu A, Gurara MK. Predictive capacity of obesity indices for high blood pressure among southern Ethiopian adult population: a WHO STEPS survey. BMC Cardiovasc Disord. 2020;20:421. doi: 10.1186/s12872-020-01686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshmukh PR, Gupta SS, Dongre AR, Bharambe MS, Maliye C, Kaur S, Garg BS. Relationship of anthropometric indicators with blood pressure levels in rural Wardha. Indian J Med Res. 2006;123:657–664. [PubMed] [Google Scholar]
- 35.Adedoyin RA, Mbada CE, Bisiriyu LA, Adebayo RA, Balogun MO, Akintomide AO. Relationship of anthropometric indicators with blood pressure levels and the risk of hypertension in Nigerian adults. Int J Gen Med. 2008;1:33–40. doi: 10.2147/IJGM.S3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma JR, Mabhida SE, Myers B, Apalata T, Nicol E, Benjeddou M, Muller C, Johnson R. Prevalence of hypertension and its associated risk factors in a Rural Black Population of Mthatha Town, South Africa. Int J Environ Res Public Health. 2021;18:1215. doi: 10.3390/ijerph18031215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shittu RO, Odeigah LO, Fakorede KO, Sikiru BA, Sule AG, Musah Y, Adeyemi FM. Prevalence and correlates of hypertension-outcome of a free medical screening in Oke-Ogun area of Oyo state, Nigeria, West Africa. J Am Soc Hypertens. 2018;12:268–274. doi: 10.1016/j.jash.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Sibomana JP, McNamara RL, Walker TD. Patient, clinician and logistic barriers to blood pressure control among adult hypertensives in rural district hospitals in Rwanda: a cross-sectional study. BMC Cardiovasc Disord. 2019;19:231. doi: 10.1186/s12872-019-1203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorato MM, Davari M, Kebriaeezadeh A, Sarrafzadegan N, Shibru T, Fatemi B. Reasons for poor blood pressure control in Eastern Sub-Saharan Africa: looking into 4P's (primary care, professional, patient, and public health policy) for improving blood pressure control: a scoping review. BMC Cardiovasc Disord. 2021;21:123. doi: 10.1186/s12872-021-01934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adler AJ, Prabhakaran D, Bovet P, Kazi DS, Mancia G, Mungal-Singh V, Poulter N. Reducing cardiovascular mortality through prevention and management of raised blood pressure: a World Heart Federation roadmap. Glob Heart. 2015;10:111–122. doi: 10.1016/j.gheart.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Morojele NK, Dumbili EW, Obot IS, Parry CDH. Alcohol consumption, harms and policy developments in sub-Saharan Africa: the case for stronger national and regional responses. Drug Alcohol Rev. 2021;40:402–419. doi: 10.1111/dar.13247. [DOI] [PubMed] [Google Scholar]
- 42.Noubiap JJ, Nansseu JR, Endomba FT, Ngouo A, Nkeck JR, Nyaga UF, Kaze AD, Bigna JJ. Active smoking among people with diabetes mellitus or hypertension in Africa: a systematic review and meta-analysis. Sci Rep. 2019;9:588. doi: 10.1038/s41598-018-37858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional descriptive analysis on participants’ characteristics and diabetes cases (old versus new cases).
Data Availability Statement
The dataset used and/or analyzed during this study is available from the corresponding author on reasonable request.