Abstract
Sepsis is a life-threatening condition characterized by an acute cytokine storm followed by prolonged dysfunction of the immune system in the survivors. Post-septic lymphopenia and functional deficits of the remaining immune cells lead to increased susceptibility to secondary infections and other morbid conditions causing late death in the patients. This state of post-septic immunoparalysis may also influence disorders stemming from inappropriate or overactive immune responses, such as autoimmune and immunoinflammatory diseases, including multiple sclerosis. In addition, ongoing autoimmunity likely influences the susceptibility to and outcome of sepsis. This review article addresses the bidirectional relationship between sepsis and multiple sclerosis, with a focus on the immunologic mechanisms of the interaction and potential directions for future studies.
Keywords: Sepsis, multiple sclerosis, gut microbiota
1. Sepsis
The devastating immune response that develops following disseminated infection leads to a life-threatening organ dysfunction called sepsis [1]. Approximately 50 million individuals developed sepsis and 11 million sepsis-related deaths were reported worldwide in 2017, which accounted for almost 20% of all global deaths [2]. Although 85% of sepsis cases and sepsis-related deaths occur in low- and middle-income countries, wealthy societies are not spared from this devastating disorder. For example, nearly 2 million people in the United States develop sepsis each year and it is the most expensive hospital-treated condition in the U.S., with >$23 billion spent on hospital treatment of sepsis annually [3]. Sepsis is characterized by the overproduction of both pro- and anti-inflammatory cytokines leading to temporary and severe lymphopenia [4–6] (Fig 1A). Sepsis survivors (∼ 75% survival rate [7]) progress into a state of prolonged immunoparalysis [8], where they become susceptible to secondary infections and viral reactivation, and have decreased 5-year survival, relative to matched individuals who did not have sepsis [9–11] (Fig 1B, C).
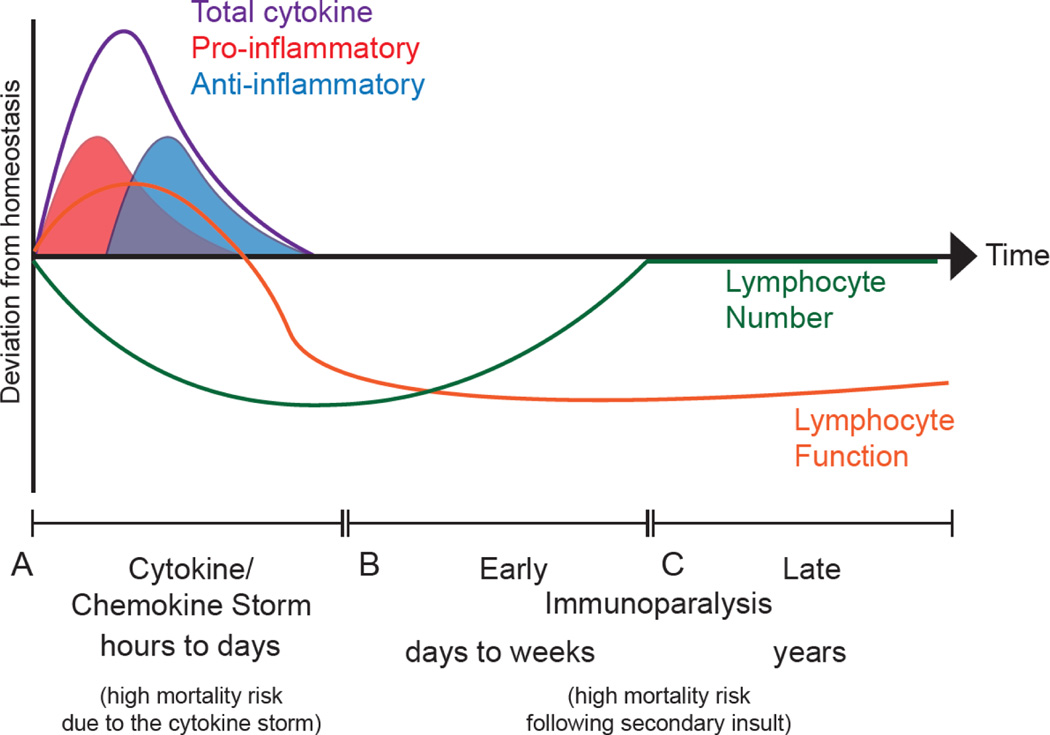
Figure 1: Influence of the sepsis-induced cytokine storm and immunoparalysis state on host immune response.
A) In the first several hours to days following a septic insult, lymphocytes become activated and begin contributing pro-inflammatory cytokines [4–6]. As the infection begins to become mismanaged lymphocytes begin to undergo apoptosis and produce anti-inflammatory cytokines to counterbalance the pro-inflammatory cytokines and limit immunopathology. The culmination of the pro- and anti-inflammatory cytokine responses is termed the cytokine storm. B) If the host survives the cytokine storm, then as the inflammation subsides the host is left with a substantially reduced number of lymphocytes and these surviving lymphocytes exhibit functional impairment. This defines the early immunoparalysis state that may last for days to weeks after the septic insult wherein hosts are now more susceptible to secondary pathogenic insults (i.e., infection or cancer). [8–11] C) With time the number of total lymphocytes recovers, however, these lymphocytes continue to exhibit functional impairment years after the septic insult due to sepsis-induced cell-intrinsic and -extrinsic changes [15]. This defines the late immunoparalysis state that is also associated with increased susceptibility to secondary pathogenic insults.
The number of peripheral blood leukocytes fluctuates significantly during the course of sepsis [12]. While a noticeable increase in neutrophil and monocyte populations is observed in the first 2–4 days from septic onset, the lymphopenic state quickly follows the resolution of the cytokine storm [13]. The marked decrease in the number of B cells, CD4+ and CD8+ T cells, and NK cells following sepsis onset is the result of apoptotic loss [12–14]. Lymphocyte numbers normalize within a month in sepsis survivors, but the functionality of the immune cells is reduced for an extended period [15] (Fig 1C). Failure to regulate either leukocytosis or lymphopenia in the early stages of sepsis correlates with increased mortality in patients [16]. Yet, it is the previously septic individual’s increased susceptibility to secondary infections and viral reactivation, due to long-lasting functional impairments, that leads to shortened life expectancy [9–11]. Experimental therapies targeting sepsis-induced immunoparalysis are thus focused not only on preventing cell loss and potentiating recovery of lymphocyte number, but also restoring function to the repopulating immune cells [17].
2. Multiple sclerosis
Multiple sclerosis (MS) is an incurable chronic inflammatory, demyelinating and neurodegenerative disease of the central nervous system (CNS) that affects more than 2.8 million individuals worldwide [18]. Current drugs target the autoimmune response that initiates and propagates CNS tissue destruction during MS [19]. Indeed, the autoimmune response during MS is initiated by CD4+ T cells specific for CNS antigens, which in turn orchestrate the activity of macrophages, CD8+ T cells, B cells and other immune cells against myelin and other structures within the CNS [20]. CD4+ T cells (i.e., T helper (Th) cells) are the accepted initiators of MS pathogenesis following recognition and activation by CNS antigens or microbial mimics of CNS antigens. These cells differentiate towards Th1 and Th17 cells and then migrate into the CNS where they are re-activated to initiate inflammation within the CNS [21]. Meanwhile, CD8+ T cells and B cells dominate the inflammatory reaction in the CNS, while macrophages and other innate immune cells are prominent contributors to the tissue damage observed in MS [20, 22, 23].
Characteristically, the disease evolves into a relapsing-remitting course where activation of the autoimmune response happens periodically provoking neurological deficits in patients. The autoimmune responses then resolve either spontaneously or after glucocorticoid therapy. However, a proportion of patients develop progressive forms of the disease, either from the start of MS manifestation (primary progressive) or following a period of relapsing-remitting course (secondary progressive). Current therapeutic strategies available for the treatment of MS are meant to modulate the immune system, thereby slowing disease progression and preventing relapses. Since demyelination and neurodegeneration are consequent to autoimmune activity, remyelination and neuroprotection are the ultimate goals of current studies in MS [24].
3. Sepsis – MS relations
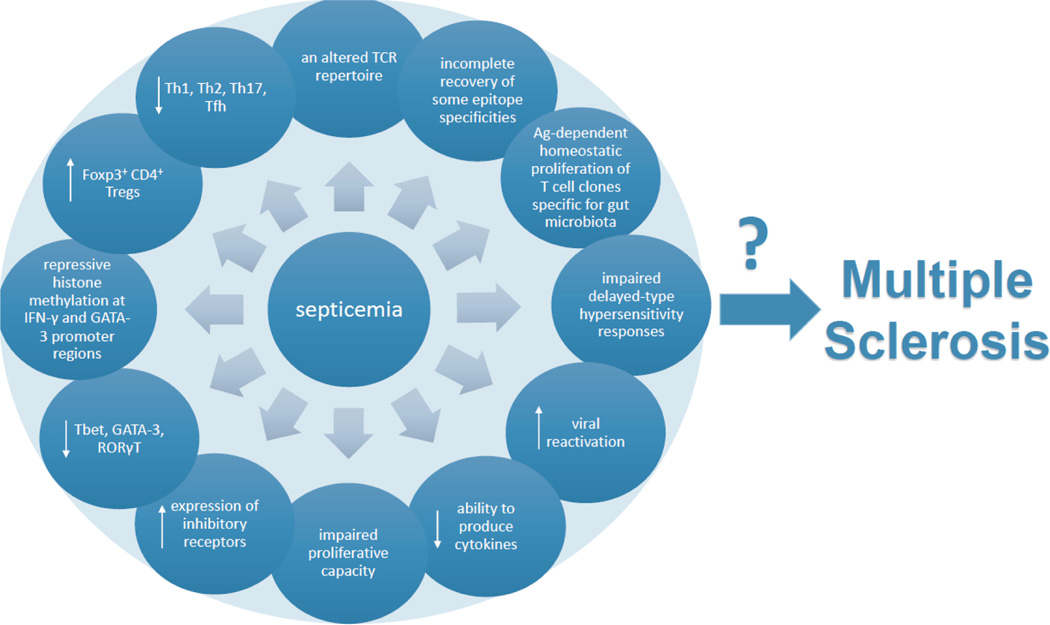
As previously stated, septicemia induces long-lasting changes in the immune system. Specifically, these changes include: an altered TCR repertoire (notably including a decreased diversity of TCR Vβ [25]), incomplete recovery of some epitope specificities [26], and antigen-dependent homeostatic proliferation of T cell clones specific for gut microbiota [27]. Additionally, T cells sustain functional deficits, reflected by impaired delayed-type hypersensitivity responses and higher viral reactivation [28–30] as well as global anergy mirrored in reduced ability to produce cytokines [31–37], impaired proliferative capacity [31, 38, 26], and increased expression of inhibitory receptors [39–46]. Further, there are changes in subset representation, including decreased transcript levels of major Th transcriptional factors Tbet, GATA-3, RORγt [47], repressive histone methylation at Ifnγ and Gata3 promoter regions [38], an increased representation of Foxp3-expressing CD4+ Tregs [35, 48–50] with a corresponding decrease in the representation of Th1, Th2, Th17 and Tfh subsets [35, 50–52]. All of these changes have the potential to affect the process of MS pathogenesis (Fig 2).
Figure 2: Effects of sepsis on T cells that are relevant for MS pathogenesis.
Septicemia induces various changes in T cell activity that could have profound influence on MS pathogenesis. [23–50]
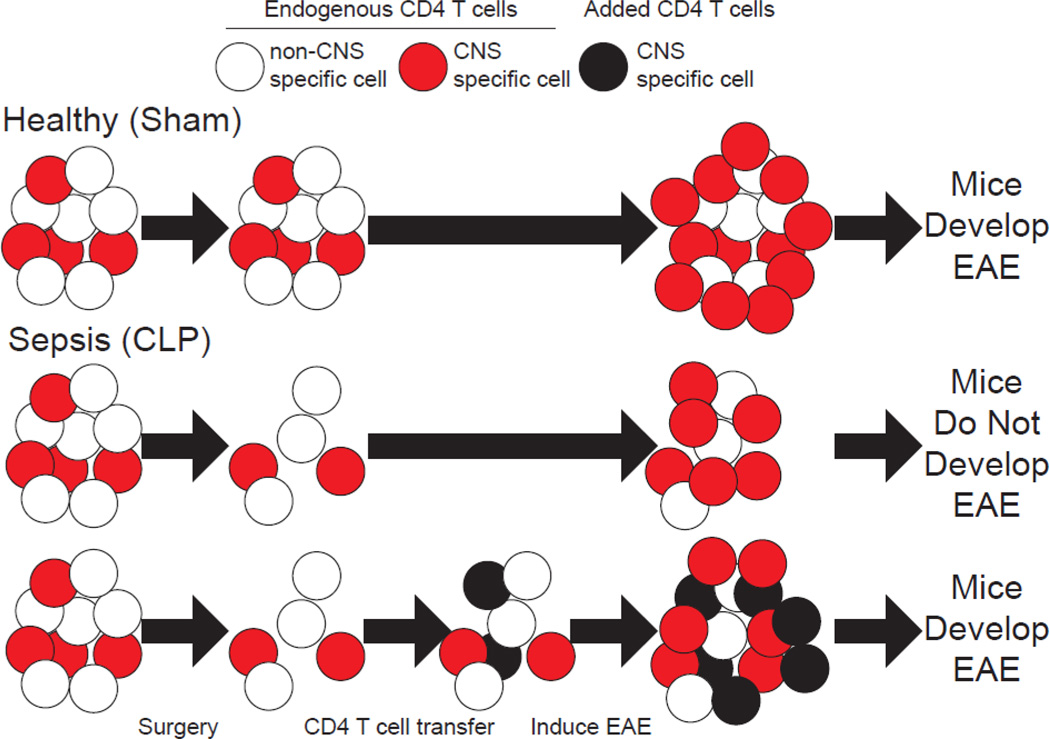
In agreement with this idea, sepsis was recently shown to impede experimental autoimmune encephalomyelitis (EAE) development in mice [53]. EAE, the most commonly used animal model in the study of MS, is induced in susceptible rodent strains by peripheral injection of CNS proteins or peptides emulsified in complete Freund’s adjuvant (CFA). The effect of sepsis on EAE was mediated through reduction in myelin oligodendrocyte protein (MOG)-specific encephalitogenic CD4+ T cell numbers in the CNS during effector phase and lymph nodes draining the site of immunization in the inductive phase of EAE. This numeric deficit even preceded immunization to induce EAE, wherein septic (cecal ligation and puncture, CLP-treated) hosts had a reduced number of naïve MOG-specific CD4+ T cells. Importantly, transfer of naïve encephalitogenic CD4+ T cells after resolution of the cytokine storm, to replace those lost during sepsis-induced lymphopenia, was able to re-establish the development of EAE disease (Fig 3). Interestingly, those MOG-specific cells that were primed and expanded in CLP hosts were not functionally impaired. Further, naïve encephalitogenic cells transferred after resolution of the cytokine storm were capable of proliferating equivalently to those in control (sham surgery-treated) counterparts suggesting the post septic environment was not limiting functionality of these cells. This observation is in stark contrast to infection scenarios after sepsis wherein the post-septic environment may limit T cells expansion and function through impairment of other cells (including dendritic cells and endothelia) [54, 55]. Yet, this may in part be the result of the substantial antigen and inflammatory bolus utilized in EAE immunization that is not present under infection conditions.
Figure 3: Sepsis ablates EAE development by reducing the number auto-antigen specific CD4+ T cells.
CLP (sepsis) induces numeric loss of CD4+ T cells, including autoantigen-specific CD4+ T cells that target the brain and spinal cord (CNS). Thus, when the autoantigen-specific cells respond following EAE induction there is reduction in the number of autoantigen-specific effectors thereby ablating EAE disease development. However, if these autoantigen specific cells are supplemented through an add back experiment, CLP hosts re-acquire the ability to develop EAE, demonstrating that the numeric deficit in autoantigen-specific CD4+ T cells is causal in the impaired capacity of septic hosts to develop EAE. [51]
Limited epidemiological data, however, do not support the notion that sepsis would impair subsequent autoimmune development. Specifically, Taiwanese septicemia patients had approximately 3-fold higher risk to develop MS [56], and more severe septicemia was associated with an increased risk to develop MS. It is, however, important to note that this study did not address whether the patient cohorts had differential HLA expression, which is strongly associated with development of MS. Further, while the selection criteria identified patients that had not presented with clinical signs prior to the septic insult, it remains formally possible that subclinical autoimmune activity may have occurred and even potentially contributed to elaboration of the septic event. Similar results were observed for non-MS demyelinating syndromes, such as neuromyelitis optica spectrum disorders, transverse myelitis, and acute disseminated encephalomyelitis [57], yet the same limitations apply. It is also notable that both of these studies were limited to Taiwanese cohorts, which may not be representative of the broad range of patients who may either experience a septic event or develop MS. Thus, more robust interrogation of the association of sepsis with subsequent autoimmune development in patient cohorts is required.
Consecutively, the discrepancy between MS and EAE sepsis-related data could be attributed to imperfections in the translational capacity of the model. Indeed, several differences in the pathogenesis of MS and EAE exist that prevent direct translation of data obtained in the model for the benefit of the patients [22, 58]. CD4+ T cells are the initiators of autoimmune response both in MS and EAE, and contribute to the disease pathogenesis in humans and experimental animals alike. Still, the immune response within the CNS is dominated by CD8+ T cells and B cells in MS, but not in EAE [22, 23]. Additionally, the use of complete CFA and pertussis toxin in EAE immunization biases the initiated immune response. Notably, CFA stimulates the immune response against non-CNS antigens on its own [59], skewing the immune response to be Th1-driven [60], and induces pain, thereby inducing glial activation and production of inflammatory mediators in the spinal cord [61]. However, novel varieties of EAE that overcome the obstacles of the classical EAE have subsequently been developed [62]. For instance, EAE models in which CD8+ T cells and B cells, in conjunction with CD4+ T cells, have a dominant role in disease pathogenesis are available for study [63, 64]. EAE can also be induced without the use of CFA in Dark Agouti rats [65]. We have also recently investigated the cellular components of CFA-free EAE in detail [66] and found there is a lower total number of cells, including CD4+ T cells in SCH-immunized rats in the inductive phase of EAE. Interestingly, CD8+ macrophages were identified as one of the leading CNS-infiltrating populations at the peak of the disease in CFA-free model of EAE. Our novel results suggest a resemblance of this model pathogenesis to that of MS. These and other non-classical varieties of EAE may assist in further elucidation of the interplay between sepsis and MS.
Alternately, the influence of ongoing autoimmunity on sepsis outcomes is also worthy of study. Yet, as with the influence of sepsis on the development of autoimmunity, epidemiological data evaluating the influence of autoimmunity on sepsis are scarce. MS patients enrolled in three studies in the U.S. had a higher overall mortality rate and increased predisposition to serious infections and sepsis than matched control individuals; this was true both with regard to controls that did not have autoimmune disease and controls with other autoimmune conditions [67–69]. Further, sepsis was identified as one of the leading reasons for MS patients’ readmission to hospitals in the U.S. [70]. An increased predisposition for serious infection and sepsis mortality was also observed for MS patients in two studies in Canada [71, 72]. Similarly, a study performed in Basque Country, Spain showed respiratory infection and sepsis were the most frequent MS-related causes of death among the examined individuals [73]. Obviously, additional epidemiological data is needed to garner insight into the relationship between sepsis and MS.
It is also important to consider the potential effects of disease-modifying drugs that are widely used in the treatment of MS on the epidemiological results. These drugs have general immunomodulatory properties, including a profound effect on peripheral leukocytes [74]. Indeed, one epidemiological study showed the disease modifying therapeutics natalizumab, fingolimod and dimethyl fumarate, unlike beta-interferon and glatiramer acetate, increased risk to infections in MS patients [75]. On the other hand, studies on animal models of sepsis showed beneficial effects of fingolimod, dimethyl fumarate, and glatiramer acetate [76–78]. Thus, it is plausible that MS drugs could influence the immune response in sepsis. This could be either to limit the ability of an individual to control an infection, which may not have otherwise become septic, or by dampening the cytokine storm to reduce the septic burden. To the best of our knowledge there has been no study conducted to date dedicated to the relationship between the risk of sepsis and specific treatment in MS. There is only one report showing slightly, yet statistically insignificant, lower risk for sepsis in the group of MS patients treated with various disease-modifying drugs [79]. It will be important to address this point in the forthcoming studies, as well. Animal models will surely be helpful in resolving the influence of the CNS autoimmunity on sepsis. Induction of sepsis during ongoing CNS inflammation or after the resolution of EAE, in appropriate EAE varieties, may be informative and hopefully opens additional angles for therapeutic intervention in patients.
4. Gut microbiota link
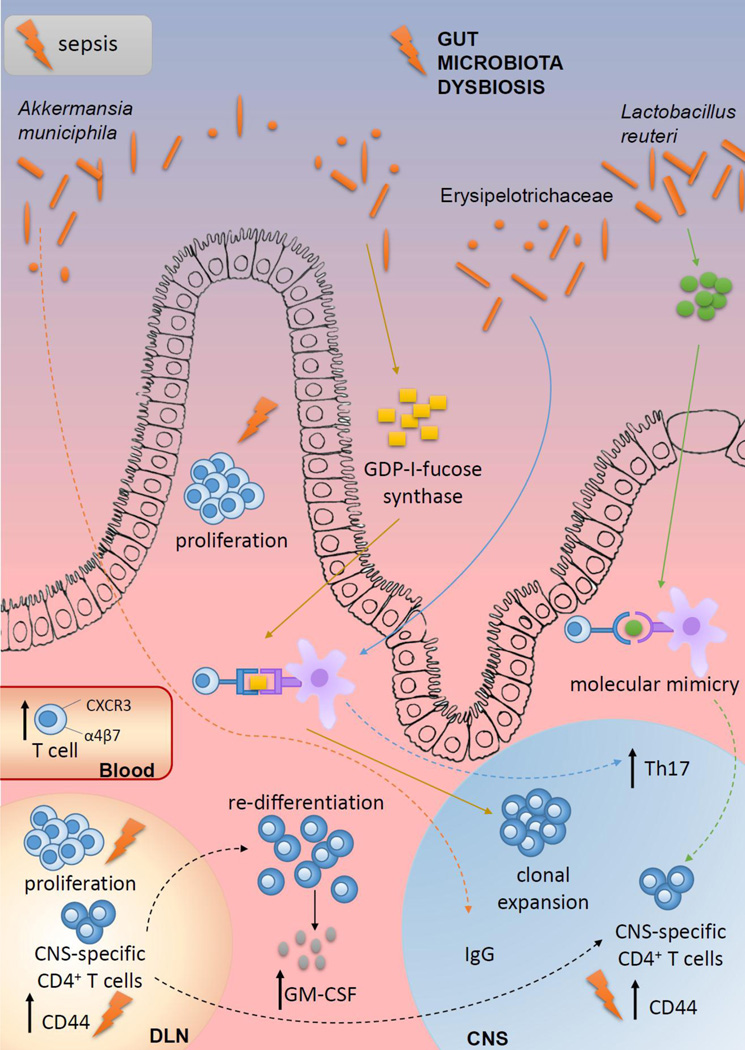
Considering the increasing interest in the contribution of gut microbiota to the pathogenesis of MS, it will be of utmost importance to determine to what extent the effects of sepsis on the CNS autoimmunity might be attributed to the changes in the gut microbiota composition and activity. Indeed, MS pathogenesis has been indirectly associated with altered gut microbiota composition [80, 81], while the importance of gut microbiota for the pathogenesis of EAE has been demonstrated in numerous studies. Notably, gut microbiota composition changes during the course of EAE and varies between the different stages and clinical subtypes of the disease [82–84]. The importance of gut microbiota in EAE was recently revealed in data showing germ-free mice did not develop disease in a model of spontaneous EAE, but disease was initiated once the mice were colonized with human gut microbiota [85]. Importantly, a higher proportion of mice developed EAE in response to MS twin-derived faecal samples than to healthy twin-derived samples. Similar results were obtained in another study, where transfer of gut microbiota from MS patients to germ-free C57Bl/6 mice increased their susceptibility to the induction of active EAE to greater extent than transfer of gut microbiota from healthy subjects [86]. These studies clearly imply the gut microbiota of MS patients is in state of dysbiosis that can be associated with disease pathogenesis (Fig 4). Indeed, reduced diversity of gut microbiota in MS patients correlated with increased abundance of CXCR3+ T cells expressing the gut-homing α4β7 integrin receptor in the peripheral blood [87]. Further, MS gut microbiota might contain microorganisms able to provoke or promote CNS autoimmunity, as elevated levels of Akkermansia muciniphila-specific IgG were present in the cerebrospinal fluid of MS patients [88]. Moreover, a CD4+ T cell clone that was clonally expanded in MS brain lesions was shown to recognize guanosine diphosphate (GDP)-l-fucose synthase, an enzyme expressed by gut microorganisms [89]. Accordingly, a recent EAE study has identified specific gut microorganisms involved in the reactivation of MOG-specific T cells [90]. Namely, peptides originating from Lactobacillus reuteri mimic MOG, while Erysipelotrichaceae can act as an adjuvant to enhance the responses of encephalitogenic Th17 cells. Thus, the observation that proliferation of lymphocytes after the acute sepsis phase can be driven in part by gut microbiota antigens [27], implies that some of the proliferating clones may also have specificity for the CNS antigens. Activation of T cell clones specific for MOG peptides in response to some gut bacterial mimic is one of the plausible explanations for the observation of Jensen et al. [53], based on data showing the numerical recovery and acquisition an activated phenotype (i.e., increased expression of CD44) of MOG-specific CD4+ T cells in some hosts with time after sepsis. Alternately, that MOG-specific cells could encounter CNS antigen as a consequence of sepsis-induced damage or are potentially acquiring an activated phenotype via homeostatic proliferation [15, 91, 92]. Regardless of the mechanism behind the acquisition of the activated phenotype, these MOG-specific CD4+ T cells may now have the potential to begin exerting their effector function within these hosts. This could explain the increased risk for sepsis survivors to develop CNS autoimmunity, but we acknowledge the possibility that other impairments to the immune system may also exist in an individual after sepsis that may temper the magnitude of the autoimmune response. It is also possible that the mechanisms by which sepsis influences subsequent autoimmunity may change with time and depend on the composition of the host gut microbiome.
Figure 4: Convergent contribution of sepsis and gut microbiota dysbiosis to MS pathogenesis.
Dysbiosis of gut microbiota in MS patients correlates with increased abundance of CXCR3+ T cells expressing the gut-homing α4β7 integrin receptor in the peripheral blood [87]. Elevated levels of Akkermansia muciniphila-specific IgG are present in the cerebrospinal fluid of MS patients [88]. CD4+ T cell clone that is clonally expanded in MS brain lesions is shown to recognize GDP-l-fucose synthase, an enzyme expressed by gut microorganisms [89]. Peptides originating from Lactobacillus reuteri mimic MOG, while Erysipelotrichaceae can act as an adjuvant to enhance the responses of encephalitogenic Th17 cells [27]. Gut dysbiosis increases abundance of GM-CSF-producing CD4+ T cells that are among the major encephalitogenic cells in EAE [99–102]. CNS-specific T cells migrate to the gut where they can undergo re-differentiation into potent encephalitogenic cells under the influence of gut microbiota dysbiosis [105, 106]. Sepsis (lightning symbol) can influence many of these processes, thus cooperating with gut microbiota dysbiosis in MS pathogenesis.
Another important point that should be addressed is the consequence of antibiotic use in sepsis and its impact on susceptibility to MS. Broad spectrum antibiotics are routinely used as the initial treatment of sepsis, and they have beneficial effects in EAE through modulation of murine gut microbiota composition [93–95]. Conversely, EAE aggravation as the consequence of broad-spectrum antibiotic application has been observed in rats [83]. The results of a recent study in spontaneous EAE clearly indicate the timing of gut microbiota perturbation by antibiotics is crucial for the consequent effects on the CNS autoimmunity. Namely, prophylactic, but not therapeutic, application of antibiotics and subsequent modulation of gut bacteria was effective in restraining CNS autoimmunity [96]. Additionally, studies on mice showed development of the regulatory arm of the gut immune system was acquired in a narrow period between the second and fourth weeks of life [97]. This “weaning reaction” of the gut immune system developed under the influence of gut microbiota and was essential for prevention of the future inflammatory pathologies in the adult organisms. Thus, the use of antibiotics in the early childhood to treat sepsis could be a predisposing factor for development of autoimmunity, particularly MS. It will be important to examine available epidemiological data on the association between childhood sepsis and development of MS later in life.
Finally, GM-CSF-producing CD4+ T cells have recently been defined as a distinct population in humans [98]. These cells were identified as the major encephalitogenic cells in EAE [99–102]. An increased proportion of GM-CSF+ CD4+ T cells was also observed in individuals with sepsis [103] and were proportionally increased in non-survivors relative to survivors. Given that the gut microbiota is essential for Th differentiation in EAE [104] and encephalitogenic T cells migrate to the gut where they can undergo re-differentiation [105, 106], it is tempting to speculate that the gut microbiota has a decisive role in GM-CSF-producing CD4+ T cell origin and function (Fig 4). Thus, tracing the gut microbiota-related fate of GM-CSF-producing CD4+ T cells in animals that survive sepsis and are subsequently immunized to develop EAE (or vice versa) seems an important direction for future study.
5. Conclusions
Sepsis and MS are devastating disorders that inflict enormous physiological and economic burdens for the society. Thus, studies on the pathogenesis of sepsis and MS are needed for the design of novel therapeutics to diminish the effects of each of these disorders. Although different in their initiation and clinical manifestation, these disorders have a common characteristic: inappropriate activity of the immune system. Given the complexity of the immune system and the numerous connections among its cellular constituents, it is logical to assume that the disbalance of the immune system imposed by one of the disorders will impact the other one. Currently, there are limited epidemiological and experimental data on the links between sepsis and MS, though they are indicative of causal interplay. Thus, novel studies on animal models, as well as novel analyses of the patient records are warranted.
Highlights.
Potential interplay between sepsis and multiple sclerosis (MS) is reviewed
Immunological mechanism(s) governing sepsis/MS interactions are proposed
The role of gut microbiota influencing the sepsis/MS interaction is discussed
Acknowledgements
This work was supported by Ministry of Education, Science and Technological Development of Republic of Serbia #451–03-9/2021–14/200007 (Đ.M., S.S.), Science Fund of the Republic of Serbia, Serbian Science and Diaspora Collaboration Program #6409283 (Đ.M., V.P.B., S.S.), NIH Grants R01AI114543 (V.P.B.), R21AI147064 (V.P.B.), R35GM134880 (V.P.B.), R21AI151183 (V.P.B.), R01GM115462 (T.S.G.), R35GM140881 (T.S.G.), T32AI007511 (I.J.J.), T32AI007485 (I.J.J.) and a Veterans Health Administration Merit Review Award I01BX001324 (T.S.G.).
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 315 (2016) 801–10. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 395(10219) (2020) 200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torio C (AHRQ), Moore B (Truven Health Analytics). National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013. HCUP Statistical Brief #204. Rockville, MD: Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf 2016 (accessed February 12, 2020). [Google Scholar]
- 4.Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016. Nov;274(1):330–353. doi: 10.1111/imr.12499. PMID: 27782333; PMCID: PMC5111634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016. Jun 30;2:16045. doi: 10.1038/nrdp.2016.45. PMID: 28117397; PMCID: PMC5538252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins RB, Raymond SL, Stortz JA, Horiguchi H, Brakenridge SC, Gardner A, Efron PA, Bihorac A, Segal M, Moore FA, Moldawer LL. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front Immunol. 2018. Jul 2;9:1511. doi: 10.3389/fimmu.2018.01511. PMID: 30013565; PMCID: PMC6036179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 41 (2013) 1916–27. doi: 10.1097/CCM.0b013e31827c09f8 [DOI] [PubMed] [Google Scholar]
- 8.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 306 (2011) 2594–605. doi: 10.1001/jama.2011.1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly JP, Hohmann SF, Wang HE. Unplanned readmissions after hospitalization for severe sepsis at academic medical center-affiliated hospitals. Crit Care Med. 43 (2015) 1916–27. doi: 10.1097/CCM.0000000000001147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 26 (1998) 1076–82. doi: 10.1086/520307 [DOI] [PubMed] [Google Scholar]
- 11.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, et al. 2014. Reactivation of multiple viruses in patients with sepsis. PLoS ONE. 9:e98819. doi: 10.1371/journal.pone.0098819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin MD, Badovinac VP, Griffith TS. CD4 T Cell Responses and the Sepsis-Induced Immunoparalysis State. Front Immunol. 11 (2020) 1364. doi: 10.3389/fimmu.2020.01364. PMID: 32733454; PMCID: PMC7358556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hohlstein P, Gussen H, Bartneck M, Warzecha KT, Roderburg C, Buendgens L, et al. Prognostic relevance of altered lymphocyte subpopulations in critical illness and sepsis. J Clin Med. 8 (2019) 353. doi: 10.3390/jcm8030353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr, Hui JJ, Chang KC, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 166 (2001) 6952–63. doi: 10.4049/jimmunol.166.11.6952 [DOI] [PubMed] [Google Scholar]
- 15.Jensen IJ, Sjaastad FV, Griffith TS, Badovinac VP. Sepsis-Induced T cell immunoparalysis: the ins and outs of impaired T cell immunity. J Immunol. 200 (2018)1543–53. doi: 10.4049/jimmunol.1701618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drewry Anne M et al. “Persistent lymphopenia after diagnosis of sepsis predicts mortality.” Shock (Augusta, Ga.) vol. 42,5 (2014): 383–91. doi: 10.1097/SHK.0000000000000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono S, Tsujimoto H, Hiraki S, Aosasa S. Mechanisms of sepsis-induced immunosuppression and immunological modification therapies for sepsis. Ann Gastroenterol Surg. 2 (2018) 351–8. doi: 10.1002/ags3.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Atlas of MS, Multiple Sclerosis International Federation https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms , 2020. (accessed 11 January 2021)
- 19.Rommer PS, Milo R, Han MH, Satyanarayan S, Sellner J, Hauer L, Illes Z, Warnke C, Laurent S, Weber MS, Zhang Y, Stuve O. Immunological Aspects of Approved MS Therapeutics. Front Immunol. 10 (2019) 1564. doi: 10.3389/fimmu.2019.01564. PMID: 31354720; PMCID: PMC6637731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sospedra M, Martin R . Immunology of Multiple Sclerosis. Semin Neurol. 36(2) (2016)115–27. doi: 10.1055/s-0036-1579739. PMID: 27116718. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, & Mills KH T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clinical and experimental immunology, 162(1) (2010) 1–11. 10.1111/j.1365-2249.2010.04143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassmann H The changing concepts in the neuropathology of acquired demyelinating central nervous system disorders. Curr Opin Neurol. 2019. Jun;32(3):313–319. doi: 10.1097/WCO.0000000000000685. PMID: 30893100. [DOI] [PubMed] [Google Scholar]
- 23.Van Kaer L, Postoak JL, Wang C, Yang G, Wu L. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol Immunol. 2019. Jun;16(6):531–539. doi: 10.1038/s41423-019-0221-5. Epub 2019 Mar 15. PMID: 30874627; PMCID: PMC6804597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 19(8) (2020) 678–688. doi: 10.1016/S1474-4422(20)30140-X. PMID: 32702337. [DOI] [PubMed] [Google Scholar]
- 25.Venet F, Filipe-Santos O, Lepape A, Malcus C, Poitevin-Later F, Grives A, Plantier N, Pasqual N, Monneret G. Decreased T-cell repertoire diversity in sepsis: a preliminary study. Crit Care Med. 41(1) (2013) 111–9. doi: 10.1097/CCM.0b013e3182657948. PMID: 23222261. [DOI] [PubMed] [Google Scholar]
- 26.Cabrera-Perez J, Condotta SA, James BR, Kashem SW, Brincks EL, Rai D, Kucaba TA, Badovinac VP, Griffith TS. Alterations in antigen-specific naive CD4 T cell precursors after sepsis impairs their responsiveness to pathogen challenge. J Immunol. 194(4) (2015) 1609–20. doi: 10.4049/jimmunol.1401711. Epub 2015 Jan 16. PMID: 25595784; PMCID: PMC4412277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrera-Perez J, Babcock JC, Dileepan T, Murphy KA, Kucaba TA, Badovinac VP, Griffith TS. Gut Microbial Membership Modulates CD4 T Cell Reconstitution and Function after Sepsis. J Immunol. 197(5) (2016) 1692–8. doi: 10.4049/jimmunol.1600940. Epub 2016 Jul 22. PMID: 27448587; PMCID: PMC4992581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 186(3) (1977) 241–50. doi: 10.1097/00000658-197709000-00002. PMID: 142452; PMCID: PMC1396336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laing KJ, Dong L, Sidney J, Sette A, Koelle DM. Immunology in the Clinic Review Series; focus on host responses: T cell responses to herpes simplex viruses. Clin Exp Immunol. 167(1) (2012) 47–58. doi: 10.1111/j.1365-2249.2011.04502.x. PMID: 22132884; PMCID: PMC3248086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, Capron F, Agut H, Gibert C, Chastre J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 175(9) (2007) 935–42. doi: 10.1164/rccm.200609-1322OC. Epub 2007 Jan 18. PMID: 17234903. [DOI] [PubMed] [Google Scholar]
- 31.De AK, Kodys KM, Pellegrini J, Yeh B, Furse RK, Bankey P, Miller-Graziano CL. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 96(1) (2000) 52–66. doi: 10.1006/clim.2000.4879. PMID: 10873428. [DOI] [PubMed] [Google Scholar]
- 32.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd , Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 306(23) (2011) 2594–605. doi: 10.1001/jama.2011.1829. PMID: 22187279; PMCID: PMC3361243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, Holzmann B. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 178(4) (1999) 288–92. doi: 10.1016/s0002-9610(99)00183-x. PMID: 10587185. [DOI] [PubMed] [Google Scholar]
- 34.Roth G, Moser B, Krenn C, Brunner M, Haisjackl M, Almer G, Gerlitz S, Wolner E, Boltz-Nitulescu G, Ankersmit HJ. Susceptibility to programmed cell death in T-lymphocytes from septic patients: a mechanism for lymphopenia and Th2 predominance. Biochem Biophys Res Commun. 308(4) (2003) 840–6. doi: 10.1016/s0006-291x(03)01482-7. PMID: 12927795. [DOI] [PubMed] [Google Scholar]
- 35.Venet F, Pachot A, Debard AL, Bohé J, Bienvenu J, Lepape A, Monneret G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25-lymphocytes. Crit Care Med. 32(11) (2004) 2329–31. doi: 10.1097/01.ccm.0000145999.42971.4b. PMID: 15640650. [DOI] [PubMed] [Google Scholar]
- 36.Wick M, Kollig E, Muhr G, Köller M. The potential pattern of circulating lymphocytes TH1/TH2 is not altered after multiple injuries. Arch Surg. 135(11) (2000) 1309–14. doi: 10.1001/archsurg.135.11.1309. PMID: 11074886. [DOI] [PubMed] [Google Scholar]
- 37.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 222(4) (1995) 482–90; discussion 490–2. doi: 10.1097/00000658-199522240-00006. PMID: 7574928; PMCID: PMC1234878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carson WF 4th, Cavassani KA, Ito T, Schaller M, Ishii M, Dou Y, Kunkel SL. Impaired CD4+ T-cell proliferation and effector function correlates with repressive histone methylation events in a mouse model of severe sepsis. Eur J Immunol. 40(4) (2010) 998–1010. doi: 10.1002/eji.200939739. PMID: 20127677; PMCID: PMC3040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 92(3) (2012) 593–603. doi: 10.1189/jlb.1211641. PMID: 22459947; PMCID: PMC3427605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 106(15) (2009) 6303–8. doi: 10.1073/pnas.0809422106. PMID: 19332785; PMCID: PMC2669369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Chéron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, Venet F. 2011. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 15(2):R99. doi: 10.1186/cc10112. PMID: 21418617; PMCID: PMC3219369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, Zhu K, Wan X, Cai Z, Deng X. 2011. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care.15(1):R70. doi: 10.1186/cc10059. PMID: 21349174; PMCID: PMC3222003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen CW, Mittal R, Klingensmith NJ, Burd EM, Terhorst C, Martin GS, Coopersmith CM, Ford ML. Cutting Edge: 2B4-Mediated Coinhibition of CD4+ T Cells Underlies Mortality in Experimental Sepsis. J Immunol. 199(6) (2017) 1961–1966. doi: 10.4049/jimmunol.1700375. PMID: 28768726; PMCID: PMC5587400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shubin NJ, Monaghan SF, Heffernan DS, Chung CS, Ayala A. 2013. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Crit Care. 17(6):R276. doi: 10.1186/cc13131. PMID: 24289156; PMCID: PMC4057112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unsinger J, Kazama H, McDonough JS, Griffith TS, Hotchkiss RS, Ferguson TA. Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J Immunol. 184(12) (2010) 6766–72. doi: 10.4049/jimmunol.0904054. PMID: 20483771; PMCID: PMC2887093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurung P, Rai D, Condotta SA, Babcock JC, Badovinac VP, Griffith TS. Immune unresponsiveness to secondary heterologous bacterial infection after sepsis induction is TRAIL dependent. J Immunol. 187(5) (2011) 2148–54. doi: 10.4049/jimmunol.1101180. PMID: 21788440; PMCID: PMC3159846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pachot A, Monneret G, Voirin N, Leissner P, Venet F, Bohé J, Payen D, Bienvenu J, Mougin B, Lepape A. Longitudinal study of cytokine and immune transcription factor mRNA expression in septic shock. Clin Immunol. 114(1) (2005) 61–9. doi: 10.1016/j.clim.2004.08.015. PMID: 15596410. [DOI] [PubMed] [Google Scholar]
- 48.Gouel-Chéron A, Venet F, Allaouchiche B, Monneret G. CD4+ T-lymphocyte alterations in trauma patients. Crit Care. 16(3) (2012) 432. doi: 10.1186/cc11376. PMID: 22734607; PMCID: PMC3580654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leng FY, Liu JL, Liu ZJ, Yin JY, Qu HP. Increased proportion of CD4(+)CD25(+)Foxp3(+) regulatory T cells during early-stage sepsis in ICU patients. J Microbiol Immunol Infect. 46(5) (2013) 338–44. doi: 10.1016/j.jmii.2012.06.012. PMID: 22921804. [DOI] [PubMed] [Google Scholar]
- 50.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 31(7) (2003) 2068–71. doi: 10.1097/01.CCM.0000069345.78884.0F. PMID: 12847405. [DOI] [PubMed] [Google Scholar]
- 51.Sharma A, Yang WL, Matsuo S, Wang P. Differential alterations of tissue T-cell subsets after sepsis. Immunol Lett. 168(1) (2015) 41–50. doi: 10.1016/j.imlet.2015.09.005. PMID: 26362089; PMCID: PMC4636913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavassani KA, Carson WF 4th, Moreira AP, Wen H, Schaller MA, Ishii M, Lindell DM, Dou Y, Lukacs NW, Keshamouni VG, Hogaboam CM, Kunkel SL. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 115(22) (2010) 4403–11. doi: 10.1182/blood-2009-09-241083. PMID: 20130237; PMCID: PMC2881495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen IJ, Jensen SN, Sjaastad FV, Gibson-Corley KN, Dileepan T, Griffith TS, Mangalam AK, Badovinac VP. 2020. Sepsis impedes EAE disease development and diminishes autoantigen-specific naïve CD4 T cells. Elife. 9:e55800. doi: 10.7554/eLife.55800. Epub ahead of print. PMID: 33191915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strother RK, Danahy DB, Kotov DI, et al. Polymicrobial Sepsis Diminishes Dendritic Cell Numbers and Function Directly Contributing to Impaired Primary CD8 T Cell Responses In Vivo. J Immunol. 197(11) (2016) 4301–4311. doi: 10.4049/jimmunol.1601463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danahy DB, Anthony SM, Jensen IJ, et al. 2017. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLoS Pathog. 13(9):e1006569. doi: 10.1371/journal.ppat.1006569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai CL, Lee JT, Lien LM, Lin CC, Tsai IJ, Sung YF, Chou CH, Yang FC, Tsai CK, Wang IK, Tseng CH, Hsu CY. The association between septicemia and the risk of multiple sclerosis: a nationwide register-based retrospective cohort study in Taiwan. QJM. 111(9) (2018) 605–611. doi: 10.1093/qjmed/hcy123. PMID: 29878253. [DOI] [PubMed] [Google Scholar]
- 57.Chou CH, Lee JT, Tsai CK, Lien LM, Yin JH, Lin CC, Tsai IJ, Sung YF, Yang FC, Tsai CL, Wang IK, Tseng CH, Hsu CY. Increased risk of non-multiple sclerosis demyelinating syndromes in patients with preexisting septicaemia: a nationwide retrospective cohort study. Postgrad Med J. 95(1124) (2019) 307–313. doi: 10.1136/postgradmedj-2019-136667. PMID: 31209183; PMCID: PMC6613738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sriram S, Steiner I. Experimental allergic encephalomyelitis: a misleading model of multiple sclerosis. Ann Neurol. 58(6) (2005) 939–45. doi: 10.1002/ana.20743. PMID: 16315280. [DOI] [PubMed] [Google Scholar]
- 59.Namer IJ, Steibel J, Poulet P, Armspach JP, Mohr M, Mauss Y, Chambron J. Blood-brain barrier breakdown in MBP-specific T cell induced experimental allergic encephalomyelitis. A quantitative in vivo MRI study. Brain. 116 ( Pt 1) (1993) 147–59. doi: 10.1093/brain/116.1.147. PMID: 7680933. [DOI] [PubMed] [Google Scholar]
- 60.Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 70(6) (2001) 849–60. PMID: 11739546. [PubMed] [Google Scholar]
- 61.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 20(2) (2004) 467–73. doi: 10.1111/j.1460-9568.2004.03514.x. PMID: 15233755. [DOI] [PubMed] [Google Scholar]
- 62.Simmons SB, Pierson ER, Lee SY, Goverman JM. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 34(8) (2013) 410–22. doi: 10.1016/j.it.2013.04.006. PMID: 23707039; PMCID: PMC3752929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner CA, Roqué PJ, Mileur TR, Liggitt D, Goverman JM. Myelin-specific CD8+ T cells exacerbate brain inflammation in CNS autoimmunity. J Clin Invest. 130(1) (2020) 203–213. doi: 10.1172/JCI132531. PMID: 31573979; PMCID: PMC6934187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.‘t Hart BA, Dunham J, Faber BW, Laman JD, van Horssen J, Bauer J, Kap YS. A B Cell-Driven Autoimmune Pathway Leading to Pathological Hallmarks of Progressive Multiple Sclerosis in the Marmoset Experimental Autoimmune Encephalomyelitis Model. Front Immunol. 2017. Jul 11;8:804. doi: 10.3389/fimmu.2017.00804. PMID: 28744286; PMCID: PMC5504154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stosic-Grujicic S, Ramic Z, Bumbasirevic V, Harhaji L, Mostarica-Stojkovic M. Induction of experimental autoimmune encephalomyelitis in Dark Agouti rats without adjuvant. Clin Exp Immunol. 136(1) (2004) 49–55. doi: 10.1111/j.1365-2249.2004.02418.x. PMID: 15030513; PMCID: PMC1808989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazarević M, Đedovic N, Stanisavljević S, Dimitrijević M, Stegnjaić G, Krishnamoorthy G, Mostarica Stojković M, Miljković Đ, Jevtić B. Complete Freund’s Adjuvant-free experimental autoimmune encephalomyelitis in Dark Agouti rats is a valuable tool for multiple sclerosis studies. J Neuroimmunol. 354 (2021) 577547. doi: 10.1016/j.jneuroim.2021.577547. PMID: 33765502. [DOI] [PubMed] [Google Scholar]
- 67.Goodin DS, Corwin M, Kaufman D, Golub H, Reshef S, Rametta MJ, Knappertz V, Cutter G, Pleimes D. 2014. Causes of death among commercially insured multiple sclerosis patients in the United States. PLoS One. 9(8):e105207. doi: 10.1371/journal.pone.0105207. PMID: 25144226; PMCID: PMC4140735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ernst FR, Pocoski J, Cutter G, Kaufman DW, Pleimes D. Analysis of Diagnoses Associated with Multiple Sclerosis-Related In-Hospital Mortality Using the Premier Hospital Database. Int J MS Care. 18(3) (2016) 154–61. doi: 10.7224/1537-2073.2014-109. PMID: 27252603; PMCID: PMC4887002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson RE, Xie Y, DuVall SL, Butler J, Kamauu AW, Knippenberg K, Schuerch M, Foskett N, LaFleur J. Multiple Sclerosis and Risk of Infection-Related Hospitalization and Death in US Veterans. Int J MS Care. 17(5) (2015) 221–30. doi: 10.7224/1537-2073.2014-035. PMID: 26472943; PMCID: PMC4599359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel S, SirDeshpande P, Desai R, Desai N, Mistry H, Patel N, Mansuri Z, Gopalkrishnan B, Mehta T, Mahuwala Z, Narwal P, Garg N. Thirty-day readmissions in multiple sclerosis: An age and gender-based US national retrospective analysis. Mult Scler Relat Disord. 2019. Jun;31:41–50. doi: 10.1016/j.msard.2019.03.012. Epub 2019 Mar 20. PMID: 30925319. [DOI] [PubMed] [Google Scholar]
- 71.Wijnands JM, Kingwell E, Zhu F, Zhao Y, Fisk JD, Evans C, Marrie RA, Tremlett H. Infection-related health care utilization among people with and without multiple sclerosis. Mult Scler. 2017 Oct;23(11):1506–1516. doi: 10.1177/1352458516681198. Epub 2016. Dec 21. PMID: . [DOI] [PubMed] [Google Scholar]
- 72.Harding K, Zhu F, Alotaibi M, Duggan T, Tremlett H, Kingwell E. Multiple cause of death analysis in multiple sclerosis: A population-based study. Neurology. 2020. Feb 25;94(8):e820–e829. doi: 10.1212/WNL.0000000000008907. Epub 2020 Jan 13. PMID: 31932517; PMCID: PMC7136054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodríguez-Antigüedad Zarranz A, Mendibe Bilbao M, Llarena González C, Audicana C. Mortality and cause of death in multiple sclerosis: findings from a prospective population-based cohort in Bizkaia, Basque Country, Spain. Neuroepidemiology. 42(4) (2014) 219–25. doi: 10.1159/000359971. Epub 2014 May 7. PMID: 24821604. [DOI] [PubMed] [Google Scholar]
- 74.Schweitzer F, Laurent S, Fink GR, Barnett MH, Hartung HP, Warnke C. Effects of disease-modifying therapy on peripheral leukocytes in patients with multiple sclerosis. J Neurol. (2020) doi: 10.1007/s00415-019-09690-6. Epub ahead of print. PMID: 32036423. [DOI] [PMC free article] [PubMed]
- 75.Wijnands JMA, Zhu F, Kingwell E, Fisk JD, Evans C, Marrie RA, Zhao Y, Tremlett H. Disease-modifying drugs for multiple sclerosis and infection risk: a cohort study. J Neurol Neurosurg Psychiatry. 89(10) (2018) 1050–1056. doi: 10.1136/jnnp-2017-317493. Epub 2018 Mar 30. PMID: 29602795. [DOI] [PubMed] [Google Scholar]
- 76.Hemdan NY, Weigel C, Reimann CM, Gräler MH. Modulating sphingosine 1-phosphate signaling with DOP or FTY720 alleviates vascular and immune defects in mouse sepsis. Eur J Immunol. 2016. Dec;46(12):2767–2777. doi: 10.1002/eji.201646417. Epub 2016 Oct 20. PMID: 27683081. [DOI] [PubMed] [Google Scholar]
- 77.Zarbato GF, de Souza Goldim MP, Giustina AD, Danielski LG, Mathias K, Florentino D, de Oliveira Junior AN, da Rosa N, Laurentino AO, Trombetta T, Gomes ML, Steckert AV, Moreira AP, Schuck PF, Fortunato JJ, Barichello T, Petronilho F. Dimethyl Fumarate Limits Neuroinflammation and Oxidative Stress and Improves Cognitive Impairment After Polymicrobial Sepsis. Neurotox Res. 2018. Oct;34(3):418–430. doi: 10.1007/s12640-018-9900-8. Epub 2018 Apr 30. PMID: 29713994. [DOI] [PubMed] [Google Scholar]
- 78.Maleki E, Sheibani M, Nezamoleslami S, Dehpour AR, Takzaree N, Shafaroodi H. Glatiramer acetate treatment inhibits inflammatory responses and improves survival in a mice model of cecal ligation and puncture-induced sepsis. J Basic Clin Physiol Pharmacol. 2021. Feb 9. doi: 10.1515/jbcpp-2020-0303. Epub ahead of print. PMID: 33559458. [DOI] [PubMed]
- 79.Capkun G, Dahlke F, Lahoz R, Nordstrom B, Tilson HH, Cutter G, Bischof D, Moore A, Simeone J, Fraeman K, Bancken F, Geissbühler Y, Wagner M, Cohan S. Mortality and comorbidities in patients with multiple sclerosis compared with a population without multiple sclerosis: An observational study using the US Department of Defense administrative claims database. Mult Scler Relat Disord. 2015. Nov;4(6):546–54. doi: 10.1016/j.msard.2015.08.005. Epub 2015 Aug 18. PMID: 26590661. [DOI] [PubMed] [Google Scholar]
- 80.Boziki MK, Kesidou E, Theotokis P, Mentis AA, Karafoulidou E, Melnikov M, Sviridova A, Rogovski V, Boyko A, Grigoriadis N. Microbiome in Multiple Sclerosis; Where Are We, What We Know and Do Not Know. Brain Sci. 10(4) (2020) 234. doi: 10.3390/brainsci10040234. PMID: 32295236; PMCID: PMC7226078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freedman SN, Shahi SK, Mangalam AK. The “Gut Feeling”: Breaking Down the Role of Gut Microbiome in Multiple Sclerosis. Neurotherapeutics. 15(1) (2018) 109–125. doi: 10.1007/s13311-017-0588-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johanson DM 2nd , Goertz JE, Marin IA, Costello J, Overall CC, Gaultier A Experimental autoimmune encephalomyelitis is associated with changes of the microbiota composition in the gastrointestinal tract. Sci Rep. 10(1) (2020) 15183. doi: 10.1038/s41598-020-72197-y. PMID: 32938979; PMCID: PMC7494894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stanisavljević S, Čepić A, Bojić S, Veljović K, Mihajlović S, Đedović N, Jevtić B, Momčilović M, Lazarević M, Mostarica Stojković M, Miljković Đ, Golić N. Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in Dark Agouti rats. Sci Rep. 9(1) (2019) 918. doi: 10.1038/s41598-018-37505-7. PMID: 30696913; PMCID: PMC6351648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colpitts SL, Kasper EJ, Keever A, Liljenberg C, Kirby T, Magori K, Kasper LH, Ochoa-Repáraz J. A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes. 8(6) (2017) 561–573. doi: 10.1080/19490976.2017.1353843. PMID: 28708466; PMCID: PMC5730387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, Kümpfel T, Hohlfeld R, Krishnamoorthy G, Wekerle H. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 114(40) (2017) 10719–10724. doi: 10.1073/pnas.1711233114. PMID: 28893994; PMCID: PMC5635914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, Crabtree-Hartman E, Sand IK, Gacias M, Zhu Y, Casaccia P, Cree BAC, Knight R, Mazmanian SK, Baranzini SE. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 114(40) (2017) 10713–10718. doi: 10.1073/pnas.1711235114. Erratum in: Proc Natl Acad Sci U S A. 2017 Oct 17;114(42):E8943. PMID: 28893978; PMCID: PMC5635915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choileáin SN, Kleinewietfeld M, Raddassi K, Hafler DA, Ruff WE, Longbrake EE. CXCR3+ T cells in multiple sclerosis correlate with reduced diversity of the gut microbiome. J Transl Autoimmun. 3 (2019) 100032. doi: 10.1016/j.jtauto.2019.100032. PMID: 32743517; PMCID: PMC7388357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vallino A, Dos Santos A, Mathé CV, Garcia A, Morille J, Dugast E, Shah SP, Héry-Arnaud G, Guilloux CA, Gleeson PJ, Monteiro RC, Soulillou JP, Harb J, Bigot-Corbel E, Michel L, Wiertlewski S, Nicot AB, Laplaud DA, Berthelot L. 2020. Gut bacteria Akkermansia elicit a specific IgG response in CSF of patients with MS. Neurol Neuroimmunol Neuroinflamm. 7(3):e688. doi: 10.1212/NXI.0000000000000688. PMID: 32123045; PMCID: PMC7136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Planas R, Santos R, Tomas-Ojer P, Cruciani C, Lutterotti A, Faigle W, Schaeren-Wiemers N, Espejo C, Eixarch H, Pinilla C, Martin R, Sospedra M. 2018. GDP-l-fucose synthase is a CD4+ T cell-specific autoantigen in DRB3*02:02 patients with multiple sclerosis. Sci Transl Med. 10(462):eaat4301. doi: 10.1126/scitranslmed.aat4301. PMID: 30305453. [DOI] [PubMed] [Google Scholar]
- 90.Miyauchi E, Kim SW, Suda W, Kawasumi M, Onawa S, Taguchi-Atarashi N, Morita H, Taylor TD, Hattori M, Ohno H. Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature. 585(7823) (2020) 102–106. doi: 10.1038/s41586-020-2634-9. PMID: 32848245. [DOI] [PubMed] [Google Scholar]
- 91.Skirecki T, Swacha P, Hoser G, Golab J, Nowis D, Kozłowska E. Bone marrow is the preferred site of memory CD4+ T cell proliferation during recovery from sepsis. JCI Insight. 2020. May 21;5(10):e134475. doi: 10.1172/jci.insight.134475. PMID: 32434988; PMCID: PMC7259529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boyman O, Krieg C, Homann D, Sprent J. Homeostatic maintenance of T cells and natural killer cells. Cell Mol Life Sci. 2012. May;69(10):1597–608. doi: 10.1007/s00018-012-0968-7. Epub 2012 Mar 30. PMID: 22460580; PMCID: PMC3909665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 183(10) (2009) 6041–50. doi: 10.4049/jimmunol.0900747. PMID: 19841183. [DOI] [PubMed] [Google Scholar]
- 94.Miller PG, Bonn MB, Franklin CL, Ericsson AC, McKarns SC. TNFR2 Deficiency Acts in Concert with Gut Microbiota To Precipitate Spontaneous Sex-Biased Central Nervous System Demyelinating Autoimmune Disease. J Immunol. 195(10) (2015) 4668–84. doi: 10.4049/jimmunol.1501664 PMID: 26475926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seifert HA, Benedek G, Nguyen H, Gerstner G, Zhang Y, Kent G, Vandenbark AA, Bernhagen J, Offner H. Antibiotics protect against EAE by increasing regulatory and anti-inflammatory cells. Metab Brain Dis. 33(5) (2018) 1599–1607. doi: 10.1007/s11011-018-0266-7. PMID: 29916184; PMCID: PMC6298859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gödel C, Kunkel B, Kashani A, Lassmann H, Arumugam M, Krishnamoorthy G. Perturbation of gut microbiota decreases susceptibility but does not modulate ongoing autoimmune neurological disease. J Neuroinflammation. 17(1) (2020) 79. doi: 10.1186/s12974-020-01766-9. PMID: 32143718; PMCID: PMC7060541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Al Nabhani Z, Dulauroy S, Marques R, Cousu C, Al Bounny S, Déjardin F, Sparwasser T, Bérard M, Cerf-Bensussan N, Eberl G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity. 50(5) (2019) 1276–1288.e5. doi: 10.1016/j.immuni.2019.02.014. PMID: 30902637. [DOI] [PubMed] [Google Scholar]
- 98.Rasouli J, Casella G, Yoshimura S, Zhang W, Xiao D, Garifallou J, Gonzalez MV, Wiedeman A, Kus A, Mari ER, Fortina P, Hakonarson H, Long SA, Zhang GX, Ciric B, Rostami A. 2020. A distinct GM-CSF+ T helper cell subset requires T-bet to adopt a TH1 phenotype and promote neuroinflammation. Sci Immunol. 5(52):eaba9953. doi: 10.1126/sciimmunol.aba9953. PMID: 33097590. [DOI] [PubMed] [Google Scholar]
- 99.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 12(6) (2011) 568–75. doi: 10.1038/ni.2031. PMID: 21516111; PMCID: PMC3116521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 12(6) (2011) 560–7. doi: 10.1038/ni.2027. Epub 2011 Apr 24. PMID: 21516112. [DOI] [PubMed] [Google Scholar]
- 101.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 178(1) (2007) 39–48. doi: 10.4049/jimmunol.178.1.39. PMID: 17182538. [DOI] [PubMed] [Google Scholar]
- 102.Stojić-Vukanić Z, Nacka-Aleksić M, Pilipović I, Vujnović I, Blagojević V, Kosec D, Dimitrijević M, Leposavić G. Aging diminishes the resistance of AO rats to EAE: putative role of enhanced generation of GM-CSF Expressing CD4+ T cells in aged rats. Immun Ageing. 12 (2015)16. doi: 10.1186/s12979-015-0044-x. PMID: 26448779; PMCID: PMC4596406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang H, Wang S, Jiang T, Fan R, Zhang Z, Mu J, Li K, Wang Y, Jin L, Lin F, Xia J, Sun L, Xu B, Ji C, Chen J, Chang J, Tu B, Song B, Zhang C, Wang FS, Xu R. High levels of circulating GM-CSF+CD4+ T cells are predictive of poor outcomes in sepsis patients: a prospective cohort study. Cell Mol Immunol. 16(6) (2019) 602–610. doi: 10.1038/s41423-018-0164-2. PMID: 30327490; PMCID: PMC6804788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 479(7374) (2011) 538–41. doi: 10.1038/nature10554. PMID: 22031325. [DOI] [PubMed] [Google Scholar]
- 105.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W Jr, Rongvaux A, Van Rooijen N, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the small intestine. Nature. 475(7357) (2011) 514–8. doi: 10.1038/nature10228. PMID: 21765430; PMCID: PMC3148838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berer K, Boziki M, Krishnamoorthy G. .2014b. Selective accumulation of pro-inflammatory T cells in the intestine contributs to the resistance to autoimmune demyelinating disease. PLoS One 9(2):e87876. doi: 10.1371/journal.pone.0087876. PMID: 24504092; PMCID: PMC3913661. [DOI] [PMC free article] [PubMed] [Google Scholar]