Abstract
Background
Early treatment with disease modifying therapies (DMTs) for multiple sclerosis (MS) has been associated with lower disability progression; the aim was to explore its association with cost of illness (COI) in MS.
Methods
All people with relapsing-remitting MS in the Swedish MS register, aged 20–57 years and receiving their first MS DMT in 2006–2009, were followed in nationwide registers for 8 years. Healthcare costs (in- and outpatient healthcare, DMTs and other prescribed drugs), and productivity losses (sickness absence and disability pension) of individuals receiving therapy in ≤6 months after diagnosis (early treatment group) were compared to those receiving therapy >6 months (late treatment group). Using Poisson regressions, the mean COI per patient per year, and per group, was estimated, adjusted for disability progression.
Results
The early treatment group comprised 74% of the 1562 individuals included in the study. The early treatment group had lower productivity losses over time. Both groups had similar healthcare costs, which first increased and then decreased over time.
Conclusions
Early DMT in MS could result in lower productivity losses possibly through maintained work capacity. COI serves as an objective measure showing the advantage of early vs. late treatment initiation in MS.
Keywords: Multiple sclerosis, cost of illness, COI, early treatment, costs
Introduction
Multiple sclerosis (MS) is an often progressive, neurological condition, 1 classified into different forms; relapsing-remitting MS, secondary-progressive MS, or primary-progressive MS. 2 Sweden has the 2nd highest prevalence of MS in Europe at 189 cases per 100,000. 3
Since MS is diagnosed in early adulthood, usually when aged 20–40 years, 1 it can affect people's work capacity. 4 About 43% of people with MS (PwMS) who not were in paid work had quit their employment within the first three years after diagnosis. 4 In fact, productivity losses account for 65%-75% of all costs in MS,5,6 due to elevated rates of sickness absence (SA) and/or disability pension (DP).7–9
Costs for disease modifying therapies (DMTs), inpatient, and specialized outpatient care are also substantial among PwMS. 7 The annual cost of illness (COI) of MS has been shown to increase with disability progression. 7 DMTs aim to slow disease progression, and therefore, can potentially slow the progression of the COI. 10
However, the timing of DMT initiation is of essence; early MS therapy can slow the accumulation of disability early-on, leading to better clinical outcomes over time.10,11 It also improves capacity to maintain work 12 and is associated with better socioeconomic outcomes. 13 Therefore, early therapy could also have a positive impact on the overall COI in MS. However, no such longitudinal studies have been conducted.
The aim of this study was to explore the association between the timing of DMT initiation in relation to MS diagnosis, i.e., early vs. late therapy, with the overall COI of MS in Sweden.
Material and methods
This was a register-based, longitudinal cohort study. Microdata, linked using the unique personal identity number that all residents in Sweden are assigned, was obtained from the following Swedish nationwide registers, kept by four authorities:
- Region Stockholm:
- Swedish MS register (SMSreg) 14 : Was used to identify the cohort members and for information on MS diagnosis date, type of MS, information on MS disability (Expanded Disability Status Scale, EDSS), and type and date of DMTs.
- National Board of Health and Welfare:
Statistics Sweden: Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA) 15 : Sex, birth year, educational level, country of birth, type of living area, and family situation.
Swedish Social Insurance Agency 15 : Micro Data for the Analysis of Social Insurance register (MiDAS): dates, diagnoses, and grade of SA and DP.
PwMS who had the relapsing-remitting form of MS (RRMS), initiated their first DMT treatment (interferons, glatiramer acetate, and natalizumab) in 2006–2009 (index year) after MS diagnosis was established, and when aged 20–57 years were identified from the SMSreg. All included were followed for 8 years in total; last year of follow-up was 2013–2016 (depending on their index year).
Never-users of DMTs, those without RRMS, individuals receiving DMT before being diagnosed with MS, and those who did not live in Sweden during the index year, were not included in the study.
Early (≤6 months) and late (>6 months) DMT groups were then defined in relation to the date of MS diagnosis. The cut-off value of 6 months was chosen arbitrarily based on information from previously published studies.11,12
The project was approved by the Regional Ethical Review Board of Stockholm, Sweden.
Study outcomes
MS disability, sociodemographic characteristics, and multi-morbidity
MS disability was defined using EDSS scores. 16 Scores range from 0 to 10, with 0.5 step intervals (0 indicating no impairment, while 10 indicating death from MS). 17 A clinically meaningful change in the EDSS score is a change of at least one point in patients with EDSS <5.5 and 0.5 point for those with EDSS of ≥5.5. 18
EDSS information is recorded in the SMSreg during visits to neurologists. 14 According, when multiple EDSS scores within a calendar year were available, the highest EDSS value was retained. If there was no EDSS information recorded in a calendar year, the average EDSS score was used for that year, computed from the scores of PwMS in the same treatment group and index year.
The following sociodemographic characteristics were measured at the year when therapy started (index year): Sex, age, educational level, country of birth, type of living area, and family situation. Multi-morbidity in the index year was derived utilizing the Rx-Risk Comorbidity Index 19 (cancer morbidity was not included), based on the type of prescribed drugs the PwMS bought, according to SPDR Using information from the Rx-Risk Comorbidity Index 19 the existence of multi-morbidity (yes/no) was established as well as whether PwMS had been diagnosed with anxiety/depression (based on prescribed drugs; yes/no).
Healthcare costs and productivity losses
The average COI per patient per year was defined from a societal perspective, quantifying the healthcare resources consumed by PwMS, as well as their SA and DP, and then multiplying them with unit costs (Table 1). All healthcare resource consumption, SA, and DP during a calendar year, were included, irrespective of whether MS was listed as the main diagnosis.
Table 1.
Unit costs used in the calculation of healthcare costs and productivity losses.
| Year | Value in 2018 SEK | Value in 2018 Eurosa | Source | |
|---|---|---|---|---|
| Average inpatient and outpatient cost per 1.0 DRG | 2006 | 50,973 kr | 4969 € | Swedish Association of Local Authorities and Regions [Sveriges Kommuner och Landsting], KPP Somatik vård 20 |
| 2007 | 50,224 kr | 4896 € | ||
| 2008 | 51,388 kr | 5009 € | ||
| 2009 | 51,785 kr | 5048 € | ||
| 2010 | 50,457 kr | 4919 € | ||
| 2011 | 50,286 kr | 4902 € | ||
| 2012 | 49,820 kr | 4857 € | ||
| 2013 | 51,468 kr | 5017 € | ||
| 2014 | 53,388 kr | 5204 € | ||
| 2015 | 55,807 kr | 5440 € | ||
| 2016 | 57,334 kr | 5589 € | ||
| Co-payment for hospital stay (cost per day of stay) | 2018 | 100 kr | 10 € | Assume 100 SEK per day, as this is the case for the majority of the regions in Sweden (including Stockholm). 21 The max co-payment amount for inpatient care was set to 1500 SEK per year (assumption for whole Sweden, based on information from the region Västra Götaland) 22 |
| Co-payment for visit in specialized care (cost per visit) | 2018 | 273 kr | 27 € | Swedish Association of Local Authorities and Regions. The max co-payment amount for outpatient care was set to 1100 SEK per year. Only one region in Sweden has a max co-payment less than 1100 SEK; so it was assumed 1100 SEK for the entire country. Swedish Association of Local Authorities and Regions 21 |
| Cost per month for natalizumab | 2018 | 15,839 kr | 1544 € | Treatment with natalizumab is every 4th week (i.e. one per month); 23 therefore, the cost per month of natalizumab was assumed to be the price for one dose of natalizumab (pharmacy's retail price), 23 excluding the cost of administration. The latter is partially captured in this study as an outpatient visit to the hospital, excluding infusion visits with a nurse, which are unfortunately not in the NPR. |
| Cost per month for rituximab | 2018 | 2022 kr | 197 € | Rituximab is used off-label in the treatment of MS; therefore, the exact treatment dosing was not available in the Swedish guidelines for MS treatment. A recent published study in Sweden regarding the use of rituximab for PwMS indicated that the drug dose is 500mg to 1000mg per treatment regime (here we assumed the mean, i.e 750mg per treatment regime), and the mean treatment interval for RRMS PwMS was 7.2 months per year. 24 The cost per month was then assumed to be 1.5 times the pharmacy's retail price per 500mg of rituximab injection, which was taken from FASS (9703.61 SEK), 25 divided with the frequency of treatment in months (frequency: every 7.2 months). 24 |
| Monthly salary including employer contributions | 2006 | 40,930 kr | 4248 € | The average age-adjusted monthly wage (2018 values) for all employment types was retrieved. 26 It was multiplied with the annual employer contributions, available from the Swedish Tax Authority. 27 The final salary was then inflated to 2018 prices using annual Harmonized Indices of Consumer Prices [HICP] for healthcare available from Eurostat. 28 |
| 2007 | 41,049 kr | 4260 € | ||
| 2008 | 40,042 kr | 4156 € | ||
| 2009 | 44,583 kr | 4627 € | ||
| 2010 | 44,080 kr | 4575 € | ||
| 2011 | 44,730 kr | 4642 € | ||
| 2012 | 44,748 kr | 4644 € | ||
| 2013 | 45,051 kr | 4676 € | ||
| 2014 | 46,120 kr | 4787 € | ||
| 2015 | 50,300 kr | 5221 € | ||
| 2016 | 48,146 kr | 4997 € |
The annual exchange rate for 2018 from SEK to Euros that was used was 10.2583. Source: Eurostat, Annual Exchange Rates Euro/ECU.
Inpatient and outpatient costs were calculated by multiplying the inpatient stays and outpatient visits during a calendar year, available in the NPR, with their observed nationwide weight for each diagnosis-related group (DRG) and the national cost per 1.0 DRG point. 5 Co-payment costs were calculated from the use of inpatient stays and outpatient visits as the sum of patient fees for inpatient and outpatient healthcare during each calendar year. The reimbursement period for patient fees was assumed to start on 1 January, and co-payments were set to zero after the accumulated fees had reached the co-payment ceiling each year (see Table 1).
The cost of prescribed dispensed drugs was derived from the SPDR, for each calendar year. These costs included both the cost reimbursed by the county and the co-payment paid by the patient. Then the annual cost of drugs was calculated by summing all costs for dispensed drugs per individual occurring during each calendar year.
The cost of DMTs not available in the SPDR (natalizumab and rituximab for the study period), was calculated using information from the SMSreg (all PwMS receiving natalizumab in Sweden are followed in the SMSreg; 29 rituximab use was also based on information from the SMSreg for patients included in the register). The number of months on these treatments, calculated taking the end minus the start date for treatment, was multiplied with the cost per month of treatment (Table 1).
The total per patient per year healthcare costs were calculated by adding the costs of inpatient and outpatient healthcare, co-payments, and drugs (including both DMTs and non-DMTs).
Productivity losses were measured based on SA and DP information, using the human capital approach. 30 All people living in Sweden (≥16 years old) with income from work or unemployment benefits can be granted SA if their work capacity is reduced due to disease or injury. The first day of a SA spell is a waiting day (100% loss of income). Income loss is reimbursed by the employer during days 2 to 14 and after that, by the Swedish Social Insurance Agency. Therefore, the Swedish Social Insurance Agency has no information on SA spells shorter than 15 days for individuals with income from work. For individuals on unemployment benefits, the Swedish Social Insurance Agency pays the benefits from day two. 31 In order to prevent bias in relation to employment status in the calculation of SA days, only SA spells >14 days were included.
Regarding DP, all people aged 19–65 years can be granted DP if their work capacity is long-term or permanently reduced. 31 Both SA and DP can be granted for full-time (100%) or part-time (75%, 50%, or 25%) of ordinary work hours. 31 Therefore, it is possible to have both partial SA and DP simultaneously. In order to handle this and not overestimate the productivity losses, we calculated the net days of SA and DP by using the percentage of the gross days, e.g., 2 absence days at 50% were combined to 1 net day.
To calculate productivity losses, the sum of SA and DP net days per year were used, multiplied with the age-adjusted mean salary, adding the social security contributions made by employers (Table 1).
All costs were inflated to 2018 prices, and were converted to Euros (EUR) (Table 1).
Analyses
Descriptive statistics were calculated regarding sociodemographic characteristics, multi-morbidity, disability progression, and average COI per patient and year for each of the two treatment groups.
Pearson's chi-squared test 32 and the likelihood ratio test 32 were used to explore whether the observed sociodemographic and multi-morbidity differences across the two groups were statistically significant (p-value<0.05).
Two-tailed Student's T-tests, with unequal variance 33 were used to assess the significance (p-value<0.05) of the disability and cost differences across the two groups.
The mean cost per patient per year, and per group, with 95% confidence intervals (95% CIs), were calculated using a single-distribution generalized linear model with a log link 34 and with the assumption that costs follow the Poisson distribution. The choice of the distribution reflected the zero costs several individuals had in different cost categories, and that data used in this study (skewed, count or cost data that will always be ≥0), and the need to have conservative cost estimates, avoiding potential overestimation.35,36 The annual cost trends were adjusted for the progression of disability over time using the average annual EDSS score per group as the disability measure.
Results
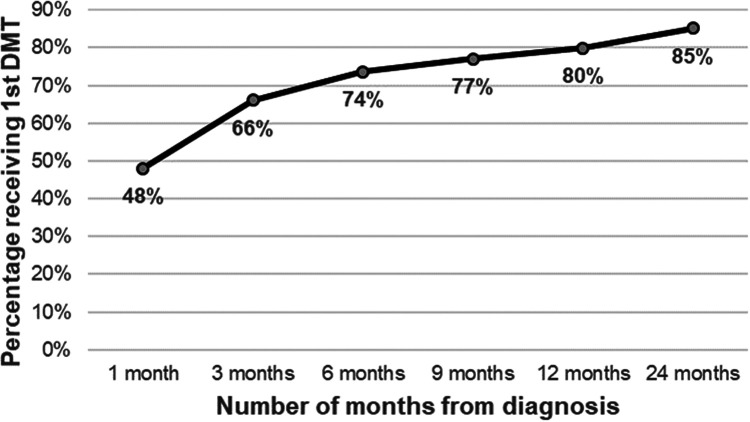
In total, 1562 individuals with RRMS receiving their first DMT in 2006–2009 were included. Of them, 74% (n = 1150) received their first DMT within 6 months of the MS diagnosis (Figure 1), while the remaining (n = 412) initiated treatment after this time point. Sex, country of birth, and family situation differed significantly across the two groups (Table 2). In the early treatment group more were women, Swedish born (vs. other nationalities), married/cohabitating with children at home and single without children (vs. married/cohabitating without children or single with children, or young individuals below the age of 21 years living at home). The baseline EDSS score was similar for both groups; higher EDSS score progression was observed for the late treatment group (Figure 1 in the supplementary material).
Figure 1.
Cumulative percentage of the study cohort receiving their 1st DMT, by months since diagnosis.
Table 2.
Sociodemographic and multi-morbidity characteristics at year of treatment start (index year), by early vs. late treatment groups.
| Early Treatment Cohort | Late Treatment Cohort | Pearson's Chi-Square (p-value) |
Log-likelihood
test Chi-Square (p-value) |
|
|---|---|---|---|---|
| N = 1150 | N = 412 | |||
| n(%)a | n(%)a | |||
| Sex | 4.57 (0.033) | 4.68 (0.03) | ||
| Women | 840 (73.04) | 323 (78.4) | ||
| Men | 310 (26.96) | 89 (21.6) | ||
| Age at year of treatment start (index year) | 1.76 (0.624) | 1.77 (0.621) | ||
| 20-29 | 307 (26.7) | 119 (28.88) | ||
| 30-39 | 437 (38) | 162 (39.32) | ||
| 40-49 | 314 (27.3) | 101 (24.51) | ||
| 50-57 | 92 (8) | 30 (7.28) | ||
| Educationb | 3.75 (0.289) | 3.68 (0.298) | ||
| 0-9 years | 116 (10.09) | 37 (8.98) | ||
| 10-12 years | 572 (49.74) | 192 (46.6) | ||
| >12 years | 445 (38.7) | 177 (42.96) | ||
| Country of birthb | 9.43 (0.0241) | 9.64 (0.022) | ||
| Sweden | 1020 (88.7) | 356 (86.41) | ||
| Nordic countries (except Sweden) | 20 (1.74) | 13 (3.16) | ||
| EU27 (except Denmark, Finland, and Sweden) | 29 (2.52) | 4 (0.97) | ||
| Rest of the world | 64 (5.57) | 33 (8.01) | ||
| Type of living areab | 1.07 (0.586) | 1.08 (0.584) | ||
| Big cities | 452 (39.89) | 164 (40.39) | ||
| Medium sized cities | 388 (34.25) | 147 (36.21) | ||
| Rural areas | 293 (25.86) | 95 (23.4) | ||
| Family situationb, c, d | 23.11 (0.0001) | 25.09 (<.0001) | ||
| Married or cohabitant, no children <18 at home | 90 (7.94) | 54 (13.3) | ||
| Married or cohabitant; children <18 at home | 445 (39.28) | 176 (43.35) | ||
| Single, no without children <18 at home | 478 (42.19) | 142 (34.98) | ||
| Single, children <18 at home | 79 (6.97) | 31 (7.64) | ||
| Youth (aged 18-20 years) living at home | 41 (3.62) | <8 (0.74) | ||
| Any commorbidities | 0.08 (0.779) | 0.08 (0.777) | ||
| Yes | 1125 (97.83) | 404 (98.06) | ||
| No | 25 (2.17) | 8 (1.94) | ||
| Anxiety/Depression | 2.10 (0.147) | 2.23 (0.135) | ||
| Yes | 66 (5.74) | 16 (3.88) | ||
| No | 1084 (94.26) | 396 (96.12) |
The percentages are calculated as n divided with the total n in each cohort if not otherwise indicated.
The total number of individuals with this type of information were n = 1133 for the early treatment group, and n = 406 for the late treatment group; 23 individuals in total had missing information.
Only cohabitants with children in common are registered as cohabitants. Otherwise they are registered as single.
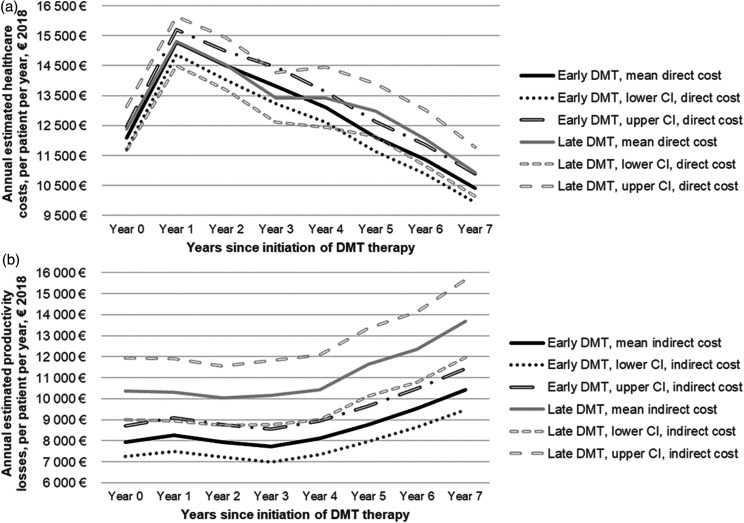
In Table 3 and in Figures 2a-b in the supplementary material, the unadjusted mean per patient per year COI for MS is presented over the 8-year follow-up period for both treatment groups. Figures 2a-b show the disability adjusted healthcare costs and productivity losses over time. Disability adjusted costs were also computed for all the cost components (Figures 3a-f in the supplementary material).
Table 3.
Mean costs per patient per year, mean costs [95% confidence intervals] in € 2018 prices (crude observed means and confidence intervals, unadjusted), by early vs. late treatment groups.
| Year 0a | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | Year 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Early treatment group (≤ 6 months from diagnosis; N = 1150) | |||||||||
| Inpatient costs | 2110 [1871-2350] | 1206 [947-1464] | 1460 [1169-1752] | 1443 [979-1907] | 1449 [1168-1731] | 1222 [965-1479] | 1150 [906-1393] | 1045 [843-1248] | |
| Outpatient costs | 1513 [1429-1598] | 1061 [994-1128] | 1169 [1086-1251] | 1212 [1136-1289] | 1420 [1319-1521] | 1450 [1357-1543] | 1561 [1462-1660] | 1659 [1535-1783] | |
| Co-payments | 110 [107-113] | 70 [67-73] | 67 [64-69] | 64 [61-67] | 68 [65-71] | 67 [65-70] | 68 [65-71] | 67 [64-70] | |
| Drug costs | 8387 [8111-8663] | 12,986 [12,696-13,276] | 11,827 [11,485-12,170] | 11,130 [10,756-11,503] | 10,202 [9804-10,600] | 9412 [8990-9833] | 8622 [8195-9050] | 7659 [7234-8085] | |
| Total healthcare costs | 12,121 [11,731-12,512] | 15,323 [14,900-15,745] | 14,523 [14,036-15,010] | 13,849 [13,225-14,474] | 13,139 [12,626-13,652] | 12,151 [11,645-12,657] | 11,401 [10,908-11,894] | 10,430 [9959-10,902] | |
| SA costs | 8379 [7632-9126] | 7844 [7030-8659] | 6110 [5397-6823] | 4643 [4006-5280] | 4302 [3679-4926] | 4297 [3687-4907] | 4068 [3469-4667] | 4129 [3511-4747] | |
| DP costs | 278 [92-464] | 1064 [716-1412] | 2349 [1848-2850] | 3623 [3007-4239] | 4535 [3848-5222] | 5254 [4514-5993] | 6359 [5532-7186] | 7299 [6395-8203] | |
| Total productivity losses | 8657 [7896-9418] | 8908 [8030-9787] | 8459 [7609-9308] | 8266 [7399-9133] | 8837 [7944-9731] | 9551 [8618-10484] | 10,427 [9419-11,436] | 11,428 [10,369-12,488] | |
| Late treatment group (> 6 months from diagnosis; N = 412) | |||||||||
| Inpatient costs | 1603 [1142-2065] | 1326 [834-1818] | 1674 [1169-2179] | 1268 [874-1662] | 1525 [800-2249] | 1580 [1058-2102] | 1342 [856-1827] | 1045 [680-1410] | |
| Outpatient costs | 1011 [919-1104] | 906 [809-1004] | 1106 [963-1248] | 1213 [1075-1352] | 1268 [1128-1407] | 1502 [1341-1664] | 1555 [1373-1737] | 1632 [1469-1795] | |
| Co-payments | 82 [77-87] | 64 [59-68] | 66 [61-71] | 64 [60-69] | 66 [61-71] | 72 [67-77] | 69 [64-73] | 68 [64-73] | |
| Drug costs | 9711 [9186-10236] | 13,036 [12,465-13,607] | 11,699 [11,049-12,350] | 10,882 [10,187-11,578] | 10,588 [9882-11,293] | 9866 [9149-10,583] | 9124 [8386-9861] | 8214 [7471-8958] | |
| Total healthcare costs | 12,407 [11,669-13,145] | 15,332 [14,509-16,155] | 14,545 [13,661-15,430] | 13,428 [12,598-14,258] | 13,446 [12,434-14,458] | 13,021 [12,114-13,928] | 12,089 [11,143-13,035] | 10,960 [10,126-11,793] | |
| SA costs | 6477 [5264-7690] | 5418 [4276-6560] | 3906 [3004-4808] | 3259 [2353-4165] | 2991 [2227-3756] | 4000 [3064-4937] | 4253 [3250-5257] | 4800 [3692-5908] | |
| DP costs | 4700 [3524-5876] | 5566 [4270-6863] | 6738 [5335-8142] | 7456 [5982-8929] | 8241 [6675-9806] | 8729 [7130-10329] | 9303 [7637-10968] | 10,081 [8319-11,842] | |
| Total productivity losses | 11,177 [9572-12,782] | 10,985 [9329-12,641] | 10,644 [9048-12,241] | 10,714 [9056-12,372] | 11,232 [9546-12,919] | 12,730 [10,953-14,507] | 13,556 [11,707-15,405] | 14,880 [12,930-16,831] | |
This is the year of initiation of the DMT therapy (index year).
Figure 2.
(a, b) COI progression (estimated mean from the regressions) from baseline (index year) to the end of follow-up, by early vs. late treatment groups, adjusted for disability progression (mean annual EDSS score for each group) during the follow-up A) Healthcare costs. B) Productivity losses.
For both groups, medications were the main cost driver for healthcare costs while DP was the driver for productivity losses. SA costs decreased over time while DP costs increased, indicating a shift from short-term to long-term productivity losses. Both treatment groups had similar healthcare costs (p-value>0.05), which increased the first year after diagnosis, and then decreased for the rest of the follow-up period. Those receiving treatment late had statistically significant higher productivity losses throughout the study period (p-value = 0.001).
Discussion
In this register-based prospective cohort study, we explored the development of the MS COI among newly diagnosed PwMS in Sweden over time in relation to how long after diagnosis PwMS received their first DMT, adjusting for MS disability progression.
Those receiving the first DMT within 6 months after diagnosis had lower productivity losses over time, possibly through maintained work capacity. Even after adjusting for differences in MS disability between the two groups, fewer PwMS in the early treatment group were on DP over time. While having DP already during the time when receiving the first DMT (at Year 0 in our study) could be related to MS symptom onset prior to the actual diagnosis of the disease,37,38 the timing of DMT initiation can still explain part of this difference. Some PwMS in the late treatment group have received their first DMT several years after diagnosis; while 85% of our total study cohort have received therapy by 24 months after diagnosis (Figure 1); 15% of individuals received it sometime afterwards. Therefore, the occurrence of DP during Year 0 for this group could potentially be linked to the presence of important MS symptoms and/or progression. In addition, intervening with a DMT as early as possible can result in fewer MS relapses, and/or postpone MS progression. The presence of MS symptoms and the consequences of MS progression, such as fatigue, weakness, and cognitive and motor impairment, have been stated as preventing remaining in paid work.4,39
Both groups had similar healthcare costs over time, indicating that it is more likely that MS symptoms requiring specialized medical attention and other comorbidities occur independent of the time of treatment initiation. The cost of drugs (referring to any drugs MS patients have received, not only DMTs) was slightly higher among those initiating treatment late (crude cost estimates; p-value>0.05) during the first year of follow-up, and similar in both groups thereafter.
The cost of drugs in this study was the main driver of healthcare costs in both groups. As previous studies have shown,8,9 new DMTs have changed the treatment landscape of MS. Accordingly, in the last two decades drugs have become main cost drivers in MS, while the need for expensive inpatient healthcare has declined.
The overall trends of healthcare costs and productivity losses observed here are in line with previous COI studies in MS with similar longitudinal designs.5–7 In addition, in line with our findings, previous studies suggest that initiating treatment as early as possible is associated with better clinical outcomes and ability to maintain employment 40 for PwMS over time.11,12 While these studies focused on treatment early from MS onset and rather than soon after diagnosis, i.e. having a different study design than the present study, they nevertheless point to the same conclusion, that early treatment is beneficial to patients by slowing MS progression.11,12
Moreover, our study aimed to present the benefit of early treatment in the overall COI of MS, which is something that has been studied sparsely so far, and not involving longitudinal COI data with multiple years of follow-up. We found no other longitudinal study which uses observed data, measuring the COI progression in relation to time of treatment initiation after MS diagnosis. One US study has shown that receiving DMTs before MS diagnosis vs. afterwards, can have a positive impact on the COI of MS. 41 However, they only followed the patients for one year after treatment initiation, 41 and they did not take into consideration any differences in the baseline MS disability of PwMS in the groups, nor its progression. In addition, one study used a health economic model, to estimate costs and effects from MS, concluding that early treatment with DMTs was cost-effective. 42
Strengths and limitations
Main strengths of this study are the prospective cohort design, with eight years of follow-up, and that data from administrative registers could be used, instead of self-reported information. All people with RRMS in the SMSreg receiving their first DMT in 2006–2009 could be followed for eight years in nationwide registers, eliminating both drop outs and recall biases that some previous MS studies had due to using self-reported information. 7 While this is an important strength, unlike those previous studies, registers did not contain information regarding primary healthcare, rehabilitation measures, home help, and home investments to improve mobility, to include in our COI calculations. Such information could complement our results, showing how MS symptoms and multi-morbidity can impact the overall healthcare costs in MS, based on the treatment timing as well. In addition, no information on multi-morbidity reported by patients was available for our study; our multi-morbidity definition was based on the Rx-Risk Comorbidity Index, 19 for which drug use data were used to measure morbidity, which can provide limited information for multi-morbidity.
While eight years of follow-up can give an indication of whether early or late therapy can have an impact on the COI in MS over time, it would be useful to have a longer follow-up to identify any future COI differences. Treatment switches and discontinuation can also play a role alongside the timing of treatment initiation in the overall COI progression in MS. However, such information was not captured in the findings of this study.
High quality and robust information from the MiDAS register in Sweden was available, capturing with accuracy the number of SA and DP net days per year. This allowed for detailed calculations of the productivity losses, which are considered the main long-term driver of the COI in MS. 7 However, a limitation of this study is that we did not have information on short-term SA-spells (≤14 days). Therefore, these shorter absences which were not quantified into productivity losses, possibly leading to the underestimation of these costs. In addition, these shorter SA spells are mainly not related to MS, it could thus be assumed not to differ substantially between the two studied groups.
Moreover, any reductions in productivity while being present at work that could potentially be related to the presence of MS were not quantified. Furthermore, productivity losses incurred by partners of PwMS, i.e., informal care costs, were not included in the COI calculations. While measuring such costs was beyond the aim of this study, they are an important cost component when defining the overall COI of MS. 7
Similar to what previous COI of MS studies have done, 7 we used the EDSS score to explore disability progression for PwMS in the two treatment groups linking it to the COI. However, EDSS captures only limited domains of disability among PwMS, e.g., fatigue is missing, and only at the point in time of the patient's disability assessment. The use of additional clinical measures could complement our analysis regarding the progression of MS disability, allowing a better correlation of disability with the timing of treatment initiation, and eventually the COI.
To allow for the difference of the timing of treatment initiation in line with our research objective, the healthcare cost outcomes and productivity losses were adjusted for by EDSS, for every year of follow-up. This method has the limitation of minimizing the potential health-improving effects of the DMTs in the resultant costs. Therefore, the unadjusted healthcare costs and productivity losses were also provided in the supplement, to demonstrate the treatment effects over time in the cost progression.
While nationwide registers with robust data were used in this study, 15 the generalizability of our findings can be limited. The study cohort was taken from SMSreg, which at that time (2006–2009) had approximately 50%-60% coverage of all PwMS in Sweden. 43 With caution and assuming no distinct differences in the sociodemographic characteristics, multi-morbidity, and disability with the PwMS that our study did not include, our findings could be generalizable to all PwMS in Sweden. Generalization and applicability of our findings to other countries may not be possible, considering the differences in the organization of healthcare and social security systems.
Conclusions
In this study, register data with nationwide coverage were used to explore the association between the timing of the first DMT initiation among PwMS with the COI. Productivity losses for PwMS who started their therapy early were significantly lower. Therefore, suggesting that by intervening as early as possible to stop MS progression, there is a lower impact on the work capacity of PwMS in terms of lower SA and DP days. COI serves as an objective measure of the burden of MS, reflecting how morbidity and disability linked to MS evolve throughout patients’ days and over many years, as well as highlighting the advantage of early vs. late DMT initiation in MS.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173221092411 for Early vs. late treatment initiation in multiple sclerosis and its impact on cost of illness: A register-based prospective cohort study in Sweden by Korinna Karampampa, Hanna Gyllensten, Chantelle Murley, Kristina Alexanderson, Andrius Kavaliunas, Tomas Olsson, Ali Manouchehrinia, Jan Hillert and Emilie Friberg in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Footnotes
Author contributions: All authors (Korinna Karampampa, KK; Hanna Gyllensten, HG; Chantelle Murley, CM; Kristina Alexanderson, KA; Jan Hillert, JH; Andrius Kavaliunas, AK; Tomas Olsson, TO; Ali Manouchehrinia, AM; Emilie Friberg, EF) contributed to the conceptualization of the research questions, the study design, and methods. KK performed the analysis of the data, interpreted the study findings, and drafted the manuscript. All remaining authors (HG, CM, KA, JH, AK, TO, AM, EF) assisted in the interpretation of the study findings and contributed with comments/suggestions and text to the manuscript.
Declaration of conflicting interests: All authors (KK, HG, CM, KA, JH, AK, TO, AM, EF) are employed or affiliated at the Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden. KK is currently employed by Celgene/Bristol Myers Squibb; she initiated this study while being employed at Karolinska Institutet (employment ended in October 2019); since then, she has not received any salary from Karolinska Institutet or other type of funding for this research. HG is currently employed part-time by Statfinn/EPID Research (which is part of IQVIA); both companies are contract research organizations that perform commissioned pharmaco-epidemiological studies, and therefore are collaborating with several pharmaceutical companies. CM's employment at Karolinska Institutet is partly funded by research grant from Biogen. AM is supported by Margaretha af Ugglas foundation. KA has received unrestricted MS research grants from Biogen. JH has received honoraria for serving on advisory boards for Biogen, Celgene, Sanofi-Genzyme, Merck KGaA, Novartis and Sandoz and speaker's fees from Biogen, Novartis, Merck KGaA, Teva and Sanofi-Genzyme. He has served as P.I. for projects, or received unrestricted research support from, Biogen, Bristol-Myers-Squibb, Merck KGaA, Novartis, Roche and Sanofi-Genzyme. His MS research is funded by the Swedish Research Council and the Swedish Brain foundation. TO has received advisory board and/or lecture honoraria, and unrestricted MS research grants from Biogen, Novartis, Sanofi, Merck and Roche. EF is partly funded by research grants from Biogen, and has received an unrestricted MS research grant from Celgene/Bristol Myers Squibb.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an unrestricted investigator-initiated trial grant from Biogen, which supported the conduct of this study. The study utilized data from the REWHARD consortium supported by the Swedish Research Council (VR; grant number 2017-00624).
Data availability statement: The data used in this study is administered by the Division of Insurance Medicine, Karolinska Institutet, and cannot be made publicly available. According to the General Data Protection Regulation, the Swedish law SFS 2018:218, the Swedish Data Protection Act, the Swedish Ethical Review Act, and the Public Access to Information and Secrecy Act, these types of sensitive data can only be made available, after legal review, for researchers who meet the criteria for access to this type of sensitive and confidential data. Readers may contact Professor Kristina Alexanderson (kristina.alexanderson@ki.se) regarding the data.
ORCID iDs: Korinna Karampampa https://orcid.org/0000-0002-2578-1865
Hanna Gyllensten https://orcid.org/0000-0001-6890-5162
Chantelle Murley https://orcid.org/0000-0003-4150-4275
Andrius Kavaliunas https://orcid.org/0000-0003-3896-7332
Ali Manouchehrinia https://orcid.org/0000-0003-4857-5762
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Korinna Karampampa, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden.
Hanna Gyllensten, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Institute of Health and Care Science, University of Gothenburg, Gothenburg, Sweden.
Emilie Friberg, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden.
References
- 1.Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol 2011; 93: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MS International Federation. Atlas of MS. 2013.
- 4.Jones N, Napier C, Baneke Pet al. et al. Global MS employment report 2016. London, UK. 2016.
- 5.Karampampa K, Gyllensten H, Yang F, et al. Healthcare, sickness absence, and disability pension cost trajectories in the first 5 years after diagnosis with multiple sclerosis: a prospective register-based cohort study in Sweden. Pharmacoecon Open 2020. Mar; 4(1): 91-103. doi:10.1007/s41669-019-0150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyllensten H, Wiberg M, Alexanderson K, et al. Costs of illness of multiple sclerosis in Sweden: a population-based register study of people of working age. Eur J Health Econ 2018; 19: 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernstsson O, Gyllensten H, Alexanderson Ket al. et al. Cost of illness of multiple sclerosis - A systematic review. PLoS One 2016; 11: e0159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieckmann P, Centonze D, Elovaara I, et al. Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: a combined perspective from the MS in the 21st century steering group. Mult Scler Relat Disord 2018; 19: 153–160. [DOI] [PubMed] [Google Scholar]
- 9.Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 2017; 23: 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerqueira JJ, Compston DAS, Geraldes R, et al. Time matters in multiple sclerosis: can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry 2018; 89: 844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavaliunas A, Manouchehrinia A, Stawiarz L, et al. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult Scler 2017; 23: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 12.Landfeldt E, Castelo-Branco A, Svedbom Aet al. et al. The long-term impact of early treatment of multiple sclerosis on the risk of disability pension. J Neurol 2018; 265: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavaliunas A, Manouchehrinia A, Gyllensten Het al. et al. Importance of early treatment decisions on future income of multiple sclerosis patients. Mult Scler J Exp Transl Clin 2020; 6: 2055217320959116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillert J, Stawiarz L. The Swedish MS registry - clinical support tool and scientific resource. Acta Neurol Scand 2015; 132: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016; 31: 125–136. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 17.Collins CD, Ivry B, Bowen JD, et al. A comparative analysis of patient-reported expanded disability Status scale tools. Mult Scler 2016; 22: 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodkin DE. EDSS Reliability. Neurology 1991; 41: 332. [DOI] [PubMed] [Google Scholar]
- 19.Pratt NL, Kerr M, Barratt JD, et al. The validity of the rx-risk comorbidity Index using medicines mapped to the anatomical therapeutic chemical (ATC) classification system. BMJ Open 2018; 8: e021122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swedish Association of Local Authorities and Regions [Sveriges Kommuner och Landsting]. Economic statistics - KPP Somatik - SKL. https://skl.se/ekonomijuridikstatistik/statistik/kostnadperpatientkpp/kppsomatik.1077.html2019.
- 21.Swedish Association of Local Authorities and Regions [Sveriges Kommuner och Landsting]. Economic statistics - Patientavgifter fr.o.m. den 1 januari 2018. https://skl.se/download/18.3ee4cc4f1611af77b89179dd/1517495154691/avgift_%C3%B6ppenv%C3%A5rd_inkl.missiv_slutenavg18_19jan18.pdf2018.
- 22.Vårdguiden 1177. Patient fees in Västra Götaland county in Sweden [Patientavgifter i Västra Götaland]. https://www.1177.se/Vastra-Gotaland/Regler-och-rattigheter/Patientavgifter-i-Vastra-Gotaland/2018.
- 23.FASS.se. Tysabri. https://www.fass.se/LIF/product?nplId=20040916001115&userType=0&docType=6&scrollPosition=751.33331298828122019.
- 24.Salzer J, Svenningsson R, Alping P, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology 2016; 87: 2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FASS.se. Rituximab - price. https://www.fass.se/LIF/product?userType=2&nplId=19980602000029&docType=30&scrollPosition=600s2019.
- 26.Statistics Sweden. Genomsnittlig månadslön 1973–2018. https://www.scb.se/hitta-statistik/statistik-efter-amne/arbetsmarknad/loner-och-arbetskostnader/lonestrukturstatistik-privat-sektor-slp/pong/tabell-och-diagram/genomsnittlig-manadslon-1973-/2019.
- 27.Swedish Tax Authority [Skatteverket]. Belopp och procent för åren 2019-2004 - privat. https://www.skatteverket.se/privat/skatter/beloppochprocent.4.18e1b10334ebe8bc80004109.html2019.
- 28.Eurostat. HICP - all items - annual average indices (2015=100). https://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&language=en&pcode=tec00027&plugin=12019.
- 29.Piehl F, Holmen C, Hillert Jet al. et al. Swedish Natalizumab (tysabri) multiple sclerosis surveillance study. Neurol Sci 2011; 31: 289–293. [DOI] [PubMed] [Google Scholar]
- 30.Drummond MF, Sculpher MJ, Claxton Ket al. et al. Methods for the economic evaluation of health care programmes. Oxford, UK: Oxford university press, 2015. [Google Scholar]
- 31.National Insurance Agency [Försäkringskassan]. Social Insurance in Figures 2017. 2017.
- 32.Moore DS. Tests of chi-squared type. Goodness-of-fit-techniques. Oxfordshire, UK: Routledge, 2017, p. 63–96. [Google Scholar]
- 33.Kim TK. T test as a parametric statistic. Korean J Anesthesiol 2015; 68: 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullagh P, Nelder J. Generalized linear models. 2nd ed. London: Chapman and Hall, Standard book on generalized linear models , 1989. [Google Scholar]
- 35.Rousson V, Rossel J-B, Eggli Y. Estimating health cost repartition among diseases in the presence of multimorbidity. Health Serv Res Manag Epidemiol 2019; 6: 2333392819891005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agresti A. Categorical data analysis. Hoboken, NJ: John Wiley & Sons, 2003. [Google Scholar]
- 37.Murley C, Karampampa K, Alexanderson Ket al. et al. Diagnosis-specific sickness absence and disability pension before and after multiple sclerosis diagnosis: an 8-year nationwide longitudinal cohort study with matched references. Mult Scler Relat Disord 2020; 42: 102077. [DOI] [PubMed] [Google Scholar]
- 38.Murley C, Tinghog P, Karampampa Ket al. et al. Types of working-life sequences among people recently diagnosed with multiple sclerosis in Sweden: a nationwide register-based cohort study. BMJ Open 2020; 10: e039228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bessing B, Hussain MA, Claflin SB, et al. Changes in multiple sclerosis symptoms are associated with changes in work productivity of people living with multiple sclerosis. Mult Scler 2021; 27: 2093–2102. [DOI] [PubMed] [Google Scholar]
- 40.Olofsson S, Wickstrom A, Hager Glenngard Aet al. et al. Effect of treatment with natalizumab on ability to work in people with multiple sclerosis: productivity gain based on direct measurement of work capacity before and after 1 year of treatment. BioDrugs 2011; 25: 299–306. [DOI] [PubMed] [Google Scholar]
- 41.Curkendall SM, Wang C, Johnson BH, et al. Potential health care cost savings associated with early treatment of multiple sclerosis using disease-modifying therapy. Clin Ther 2011; 33: 914–925. [DOI] [PubMed] [Google Scholar]
- 42.Tinelli M, Pugliatti M, Antonovici A, et al. Averting multiple sclerosis long-term societal and healthcare costs: the value of treatment (VoT) project. Mult Scler Relat Disord 2021; 54: 103107. [DOI] [PubMed] [Google Scholar]
- 43.Swedish Neuroregistries MS. Visualisation and Analysis platform (VAP) for Multiple Sclerosis - Coverage ratio broken down by county, gender and the last follow-up date [Täckningsgrad uppdelat för län, kön och sista uppföljningsdatum], Coverage ratio against the patient register, by year [Täckningsgrad mot patientregistret, år]. https://www.neuroreg.se/en.html/multiple-sclerosis-realtime-data-and-results-vap: Swedish Neuroregistries - Multiple Sclerosis, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173221092411 for Early vs. late treatment initiation in multiple sclerosis and its impact on cost of illness: A register-based prospective cohort study in Sweden by Korinna Karampampa, Hanna Gyllensten, Chantelle Murley, Kristina Alexanderson, Andrius Kavaliunas, Tomas Olsson, Ali Manouchehrinia, Jan Hillert and Emilie Friberg in Multiple Sclerosis Journal – Experimental, Translational and Clinical