Abstract
RIPK3 (receptor-interacting protein kinase 3) is a serine/threonine-protein kinase. As a key component of necrosomes, RIPK3 is an essential mediator of inflammatory factors (such as TNFα-tumor necrosis factor α) and infection-induced necroptosis, a programmed necrosis. In addition, RIPK3 signaling is also involved in the regulation of apoptosis, cytokine/chemokine production, mitochondrial metabolism, autophagy, and cell proliferation by interacting with and/or phosphorylating the critical regulators of the corresponding signaling pathways. Similar to apoptosis, RIPK3-signaling-mediated necroptosis is inactivated in most types of cancers, suggesting RIPK3 might play a critical suppressive role in the pathogenesis of cancers. However, in some inflammatory types of cancers, such as pancreatic cancers and colorectal cancers, RIPK3 signaling might promote cancer development by stimulating proliferation signaling in tumor cells and inducing an immunosuppressive response in the tumor environment. In this review, we summarize recent research progress in the regulators of RIPK3 signaling, and discuss the function of this pathway in the regulation of mixed lineage kinase domain-like (MLKL)-mediated necroptosis and MLKL-independent cellular behaviors. In addition, we deliberate the potential roles of RIPK3 signaling in the pathogenesis of different types of cancers and discuss the potential strategies for targeting this pathway in cancer therapy.
Keywords: RIPK3 signaling, MLKL necroptosis, MLKL-independent signaling, Cancer pathogenesis
Introduction
RIPK3 (receptor-interacting protein kinase 3) is the key component of the RIPK1–RIPK3–MLKL (mixed lineage kinase domain-like) complex called the necrosome [1–4]. The canonical function of RIPK3 signaling is to stimulate MLKL activation and to trigger necroptosis [5–8]. In addition, RIPK3 has many non-canonical functions, including triggering inflammasome activation and cytokine production, stimulating mitochondrial metabolism and ROS production, as well as regulating autophagy and cell proliferation [9–12]. Moreover, the cells dying of RIPK3-mediated necroptosis induce sterile inflammation reactions in the tissue environment by stimulating both innate and adaptive immune responses. Furthermore, the immune responses induced by necroptotic cells are not limited to local tissue, but can be expanded and cause systematic responses [13, 14].
RIPK3 signaling has been implicated in the pathogenesis of many types of cancers [15–17]. Many chemicals that can either activate or repress RIPK3 signaling have been identified and used in experimental studies. However, due to the complicated functions of RIPK3 signaling both intrinsic to the target cells and extrinsic to the neighboring cells and accumulated immune cells, the role of RIPK3 signaling in cancer pathogenesis is very complex. Both tumor-repressive and tumor-promoting activities of RIPK3 signaling have been observed. In addition, the roles of RIPK3 in cancer development, progression, metastasis and relapse might be not the same. Thus targeting RIPK3 signaling for cancer therapy is still in the pre-clinical stage. Detailed understanding of the roles of RIPK3 signaling in the pathogenesis of different types of cancers is required. In this review, we summarize the recent research on the regulatory mechanisms of RIPK3 signaling, discuss the roles of RIPK3 signaling in different types of cancers and elaborate on the potential strategies to target RIPK3 signaling for cancer therapy.
RIPK3 gene and its encoded protein
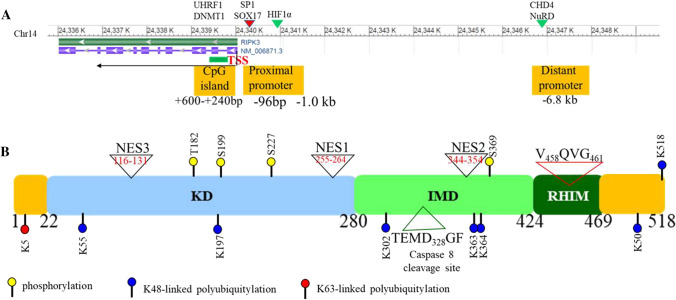
The human RIPK3 gene is located at chromosome 14q12. It contains 10 exons and spans about 40 kb of genomic DNA (Fig. 1a) [18]. The region from − 95 bp to 0 bp of the TSS (transcription start site) in RIPK3 showed strong promoter activity [19]. Transcription factors SOX17 (SRY-box 17) and zinc finger protein SP1 were found to regulate the transcriptional activity of RIPK3 by binding to this promoter region. The SNP (single nucleotide polymorphism) rs3212247-C in the RIPK3 promoter region was identified in a Chinese population which increases the affinity of SOX17 and SP1 binding to the RIPK3 gene promoter, resulting in enhanced RIPK3 expression. Individuals with this SNP exhibit an increased susceptibility to heart failure with poor prognosis [20]. RIPK3 expression is also regulated by epigenetic modification. A CpG island was identified at the 240–600 bp region downstream of the TSS in the human RIPK3 gene. The epigenetic regulator UHRF1 (ubiquitin-like, containing PHD and RING finger domains 1) recognizes hemi-methylated DNA and regulates RIPK3 expression by maintaining the methylation levels in this CpG island by recruiting DNMT1 (DNA methyltransferase 1) [19, 21]. In addition, a conserved upstream promoter was identified in the RIPK3 gene which is located at − 6.8 kb of the TSS. This promoter could be recognized by CHD4 (chromodomain helicase DNA binding protein 4), an enzyme of the NuRD (nucleosome remodeling and deacetylase) chromatin-remodeling complex. CHD4 represses RIPK3 transcription through deacetylation of the RIPK3 promoter region [22, 23].
Fig. 1.
RIPK3 gene and protein structure. A Two promoters, proximal and distal, have been identified in the RIPK3 gene. Transcription factors SP1 and SOX17 bind to the proximal promoter to up-regulate RIPK3 expression, whereas CHD4, in the chromatin-remodeling complex NuRD, recognizes the distal promoter and represses RIPK3 expression by deacetylating H3K27. In addition, a HIF1 binding site was identified at 1 kb upstream of the TSS. In hypoxic conditions, HIF1α binds to this site and represses RIPK3 expression. Furthermore, a CpG island was identified approximately 240–600 bp downstream of the TSS. Epigenetic regulator UHRF1 represses RIPK3 expression by recruiting DNMT1 and maintaining the methylation state of this CpG island. B RIPK3 protein is composed of a kinase domain (KD) at the N terminus and a RHIM domain at the C terminus connected by an intermediate domain (IMD). The caspase 8 cleavage site, three NESs and several key phosphorylation and ubiquitination sites are indicated
Human RIPK3 is a 518 amino acid protein which contains a kinase domain (22-280aa) at the N terminus and a RHIM (RIP homotypic interaction motif, 424-469aa) at the C terminus, which are linked by an IMD (intermediate domain) (Fig. 1b) [24, 25]. Both the kinase domain and RHIM are indispensable for RIPK3’s activity [26]. RHIM mediates the interaction of RIPK3 with other RHIM-containing proteins including RIPK1, TRIF (TIR-domain-containing adapter inducing interferon-β, as called TICAM1) and the cytoplasmic nucleic acid sensor ZBP1 (Z-DNA binding protein 1, also known as DAI) [4, 26, 27]. RIPK3 is predominantly localized in the cytoplasm but can shift between the nucleus and cytoplasm [28]. A nuclear localization-like sequence was identified in the 224-518aa region, and two NESs (leucine-rich canonical nuclear export signals), NES1 (255-264aa) and NES2 (344-354aa), mediate the nucleocytoplasmic shuttling of RIPK3 in a CRM1-dependent manner. Another NES (NES3, 116-131aa) was found to be controlling the cytoplasmic distribution of RIPK3 [28].
RIPK3 protein levels are controlled by both caspase 8-mediated cleavage and ubiquitin-mediated degradation mechanisms [29–36]. Several ubiquitin ligases such as CHIP [33], PELI1 [34], TRIM11 [35], TRIM25 [36], Parkin [30], and vIRD (viral inducer of RIPK3 degradation) [32], as well as deubiquitinases including A20 and USP22 [31, 37], have been identified as RIPK3 binding proteins, regulating ubiquitination and stability of RIPK3 protein. RIPK3 signaling activity is also regulated by phosphorylation and polymerization stimulated by upstream signaling [31, 34, 37]. The key post-translational modification sites of RIPK3 are summarized in Fig. 1B.
Upstream signaling pathways that stimulate the activation of RIPK3
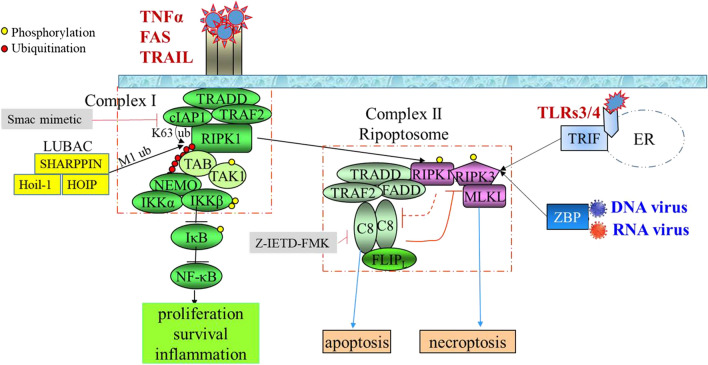
RIPK3 signaling is primarily studied in the context of TNFα stimulation (Fig. 2). Upon TNFα stimulation, TNFR1 forms homo-trimers which rapidly recruit TRADD, RIPK1, TRAF2/5 and cIAP1/2 to assemble a complex in the lipid rafts of the plasma membrane named complex I [38]. In this complex, cIAP1/2 and TRAF2/5 add a K63-linked polyubiquitination chain on RIPK1 at the K376 residue [39–46]. The polyubiquitination chain provides a unique dock to recruit the TAB2/3-TAK1 complex and IKK complex. The IKK complex is formed by active IκB kinase (IKK) α, IKKβ and IKKγ (as named NF-kB essential modifier, NEMO) [40, 45–50]. A linear ubiquitin chain assembly complex (LUBAC, composed of HOIP, HOIL-1 and SHARPIN) is also recruited to complex I, which synthesizes M1 linear ubiquitin chains on cIAP1/2, RIPK1, NEMO and IκBα to enhance the activation of TAK1 and its downstream signaling [51–58]. Activated TAK1 induces the activation of the IKK complex by direct phosphorylation of IKKβ which then activates NF-κB signaling by phosphorylation of IκBα, the negative regulator of NF-κB. In addition, active TAK1 also induces MKK4/7-JNK and MKK3/6-P38 MAPK pathways by directly phosphorylating the corresponding MKKs. NF-κB and JNK signaling regulate the expression of many pro-survival genes such as BCL-xL, cFLIP and cIAPs as well as many inflammatory cytokines such as IL1β and TNFα [46, 47, 59]. Thus complex I primarily mediates TNFα-stimulated survival and pro-inflammatory signals.
Fig. 2.
RIPK3-mediated necroptotic pathway. TNF family members stimulate RIPK3 activation through inducing RIPK1 activation and RIPK1–RIPK3 interaction, whereas bacterial and viral infections activate RIPK3 through stimulating either TLR3/4 signal-triggered TRIF-RIPK3 interaction or pathogen DNA/RNA-triggered ZBP1-RIPK3 interaction. Upon TNFα stimulation, RIPK3-mediated necroptosis can be experimentally induced by Smac-mimetic + Caspase 8 inhibitor combination treatment. Smac-mimetic treatment releases RIPK1 from complex I and promotes the formation of complex II. Caspase 8 inhibitor treatment enhances RIP-mediated necroptosis by preventing the degradation of RIPK1 and RIPK3. C8 and C10 represent caspase 8 and caspase 10, respectively. ER endoplasmic reticulum
The dissociation of complex I is required for TNFα-stimulated activation of RIPK3 signaling, which is mediated by DUBs such as OTULIN (OTU deubiquitinase with linear linkage specificity), CYLD (cylindromatosis) or A20 (TNFAIP3) [60–64]. The DUBs remove the ubiquitin chains from RIPK1 and release RIPK1 from complex I. In the cytosol, RIPK1 can be activated by auto-phosphorylation of its Ser14/15, 20, 161 and 166 residues (Ser 14/15, 161 and 166, as well as Thr169 in mice) [65–67]. Among these sites, auto-phosphorylation of Ser166 is critical for RIPK1-mediated apoptosis and necroptosis [67–69]. RIPK1 then forms a new complex called complex II (or Ripoptosome) [38, 70, 71]. The complex II is composed of a complex IIa and a necrosome. The complex IIa is formed by TRADD, RIPK1, pro-caspase-8, FADD and c-FLIP which regulates caspase 8-mediated extrinsic apoptotic signaling. The necrosome complex is composed of RIPK1, RIPK3 and MLKL and activates MLKL-mediated necroptosis through serial phosphorylation cascade [72]. Thus, the complex II mediates two TNFα-induced death signals. RIPK1 is involved in both complex I and complex II, and plays a critical role in regulating the balance of TNFα-induced survival and death signals [73]. Such functions of RIPK1 are primarily controlled by cIAP and LUBAC-mediated ubiquitination, and finely regulated by several kinases such as TAK1, ULK1, IKKα/β, TBK1 and P38-MK2, which phosphorylate human RIPK1 at residues Ser320, Ser357, Ser25, Thr189/Thr190 and Ser320/Ser335, respectively (corresponding to residues Ser321, Ser356, Ser25, Thr189/Thr190 and Ser321/Ser336 in mouse RIPK1) [66]. Phosphorylation at of these residues will reduce the kinase activity of RIPK1 and prevent RIPK1 from integrating into complex II. In addition, RIPK1 kinase activity is also regulated by ROS via oxidation of cysteines 257, 268 and 586 in RIPK1 [74–76].
After complex II is assembled, the activity of the necrosome is tightly restricted by pro-caspase 8 and cFLIPL heterodimers. The pro-caspase 8/cFLIPL heterodimers inhibit necroptosis by cleaving RIPK1 (after residue Asp324 for human and Asp325 for mouse) and RIPK3 (after residue Asp328 for human and Asp333 for mouse) [77–80]. Interestingly, cFLIPS, a short splice isoform of the cFLIP gene, competes with cFLIPL to form heterodimers with pro-caspase 8. The pro-caspase 8/cFLIPs heterodimers promote necrosome assembly and necroptosis. Therefore, cFLIP isoforms in complex II determine whether cell death occurs by RIPK3-dependent necroptosis or caspase-dependent apoptosis. cFLIP is a NF-κB target gene. Thus necroptosis is also regulated by NF-κB signaling through regulating cFLIP expression [70, 71]. In addition, TNFα receptor signaling through PDK1 activates p90 ribosomal S6 kinase 1 and 2 (RSK1/2). RSK1/2 represses the activity of pro-caspase8 and permits necroptosis by phosphorylating pro-caspase8 at Thr265 for mouse and Thr263 for human [81].
Within the necrosome, activated RIPK1 is able to bind to RIPK3 through the interaction of their RHIM motifs [82]. Once RIPK1–RIPK3 interaction occurs, more RIPK3 is recruited into the complex leading to RIPK3–RIPK3 homo-oligomerization/aggregation and RIPK3 phosphorylation for activation [82]. Although RIPK1 kinase does not directly phosphorylate RIPK3, RIPK1 kinase activation is required for induction of RIPK1 and RIPK3 interaction. Thus, RIPK1 kinase activity is indispensable for IFNα-induced RIPK3 activation and necroptosis. Interestingly, a recent study suggested that in RIPK1-deficient cells, a death domain containing adaptor TRADD can also directly interact with RIPK3 to facilitate TNFα-stimulated necroptosis [83]; however, other studies suggested that TRADD mediates TNFα-induced apoptosis by competing with RIPK1 [84, 85]. In addition to TNFα, FAS and TRAIL might also use a RIPK1-dependent mechanism in the induction of necrosome formation and RIPK3 activation [86].
TRIF (TIR-domain-containing adapter inducing interferon-β) and ZBP1 (Z-DNA binding protein 1, also known as DAI) are other two RHIM-containing proteins in mammals which can directly interact and activate RIPK3 signaling [4, 27]. TRIF is an adaptor protein for both TLR3 (receptor of double-stranded RNA) and TLR4 (bacterial lipopolysaccharide—LPS receptor) signals [4, 87], while ZBP1 is a cytoplasmic nucleic acid sensor which recognizes viral and endogenous DNA and RNA in cytosol [3, 88]. Bacterial and viral infections, through activation of TLR3 and TLR4 signaling, induce TRIF and RIPK3 interaction to activate RIPK3 [89, 90]. Viral infections induce interaction of RIPK3 and ZBP1 to activate RIPK3 signaling [91–93]. In addition, the ZBP1 and RIPK3 interaction can be stimulated by endogenous cytoplasmic DNA and RNA induced by DNA damage reagents such as radiation and chemotherapy [94]. Binding of TRIF or ZBP1 to RIPK3 will trigger the activation of RIPK3 to assemble the necrosome by recruiting MLKL. Both TRIF and ZBP1-mediated RIPK3 activation are enhanced when RIPK1 is deleted, suggesting that RIPK1 competes with TRIF and ZBP1 for RIPK3 interaction [95–97] (Fig. 2). Furthermore, some viruses produce RHIM-containing proteins such as ICP6 protein in HSV-1 (herpes simplex virus 1) infection which directly activate RIPK3 necroptotic signaling and induce necroptosis in infected cells [98, 99]. Interestingly, the ICP6 protein activates necroptosis only in mouse cells but not human cells [99].
Signaling pathway cascades downstream of RIPK3
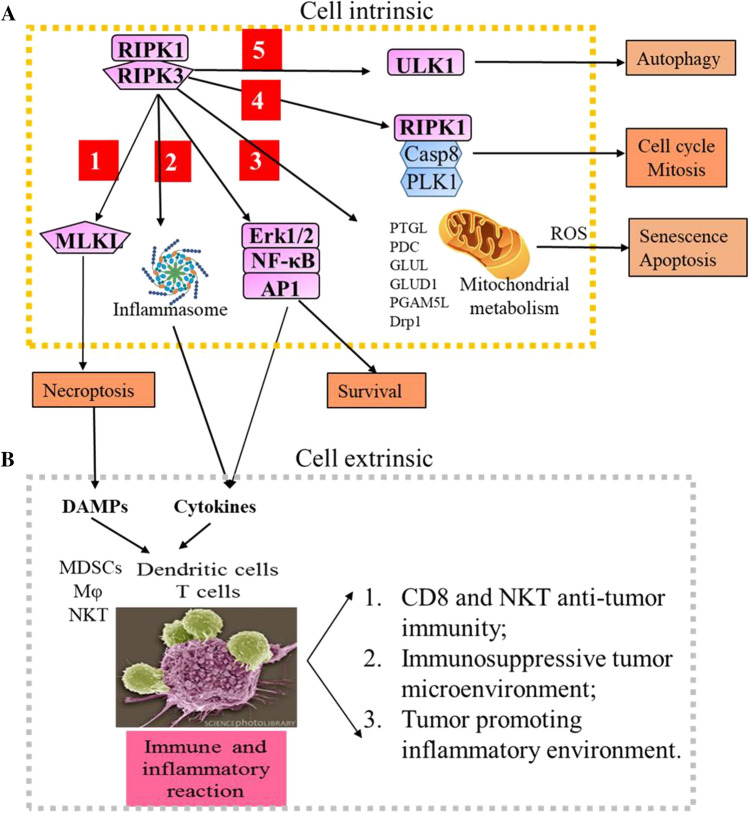
Multiple pathways downstream of RIPK3 signaling have been identified, including MLKL-necroptosis, inflammasome-cytokine, mitochondrial-metabolism, and autophagy (Fig. 3A). Active RIPK3 regulates these pathways by directly binding and/or phosphorylating the key molecular substrates.
RIPK3-necroptosis. In low active caspase 8 conditions, the active RIPK3 polymerizes and recruits MLKL to assemble the necrosome. Within the necrosome, the active RIPK3 phosphorylates MLKL at residues Thr357 and Ser358 (Ser352 and Thr349 in mouse RIPK3) within the activation loop [65, 82, 100–102]. The phosphorylation of these residue results in a conformation change in the MLKL protein, leading to dislocation of the N-terminal four helical bundle domain (4HB) away from the kinase domain. The 4HB domain has lipid-binding capacity, and the exposure of the 4HB domain permits the plasma membrane translocation and binding of MLKL to execute necroptosis [102–105]. After being recruited to the plasma membrane, the Ser358-phosphorylated MLKL is further phosphorylated by TAM (Tyro3, Axl, and Mer) kinase at Tyr376 residue, which resulting in MLKL oligomerization, formation of membrane-rupturing pores and cytolytic death [106, 107]. Apart from the constitution of the RIPK3/MLKL complex, in cardiomyocytes, active RIPK3 also induces necroptosis by phosphorylating CaMKII and induces cyclophilin D (CypD)-mediated-mPTP (mitochondrial permeability transition pore) in the myocardium [108]. RIPK3 deficiency prevents ischemia/reperfusion injury and doxorubicin-induced CaMKII activation and blocks necroptosis in the myocardium.
-
RIPK3-cytokine production. RIPK3 signaling regulates the production of many cytokines and chemokines. Most early studies demonstrated that RIPK3 signaling induces necroptosis-dependent extrinsic cytokine/chemokine production [109, 110]. The necroptotic cells “spill out” intracellular molecules called DAMPs (damage-associated molecular patterns), including HMGB1 (high mobility group box-1), IL1 family cytokines (IL1α, IL1β, IL18 and IL33), heat-shock proteins, ribonucleoproteins, U1 snRNP, mtDNA, the S100 calcium-binding proteins S100A8 and S100A9, uric acid, ATP and mitochondrial factors. DAMPs are then recognized by PRRs (pattern-recognition receptors), such as TLRs and IL-1 receptors on bystander cells, thereby stimulating an inflammatory response and inducing the production of cytokines and chemokines [10, 12, 111–113].
However, recent studies have also shown that RIPK3 plays a cell death-independent role in cytokine/chemokine production by inducing intrinsic inflammatory signaling [89, 114, 115]. In many types of cells, such as macrophages and dendritic cells (DCs), RIPK3 regulates cytokine/chemokine production by regulating NF-κB and MAPK pathways to induce transcription of cytokine/chemokine genes and promote NLRP3 (Nod-like receptor family pyrin domain-containing 3) inflammasome activation for processing and maturation of cytokines including IL1β and IL18 [89, 116–119]. To date, the detailed mechanism of how RIPK3 controls NF-κB activity remains a mystery. However, two mechanisms have been proposed to explain how RIPK3-signaling regulates inflammasome activity: (1) In caspase 8-expressing cells, RIPK3 protein promotes inflammasome activation through a caspase 8–RIPK1–NLRP3 axis, independent of its kinase activity [25, 29, 120]. (2) In caspase 8 inactivated cells, RIPK3 promotes inflammasome activation via its kinase-mediated MLKL phosphorylation. Phospho-MLKL can directly induce NLRP3-inflammasome activation via potassium efflux after membrane rupture [111, 121, 122]. In addition, RIPK3 also drives cytokine translation by activating the cap-dependent translation initiation pathway components AKT, mTORC1 and eIF4E [123]. Such RIPK3-induced mRNA translation and cytokine synthesis can be observed even in cells with ruptured plasma membranes [13, 124].
RIPK3-mitochondrial metabolism. In addition to the MLKL-necroptotic pathway, RIPK3 also serves as a major component of other signaling pathways independent of necroptosis. For examples, RIPK3 mediates TNF-stimulated tricarboxylic acid cycle and oxidative phosphorylation, resulting in enhanced ROS (reactive oxygen species) production. RIPK3 plays this role by directly interacting and phosphorylating the rate-limiting enzymes for glycolysis such as PYGL (liver form of glycogen phosphorylase) and the E3 subunit of PDC (pyruvate dehydrogenase complex), as well as the key enzymes for glutamine catabolism including GLUL (glutamate-ammonia ligase) and GLUD1 (glutamate dehydrogenase 1) [8, 75, 125]. In addition, RIPK3 directly phosphorylates PGAM5, a mitochondrial phosphatase [126]. PGAM5 activates DRP1-mediated mitochondrial fission and ROS production by dephosphorylating DRP1 at Ser637. RIPK1 also activates DRP1 by phosphorylating DRP1 at Ser616 in a RIPK3-dependent fashion independent of MLKL. Thus, the RIP3–RIPK1 complex regulates DRP1-mediated mitochondrial fission and ROS production by both removing phospho-Ser637 and adding phospho-Ser616 on DRP1. ROS in turn stimulates activation of the NLRP3 inflammasome [116, 126, 127]. However, note that some of these results have not been independently validated.
RIPK3-cell cycle. It was found that RIPK3 regulates cell growth by controlling cell cycle progression and cell division. RIPK3 plays this role by directly or indirectly phosphorylating proteins associated with the cell cycle, metabolism and development, as demonstrated by proteome-wide analysis [128, 129]. Knock-out of RIPK3 in MEFs caused obvious arrest of cell cycle progression and thus cell division [129]. RIPK3 is also reported to maintain chromosome stability by interacting with caspase-8, RIPK1 and PLK1 (Polo-like kinase 1) in the mitosis-associated ripoptosome [130]. PLK1 is a pleiotropic master regulator of mitosis and regulates DNA replication after stress. Within the mitosis-associated ripoptosome, PLK1 levels are controlled by caspase8- mediated cleavage in a RIPK1-dependent manner. Deletion and inhibition of RIPK1 or caspase8 led to elevated PLK1 levels and chromosomal instability. Furthermore, PLK1 phosphorylates RIPK3 at Ser369 to prevent RIPK3 from caspase-8 cleavage. It was speculated that the elevated RIPK3 in the G2/M phase provides cells an alternative to die if a mitosis error should occur [131].
RIPK3-autophagy. In response to genotoxic stress, such as etoposide treatment- induced alternative autophagy, RIPK3 phosphorylates Ser746 on ULK1, an essential initiator of both canonical autophagy and alternative autophagy. Phospho-ULK1 (Ser746) localizes exclusively to the Golgi, which is required for alternative autophagy, but not for canonical autophagy. The loss of RIPK3 or inhibition of RIPK3 kinase activity abolished ULK1 Ser746 phosphorylation and alternative autophagy [132, 133].
Fig. 3.
RIPK3 signaling stimulates inflammation and immunity. A In addition to stimulating MLKL-mediated necroptosis (1), RIPK3 signaling also induces: cytokine production by activating ERK/NF-kB/AP1-mediated transcription and inflammasome-mediated pro-cytokine processing (2); mitochondrial metabolism and senescence/apoptosis by phosphorylating several mitochondrial proteins (3); cell cycle and mitosis by regulating the RIPK1/caspase 8/PLK1 mitosis-associated ripoptosome (4); and autophagy by phosphorylating ULK1 (5). Thus RIPK3 activation can cause at least 5 types of cell-intrinsic effects. B In addition, RIPK3 activation also causes amplified damage in local tissues and significant systematic symptoms which are cell extrinsic effects. The necroptotic cells induce immune and inflammatory reactions in tissues by secreting cytokines to recruit both innate and adaptive immune cells, and releasing DAMPs to activate immune cells
RIPK3 signaling stimulates immunogenicity and inflammatory reactions
Cells dying via apoptosis send “eat-me” signals to macrophages, thus apoptotic cells are quickly removed by macrophages and cause limited influence to other tissue cells. In contrast to apoptotic cells, cells dying via necroptosis induce significant inflammatory reactions and immune responses. Necroptotic cells secrete substantial amounts of cytokines and chemokines including IL6, CXCL1, CXCL2, CCL8 and CCL2. Such cytokine/chemokine secretion starts when RIPK1-NF-κB and RIPK1–RIPK3 signaling is activated and continues even after the cell membrane ruptured [13, 124]. In addition, necroptotic cells release DAMPs into the surrounding environment which can activate immune cells and promote inflammation [134] (Fig. 3B).
The cytokines/chemokines induce the accumulation of myeloid cells including granulocytes, monocytes and DCs, as well as T and B lymphocytes. The DAMPs can be taken up by DCs which process and present the DAMPs to CD4+ T cells and CD8+ T cells to stimulate adaptive immunity through a phenomenon known as antigenic cross-priming [13, 135]. Some of the DAMPs may have cell-specific immunogenic activity which induce cell-specific adaptive immune responses. For example, DAMPs released from necrotic cancer cells can be sensed by various PRRs in immune cells and lead to anti-cancer immune surveillance [8, 13, 115, 135–142]. Recently it has been reported that RIPK3-mediated necroptotic cells also stimulate the activation of NKT cell-mediated immune responses [143]. The accumulated myeloid cells induce inflammatory reactions in local tissues by stimulating innate immune responses. Such innate immune responses could either promote an adaptive immune response by inducing a M1 type of macrophage, or repress an adaptive immune response by stimulating a M2 type of macrophage and MDSCs (myeloid-derived suppressive cells) [144, 145]. Thus in cancer tissues, the RIPK3-dependent cytokine synthesis within necroptotic dying cells plays critical roles in antitumor CD8+ T cell responses [13, 115, 135, 142].
RIPK3 signaling in the pathogenesis of cancers
It is well-accepted that the escape of apoptosis is an indisputable hallmarks of cancers. Recent studies demonstrated that necroptosis resistance is also very common in cancer cells, suggesting that escape from necroptosis could be also a potential hallmark of cancers. Necroptosis has been suggested to play a pivotal role in multiple facets of cancer biology, including oncogenesis, cancer metastasis and cancer immunity. However, distinct from apoptosis, necroptotic cells induce significant anti-tumor immune response by inducing DC-mediated anti-tumor T cells. In addition, necroptotic cells also stimulate inflammatory reactions in the tumor tissues by releasing cytokines and DAMPs. The inflammatory reactions can either enhance or repress an anti-tumor microenvironment. Furthermore, RIPK3 signaling stimulates mitochondrial oxidative phosphorylation and other cellular activities. All these functions explain the complex roles of RIPK3 signaling in the pathogenesis of different types of cancers. Both tumor-repressive and tumor-promoting activities of RIPK3 signaling have been reported (summarized in Table 1). The pro-carcinogenesis or anti-carcinogenesis functions of RIPK3 signaling are primarily dependent on the balance of cytokines and chemokines produced, as demonstrated in some cancer models. However, how does this exquisite balance work remains poorly understood.
Table 1.
Summary of literature on tumor-repressive and tumor-promoting activity of RIPK3
| Cancers with RIPK3 down-regulation | Cancers with RIPK3 over-expression |
|---|---|
|
Breast cancer [148] Acute myeloid leukemia [151, 152] Head and neck squamous cell carcinoma [153] Melanoma [154] Mesothelioma [147] Prostate cancer [155] |
Pancreatic ductal adenocarcinoma [167–169] Colon cancer [170] Esophageal cancer [170] HOXA-expressing acute myeloid leukemia |
| Cancer models showing tumor-repressive activity of RIPK3 | Cancer models showing tumor-promoting activity of RIPK3 |
|---|---|
|
RUNX-1-ETO acute myeloid leukemia [151] FLT3-ITD acute myeloid leukemia [151] |
HOXAs-expressing acute myeloid leukemias [182] |
|
TAK1 knockout Hepatocellular carcinoma [165] Myc/NrasG12V Hepatocellular carcinoma Myc/AKT1 Hepatocellular carcinoma [166] |
Myc/NrasG12V intrahepatic cholangiocarcinoma Myc/AKT1intrahepatic cholangiocarcinoma [166] |
|
B16 melanoma [173] Lewis lung carcinoma [173] |
|
|
AOM-DSS-induced colitis-associated colorectal cancer [167, 175, 176] ApcMin/+ colon cancer [179] MC38 colon cancer [179] |
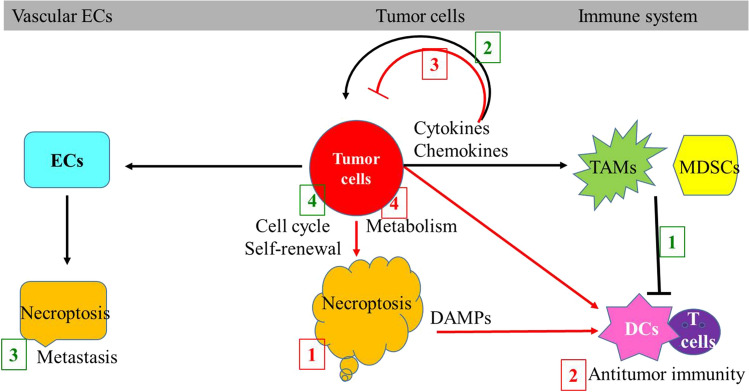
Based on current knowledge, the mechanisms by which RIPK3 signaling represses tumor development including: (1) killing cancer cells via necroptosis; (2) inducing T cell-mediated cancer immune surveillance; (3) secreting tumor-repressive cytokines; and (4) restricting tumor development by inducing mitochondrial metabolism and ROS production. The mechanisms by which RIPK3 signaling promotes tumor development and progress include (1) inducing the accumulation of immune-repressive myeloid cells such as MDSCs and TAMs (tumor-associated macrophages) to promote cancer cells escape from the immune surveillance; (2) secreting tumor-promoting cytokines and inducing tumor-promoting microenvironment and angiogenesis; (3) promoting tumor metastasis by inducing death of vascular endothelial cells (ECs); and (4) promoting tumor relapse by inducing proliferation and self-renewal signaling (Fig. 4).
-
Tumor repressive activity of RIPK3 signaling. Although deletion and/or mutation of the RIPK3 gene has not been reported in any cancers, loss of RIPK3 expression has been detected in > 80% of human cancer cell lines [21, 146, 147]. A necroptosis sensitivity screen in 941 human cancer cell lines demonstrated that 780/941 (83%) of human cancer cell lines are fully resistant to TSZ (TNFα + Cyclohexamide + zVAD.fmk) -induced necroptosis due to the loss of RIPK3 expression. Ectopic RIPK3 expression or pharmacological restoration of RIPK3 expression in some of these cancer cell lines reduce tumor growth in vitro culture and in a xenograft models [19], suggesting a tumor-repressive function of RIPK3.
Decreased RIPK3 expression has also been reported in many types of primary cancers, such as breast cancer [148], colorectal cancer [149, 150], acute myeloid leukemia (AML) [151, 152], head and neck squamous cell carcinoma [153], melanoma [154], primary malignant mesotheliomas [147] and prostate tumors [155]. Studies of patient tumor biopsies and tumor xenograft models demonstrated that loss of RIPK3 expression occurs progressively during tumor progression and disease metastasis in melanoma, colorectal, gastric, and ovarian cancers. In these types of cancers, the reduced expression of RIPK3 is associated with disease progression and metastasis as well as shortened overall survival (OS) of the patients [21, 152, 154–156]. Decreased expression of MLKL is also observed in patients with gastric cancer [157], ovarian carcinoma [158], cervical squamous cell carcinoma [159], colon cancer [160], and early-stage resected pancreatic adenocarcinoma which is correlated with a decreased OS [161]. These findings suggest that, like apoptosis, necroptosis also functions as a natural barrier that protects against the development in many cancer types. Reduction of RIPK3 and/or MLKL expression are candidate prognostic biomarkers in those cancers.
In most types of cancers, RIPK3 expression is silenced by hypermethylation of the CpG island that localized at 240–600 bp downstream of the TSS [21, 150, 162]. The methylation of this CpG island is maintained by DNMT1. Thus RIPK3 expression can be restored by DNMT1 inactivation or demethylation agents such as 5-azacitidine and decitabine [21]. In cancer types that harbor activing BRAF mutations and/or express high levels of AXL/TYRO3, RIPK3 expression is repressed by the oncoprotein kinases [19, 146]. Such oncoprotein kinases are known to regulate many transcription factors, including JUN, FOS, ETS, SP1 and MYC. It is possible that they repress RIPK3 expression by inactivating the activity of transcription factors such as SP1 and promoting promoter methylation of RIPK3 gene. In addition, in colon cancer tissues, RIPK3 expression is repressed by a hypoxic environment through promoting HIF1α activity [150, 163].
The tumor-suppressive effects of RIPK3 signaling have been only documented to date in certain types of leukemia and liver cancer animal models. In RUNX1-ETO or FLT3-ITD-induced leukemia models, TNFα-RIPK3 signaling represses AML development primarily by stimulating inflammasome-IL1β signaling-induced differentiation of the pre-leukemia cells. Genetic inactivation of TNF-RIPK3-inflammasome-IL1β signaling markedly accelerated leukemogenesis in these two leukemic models. MLKL-mediated necroptosis has a minor contribution to RIPK3-mediated leukemia repression. Interestingly, it seems that the tumor repressive function of RIPK3 is only limited to leukemia induced by certain genetic mutations, because deletion of RIPK3 fails to accelerate leukemia development in MLL-ENL and Eμ-MYC-induced leukemia models [151, 164].
In the liver-specific TAK1 knockout HCC (hepatocellular carcinoma) model, RIPK3 controls the transition from inflammation to cancer by inhibiting caspase8-induced chromosomal aberrations associated with immortalization of hepatocytes. RIPK3 deletion accelerates inflammatory hepatocarcinogenesis but inhibits cholestasis by promoting caspase8- and JNK-dependent compensatory cell proliferation [165]. In transposon-mediated co-expressing oncogenic Myc/NrasG12V (pCaMIN) or Myc/AKT1 (pCaMIA) models, Seehawer et al. demonstrated that hydrodynamic tail-vein injection-induced apoptosis in the microenvironment promotes HCC development, whereas electroporation-triggered necroptosis promotes ICC (intrahepatic cholangiocarcinoma) development in animals. Pharmacological or genetic inhibition of necroptosis converts ICC to HCC. These studies suggest that necroptotic cells may reshape the microenvironment and direct the lineage commitment of liver cancer. This process is independent of the oncogenic drivers but may be involved in the epigenetic regulation of the genes Tbx3 and Prdm5 [166].
Tumor promoting activity of RIPK3 signaling. In some of the inflammatory cancer types, such as human PDA (pancreatic ductal adenocarcinoma), RIPK1, RIPK3 and MLKL, the key components of necrosome, are highly expressed in cancer tissues compared with normal pancreas [167–169]. Chemotherapy such as gemcitabine further enhances the expression of RIPK1 and RIPK3 in PDA cells in vivo. In patients with colon and esophageal cancers, the higher phosphorylation levels of MLKL are correlated with a poorer prognosis and shorter OS [170]. In some breast cancer cell lines, the knockout of RIPK1, RIPK3, or MLKL genes in cancer cells markedly reduced their tumorigenicity and appeared to sensitize the cells to radiotherapy. Moreover, in a xenograft model, the necroptosis inhibitor NSA (necrosulfonamide) greatly delayed tumor growth [170]. Two recent studies demonstrated that although RIPK3 is frequently suppressed in primary tumors, it is dramatically re-expressed in recurrent breast tumor cells by a histone methyltransferase G9a-mediated epigenetic mechanism [171, 172]. One study demonstrated that, high RIPK3 expression rendered recurrent tumor cells extracellular cysteine-dependent proliferation and G9a-mediated inflammation repression. As a consequence, recurrent tumor cells were highly sensitive to cysteine deprivation- induced ferroptosis and G9a inhibition-induced necroptosis [172]. Another study showed that RIPK3 was critical in preventing chromosome instability and aneuploidy in recurrent tumor cells as RIPK3 knockdown triggers mitotic defects and p53 activation. Thus RIPK3 knockdown in recurrent tumor cells reduced clonogenic growth, causing cytokinesis failure, p53 stabilization, and repressed the activities of YAP/TAZ [171].
Fig. 4.
RIPK3 signaling has both tumor-promoting and tumor-repressive activities. RIPK3 signaling represses cancer development by (1) killing the cancer cells through necroptosis; (2) inducing DC/T cell-mediated antitumor immunity; (3) secreting tumor-suppressive cytokines; and (4) inducing cancer-restrictive mitochondrial metabolism and ROS production (the numbers are indicated in red). RIPK3 signaling promotes cancer development by: (1) inducing immune-suppressive myeloid cells; (2) producing tumor-promoting cytokines; (3) inducing death in vascular endothelial cells; and (4) regulating cell cycle and/or self-renewal signaling (the numbers are indicated in green)
The tumor-promoting activity of RIPK3 signaling has been assessed in several animal models. In both p48CreKrasG12D(KC) and Pdx1CreKrasG12DTp53R172H(KPC) murine PDA models, RIPK3-mediated necroptosis in cancer cells promotes cancer cell growth and disease progression primarily by releasing CXCL1 chemokine and SAP130 nuclear protein [167]. CXCL1 and SAP130 recruit MDSCs and TAMs to the tumor tissues through activation of CXCR2 and Mincle signaling. MDSCs and TAMs inhibit T cell-mediated antitumor immune reactions. Therefore, blockade of necroptosis by RIPK3 knockout or inactivation of CXCL1/Mincle signaling protects against pancreatic oncogenesis in PDA. In addition, necroptotic PDA cells also produce the chemokine CXCL5 which may promote the migration and invasion of tumor cells by activation of CXCR2 signaling in tumor cells [168]. An additional study demonstrated that TAMs in PDA tissue also express high levels of RIPK1. RIPK1 in TAMs is required for the tumor-promoting activity of the TAMs. Inhibition of RIPK1 reprograms TAMs from a tumor-promoting M2 type to a tumor-protective M1 type, which provides T cell-mediated adaptive anti-tumor immunity in PDA. Targeting RIPK1 synergized with a PD1 blocker promoting co-stimulator-based immunotherapies [169]. In B16 melanoma or LLC1 (Lewis lung carcinoma line 1) lung carcinoma metastasis models, tumor cells express amyloid precursor protein (APP). APP promotes tumor cell extravasation and metastasis by inducing death receptor 6 (DR6)-mediated necroptosis of vascular ECs. Targeting DR6-mediated necroptosis in ECs inhibits the development of tumor metastasis [173]. However, such conclusions were challenged by a recent study which demonstrated that inhibition of RIPK1 activity had no effect on tumor growth/survival in a mutant Kras-driven PDA model, nor did it reduce lung metastases in a B16 melanoma model [174]. Whether such discrepancies are due to the different role of RIPK1 and RIPK3 activity in tumor pathogenesis need to been studied further.
In the AOM-DSS-induced CAC (colitis-associated colorectal cancer) model, several labs demonstrated that RIPK3 promotes cancer development by two potential mechanisms: (1) inducing JNK signaling-mediated proliferation in premalignant intestinal epithelial cells (IECs); and (2) repressing anti-cancer T cell immunity by CXLC1-dependent recruitment of immunosuppressive myeloid cells. Thus, RIPK3 deletion may represses CAC development in this model by repressing tumor cell proliferation and reducing infiltration of immunosuppressive subsets of myeloid cells [167, 175, 176]. In addition, in a CAC xenograft model, radiation induces both apoptosis and necroptosis in cancer cells and normal tissue cells. Necroptotic cells stimulate tumor repopulation through secreting IL-8 and HMGB1 in a MLKL/JNK axis-dependent manor. The elevated expression of IL-8 in tumor tissue is associated with a worse prognosis in CAC patients. Blockage MLKL/JNK/IL-8 during conventional radiotherapy may enhance the efficacy of radiotherapy in CAC [177, 178]. Furthermore, in both ApcMin/+mice and MC38 transplantable tumor models, RIPK3 signaling in MDSCs in tumor tissues promotes tumor growth by stimulating the expansion of IL17-producing T cells. All these studies suggest RIPK3 signaling as a potential therapeutic target in colorectal cancer[179]. However, other studies suggest that loss of RIPK3 does not affect or can even enhance DSS-induced colitis and CRC development [115, 180, 181]. Such differences are most likely due to variation in the gut microbiota.
Modifying RIPK3-MLKL signaling for cancer therapy
Radiotherapy, chemotherapy, and immunotherapy are administered to kill cancer cells primarily by inducing apoptosis. However, some cancer cells are resistant to these therapies due to the inactivation of apoptosis signaling pathways. Inducing necroptosis has been proposed as an alternative strategy for cancer treatment to overcome apoptosis-resistant cancer cells [17]. In addition, due to the immunogenicity of necroptotic cells, induction of necroptosis in cancer cells has been used as a “vaccine” to trigger specific anti-cancer T cell responses. However, in some of the cancers, RIPK3-signaling might promote cancer development and progression. Specifically, inhibition of RIPK3 signaling might be a useful strategy to treat such cancers. Many RIPK3 inhibitors have been developed and are currently being used in clinical trials for autoimmune and inflammatory diseases.
-
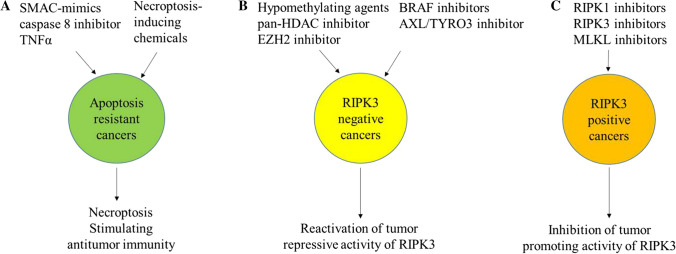
Overcome apoptotic resistance in cancer cells by inducing necroptosis. SMAC mimetics are small-molecule antagonists of IAPs, and can induce necroptosis when combined with TNFα and a caspase 8 inhibitor. The basic element of SMAC-mimics is a Ala-Val-Pro-Ile peptide. Five monomeric SMAC-mimics (the peptidomimetics BI891065, CUDC-427, DEBIO 1143, and LCL-161 and the non-peptidomimetic antagonist ASTX660) and three peptidomimetic dimeric SMAC-mimics (APG-1387, birinapant, and AEG40826/HGS1029) have been developed and tested in phase 1 and 2 clinical trials for the management of various malignancies, including breast, ovarian, fallopian, non-small cell lung cancer, renal cell carcinoma, colorectal and peritoneal cancer, myeloma, and leukemia [182, 183]. However, due to the significant side effects of TNFα, including TNFα in the regimen is limited. Nevertheless, in TNFα expressing cancers, SMAC-mimics + caspase 8 inhibitor combination might be sufficient to induce necroptosis. In addition, in some cancer types such as HOXA-expressing AML, endogenous TNFα can be induced by inhibition of p38 MAPK–MK2 pathway [184]. Thus addition of p38 MAPK-MK2 inhibitior to the regimen has been suggested. Recently, several necroptosis-inducing chemicals have been identified. For example, Shikonin (SHI) is a natural compound extracted from medicinal Chinese herbs which inhibits tumor growth mainly by inducing necroptosis [185–188]. SHI plays this role by repressing autophagy and CYLD, inhibiting PKM2 and inducing ROS production [30, 37, 100, 164, 189–197]. SHI synergistically kills certain types of cancers when combined with canonical chemotherapy drugs such as gemcitabine, erlotinib, docetaxel, cisplatin and paclitaxel [188, 196–204]. However, due to the loss of RIPK3 expression in most types of cancers, this treatment might only work in certain types of cancers, for example recombinant MLL-AML and other HOXAs-expressing AMLs [184]. Such chemicals might yield synergistic effect when combined with RIPK3 restoring agents [205–207]. (Fig. 5A)
In most types of cancer cells, RIPK3 expression can be restored by hypomethylating agents such as decitabine, 5-azacytidine and RG108, as well as pan-HDAC inhibitors, such as SAHA, and EZH2 inhibitors, such as EPZ6438 [205]. Such agents induce the re-expression of RIPK3 in tumor cells by demethylation of the CpG islands in the RIPK3 gene. In cancer types harboring mutations or over-expression of oncoprotein kinases, such as BRAF, AXL or FLT3, RIPK3 expression can be reactivated by specific and non-specific kinase inhibitors [146, 151]. For examples, the BRAF inhibitor TAK-632 and the AXL/TYRO3 inhibitor BMS-777607 reactivate RIPK3 expression in tumor cells with activating BRAF V600E mutations and levels of AXL/TYRO3, respectively [146]. HS-173, a phosphoinositide 3-kinase (PI3K) inhibitor can induce RIPK3-mediated necroptosis in lung cancer cells via upregulation of RIPK3 [208]. In melanoma patient tumors, the BRAF inhibitors dabrafenib and vemurafenib increased RIPK3 expression by at least 1.2-fold in 58.3% of the patients [209]. The molecular mechanism by which all these kinase inhibitors reactivate RIPK3 expression need further investigation. Nevertheless, in RIPK3-negative cancer cells, ectopic RIPK3 expression or pharmacological restoration of RIPK3 expression reduces tumor growth and increases their sensitivity to chemotherapy in a xenograft model, suggesting reactivation of RIPK3 might be a promising novel anti-cancer strategy [19, 21] (Fig. 5B).
Due to the strong immunogenic capacity of necroptotic cells, several studies assessed the potential of triggering anti-cancer immunity by inducing necroptosis in tumor cells. It was found that inducing necroptosis in local tumor cells could trigger anti-cancer immunity and inhibit metastasis to in distant organs. Furthermore, it was reported that necroptotic cancer cells or a nanovaccine which mimics necroptotic cancer cells could boost efficient anti-tumor immunity in animal models [140, 142, 210, 211]. Future studies need to determine how to specifically use necroptotic cancer cells to stimulate anti-cancer immunity without inducing immune-repressive inflammatory reactions.
Inhibit the RIPK3 pathway for cancer therapy. For inflammatory cancer types, necroptotic cells stimulate immune-repressive inflammatory reactions in the tumor environment. In addition, in some types of cancer cells, such as recurrent breast cancers, RIPK3 signaling promotes tumor growth. We found in most acute monocytic leukemia subtypes, RIPK3 is expressed and activated in the nucleus as demonstrated by phospho-RIPK3 and phospho-MLKL staining. RIPK3 signaling in these subtypes of leukemic cells promotes leukemia progression by stimulating STAT3 signaling [212]. In all these situations, inhibition of RIPK3 signaling might benefit patients and repress cancer growth. Currently, many types of selective inhibitors have been developed for RIPK3 including GSK'840, GSK'843, GSK'872, GW440139B, HS-1371, ponatinib and dabrafenib (a BRAF inhibitor); for RIPK1 such as necrostatins, GSK2982772, GSK3145095, RIPA-56, DNL747, ponatinib and pazopanib; for MLKL such as necrosulfonamide (NSA), GW806742X and protein Trx1 (Fig. 5C). Future studies will need to test whether such inhibitors can selectively repress the inflammatory cancer types when combined with other canonical chemotherapies and immune checkpoint inhibitors. In addition, the difference between the effect of RIPK1 inhibition and RIPK3 inhibition on cancer development and progression needs to be evaluated.
Fig. 5.
Potential strategies to target RIPK3 signaling for cancer treatment. A In apoptosis-resistant cancers, RIPK3 signaling activators overcome apoptosis resistance by inducing necroptosis and potentially stimulating anti-tumor immunity. B In RIPK3-negative cancers, epigenetic drugs and kinase inhibitors reactivate the tumor repressive activity of RIPK3 by inducing RIPK3 expression. C In RIPK3-positive cancers, RIPK1 and RIPK3 inhibitors might repress cancer development and progression by inhibiting the tumor-promoting activity of RIPK3
Prospective
During last decade, the upstream stimuli and downstream pathways of RIPK3 signaling have been studied intensively. Many interacting partner proteins for RIPK3 have been identified. These partner proteins regulate RIPK3 activity by mediating phosphorylation or ubiquitination modifications. The roles of RIPK3 in regulating MLKL-mediated necroptosis and several other MLKL-cell death-independent cellular behaviors have been well-documented. In addition, studies suggest that RIPK3 signaling is involved in the pathophysiology of many types of human diseases, including sepsis/systemic inflammatory response syndrome, chronic pulmonary diseases, renal diseases (such as I/R–induced AKI and kidney fibrosis), nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, cardiovascular diseases (such as heart failure, myocardial injury and aortic aneurysm), neurodegenerative diseases (such as multiple sclerosis, amyotrophic lateral sclerosis, Parkinson’s and Alzheimer’s disease, spinal cord injury, and traumatic brain injury). The roles of RIPK3 signaling in all these diseases have been well-documented by both RIPK3 knockout models and specific RIPK1–RIPK3 inhibitors [16]. However, there are still many unanswered questions need to be addressed in future.
The role of RIPK3 signaling in cancer pathogenesis is beginning to be recognized. However, cancer animal models in germ-free environment might primarily assess the cancer cell-intrinsic role of RIPK3, whereas the role of RIPK3 in the tumor microenvironment and immune response is under-evaluated. Future studies need to determine the influence of the microbiota and environmental factors on RIPK3 signaling in cancer pathogenesis by comparative studies of animal models in both regular and germ free environments.
It was demonstrated that RIPK3 and MLKL continuously shuttle between the nucleus and the cytoplasm. Within the nucleus, RIPK3 and MLKL signaling is activated as demonstrated by phosphorylation of both RIPK3 and MLKL. Studies suggest that the nuclear translocation of RIPK3 and MLKL is preceded to the signaling induction and is required for cytosolic necrosome assembly and cellular necroptosis [213, 214]. We found that in some types of AML cells especially HOXA+ types of AML cells, phospho-RIPK3 and phospho-MLKL are localized in the nucleus during interphase and localized at the mitotic spindle during G2/M phases [212, 215]. However, the detailed functions of nuclear RIPK3 in cellular behaviors have not been studied.
Both tumor-promoting and tumor-repressive activities of RIPK3-signaling have been observed. Most of these observations were obtained from studies of cancer cell lines. Although loss of RIPK3 expression is commonly detected in the primary cancer samples, high level expression of RIPK3 is also detected in some types of cancers. In addition, re-expression of RIPK3 is observed in specific cancer types from relapsed patients. Future studies need to determine whether the distinct roles of RIPK3 in cancer pathogenesis are correlated with genetic mutations, tumor subtypes, or developmental stage of the cancers. For example, we and others found that high levels of RIPK3 signaling are detected in bone marrow samples from low risk MDS (myelodysplasia syndromes) patients but are reduced during disease progression and AML transformation [215]. However, RIPK3 signaling is not decreased in HOXA+ AML patient samples, which account approximately 50% of all AML [184, 212]. In these RIPK3-expressing types of AML cells, inhibition of RIPK3 signaling represses AML cell growth, suggesting a tumor-promoting activity. Our studies suggest that in aplastic anemia and MDS bone marrow tissue, a small proportion of hematopoietic cells die of RIPK3-mediated necroptosis. Necroptotic cells in these patients stimulate T cell-mediated hematopoietic repression, which resembles the pathogenesis of immune-related aplastic anemia, or stimulate the expansion of MDSCs, which induces myelodysplastic features of MDS. In HOXA+ AML, RIPK3 signaling promotes the proliferation and maintains the undifferentiated state of AML cells. However, in HOXA− AML, RIPK3 signaling plays the opposite role [151]. These studies suggest that the roles of RIPK3 signaling in AML development and progression are associated with the disease subtypes and stages.
Most importantly, since RIPK3 signaling can be stimulated by bacterial and viral infections, the animal facility environment is very important for the study and comparison of the role of RIPK3 signaling in the pathophysiology of diseases in animal models. For examples, in many animal models, obvious diseases were observed when animals are maintained in animal facilities with viral and bacterial infections; however, these animals failed to develop diseases when maintained in germ-free animal facilities. These environmental factors may explain the contradictory conclusions of RIPK3 signaling in AOM-DSS-induced colitis- associated colorectal cancer model from different laboratories [109, 115, 167, 175, 180, 216].
Author contributions
SL drafted the first version of this review. All authors contributed to the writing of this manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by NIH Grants R01 HL133560-01 and R01 CA223194-01 through Loyola University Chicago, as well as Loyola program development funds to Jiwang Zhang.
Availability of data and materials
This is not applicable for this review.
Code availability
This is not applicable for this review.
Declarations
Conflict of interest
The authors declare that they have no competing financial or professional interests.
Ethics approval
This is not applicable for this review.
Consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shanhui Liu and Kanak Joshi contributed equally to this work.
References
- 1.Mulay SR, Desai J, Kumar SV, Eberhard JN, Thomasova D, Romoli S, Grigorescu M, Kulkarni OP, Popper B, Vielhauer V, et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun. 2016;7:10274. doi: 10.1038/ncomms10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157(5):1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Daniels BP, Kofman SB, Smith JR, Norris GT, Snyder AG, Kolb JP, Gao X, Locasale JW, Martinez J, Gale M, Jr, et al. The nucleotide sensor ZBP1 and kinase RIPK3 induce the enzyme IRG1 to promote an antiviral metabolic state in neurons. Immunity. 2019;50(1):64–76e64. doi: 10.1016/j.immuni.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27(15):1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feoktistova M, Leverkus M. Programmed necrosis and necroptosis signalling. FEBS J. 2015;282(1):19–31. doi: 10.1111/febs.13120. [DOI] [PubMed] [Google Scholar]
- 7.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 9.Shlomovitz I, Zargrian S, Gerlic M. Mechanisms of RIPK3-induced inflammation. Immunol Cell Biol. 2017;95(2):166–172. doi: 10.1038/icb.2016.124. [DOI] [PubMed] [Google Scholar]
- 10.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18(2):127–136. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- 11.Wegner KW, Saleh D, Degterev A. Complex pathologic roles of RIPK1 and RIPK3: moving beyond necroptosis. Trends Pharmacol Sci. 2017;38(3):202–225. doi: 10.1016/j.tips.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 13.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A, Albert ML. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350(6258):328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries F, Yang S, Wang B, Moynagh PN. RIP kinases: key decision makers in cell death and innate immunity. Cell Death Differ. 2015;22(2):225–236. doi: 10.1038/cdd.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najafov A, Chen H, Yuan J. Necroptosis and cancer. Trends Cancer. 2017;3(4):294–301. doi: 10.1016/j.trecan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight 2019, 4(15) [DOI] [PMC free article] [PubMed]
- 17.Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, Cheng H, Jin K, Ni Q, Yu X, et al. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019;18(1):100. doi: 10.1186/s12943-019-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473(3):285–291. doi: 10.1016/S0014-5793(00)01473-3. [DOI] [PubMed] [Google Scholar]
- 19.Yang C, Li J, Yu L, Zhang Z, Xu F, Jiang L, Zhou X, He S. Regulation of RIP3 by the transcription factor Sp1 and the epigenetic regulator UHRF1 modulates cancer cell necroptosis. Cell Death Dis. 2017;8(10):e3084. doi: 10.1038/cddis.2017.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu D, Huang J, Hu S, Zhang Y, Li S, Sun Y, Li C, Cui G, Wang DW. A common variant of RIP3 promoter region is associated with poor prognosis in heart failure patients by influencing SOX17 binding. J Cell Mol Med. 2019;23(8):5317–5328. doi: 10.1111/jcmm.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS, Kim SI, Kim SJ, Son MK, Hong SS, et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25(6):707–725. doi: 10.1038/cr.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colijn S, Gao S, Ingram KG, Menendez M, Muthukumar V, Silasi-Mansat R, Chmielewska JJ, Hinsdale M, Lupu F, Griffin CT. The NuRD chromatin-remodeling complex enzyme CHD4 prevents hypoxia-induced endothelial Ripk3 transcription and murine embryonic vascular rupture. Cell Death Differ. 2020;27(2):618–631. doi: 10.1038/s41418-019-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreenivasan K, Ianni A, Kunne C, Strilic B, Gunther S, Perdiguero E, Kruger M, Spuler S, Offermanns S, Gomez-Del Arco P, et al. Attenuated epigenetic suppression of muscle stem cell necroptosis is required for efficient regeneration of dystrophic muscles. Cell Rep. 2020;31(7):107652. doi: 10.1016/j.celrep.2020.107652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274(24):16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 25.Yu PW, Huang BC, Shen M, Quast J, Chan E, Xu X, Nolan GP, Payan DG, Luo Y. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol. 1999;9(10):539–542. doi: 10.1016/S0960-9822(99)80239-5. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277(11):9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 27.Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10(8):916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Ma J, Chen Y, Wu M. Nucleocytoplasmic shuttling of receptor-interacting protein 3 (RIP3): identification of novel nuclear export and import signals in RIP3. J Biol Chem. 2004;279(37):38820–38829. doi: 10.1074/jbc.M401663200. [DOI] [PubMed] [Google Scholar]
- 29.Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19(10):2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Lee SB, Kim JJ, Han SA, Fan Y, Guo LS, Aziz K, Nowsheen S, Kim SS, Park SY, Luo Q, et al. The AMPK-Parkin axis negatively regulates necroptosis and tumorigenesis by inhibiting the necrosome. Nat Cell Biol. 2019;21(8):940–951. doi: 10.1038/s41556-019-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, Prodhomme T, Duong B, Whang MI, Advincula R, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16(6):618–627. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Nailwal H, Rector J, Rahman MM, Sam R, McFadden G, Chan FK. A class of viral inducer of degradation of the necroptosis adaptor RIPK3 regulates virus-induced inflammation. Immunity. 2021;54(2):247–258e247. doi: 10.1016/j.immuni.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo J, Lee EW, Sung H, Seong D, Dondelinger Y, Shin J, Jeong M, Lee HK, Kim JH, Han SY, et al. CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat Cell Biol. 2016;18(3):291–302. doi: 10.1038/ncb3314. [DOI] [PubMed] [Google Scholar]
- 34.Choi SW, Park HH, Kim S, Chung JM, Noh HJ, Kim SK, Song HK, Lee CW, Morgan MJ, Kang HC, et al. PELI1 selectively targets kinase-active RIP3 for ubiquitylation-dependent proteasomal degradation. Mol Cell. 2018;70(5):920–935e927. doi: 10.1016/j.molcel.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Xie Y, Zhao Y, Shi L, Li W, Chen K, Li M, Chen X, Zhang H, Li T, Matsuzawa-Ishimoto Y, et al. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J Clin Invest. 2020;130(4):2111–2128. doi: 10.1172/JCI133264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mei P, Xie F, Pan J, Wang S, Gao W, Ge R, Gao B, Gao S, Chen X, Wang Y et al. (2021) E3 ligase TRIM25 ubiquitinates RIP3 to inhibit TNF induced cell necrosis. Cell Death Differ [DOI] [PMC free article] [PubMed]
- 37.Roedig J, Kowald L, Juretschke T, Karlowitz R, Ahangarian Abhari B, Roedig H, Fulda S, Beli P, van Wijk SJ. USP22 controls necroptosis by regulating receptor-interacting protein kinase 3 ubiquitination. EMBO Rep. 2021;22(2):e50163. doi: 10.15252/embr.202050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30(6):689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26(22):3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 41.Tang Y, Tu H, Zhang J, Zhao X, Wang Y, Qin J, Lin X. K63-linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat Commun. 2019;10(1):4157. doi: 10.1038/s41467-019-12033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283(36):24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA. 2008;105(33):11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131(4):682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Kobayashi M, Blonska M, You Y, Lin X. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. J Biol Chem. 2006;281(19):13636–13643. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- 46.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22(2):245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, et al. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J Biol Chem. 2010;285(8):5347–5360. doi: 10.1074/jbc.M109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK, Nuchtern JG, Zhang D, Fu S, Schneider MD, et al. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFkappaB and AP-1 activation as well as IL-6 gene expression. J Biol Chem. 2008;283(36):24497–24505. doi: 10.1074/jbc.M802825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Besse A, Lamothe B, Campos AD, Webster WK, Maddineni U, Lin SC, Wu H, Darnay BG. TAK1-dependent signaling requires functional interaction with TAB2/TAB3. J Biol Chem. 2007;282(6):3918–3928. doi: 10.1074/jbc.M608867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17(5):418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elton L, Carpentier I, Verhelst K, Staal J, Beyaert R. The multifaceted role of the E3 ubiquitin ligase HOIL-1: beyond linear ubiquitination. Immunol Rev. 2015;266(1):208–221. doi: 10.1111/imr.12307. [DOI] [PubMed] [Google Scholar]
- 52.Emmerich CH, Schmukle AC, Walczak H. The emerging role of linear ubiquitination in cell signaling. Sci Signal. 2011;4(204):re5. doi: 10.1126/scisignal.2002187. [DOI] [PubMed] [Google Scholar]
- 53.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 54.Liang L, Fan Y, Cheng J, Cheng D, Zhao Y, Cao B, Ma L, An L, Jia W, Su X, et al. TAK1 ubiquitination regulates doxorubicin-induced NF-kappaB activation. Cell Signal. 2013;25(1):247–254. doi: 10.1016/j.cellsig.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peltzer N, Darding M, Walczak H. Holding RIPK1 on the ubiquitin leash in TNFR1 signaling. Trends Cell Biol. 2016;26(6):445–461. doi: 10.1016/j.tcb.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, Spilgies L, Surinova S, Taraborrelli L, Hartwig T, et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13(10):2258–2272. doi: 10.1016/j.celrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peltzer N, Rieser E, Taraborrelli L, Draber P, Darding M, Pernaute B, Shimizu Y, Sarr A, Draberova H, Montinaro A, et al. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Rep. 2014;9(1):153–165. doi: 10.1016/j.celrep.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 58.Peltzer N, Darding M, Montinaro A, Draber P, Draberova H, Kupka S, Rieser E, Fisher A, Hutchinson C, Taraborrelli L, et al. LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature. 2018;557:112. doi: 10.1038/s41586-018-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao R, Fan Y, Mou Y, Zhang H, Fu S, Yang J. TAK1 lysine 158 is required for TGF-beta-induced TRAF6-mediated Smad-independent IKK/NF-kappaB and JNK/AP-1 activation. Cell Signal. 2011;23(1):222–227. doi: 10.1016/j.cellsig.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, Leonard TO, Norbury CC, Fitzpatrick L, Zhang M, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204(6):1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed N, Zeng M, Sinha I, Polin L, Wei WZ, Rathinam C, Flavell R, Massoumi R, Venuprasad K. The E3 ligase Itch and deubiquitinase Cyld act together to regulate Tak1 and inflammation. Nat Immunol. 2011;12(12):1176–1183. doi: 10.1038/ni.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 63.Komander D, Reyes-Turcu F, Licchesi JD, Odenwaelder P, Wilkinson KD, Barford D. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10(5):466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato Y, Goto E, Shibata Y, Kubota Y, Yamagata A, Goto-Ito S, Kubota K, Inoue J, Takekawa M, Tokunaga F, et al. Structures of CYLD USP with Met1- or Lys63-linked diubiquitin reveal mechanisms for dual specificity. Nat Struct Mol Biol. 2015;22(3):222–229. doi: 10.1038/nsmb.2970. [DOI] [PubMed] [Google Scholar]
- 65.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci USA. 2019;116(20):9714–9722. doi: 10.1073/pnas.1901179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10(11):1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laurien L, Nagata M, Schunke H, Delanghe T, Wiederstein JL, Kumari S, Schwarzer R, Corona T, Kruger M, Bertrand MJM, et al. Autophosphorylation at serine 166 regulates RIP kinase 1-mediated cell death and inflammation. Nat Commun. 2020;11(1):1747. doi: 10.1038/s41467-020-15466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, Macfarlane M, Cain K, et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:689. doi: 10.1016/j.molcel.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, Macfarlane M, Hacker G, Leverkus M. cIAPs block ripoptosome formation, a RIP1/Caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murphy JM, Vince JE. Post-translational control of RIPK3 and MLKL mediated necroptotic cell death. F1000Research. 2015;4:1297. doi: 10.12688/f1000research.7046.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133(4):693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ, Chen X, Cai Q, Yang ZH, Huang D, Wu R, et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat Commun. 2017;8:14329. doi: 10.1038/ncomms14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schenk B, Fulda S. Reactive oxygen species regulate Smac mimetic/TNFalpha-induced necroptotic signaling and cell death. Oncogene. 2015;34(47):5796–5806. doi: 10.1038/onc.2015.35. [DOI] [PubMed] [Google Scholar]
- 76.Wu W, Wang X, Berleth N, Deitersen J, Wallot-Hieke N, Bohler P, Schlutermann D, Stuhldreier F, Cox J, Schmitz K, et al. The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep. 2020;31(3):107547. doi: 10.1016/j.celrep.2020.107547. [DOI] [PubMed] [Google Scholar]
- 77.Lalaoui N, Boyden SE, Oda H, Wood GM, Stone DL, Chau D, Liu L, Stoffels M, Kratina T, Lawlor KE, et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature. 2020;577(7788):103–108. doi: 10.1038/s41586-019-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tao P, Sun J, Wu Z, Wang S, Wang J, Li W, Pan H, Bai R, Zhang J, Wang Y, et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature. 2020;577(7788):109–114. doi: 10.1038/s41586-019-1830-y. [DOI] [PubMed] [Google Scholar]
- 79.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278(51):51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 80.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13(19):2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang ZH, Wu XN, He P, Wang X, Wu J, Ai T, Zhong CQ, Wu X, Cong Y, Zhu R, et al. A non-canonical PDK1-RSK signal diminishes pro-caspase-8-mediated necroptosis blockade. Mol Cell. 2020;80(2):296–310e296. doi: 10.1016/j.molcel.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150(2):339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Chang X, Feng J, Yu J, Chen G. TRADD mediates RIPK1-independent necroptosis induced by tumor necrosis factor. Front Cell Dev Biol. 2019;7:393. doi: 10.3389/fcell.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dowling JP, Alsabbagh M, Del Casale C, Liu ZG, Zhang J. TRADD regulates perinatal development and adulthood survival in mice lacking RIPK1 and RIPK3. Nat Commun. 2019;10(1):705. doi: 10.1038/s41467-019-08584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng L, Bidere N, Staudt D, Cubre A, Orenstein J, Chan FK, Lenardo M. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol Cell Biol. 2006;26(9):3505–3513. doi: 10.1128/MCB.26.9.3505-3513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D, Vandenabeele P, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19(12):2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takizawa H, Fritsch K, Kovtonyuk LV, Saito Y, Yakkala C, Jacobs K, Ahuja AK, Lopes M, Hausmann A, Hardt WD, et al. Pathogen-induced TLR4-TRIF innate immune signaling in hematopoietic stem cells promotes proliferation but reduces competitive fitness. Cell Stem Cell. 2017;21:225. doi: 10.1016/j.stem.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, Kanneganti TD. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1(2):aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Najjar M, Saleh D, Zelic M, Nogusa S, Shah S, Tai A, Finger JN, Polykratis A, Gough PJ, Bertin J, et al. RIPK1 and RIPK3 kinases promote cell-death-independent inflammation by toll-like receptor 4. Immunity. 2016;45(1):46–59. doi: 10.1016/j.immuni.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174(8):4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 91.Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem. 2008;283(25):16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kitur K, Wachtel S, Brown A, Wickersham M, Paulino F, Penaloza HF, Soong G, Bueno S, Parker D, Prince A. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 2016;16(8):2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ingram JP, Thapa RJ, Fisher A, Tummers B, Zhang T, Yin C, Rodriguez DA, Guo H, Lane R, Williams R, et al. ZBP1/DAI drives RIPK3-mediated cell death induced by IFNs in the absence of RIPK1. J Immunol. 2019;203(5):1348–1355. doi: 10.4049/jimmunol.1900216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen D, Tong J, Yang L, Wei L, Stolz DB, Yu J, Zhang J, Zhang L. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc Natl Acad Sci USA. 2018;115(15):3930–3935. doi: 10.1073/pnas.1717190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vanden Berghe T, Kaiser WJ. RIPK1 prevents aberrant ZBP1-initiated necroptosis. Oncotarget. 2017;8(1):1–2. doi: 10.18632/oncotarget.13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newton K, Wickliffe KE, Maltzman A, Dugger DL, Strasser A, Pham VC, Lill JR, Roose-Girma M, Warming S, Solon M, et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540(7631):129–133. doi: 10.1038/nature20559. [DOI] [PubMed] [Google Scholar]
- 97.Lin J, Kumari S, Kim C, Van TM, Wachsmuth L, Polykratis A, Pasparakis M. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540(7631):124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci USA. 2014;111(43):15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17(2):229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 101.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39(3):443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 102.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7(4):971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 104.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24(1):105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16(1):55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Najafov A, Mookhtiar AK, Luu HS, Ordureau A, Pan H, Amin PP, Li Y, Lu Q, Yuan J. TAM kinases promote necroptosis by regulating oligomerization of MLKL. Mol Cell. 2019;75(3):457–468e454. doi: 10.1016/j.molcel.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 107.Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, Oberst A, Quarato G, Low J, Cripps JG, et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016;23(1):76–88. doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22(2):175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 109.Moriwaki K, Balaji S, Bertin J, Gough PJ, Chan FK. Distinct kinase-independent role of RIPK3 in CD11c(+) mononuclear phagocytes in cytokine-induced tissue repair. Cell Rep. 2017;18(10):2441–2451. doi: 10.1016/j.celrep.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alvarez-Diaz S, Dillon CP, Lalaoui N, Tanzer MC, Rodriguez DA, Lin A, Lebois M, Hakem R, Josefsson EC, O'Reilly LA, et al. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity. 2016;45(3):513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 112.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16(7):689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 113.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370(5):455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wallach D, Kovalenko A, Kang TB. ‘Necrosome’-induced inflammation: must cells die for it? Trends Immunol. 2011;32(11):505–509. doi: 10.1016/j.it.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 115.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41(4):567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, Zhou R. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15(12):1126–1133. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- 117.McKee CM, Coll RC. NLRP3 inflammasome priming: a riddle wrapped in a mystery inside an enigma. J Leukoc Biol. 2020;108(3):937–952. doi: 10.1002/JLB.3MR0720-513R. [DOI] [PubMed] [Google Scholar]
- 118.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moriwaki K, Bertin J, Gough PJ, Chan FK. A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. J Immunol. 2015;194(4):1938–1944. doi: 10.4049/jimmunol.1402167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Nunez G, Masters SL, Murphy JM, Schroder K, Vaux DL, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci USA. 2017;114(6):E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38(1):27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 123.Muendlein HI, Sarhan J, Liu BC, Connolly WM, Schworer SA, Smirnova I, Tang AY, Ilyukha V, Pietruska J, Tahmasebi S, et al. Constitutive interferon attenuates RIPK1/3-mediated cytokine translation. Cell Rep. 2020;30(3):699–713e694. doi: 10.1016/j.celrep.2019.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Orozco SL, Daniels BP, Yatim N, Messmer MN, Quarato G, Chen-Harris H, Cullen SP, Snyder AG, Ralli-Jain P, Frase S, et al. RIPK3 activation leads to cytokine synthesis that continues after loss of cell membrane integrity. Cell Rep. 2019;28(9):2275–22872275. doi: 10.1016/j.celrep.2019.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Z, Wang Y, Zhang Y, He X, Zhong CQ, Ni H, Chen X, Liang Y, Wu J, Zhao S, et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat Cell Biol. 2018;20(2):186–197. doi: 10.1038/s41556-017-0022-y. [DOI] [PubMed] [Google Scholar]
- 126.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148(1–2):228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 127.Moriwaki K, Farias Luz N, Balaji S, De Rosa MJ, O'Donnell CL, Gough PJ, Bertin J, Welsh RM, Chan FK. The mitochondrial phosphatase PGAM5 is dispensable for necroptosis but promotes inflammasome activation in macrophages. J Immunol. 2016;196(1):407–415. doi: 10.4049/jimmunol.1501662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu X, Tian L, Li J, Zhang Y, Han V, Li Y, Xu X, Li H, Chen X, Chen J, et al. Investigation of receptor interacting protein (RIP3)-dependent protein phosphorylation by quantitative phosphoproteomics. Mol Cell Proteomics. 2012;11(12):1640–1651. doi: 10.1074/mcp.M112.019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al-Moujahed A, Tian B, Efstathiou NE, Konstantinou EK, Hoang M, Lin H, Miller JW, Vavvas DG. Receptor interacting protein kinase 3 (RIP3) regulates iPSCs generation through modulating cell cycle progression genes. Stem Cell Res. 2019;35:101387. doi: 10.1016/j.scr.2019.101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liccardi G, Ramos Garcia L, Tenev T, Annibaldi A, Legrand AJ, Robertson D, Feltham R, Anderton H, Darding M, Peltzer N, et al. RIPK1 and caspase-8 ensure chromosome stability independently of their role in cell death and inflammation. Mol Cell. 2019;73(3):413–428e417. doi: 10.1016/j.molcel.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gupta K, Liu B. PLK1-mediated S369 phosphorylation of RIPK3 during G2 and M phases enables its ripoptosome incorporation and activity. iScience. 2021;24(4):102320. doi: 10.1016/j.isci.2021.102320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Torii S, Yamaguchi H, Nakanishi A, Arakawa S, Honda S, Moriwaki K, Nakano H, Shimizu S. Identification of a phosphorylation site on Ulk1 required for genotoxic stress-induced alternative autophagy. Nat Commun. 2020;11(1):1754. doi: 10.1038/s41467-020-15577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Torii S, Shimizu S. Involvement of phosphorylation of ULK1 in alternative autophagy. Autophagy. 2020;16(8):1532–1533. doi: 10.1080/15548627.2020.1776476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chan FK, Luz NF, Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol. 2015;33:79–106. doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ren J, Jia X, Zhao Y, Shi W, Lu J, Zhang Y, Wu J, Liang B, Wu R, Fu G, et al. The RIP3-RIP1-NF-kappaB signaling axis is dispensable for necroptotic cells to elicit cross-priming of CD8(+) T cells. Cell Mol Immunol. 2017;14(7):639–642. doi: 10.1038/cmi.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J Immunol. 2008;181(12):8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184(8):4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Krysko DV, Brouckaert G, Kalai M, Vandenabeele P, D'Herde K. Mechanisms of internalization of apoptotic and necrotic L929 cells by a macrophage cell line studied by electron microscopy. J Morphol. 2003;258(3):336–345. doi: 10.1002/jmor.10161. [DOI] [PubMed] [Google Scholar]
- 139.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kang T, Huang Y, Zhu Q, Cheng H, Pei Y, Feng J, Xu M, Jiang G, Song Q, Jiang T, et al. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials. 2018;164:80–97. doi: 10.1016/j.biomaterials.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 141.Schmidt SV, Seibert S, Walch-Ruckheim B, Vicinus B, Kamionka EM, Pahne-Zeppenfeld J, Solomayer EF, Kim YJ, Bohle RM, Smola S. RIPK3 expression in cervical cancer cells is required for PolyIC-induced necroptosis, IL-1alpha release, and efficient paracrine dendritic cell activation. Oncotarget. 2015;6(11):8635–8647. doi: 10.18632/oncotarget.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Snyder AG, Hubbard NW, Messmer MN, Kofman SB, Hagan CE, Orozco SL, Chiang K, Daniels BP, Baker D, Oberst A. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci Immunol. 2019;4(36):eaaw2004. doi: 10.1126/sciimmunol.aaw2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kang YJ, Bang BR, Han KH, Hong L, Shim EJ, Ma J, Lerner RA, Otsuka M. Regulation of NKT cell-mediated immune responses to tumours and liver inflammation by mitochondrial PGAM5-Drp1 signalling. Nat Commun. 2015;6:8371. doi: 10.1038/ncomms9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li L, Yu S, Zang C. Low necroptosis process predicts poor treatment outcome of human papillomavirus positive cervical cancers by decreasing tumor-associated macrophages M1 polarization. Gynecol Obstet Invest. 2018;83(3):259–267. doi: 10.1159/000487434. [DOI] [PubMed] [Google Scholar]
- 145.Wu L, Zhang X, Zheng L, Zhao H, Yan G, Zhang Q, Zhou Y, Lei J, Zhang J, Wang J, et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol Res. 2020;8(5):710–721. doi: 10.1158/2326-6066.CIR-19-0261. [DOI] [PubMed] [Google Scholar]
- 146.Najafov A, Zervantonakis IK, Mookhtiar AK, Greninger P, March RJ, Egan RK, Luu HS, Stover DG, Matulonis UA, Benes CH, et al. BRAF and AXL oncogenes drive RIPK3 expression loss in cancer. PLoS Biol. 2018;16(8):e2005756. doi: 10.1371/journal.pbio.2005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tan Y, Sementino E, Cheung M, Peri S, Menges CW, Kukuyan AM, Zhang T, Khazak V, Fox LA, Ross EA, et al. Somatic epigenetic silencing of RIPK3 inactivates necroptosis and contributes to chemoresistance in malignant mesothelioma. Clin Cancer Res. 2021;27(4):1200–1213. doi: 10.1158/1078-0432.CCR-18-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stoll G, Ma Y, Yang H, Kepp O, Zitvogel L, Kroemer G. Pro-necrotic molecules impact local immunosurveillance in human breast cancer. Oncoimmunology. 2017;6(4):e1299302. doi: 10.1080/2162402X.2017.1299302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Feng X, Song Q, Yu A, Tang H, Peng Z, Wang X. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma. 2015;62(4):592–601. doi: 10.4149/neo_2015_071. [DOI] [PubMed] [Google Scholar]
- 150.Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015;6:e1636. doi: 10.1038/cddis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hockendorf U, Yabal M, Herold T, Munkhbaatar E, Rott S, Jilg S, Kauschinger J, Magnani G, Reisinger F, Heuser M, et al. RIPK3 restricts myeloid leukemogenesis by promoting cell death and differentiation of leukemia initiating cells. Cancer Cell. 2016;30(1):75–91. doi: 10.1016/j.ccell.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 152.Nugues AL, El Bouazzati H, Hetuin D, Berthon C, Loyens A, Bertrand E, Jouy N, Idziorek T, Quesnel B. RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis. 2014;5:e1384. doi: 10.1038/cddis.2014.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCormick KD, Ghosh A, Trivedi S, Wang L, Coyne CB, Ferris RL, Sarkar SN. Innate immune signaling through differential RIPK1 expression promote tumor progression in head and neck squamous cell carcinoma. Carcinogenesis. 2016;37(5):522–529. doi: 10.1093/carcin/bgw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Geserick P, Wang J, Schilling R, Horn S, Harris PA, Bertin J, Gough PJ, Feoktistova M, Leverkus M. Absence of RIPK3 predicts necroptosis resistance in malignant melanoma. Cell Death Dis. 2015;6:e1884. doi: 10.1038/cddis.2015.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang KJ, Wang KY, Zhang HZ, Meng XY, Chen JF, Wang P, Jiang JH, Ma Q. Up-regulation of RIP3 alleviates prostate cancer progression by activation of RIP3/MLKL signaling pathway and induction of necroptosis. Front Oncol. 2020;10:1720. doi: 10.3389/fonc.2020.01720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Conev NV, Dimitrova EG, Bogdanova MK, Kashlov YK, Chaushev BG, Radanova MA, Petrov DP, Georgiev KD, Bachvarov CH, Todorov GN, et al. RIPK3 expression as a potential predictive and prognostic marker in metastatic colon cancer. Clin Invest Med. 2019;42(1):E31–E38. doi: 10.25011/cim.v42i1.32390. [DOI] [PubMed] [Google Scholar]
- 157.Ertao Z, Jianhui C, Kang W, Zhijun Y, Hui W, Chuangqi C, Changjiang Q, Sile C, Yulong H, Shirong C. Prognostic value of mixed lineage kinase domain-like protein expression in the survival of patients with gastric cancer. Tumour Biol. 2016;37(10):13679–13685. doi: 10.1007/s13277-016-5229-1. [DOI] [PubMed] [Google Scholar]
- 158.He L, Peng K, Liu Y, Xiong J, Zhu FF. Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. Onco Targets Ther. 2013;6:1539–1543. doi: 10.2147/OTT.S52805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ruan J, Mei L, Zhu Q, Shi G, Wang H. Mixed lineage kinase domain-like protein is a prognostic biomarker for cervical squamous cell cancer. Int J Clin Exp Pathol. 2015;8(11):15035–15038. [PMC free article] [PubMed] [Google Scholar]
- 160.Li X, Guo J, Ding AP, Qi WW, Zhang PH, Lv J, Qiu WS, Sun ZQ. Association of mixed lineage kinase domain-like protein expression with prognosis in patients with colon cancer. Technol Cancer Res Treat. 2017;16(4):428–434. doi: 10.1177/1533034616655909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Colbert LE, Fisher SB, Hardy CW, Hall WA, Saka B, Shelton JW, Petrova AV, Warren MD, Pantazides BG, Gandhi K, et al. Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer. 2013;119(17):3148–3155. doi: 10.1002/cncr.28144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang CY, Kuo WT, Huang YC, Lee TC, Yu LC. Resistance to hypoxia-induced necroptosis is conferred by glycolytic pyruvate scavenging of mitochondrial superoxide in colorectal cancer cells. Cell Death Dis. 2013;4:e622. doi: 10.1038/cddis.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fukasawa M, Kimura M, Morita S, Matsubara K, Yamanaka S, Endo C, Sakurada A, Sato M, Kondo T, Horii A, et al. Microarray analysis of promoter methylation in lung cancers. J Hum Genet. 2006;51(4):368–374. doi: 10.1007/s10038-005-0355-4. [DOI] [PubMed] [Google Scholar]
- 164.Thijssen R, Alvarez-Diaz S, Grace C, Gao MY, Segal DH, Xu Z, Strasser A, Huang DCS. Loss of RIPK3 does not impact MYC-driven lymphomagenesis or chemotherapeutic drug-induced killing of malignant lymphoma cells. Cell Death Differ. 2020;27(8):2531–2533. doi: 10.1038/s41418-020-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vucur M, Reisinger F, Gautheron J, Janssen J, Roderburg C, Cardenas DV, Kreggenwinkel K, Koppe C, Hammerich L, Hakem R, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013;4(4):776–790. doi: 10.1016/j.celrep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 166.Seehawer M, Heinzmann F, D'Artista L, Harbig J, Roux PF, Hoenicke L, Dang H, Klotz S, Robinson L, Dore G, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562(7725):69–75. doi: 10.1038/s41586-018-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532(7598):245–249. doi: 10.1038/nature17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ando Y, Ohuchida K, Otsubo Y, Kibe S, Takesue S, Abe T, Iwamoto C, Shindo K, Moriyama T, Nakata K, et al. Necroptosis in pancreatic cancer promotes cancer cell migration and invasion by release of CXCL5. PLoS ONE. 2020;15(1):e0228015. doi: 10.1371/journal.pone.0228015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, Taunk PS, Wu N, Su W, Wu J, et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell. 2018;34(5):757–774e757. doi: 10.1016/j.ccell.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu X, Zhou M, Mei L, Ruan J, Hu Q, Peng J, Su H, Liao H, Liu S, Liu W, et al. Key roles of necroptotic factors in promoting tumor growth. Oncotarget. 2016;7(16):22219–22233. doi: 10.18632/oncotarget.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin CC, Mabe NW, Lin YT, Yang WH, Tang X, Hong L, Sun T, Force J, Marks JR, Yao TP, et al. RIPK3 upregulation confers robust proliferation and collateral cystine-dependence on breast cancer recurrence. Cell Death Differ. 2020;27(7):2234–2247. doi: 10.1038/s41418-020-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mabe NW, Garcia NMG, Wolery SE, Newcomb R, Meingasner RC, Vilona BA, Lupo R, Lin CC, Chi JT, Alvarez JV. G9a promotes breast cancer recurrence through repression of a pro-inflammatory program. Cell Rep. 2020;33(5):108341. doi: 10.1016/j.celrep.2020.108341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, Pasparakis M, Offermanns S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536(7615):215–218. doi: 10.1038/nature19076. [DOI] [PubMed] [Google Scholar]
- 174.Patel S, Webster JD, Varfolomeev E, Kwon YC, Cheng JH, Zhang J, Dugger DL, Wickliffe KE, Maltzman A, Sujatha-Bhaskar S, et al. RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ. 2020;27(1):161–175. doi: 10.1038/s41418-019-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou M, He J, Shi Y, Liu X, Luo S, Cheng C, Ge W, Qu C, Du P, Chen Y. ABIN3 negatively regulates necroptosis-induced intestinal inflammation through recruiting A20 and restricting the ubiquitination of RIPK3 in inflammatory bowel disease. J Crohns Colitis. 2021;15(1):99–114. doi: 10.1093/ecco-jcc/jjaa131. [DOI] [PubMed] [Google Scholar]
- 176.Tortola L, Nitsch R, Bertrand MJM, Kogler M, Redouane Y, Kozieradzki I, Uribesalgo I, Fennell LM, Daugaard M, Klug H, et al. The tumor suppressor Hace1 is a critical regulator of TNFR1-mediated cell fate. Cell Rep. 2016;16(12):3414. doi: 10.1016/j.celrep.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Y, Zhao M, He S, Luo Y, Zhao Y, Cheng J, Gong Y, Xie J, Wang Y, Hu B, et al. Necroptosis regulates tumor repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway. J Exp Clin Cancer Res. 2019;38(1):461. doi: 10.1186/s13046-019-1423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.He S, Cheng J, Sun L, Wang Y, Wang C, Liu X, Zhang Z, Zhao M, Luo Y, Tian L, et al. HMGB1 released by irradiated tumor cells promotes living tumor cell proliferation via paracrine effect. Cell Death Dis. 2018;9(6):648. doi: 10.1038/s41419-018-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jayakumar A, Bothwell ALM. RIPK3-induced inflammation by I-MDSCs promotes intestinal tumors. Cancer Res. 2019;79(7):1587–1599. doi: 10.1158/0008-5472.CAN-18-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bozec D, Iuga AC, Roda G, Dahan S, Yeretssian G. Critical function of the necroptosis adaptor RIPK3 in protecting from intestinal tumorigenesis. Oncotarget. 2016;7(29):46384–46400. doi: 10.18632/oncotarget.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alvarez-Diaz S, Preaudet A, Samson AL, Nguyen PM, Fung KY, Garnham AL, Alexander WS, Strasser A, Ernst M, Putoczki TL, et al. Necroptosis is dispensable for the development of inflammation-associated or sporadic colon cancer in mice. Cell Death Differ. 2020;28:1466. doi: 10.1038/s41418-020-00673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jensen S, Seidelin JB, LaCasse EC, Nielsen OH. SMAC mimetics and RIPK inhibitors as therapeutics for chronic inflammatory diseases. Sci Signal. 2020;13(619):eaax8295. doi: 10.1126/scisignal.aax8295. [DOI] [PubMed] [Google Scholar]
- 183.Brumatti G, Ma C, Lalaoui N, Nguyen NY, Navarro M, Tanzer MC, Richmond J, Ghisi M, Salmon JM, Silke N, et al. The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia. Sci Transl Med. 2016;8(339):339ra369. doi: 10.1126/scitranslmed.aad3099. [DOI] [PubMed] [Google Scholar]
- 184.Lalaoui N, Hanggi K, Brumatti G, Chau D, Nguyen NY, Vasilikos L, Spilgies LM, Heckmann DA, Ma C, Ghisi M, et al. Targeting p38 or MK2 enhances the anti-leukemic activity of smac-mimetics. Cancer Cell. 2016;29(2):145–158. doi: 10.1016/j.ccell.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 185.Markowitsch SD, Juetter KM, Schupp P, Hauschulte K, Vakhrusheva O, Slade KS, Thomas A, Tsaur I, Cinatl J, Jr, Michaelis M, et al. Shikonin reduces growth of docetaxel-resistant prostate cancer cells mainly through necroptosis. Cancers (Basel) 2021;13(4):882. doi: 10.3390/cancers13040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Matias D, Balca-Silva J, Dubois LG, Pontes B, Ferrer VP, Rosario L, DoCarmo A, Echevarria-Lima J, Sarmento-Ribeiro AB, Lopes MC, et al. Dual treatment with shikonin and temozolomide reduces glioblastoma tumor growth, migration and glial-to-mesenchymal transition. Cell Oncol. 2017;40(3):247–261. doi: 10.1007/s13402-017-0320-1. [DOI] [PubMed] [Google Scholar]
- 187.Lu L, Qin A, Huang H, Zhou P, Zhang C, Liu N, Li S, Wen G, Zhang C, Dong W, et al. Shikonin extracted from medicinal Chinese herbs exerts anti-inflammatory effect via proteasome inhibition. Eur J Pharmacol. 2011;658(2–3):242–247. doi: 10.1016/j.ejphar.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ding Y, He C, Lu S, Wang X, Wang C, Wang L, Zhang J, Piao M, Chi G, Luo Y, et al. MLKL contributes to shikonin-induced glioma cell necroptosis via promotion of chromatinolysis. Cancer Lett. 2019;467:58–71. doi: 10.1016/j.canlet.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 189.Hanna-Addams S, Liu S, Liu H, Chen S, Wang Z. CK1alpha, CK1delta, and CK1epsilon are necrosome components which phosphorylate serine 227 of human RIPK3 to activate necroptosis. Proc Natl Acad Sci USA. 2020;117(4):1962–1970. doi: 10.1073/pnas.1917112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013;288(23):16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xie T, Peng W, Yan C, Wu J, Gong X, Shi Y. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep. 2013;5(1):70–78. doi: 10.1016/j.celrep.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 192.McQuade T, Cho Y, Chan FK. Positive and negative phosphorylation regulates RIP1- and RIP3-induced programmed necrosis. Biochem J. 2013;456(3):409–415. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee SY, Kim H, Li CM, Kang J, Najafov A, Jung M, Kang S, Wang S, Yuan J, Jung YK. Casein kinase-1gamma1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3. Cell Death Dis. 2019;10(12):923. doi: 10.1038/s41419-019-2146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang F, Mayca Pozo F, Tian D, Geng X, Yao X, Zhang Y, Tang J. Shikonin inhibits cancer through P21 upregulation and apoptosis induction. Front Pharmacol. 2020;11:861. doi: 10.3389/fphar.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thonsri U, Seubwai W, Waraasawapati S, Wongkham S, Boonmars T, Cha'on U, Wongkham C. Antitumor effect of shikonin, a PKM2 inhibitor, in Cholangiocarcinoma Cell Lines. Anticancer Res. 2020;40(9):5115–5124. doi: 10.21873/anticanres.14515. [DOI] [PubMed] [Google Scholar]
- 196.Liu T, Sun X, Cao Z. Shikonin-induced necroptosis in nasopharyngeal carcinoma cells via ROS overproduction and upregulation of RIPK1/RIPK3/MLKL expression. Onco Targets Ther. 2019;12:2605–2614. doi: 10.2147/OTT.S200740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou Z, Lu B, Wang C, Wang Z, Luo T, Piao M, Meng F, Chi G, Luo Y, Ge P. RIP1 and RIP3 contribute to shikonin-induced DNA double-strand breaks in glioma cells via increase of intracellular reactive oxygen species. Cancer Lett. 2017;390:77–90. doi: 10.1016/j.canlet.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 198.Liu ZY, Zheng M, Li YM, Fan XY, Wang JC, Li ZC, Yang HJ, Yu JM, Cui J, Jiang JL, et al. RIP3 promotes colitis-associated colorectal cancer by controlling tumor cell proliferation and CXCL1-induced immune suppression. Theranostics. 2019;9(12):3659–3673. doi: 10.7150/thno.32126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim HJ, Hwang KE, Park DS, Oh SH, Jun HY, Yoon KH, Jeong ET, Kim HR, Kim YS. Shikonin-induced necroptosis is enhanced by the inhibition of autophagy in non-small cell lung cancer cells. J Transl Med. 2017;15(1):123. doi: 10.1186/s12967-017-1223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shahsavari Z, Karami-Tehrani F, Salami S. Shikonin induced necroptosis via reactive oxygen species in the T-47D breast cancer cell line. Asian Pacific J Cancer Prevent APJCP. 2015;16(16):7261–7266. doi: 10.7314/APJCP.2015.16.16.7261. [DOI] [PubMed] [Google Scholar]
- 201.Han W, Xie J, Li L, Liu Z, Hu X. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis. 2009;14(5):674–686. doi: 10.1007/s10495-009-0334-x. [DOI] [PubMed] [Google Scholar]
- 202.Zhao Q, Kretschmer N, Bauer R, Efferth T. Shikonin and its derivatives inhibit the epidermal growth factor receptor signaling and synergistically kill glioblastoma cells in combination with erlotinib. Int J Cancer. 2015;137(6):1446–1456. doi: 10.1002/ijc.29483. [DOI] [PubMed] [Google Scholar]
- 203.Wu H, Xie J, Pan Q, Wang B, Hu D, Hu X. Anticancer agent shikonin is an incompetent inducer of cancer drug resistance. PLoS ONE. 2013;8(1):e52706. doi: 10.1371/journal.pone.0052706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lu B, Gong X, Wang ZQ, Ding Y, Wang C, Luo TF, Piao MH, Meng FK, Chi GF, Luo YN, et al. Shikonin induces glioma cell necroptosis in vitro by ROS overproduction and promoting RIP1/RIP3 necrosome formation. Acta Pharmacol Sin. 2017;38(11):1543–1553. doi: 10.1038/aps.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Steinhart L, Belz K, Fulda S. Smac mimetic and demethylating agents synergistically trigger cell death in acute myeloid leukemia cells and overcome apoptosis resistance by inducing necroptosis. Cell Death Dis. 2013;4:e802. doi: 10.1038/cddis.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Steinwascher S, Nugues AL, Schoeneberger H, Fulda S. Identification of a novel synergistic induction of cell death by Smac mimetic and HDAC inhibitors in acute myeloid leukemia cells. Cancer Lett. 2015;366(1):32–43. doi: 10.1016/j.canlet.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 207.Gerges S, Rohde K, Fulda S. Cotreatment with Smac mimetics and demethylating agents induces both apoptotic and necroptotic cell death pathways in acute lymphoblastic leukemia cells. Cancer Lett. 2016;375(1):127–132. doi: 10.1016/j.canlet.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 208.Park JH, Jung KH, Kim SJ, Yoon YC, Yan HH, Fang Z, Lee JE, Lim JH, Mah S, Hong S, et al. HS-173 as a novel inducer of RIP3-dependent necroptosis in lung cancer. Cancer Lett. 2019;444:94–104. doi: 10.1016/j.canlet.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 209.Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J, Haydu LE, Mijatov B, Becker TM, Boyd SC, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20(7):1965–1977. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- 210.Ci T, Li H, Chen G, Wang Z, Wang J, Abdou P, Tu Y, Dotti G, Gu Z (2020) Cryo-shocked cancer cells for targeted drug delivery and vaccination. Sci Adv 6(50) [DOI] [PMC free article] [PubMed]
- 211.Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L, Delrue I, Taminau J, Wiernicki B, De Groote P, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15(2):274–287. doi: 10.1016/j.celrep.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 212.Xin J, You D, Breslin P, Li J, Zhang J, Wei W, Cannova J, Volk A, Gutierrez R, Xiao Y, et al. Sensitizing acute myeloid leukemia cells to induced differentiation by inhibiting the RIP1/RIP3 pathway. Leukemia. 2017;31(5):1154–1165. doi: 10.1038/leu.2016.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Weber K, Roelandt R, Bruggeman I, Estornes Y, Vandenabeele P. Nuclear RIPK3 and MLKL contribute to cytosolic necrosome formation and necroptosis. Commun Biol. 2018;1:6. doi: 10.1038/s42003-017-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yoon S, Bogdanov K, Kovalenko A, Wallach D. Necroptosis is preceded by nuclear translocation of the signaling proteins that induce it. Cell Death Differ. 2016;23(2):253–260. doi: 10.1038/cdd.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xin J, Breslin P, Wei W, Li J, Gutierrez R, Cannova J, Ni A, Ng G, Schmidt R, Chen H, et al. Necroptosis in spontaneously-mutated hematopoietic cells induces autoimmune bone marrow failure in mice. Haematologica. 2017;102(2):295–307. doi: 10.3324/haematol.2016.151514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, Xu BQ, Li JH, Jing L, Jiang JL, Tang J, et al. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am J Cancer Res. 2015;5(10):3174–3185. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is not applicable for this review.
This is not applicable for this review.