Abstract
Background
Methods
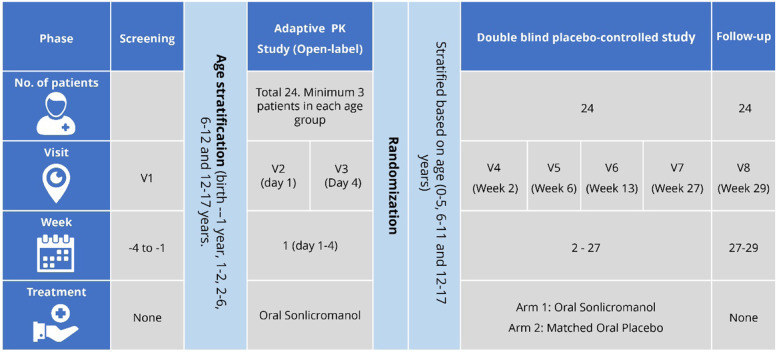
The KHENERGYC trial will be a phase II, randomised, double-blinded, placebo-controlled (DBPC), parallel-group study in the paediatric population (birth up to and including 17 years). The study will be recruiting 24 patients suffering from motor symptoms due to genetically confirmed PMD. The trial will be divided into two phases. The first phase of the study will be an adaptive pharmacokinetic (PK) study with four days of treatment, while the second phase will include randomisation of the participants and evaluating the efficacy and safety of sonlicromanol over 6 months.
Discussion
Effective novel therapies for treating PMDs in children are an unmet need. This study will assess the pharmacokinetics, efficacy, and safety of sonlicromanol in children with genetically confirmed PMDs, suffering from motor symptoms.
Trial registration
clinicaltrials.gov: NCT04846036, registered April 15, 2021. European Union Clinical Trial Register (EUDRACT number: 2020–003124-16), registered October 20, 2020. CCMO registration: NL75221.091.20, registered on October 7, 2020.
Keywords: Mitochondrial diseases, OXPHOS, Redox metabolism, Children, Sonlicromanol, GMFM, Clinical study protocol, KH176
Background
Mitochondria, being the energy generators of the cell, are involved in intermediary metabolism via several pathways, a major one being oxidative phosphorylation (OXPHOS) [1–4]. Energy generation (Adenosine triphosphate (ATP)) through OXPHOS comprises more than 80% of the cell requirement and denotes the most important function of this organelle [4–6].
Primary mitochondrial diseases (PMDs) are clinically heterogeneous metabolic disorders occurring due to disruption of the mitochondrial function [7]. This disruption may be attributed to genetic mutations in either nuclear deoxyribonucleic acid (nDNA) or mitochondrial DNA (mtDNA), causing a decrease in one or more OXPHOS enzyme complexes [1]. PMDs exist in several phenotypes along with a wide variety of clinical symptoms. These symptoms include cognitive decline, mental retardation, epilepsy, migraine, perceived fatigue, exercise intolerance, cardiomyopathy, conduction abnormality, diabetes mellitus, myopathy, and stunted growth [1]. Leigh Disease, MELAS (Mitochondrial Encephalomyopathy, Lactic acidosis, and Stroke-like episodes), MIDD (Maternally Inherited Diabetes and Deafness), and LHON (Leber’s Hereditary Optic Neuropathy) are some of the PMDs commonly observed [8]. Leigh syndrome is the most common PMD prevalent among children and may involve more than 75 gene mutations in the OXPHOS system [9]. The mitochondrial DNA 3243A > G mutation in the MT-TL1 gene is the most observed PMD defect in adults. However, the age of onset of the disease has been reported already in young children [1, 10, 11]. This syndrome is often persistently progressive, resulting in substantial morbidity and mortality. Further, this mutation is not limited to classical MELAS syndrome and may include other phenotypes like MIDD, chronic progressive external ophthalmoplegia (CPEO) and mixed phenotypes [1], all together described as MELAS spectrum disorders or alternatively m.3243 A > G MELAS with strokes and m.3243A > G non-MELAS (minus strokes) [12, 13].
Further, genetic defects of the OXPHOS system’s subunits often lead to increased generation of reactive oxygen species (ROS), causing abnormalities in the redox-controlled cell signalling in addition to irreversible damage to macromolecules via lipid peroxidation and protein carbonylation. It also shifts the cellular redox equilibrium to a more oxidising environment. Since oxidative distress and disparity in redox regulation are common pathological features in PMDs and play a significant role in the development of its clinical manifestations, they denote a crucial target for the advancement of therapy [14]. Regardless of advances in understanding these disorders, there are only a few treatment options available, and they are mostly supportive. Hence, there is an unmet need for novel treatment.
Small molecules targeting ROS may help to improve the regulation of cellular energy metabolism and, thus, be effective in PMDs. Such antioxidants, either in clinical use or clinical trials, include glutathione, N-acetylcysteine, lipoic acid, vitamin C, vitamin E, coenzyme Q10, Idebenone, MitoQ, etc. [15]. Certain redox-active molecules are also being studied in PMDs, such as EPI-743 (vatiquinone) [16], JP4–039 [17], SKQ1 [18] and KL1333 [19]. EPI-743 may halt disease progression and/or reversal in children with genetically confirmed Leigh syndrome in an open-label study [16]. JP4–039 has shown ROS scavenging effects in animal models and tumour cell lines [20, 21]. Further, SKQ1, a mitochondria-targeted antioxidant, has been studied in a mouse PMD model [18]. KL-1333, an NAD+ modulator has been shown to increase ATP levels and decrease ROS and lactate levels in human MELAS fibroblasts [19]. Future explorations of these small molecules may result in effective answers to the treatment of PMDs.
Sonlicromanol (KH176) is a small, orally available molecule being developed to treat PMDs. It has strong radical trapping activity in the cell, efficiently reduces increased levels of cellular ROS, and protects OXPHOS deficient cells from ROS-induced cell death. Furthermore, by targeting and activating the thioredoxin/peroxiredoxin enzyme machinery, it restores the redox balance. Sonlicromanol’s triple mode of action finally includes the specific inhibition of the microsomal PGES-1 enzyme explaining its anti-inflammatory properties [22]. It provides a novel approach for PMDs treatment due to its reductive and oxidative distress modulating, and anti-inflammatory properties [14, 22]. The EMA has granted sonlicromanol “Orphan Medicinal Drug Designation” to treat Leigh disease, MIDD, and MELAS syndrome. It has also received Orphan Drug Designation to manage all forms of inherited mitochondrial respiratory chain disorders and Rare Paediatric Disease (RPD) label to treat paediatric patients with MELAS syndrome by the US Food and Drug Administration (FDA).
A phase I placebo-controlled trial showed sonlicromanol to be well tolerated up to 800 mg (single dose or up to 400 mg in multiple doses). Its pharmacokinetics (PK) demonstrated a rapid absorption profile with a time to reach maximum concentration (Tmax) of about 2 h. The drug has a half-life of about 9 h, and its active metabolite has a half-life of about 15 h. Steady-state concentrations are achieved within three days of dosing [23]. Subsequently, a Phase IIa randomised, double-blind placebo-controlled trial (KHENERGY Study) was conducted with sonlicromanol in adults with confirmed mtDNA m.3243A > G mutation in transfer RNALeu(UUR). The results showed adequate safety and tolerability of twice-daily oral dosing of 100 mg of sonlicromanol. Importantly, indications of a possible treatment effect were observed on parameters relating to cognition and mood/depression. Present ongoing trials: Phase IIb (CTRI: NCT04165239) and 1-year open-label extension study (CTRI: NCT04604548) may confirm the efficacy of sonlicromanol in adult patients with PMDs [24].
As PMDs frequently occur in the paediatric population, there is a high need for effective treatments. For this reason, the paediatric study with sonlicromanol presented in this work was initiated relatively early in the development program for sonlicromanol. In line with the previous studies, the present study is designed to evaluate the PK, safety, and efficacy of sonlicromanol in children (aged from birth up to and including 17 years) who have genetically confirmed PMDs and suffering from motor symptoms.
Methods
Study design and overview
The KHENERGYC trial is a phase II, randomised, double-blinded, placebo-controlled (DBPC), parallel-group study to evaluate the safety, efficacy, and pharmacokinetics of sonlicromanol among the paediatric population with PMD and motor symptoms. The trial will be divided into two phases. The first phase of the study will be a 4-day adaptive pharmacokinetic (PK) study, while the second phase will include randomisation of the participants and evaluating the efficacy and safety of sonlicromanol over a 6-month period. The study design is depicted in Fig. 1. This study will be conducted at the Radboud University Medical Centre, Nijmegen, Netherlands. Approval of the trial was obtained from the local ethics committee (NL75221.091.20). The trial has been registered at the United States trial registry (clinicaltrials.gov: NCT04846036) as well as the European Union Clinical Trial Register (EudraCT number: 2020–003124-16).
Fig. 1.
Study design
During the first phase of the trial, four days of treatment will be provided to the children recruited in the following age groups: birth to 1 year, 1–2 years, 2–6 years, 6–12 years, and 12–17 years. A minimum of three participants will be enrolled in each age group. All participants will be provided with oral sonlicromanol for four days with the anticipated paediatric-equivalent dose (PED) (i.e., the dose which results in similar plasma sonlicromanol concentrations to adult patients with PMDs who receive a dose of 100 mg BID) from a Physiologically Based Pharmacokinetic analysis (PBPK model, data on file). The PK data obtained after completion of this phase will be analysed to confirm the PED to be used in the second phase of the study. Elder age groups will be studied before their younger counterparts.
The second phase of the trial will be the randomised DBPC study. It will involve age-based stratification of the participants and randomisation into treatment and placebo groups. The trial participants, caretakers, as well as the researchers will be blinded to the allocation of the study group. The treatment group will receive oral sonlicromanol (PED) twice daily, while the placebo group will be given a matching placebo treatment twice daily for 26 weeks (6 months). To avoid unblinding due to the bitterness of sonlicromanol, 50 mg or 100 mg mannitol containing 0.5 μg or 1 μg BitrexR (denatonium benzoate) will be added to the placebo formulation. Unblinding will be permissible only on the occurrence of serious or unexpected adverse events. A follow-up visit will be planned two weeks after the last dose of the treatment period. Each participant will receive a minimum of 178 days of treatment in the DBPC period.
Study objectives
Primary objective
To assess the efficacy of sonlicromanol on the gross motor function over 6 months in children with genetically confirmed mitochondrial defects that affect the OXPHOS process.
Secondary objectives
To assess the effects of sonlicromanol over six months on growth (weight, height), fine manual motor skills, physical performance, spasticity, dystonia, ataxia, disability, signs, and symptoms of PMD, the burden on the caregiver, quality of life, clinician-scored and patient/caregiver scored global impression of change and patient/caregiver-identified 3 most bothersome symptoms of PMD.
Safety objectives
To assess the safety of sonlicromanol by assessing Treatment-emergent adverse events (TEAEs), vital signs (weight, temperature, systolic and diastolic blood pressure [SBP/DBP], heart rate [HR]), laboratory tests (chemistry, haematology), and electrocardiogram (ECG) will be monitored to evaluate the safety and tolerability over 6 months.
Additional objectives
To establish the PED of sonlicromanol and examine the multidose PK of sonlicromanol along with its active metabolite (KH176m) after 4 days and after 6 months; to analyse urine and blood samples for biomarkers (including the percentage of mtDNA heteroplasmy in urinary epithelial cells in patients with a mutation of mitochondrial transfer ribonucleic acid, fibroblast growth factor-21, growth/differentiation factor-15, and metabolomics endpoints); to collect information on Health Economics and Outcomes Research (HEOR) parameters, and to examine the palatability and acceptability of sonlicromanol.
Outcome measures
Clinical efficacy outcome measures
All clinical efficacy assessments will be performed at all visits in the DBPC study. They will be conducted before the first dose, during V4, V5, V6, V7, and at follow-up (V8) of the trial. The testing procedure and timing of the assessments will be standardised as much as possible. The same rater/trial staff member will perform the assessments for clinician-reported assessments, wherever needed. All the measurements conducted for achieving the primary, secondary, and additional objectives are listed in Table 1.
Table 1.
Outcome measures to be employed for evaluating the effect of sonlicromanol on the study objectives
| Objective | Outcome measure | Comments | Reference |
|---|---|---|---|
| Primary outcome measure | |||
| Gross motor symptoms of the genetic PMD in children | Gross Motor Function Measure −88 (GMFM-88) |
• 88 items, 5 categories: lying and rolling; sitting; crawling and kneeling; standing; walking, running, and jumping. • 4-point Likert scale. |
[25] |
| Secondary outcome measures | |||
| Fine manual dexterity | 9-Hole Peg Test (NHPT) | • Practice session at the screening visit. | [26] |
| The physical ability of the patient | 10 Meter Walk Test (10MWT) | • 7-point scale ranging from total assistance to no assistance for walking/running. | [27, 28] |
| Muscle spasticity | Modified Tardieu Scale for Spasticity (MTS) | • Quality (on a 5-grade scale) and angle of muscle reaction. | [29] |
| Dystonia | Barry-Albright Dystonia Scale (BAD) |
• 8 body parts will be evaluated: eyes, mouth, neck, trunk, and four limbs. • 5-point ordinal scale. |
[30] |
| Ataxia | Scale for the Assessment and Rating of Ataxia (SARA) | • 8 items: gait, stance, sitting, speech, finger-chase test, nose-finger test, fast alternating movements, and heel-shin test. | [31] |
| Disability | Paediatric Evaluation of Disability Inventory (PEDI-CAT) |
• Interview-based • 3 main domains: responsibility (51 items, 5-point scale), mobility (75 items), and social/cognitive ability (60 items, 4-point scale). |
[32] |
| PMD signs and symptoms | International Paediatric Mitochondria Disease Scale (IPMDS) | • 3 domains of the disease: complaints and symptoms (23 items), physical examination (21 items), and functional tests (13 items, some are only for children ≥6 years). | [33] |
| Caregiver’s burden | ZARIT-12 Burden Scale | • Ranges from ‘not at all’ to ‘extremely’. | [34] |
| Quality of life | Neuro Quality of life Fatigue-Short Form (NeuroQL-SF) Paediatric version |
• Physical, mental, and social effects. • 8-item self-assessment questionnaire recommended for children of 8–17 years. |
[35, 36] |
| Clinician’s perception or improvement or worsening of the PMD | Clinician-scored Global Impression of Change (CGIC) | • 7-point Likert scale. | [36, 37] |
| Patient’s perception of change due to intervention | Patient/Caregiver scored Global Impression of Change (PGIC) |
• 7-point Likert scale. • Will be completed by 12–18-year-old children. • For children younger than 12 years or cognitively disabled, parents/caregivers will be completing the test. |
[36, 38] |
| Patient/Caregiver scored impression of change on patient-identified 3 most bothersome symptoms caused by PMD | Most Bothersome Symptom Assessment (MBSA) |
• 7-point Likert scale. • Will be completed by 12–18-year-old children. • For children younger than 12 years or cognitively disabled, parents/caregivers will be completing the test. |
[36] |
| Growth | Growth | • Overall body growth will be measured (height, weight, head circumference, and weight-for-height). | |
| Additional outcome measures | |||
| Health Economics and Outcomes Research | EQ-5D-Y (Proxy 1) |
• HRQoL via 5 dimensions: mobility, self-care, daily activities, pain/discomfort, and anxiety/depression. • Each dimension will have 5 levels: no, slight, moderate, severe, and extreme problems. |
[39, 40] |
| Health Utilities Index (HUI) | • Comprehensive health status and HRQoL. | [41, 42] | |
| Acceptability of sonlicromanol | Entry in the diary by the parent/caregiver | • Evaluation will be based on whether the patient has swallowed the complete dose, spat out part of the dose, or refused to take the dose. | [43] |
| Palatability of sonlicromanol | Facial Hedonic Scale/Visual Analogue Scale-5 (FHS/VAS-5) | [43] | |
PMD Primary Mitochondrial Diseases, GMFM-88 Gross Motor Function Measure −88, NHPT 9-Hole Peg Test, 10MWT 10 Meter Walk Test, MTS Modified Tardieu Scale for Spasticity, BAD Barry-Albright Dystonia Scale, SARA Scale for the Assessment and Rating of Ataxia, PEDI-CAT Paediatric Evaluation of Disability Inventory, IPMDS International Paediatric Mitochondria Disease Scale, NeuroQL-SF Neuro Quality of life Fatigue-Short Form, HRQoL health-related quality of life, CGIC Clinician-scored Global Impression of Change, PGIC Patient/Caregiver scored Global Impression of Change, MBSA Most Bothersome Symptom Assessment, HUI Health Utilities Index, FHS/VAS-5 Facial Hedonic Scale/Visual Analogue Scale-5, EQ-5D-Y EuroQol 5D youth version proxy version 1
Safety outcome measures
Vital signs, SBP/DBP, HR, ECG will be recorded before the first dose during all the visits (V1-V8) before and on PK-assessment days (just before taking a PK sample). Clinically significant abnormal findings in ECG will be recorded as adverse events (AE). A 2D echocardiography (left ventricular ejection fraction, left ventricular wall thickness, and left atrium dilatation) will be performed during the screening visit and at week 27.
Blood and other biological samples will be collected before the first dose at screening (V1) and V3-V8, in recommended volume based on the patient’s age and weight. Haematology and clinical chemistry assessments will be conducted to assess any TEAE. Depending on the event, follow-up may require additional tests or medical procedures as indicated and/or referral to the general physician or a medical specialist. Serious adverse events (SAEs) will be reported until the end of the study.
Study participants
The study will be conducted in children (from birth up to and including 17 years) suffering from motor symptoms due to genetically confirmed PMD for which the gene defect is identified to decrease one or more OXPHOS system enzymes. Twenty-four (24) patients are planned to be randomised in the study.
Inclusion criteria
The study population will be children (from birth up to and including 17 years) suffering from genetic PMD for which the gene defect is identified to reduce one or more OXPHOS enzymes; with abnormal gross motor function and/or at least one of the clinically significant motor symptoms (hypotonia, dystonia, reduced muscle power, ataxia, chorea and/or spasticity) depending on the judgement by the investigator; having a score of ≤96% in Gross Motor Function Measure-88 (GMFM-88) and ≥ 10 in the International Paediatric Mitochondria Disease Scale (IPMDS), before allotment in the adaptive PK phase and randomisation into DBPC phase; with stable symptoms since the previous routine control visit based on the judgement by the investigator; those who will provide written informed (patient/parental/caregiver) consent, co-consent (consent provided by children aged between 12–16 years, in addition to consent from the parent/caregiver) or assent in addition to consent from the parent/caregiver (subjects aged <12 years) and who are able and willing to abide with the requirements of the study protocol. Further, only those women of childbearing age (WOCBP) who will be willing to use highly effective contraception methods for the duration of the study will be included. Male subjects with female partners of childbearing potential must be willing to use condoms.
Exclusion criteria
The exclusion criteria of the study are described below:
Participants who have undergone gastrointestinal surgery with removal of stomach or duodenum/jejunum segments that interfere with absorption (feeding via gastrostomy tube will be permitted).
Prior treatment with an investigational drug within 3 months or 5 times the half-lives of the investigational drug (whichever is longer) before the first dose of the study drug.
- Clinically significant cardiovascular disease or risk factors for arrhythmia based on judgement by the investigator like
- Abnormal ECG (including QTcF exceeding the 95th percentile for the age- and sex-dependent QTc interval) and/or
- Abnormality detected in 2D ECHO.
- Systolic Blood Pressure (SBP) above the 95th percentile for the sex, age group, and height percentile at screening or baseline on a single measurement.
- Any history of acute/chronic heart failure.
- History of unexplained syncope.
- Family history or medical history of congenital long and short QT syndrome or sudden death.
- Hyper- or hypo-kalaemia; hyper- and hypomagnesaemia; hyper or hypocalcaemia.
- Clinically significant abnormal laboratory findings such as
- Aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), or bilirubin >3 times the upper limit of normal (ULN) (If ASAT or ALAT >3 times but <3.5 times the ULN, re-assessment will be performed at the investigator’s decision).
- Estimated glomerular filtration rate (eGFR) (based on the formula: 40.9*((1.8/Cystatine C) 0.93) below age-appropriate limits: < 2 months: < 25 ml/min/1.73 m2, 2 months to 1 year: < 35 ml/min/1.73 m2, > 1 year: < 60 ml/min/1.73 m2.
- The investigator will judge other clinically significant parameters at screening or baseline.
History of hypersensitivity to any of the components of the investigational drug.
Medical history of drug abuse (use of narcotic agents such as cannabinoids, amphetamines, cocaine, opiates, or misuse of prescription drugs such as benzodiazepines, opiates).
- The use of any drug and/or supplements within 4 weeks or 5 times the half-life (whichever will be longer) before the first dose of the study drug, unless stable for at least one month before first dosing and remaining stable throughout the study. These includes
- (Multi)vitamins, coenzyme Q10, vitamin E, riboflavin, and antioxidant supplements (including, but not limited to idebenone/EPI-743, mitoQ).
- Any medication that influences the mitochondrial functioning negatively (including but not limited to valproic acid, glitazones, statins, anti-virals, amiodarone, and non-steroidal anti-inflammatory drugs),
- Any strong cytochrome P450 (CYP)3A4 inhibitors (all conazoles-anti-fungal, HIV antivirals, grapefruit) or CYP3A4 inducers (including HIV antivirals, carbamazepine, phenobarbital, phenytoin, rifampicine, St. John’s wort, pioglitazone, troglitazone).
- Any drug known to disturb cardiac repolarisation, unless the QTc interval at screening is normal during stable treatment for a period of two weeks, or 5 half-lives of the drug and its major metabolite(s), whichever is shorter (all antipsychotics, certain anti-depressants such as nortriptyline or amitriptyline, fluoxetine, anti-emetics like domperidone, granisetron, ondansetron).
- Any drug metabolised by CYP3A4 with a narrow therapeutic index.
Study procedures
Screening procedure
Informed consent by the parent/guardian, informed consent by the children older than 16 years, and child co-consent and assent (wherever applicable) will be obtained before the screening. The potential patients will be screened for the eligibility criteria (inclusion and exclusion criteria), including the medical history, physical examination (vital signs and cardiac health), clinical chemistry, haematology, and beta-human chorionic gonadotropin (for assessing pregnancy, WOCBP only). Further, 9 Hole Peg Test training will also be performed to reduce the testing burden at subsequent visits. Parents/caregivers will be provided diaries to keep daily records of study medication, seizures, or headache/migraine frequency. The details are mentioned in Table 2.
Table 2.
Summary of study procedures
| Study Period | Screening | Adaptive PK study | Double-blind Treatment Period | Follow up | ||||
|---|---|---|---|---|---|---|---|---|
| Timing (weeks) | −4 to −1 | Day 1 | Day 4 | 2 (pre-dose) – 27 | 27–29 | |||
| Window (days) | +/−7 (except +7 for V7) | +/− 2 | ||||||
| Visit number | V1 | V2 | V3 | V4a | V5 | V6 | V7b | V8 |
| Day/Week | −4 to −1 | Day 1 | Day 4 | Wk 2 / Day 1 | Wk 6 | Wk 13 | Wk 27 | Wk 29 |
| Informed Consent | x | |||||||
| Inclusion/Exclusion criteria | x | x | x | |||||
| Demographics | x | xc | ||||||
| Medical history | x | x | xc | |||||
| Laboratoryd | x | x | xe | x | x | x | x | |
| Pregnancy testf | x | x | x | x | Monthly | |||
| Physical examination | x | x | x | x | x | x | ||
| Bodyweight and heightg | x | x | xh | xh | x | |||
| Vital signsi | x | x | xg | x | x | x | x | x |
| ECGk | x | xj | xj | x | x | x | x | x |
| 2D-Echocardiographyl | x | x | ||||||
| GMFM-88 | x | x | x | x | x | x | ||
| IPMDS | x | x | x | x | x | x | ||
| Study medication | Daily | |||||||
| Randomisation | xm | |||||||
| PEDI-CAT | xm | x | x | x | x | |||
| BAD | xm | x | x | x | x | |||
| Tardieu Spasticity test | xm | x | x | x | x | |||
| SARA | xm | x | x | x | x | |||
| 9 Hole Peg Test | xn | xm | x | x | x | x | ||
| 10 MWT | xm | x | x | x | x | |||
| Zarit-12 Burden scale | xm | x | x | x | x | |||
| NeuroQL-SF | xm | x | x | x | x | |||
| Clinician-scored and Patient/Caregiver scored GIC, MBSAo | x | x | x | x | x | |||
| EQ-5D-Y | xm | x | x | x | x | |||
| Health Utilities Index | xm | x | x | x | x | |||
| Palatability | x | |||||||
| Acceptability | Continuously | |||||||
| PK samplingp | x | x | x | |||||
| Telephone compliance Checksq | Weekly | |||||||
| AE recording | Continuously | |||||||
| Concomitant medication | Continuously | |||||||
| Diaryr | Continuously | |||||||
| Study medication dispensing | x | x | x | x | ||||
| Return study medication and Drug accountability | x | x | x | x | ||||
aVisit not earlier than 10 days after the last dose in the adaptive PK study phase to avoid carry-over effects
bAssessments are also to be performed in case of premature discontinuation
cDemographics, Medical history on V4 only for patients not participating in the Adaptive PK study
dIncluding haematology and clinical chemistry parameters. Metabolomics and biomarkers in plasma and urine (overnight sampled portion or first-morning urine portion to be collected before early morning food/drinks intake) at V4 and V7. For patients with heteroplasmic mitochondrial DNA mutations: mtDNA heteroplasmy assessment in urine at V4 and V7
eOnly for patients with heteroplasmic mitochondrial DNA mutations: mtDNA heteroplasmy assessment in urine at V4
fIn females with childbearing potential only, as defined in section 3.2.9. Pregnancy blood test at screening, urine (dipstick) tests at monthly intervals, and at the Follow-up visit. Females of childbearing potential will be provided with urine (dipstick) pregnancy tests and will be instructed to perform the pregnancy tests at home, at monthly intervals throughout the double-blind study treatment period. The female subjects (or parent/caregiver) will be contacted by the study staff each month to report the results of the pregnancy tests
gFor subjects <3 years: height, weight, skull circumference, and weight-for-height will be assessed. For subjects>3 years, weight, height, and BMI will be assessed
hBodyweight only
iIncluding supine blood pressure and heart rate. Vital signs are to be recorded as close as possible to each PK assessment at V2, 3, and 7
jAs close as possible before each sampling timepoint of the PK assessment
kECGs will be recorded at Screening, Day 1 (V2) before first dosing on day 1; on Day 4 (V3) and Visit 7 just before the PK sample assessments; at day 1 (V4), at month 1 (V5), month 3 (V6), at month 6 (V7, just before the PK sample assessments) and Follow-up (V8)
lNot to be done if documented (favourable) result dated less than 6 months prior to screening is available
mBefore trial, medication intake
nThis is a training session to reduce the learning effect. By conducting the learning session at the screening visit, the testing burden at V4 is reduced
oThis includes the Patient / Caregiver scored global impression of change for the patient/caregiver chosen 3 most bothersome symptoms. Patient scored global impression of change to be assessed by parent/caregiver for all children under 12 years of age and children aged 12–18 considered unable to provide a reliable assessment. A baseline situation is recorded to document the most important signs and symptoms to base the impression of change
pOn days 1 and 4 and Visit 7, the PK sampling schedule depends on age
qTelephone contacts will be conducted starting from Day 1 of the double-blind treatment period to verify the subject’s compliance with medication intake and the correct completion of the daily diaries. In addition, female subjects of childbearing potential and/or parent/caregiver will be contacted each month and asked to report the results of the urine (dipstick) pregnancy tests to confirm the absence of pregnancy
rParent/caregiver of the subject will keep a diary during the study, for daily recording of intake of study medication, including seizures, migraine frequency
Adaptive PK study procedure
After screening, subject eligibility will be reconfirmed on the Day 1 of V2, before the first dose of the study medication. The blood sampling for PK studies will be done at V2 (Day 1) and V3 (Day 4). The amount of blood withdrawn will depend on the age and weight of the patient and mostly follow the 5-sample schedule for PK studies. Further, the blood samples will also be used for safety laboratory assessments (haematology and clinical chemistry tests) (Table 2).
The patients will be enrolled in specific age groups (birth to 1 year, 1 to 2 years, 2 to 6 years, 6 to 12 years, and 12 up to and including 17 years) (Fig. 1). The PK and safety data of the patients (at least 3 patients) in each age group will be analysed to establish the paediatric-equivalent dose (PED) that will be used in the DBPC phase of the study. After completion of the PK assessments and analysis per age group (minimum 3 patients), a Data and Safety Monitoring Board (DSMB) will review the safety and PK data. It will issue an official recommendation on the safety of the study subjects and the proposed dose in the specific age group. This may lead to a change in sonlicromanol dose for one or more age groups. This DSMB recommendation letter will be submitted to the Medical Research Ethics Committee (MREC) for approval. After receiving MREC’s approval on the DSMB recommendation, the patients in the specific age group will be enrolled in the DBPC phase of the study. The adaptive PK study with the anticipated PED will be initially conducted in the elder children: older age groups will be studied before younger age groups.
Double-blind, placebo-controlled phase
All the patients completing the first phase of the study (the Adaptive PK study) will be offered participation in the DBPC phase. When a minimum of three subjects in each age cohort has completed the Adaptive PK phase, new participants from that age group may be enrolled, directly in the DBPC phase without having to participate in the Adaptive PK study.
In this second phase, subjects will be randomised (by age group) in a 1:1 ratio (using block size of 4) over two arms using Lifesphere EDC and Lifesphere Central Coding (vendor: ArisGlobal) [44] The allocation of randomization sequence is fully integrated into the LifeSphere EDC system and is blinded to the patient, caretaker and the researcher. The randomization number will be automatically generated by Lifeshpere EDC [44] and will be used for assigning the subjects to the treatment groups. Arm 1 will receive oral sonlicromanol (PED) twice daily for 26 weeks (6 months), while Arm 2 will receive a matching placebo twice daily for 26 weeks (6 months). The patients and/or parents/caregivers will be required to maintain the patients’ daily diaries. The patients’ eligibility will be reconfirmed on Day 1 of the treatment period (V4) before randomisation. Further, the patients will be assessed for all the clinical and safety outcome measures at each visit during the treatment phase. Table 2 describes the detailed study procedures. A final follow-up visit (V8) will be scheduled two weeks after the intake of the last dose of the treatment period. Study medication will be collected at each visit, after which, new study medication kits will be provided. All the remaining study medications will be collected during the final visit.
Withdrawal procedure
Premature discontinuation from the study will occur whenever the participant does not finish the scheduled 26 weeks treatment period and the follow-up assessment. The participants will be allowed to prematurely terminate the study at any time or be excluded from the study at the investigator’s decision if continuous administration of the study drug is thought not to be in the participant’s best interests. Also, the participant will be discontinued by the investigator or the sponsor (designee) if enrolment into the study is found inappropriate, the study plan is violated, and/or for any other safety reasons. Mandatory withdrawal criteria will be applicable when QTc exceeds the 95th percentile for the age- and sex-dependent QTc interval or if female participants become pregnant. Withdrawal of the assent/(co-)consent by subject/parents/caregiver or loss to follow-up will also lead to premature termination. Failure or refusal of blood sampling for the PK assessment at Day 4 (V3) of the PK study will also be treated as a withdrawal of consent and result in termination of the participant.
Study medication
The medicinal product under investigation is sonlicromanol, administered as an oral liquid. It will be manufactured and provided under the responsibility of the sponsor. Sonlicromanol, as well as the placebo, will be provided as a powder for oral solution. To maintain the blinding of the study, the placebo formulation will be matched to the active study medication in size, colour, shape, and taste. The investigational medicinal product and its matching placebo will be packaged in the same way, in glass bottles according to a randomisation list.
Route of administration and Dosage
Study medications (sonlicromanol and placebo) will be made available as a powder that must be dissolved in water. An oral syringe will be used to administer the solution in the correct volume. Patients with a nasogastric/ percutaneous endoscopic gastrostomy tube will be dosed through the stoma. The first daily dose should be taken in the morning, followed by the second after 12 h. A minimum 6-h allowable intake window will be provided, i.e., two doses must not be taken within 6 h of one another and not more than 18 h apart.
Sonlicromanol will be given in the anticipated PED for four days (Adaptive PK study), followed by sonlicromanol or placebo in the confirmed PED twice daily for 26 weeks (DBPC phase). According to Physiologically Based Pharmacokinetics (PBPK) model and the plasma concentration results from the PK phase, the anticipated PED will be administered in fixed doses, comparable in exposure to adults with PMDs treated at 100 mg, twice daily (Table 3).
Table 3.
Anticipated Paediatric-equivalent dose comparable to adults with mitochondrial disease treated at 100 mg twice daily
| Population | Dose (mg) per administration (BID) |
|---|---|
| Neonates (0–28 days) | 2 |
| Infants (1–2.5 months) | 4 |
| Infants (2.5–12 months) | 12 |
| Toddlers (1–2 years) | 23 |
| Young Children (2–6 years) | 33 |
| Middle-Aged Children (6–12 Years) | 55 |
| Adolescents (12–17 years) | 80 |
Power and sample size calculation
There is limited information on GMFM-88 scores in the target population [45–48]. Based on the available information, the sample calculations were conducted assuming the standard deviation (SD) range between 2 and 4.5% and an effect size of 4% or 5%. A sample size of 12 (in each group) was calculated to achieve a power of 80%. This could detect a mean change from a baseline difference of 4% between the groups for the total GMFM-88 score.
Statistical analysis
All statistical analyses will be performed using the SAS® System (SAS Institute Inc., Cary, NC, USA). Continuous data will be presented using descriptive statistics where the parameters will be reported such as n (number of observations), mean, SD, minimum (Min), median and maximum (Max). Geometric means will be used for PK variables (AUC and Cmax). Absolute and relative frequencies will be utilised to analyse categorical variables. Unless otherwise specified, all tests will be two-sided at the 5% level, and all the confidence intervals will be two-sided at the 95% level. Given the number of assessments conducted in this trial, the SAP will include appropriate corrections for multiple testing using a hierarchical procedure and/or alpha correction.
Four different analyses sets will be applied for performing the statistical analysis, namely: all-randomised, all-treated, safety, and per-protocol (PP) sets. The populations used in the efficacy analyses of the DBPC phase will be the all-randomised and PP populations. The all-randomised population will consist of all patients randomised for the double-blinded treatment, regardless of whether they received the study medication or not, consistent with the Intention-to-Treat (ITT) principle. The primary efficacy endpoint (change in GMFM-88 score) at weeks 6, 13, 27, and 29 will be performed using the all-randomized and PP population. The PP population will consist of all patients in the all-treated set who will not violate the protocol in a way that could affect the primary outcome measure. The population for assessing the safety will consist of all patients in the safety set. This population will include all randomised patients receiving the study medication and for whom at least one safety assessment (after first drug intake) is available (patients are assigned the treatment actually received). Descriptive statistics will be used to analyse the safety data. The demographics, subject disease characteristics, and baseline data (including medical history) will be presented descriptively for the PK study (V1 or V2) and DBPC study (V4).
Data collection, management and monitoring
All data are either transferred from third parties e.g., labs, ECG analysis provider, etc., or entered directly into the e-CRF by site personnel. Reference ranges are imported in the Lifesphere EDC [44]. Further, all the data from the database, external data, coded data, and any other derived data files used for the analysis of the clinical project will be stored on secure electronic storage media. The data management and monitoring will be performed using Lifesphere EDC and Lifeshpere Central Coding system [44]. Verbatim terms will be coded using the LifeSphere Central Coding system. However, manual coding will be done for terms that are not automatically coded by the LifeSphere Central Coding system and will be reviewed by a medical reviewer. Adverse events, medical history and concomitant treatment verbatim terms will be coded according to MedDRA® and WHO Drug Global thesauri. Further details of data management procedures are described in the Data Management Plan.
Moreover, the safety management plan will be in place for collecting, assessing, reporting, and managing solicited and spontaneously reported adverse events and other unintended effects of trial interventions or trial conduct. The periodic safety monitoring of the participants will be performed by DSMB committee consisting of 3 members, independent from the sponsor, without any competing interests. The DSMB will be providing its recommendations to the sponsor.
Dissemination plans
The trial results will be communicated to participants, healthcare professionals, the public, and other relevant groups via a publication, or at the conferences/meetings.
Discussion
Mitochondrial diseases are a group of disorders with no proven effective treatment to date, except for idebenone for LHON authorized under exceptional circumstances in the European Union (EU) [8, 49, 50]. Novel experimental strategies that fall into two broad categories, pharmacological and genetic approaches, are being investigated. Several recent reviews have focused on these emerging therapies [7, 51, 52]. Based on the current understanding of the effects of OXPHOS system flaws at the cellular level, several novel small molecules potentially capable of mitigating the consequences of hampered oxidative phosphorylation are in the preclinical developmental stage, and few of them have reached the clinical development stage [8, 15, 51, 53].
The prevalence of childhood-onset (age less than 17 years) PMDs ranges between 5–15 cases per 100,000 individuals [1]. Mitochondrial disease presenting in childhood is characterized by clinical, biochemical, and genetic complexity. Some children are affected by distinct syndromes, but the majority have nonclassical multisystemic disease presentations involving virtually any organ in the body. Each child has a unique constellation of clinical features and disease trajectory, leading to enormous challenges in the diagnosis and management of these heterogeneous disorders [8].
The study drug sonlicromanol has shown beneficial properties on motor coordination and motor learning in preclinical models of Leigh disease [54] and potential efficacy signals on brain involvement in adult patients with PMDs [24]. Since PMDs hinder the development of children, effective therapies for treating specific PMDs in children are urgently needed. The present study will help to characterise the pharmacokinetics, efficacy, and safety of sonlicromanol in children (up to 17 years old) with genetically confirmed PMDs and who suffer from motor symptoms.
The study has been initiated in February 2021 and is currently actively recruiting patients.
Acknowledgements
We are grateful to all children and their families participating in this trial. We especially thank Gonnie van Osta for her help for the statistical plan of the project.
Abbreviations
- ALAT
Alanine aminotransferase
- ASAT
Aspartate aminotransferase
- ATP
Adenosine triphosphate
- BAD
Barry-Albright Dystonia scale
- DBP
Diastolic Blood pressure
- DBPC
Double-blind, placebo-controlled
- ECG
Electrocardiogram
- e-CRF
Electronic Case Report Form
- EMA
European Medicines Agency
- EU
European Union
- FDA
Food and Drug Administration
- HEOR
Health Economics and Outcomes Research
- HR
Heart rate
- IPMDS
International Paediatric Mitochondrial Disease Scale
- ITT
Intention-to-Treat
- LHON
Leber’s Hereditary Optic Neuropathy
- MELAS
Mitochondrial Encephalomyopathy, Lactic acidosis, and Stroke-like episodes
- MIDD
Maternally Inherited Diabetes and Deafness
- MREC
Medical Research Ethics Committee
- mtDNA
Mitochondrial DNA
- nDNA
Nuclear DNA
- NeuroQL-SF
Neurology Quality of life short Form
- OXPHOS
Oxidative phosphorylation
- PED
Paediatric-equivalent dose
- PEDI-CAT
Paediatric Evaluation of Disability Inventory
- PMDs
Primary mitochondrial diseases
- ROS
Reactive oxygen species
- RPD
Rare Paediatric Disease
- SAP
Statistical Analysis Plan
- SARA
Scale for the Assessment and Rating of Ataxia
- SBP
Systolic Blood Pressure
- TEAEs
Treatment-emergent adverse events
- ULN
Upper limit of normal
Authors’ contributions
The protocol was developed and agreed by RvM, LdB, GR and HR. RvM developed the statistical plan. RvM and JS manages the project. RvM, GR, and JS drafted the manuscript and RvM, LdB, GR, HR, and JS reviewed the final manuscript. All contributors have read and approved the final manuscript.
Funding
Part of this study is funded by the European Regional Development Fund (EFRO-OP Oost) nr. UP-16-00901 and the Foundations Energy4All, Join4Energy, Tim Foundation Ride4Kids, and Road4Energy. The funders have no role in study design, data collection and analysis, data interpretation, decision to publish, or in preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The study has undergone full external peer review by the European Regional Development Fund (EFRO-OP Oost) nr. UP-16-000910 as part of the peer review process.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study. However, the datasets generated from the current study will be made available from the corresponding author upon reasonable request and in agreement with the research collaboration and data transfer guidelines of the UMC Nijmegen.
Declarations
Ethics approval and consent to participate
Before initiating the study, the investigator will submit the protocol, protocol amendments, ICFs (separate ICFs for children younger than 12 years, children aged 12 to15 years, children aged 16 up to and including 17 years, and for parents/caregivers), investigator’s brochure, and other relevant documents such as patient-facing materials, patient instructions’, DSMB charter, etc. to an IEC/IRB for review and approval. Except for changes required to eliminate an immediate hazard to study subjects, any amendments to the protocol will require IEC/IRB approval before implementing changes made to the study design. The protocol has been approved by the local ethics committee, CCMO [Central Committee on Research Involving Human Subjects; registration number: NL75221.091.20].
The investigator or his/her representative will explain the nature of the study to the potential subject and the parent/caregiver and answer all the questions regarding the study. Informed consent, co-consent, and, wherever possible, assent will be obtained in written form by the investigator or appropriate designee and documented by a dated signature of the parent/caregiver, the subject (wherever feasible), and the investigator or appropriate designee on the ICF. Subjects and their parents/caregiver will be requested to sign a statement of informed consent that meets the applicable regulatory requirements and local regulations of the IEC/IRB or study centre (i.e., the ICF). The authorized person obtaining the informed consent (i.e., investigator or representative/delegate) will also sign the ICF The subject and their parent/caregiver will receive a copy of the signed ICF A statement that written informed consent and/or assent was obtained before the subject was enrolled in the study and the date of the written consent will be added in the subject’s medical record.
A written informed consent to participate will be obtained from the parents or legal representatives of any subject under the age of 16. In the Netherlands, children between the age of 12 and 16 years, however, have an official role in deciding about participation in medical-scientific research. They must provide a shared consent (co-consent) together with their parents/legal representatives. Patients below the age of 12 years can provide ‘assent’ in addition to the written informed consent provided by parents/legal representatives. Informed consent will be obtained directly from subjects aged 16 years and older. A separate, age appropriate, subject information form (in addition to the information form provided to the parents/legal representatives) for all age groups is legally required.
The research data will be stored using a study identification code for each participant. It will be documented and safeguarded by the principal investigator according to research guidelines after completion of the study. Any publications related to the trial will not report patient identification details.
Consent for publication
Not applicable as the manuscript does not contain individual personal data.
Competing interests
Gerrit Ruiterkamp and Herma Renkema are employees of Khondrion, Rob van Maanen is a former employee of Khondrion, and Jan Smeitink is the founding CEO of this mitochondrial medicine company.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 2.Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med. 2012;366(12):1132–1141. doi: 10.1056/NEJMra1012478. [DOI] [PubMed] [Google Scholar]
- 3.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, et al. The oxidative phosphorylation system in mammalian mitochondria. Adv Exp Med Biol. 2012;942:3–37. doi: 10.1007/978-94-007-2869-1_1. [DOI] [PubMed] [Google Scholar]
- 5.Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Asp Med. 2004;25(4):365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Schlieben LD, Prokisch H. The dimensions of primary mitochondrial disorders. Front Cell Dev Biol. 2020;8:600079. doi: 10.3389/fcell.2020.600079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinker RJ, Lim AZ, Stefanetti RJ, McFarland R. Current and emerging clinical treatment in mitochondrial disease. Mol Diagn Ther. 2021;25(2):181–206. doi: 10.1007/s40291-020-00510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman S. Mitochondrial disease in children. J Intern Med. 2020;287(6):609–633. doi: 10.1111/joim.13054. [DOI] [PubMed] [Google Scholar]
- 9.Lake NJ, Compton AG, Rahman S, Thorburn DR. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann Neurol. 2016;79(2):190–203. doi: 10.1002/ana.24551. [DOI] [PubMed] [Google Scholar]
- 10.Hirano M, Pavlakis SG. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): current concepts. J Child Neurol. 1994;9(1):4–13. doi: 10.1177/088307389400900102. [DOI] [PubMed] [Google Scholar]
- 11.Okhuijsen-Kroes EJ, Trijbels JM, Sengers RC, Mariman E, van den Heuvel LP, Wendel U, et al. Infantile presentation of the mtDNA A3243G tRNA(Leu (UUR)) mutation. Neuropediatrics. 2001;32(4):183–190. doi: 10.1055/s-2001-17372. [DOI] [PubMed] [Google Scholar]
- 12.de Laat P, Rodenburg RR, Roeleveld N, Koene S, Smeitink JA, Janssen MC. Six-year prospective follow-up study in 151 carriers of the mitochondrial DNA 3243 A>G variant. J Med Genet. 2021;58(1):48–55. doi: 10.1136/jmedgenet-2019-106800. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R, Reinstadler B, Engelstad K, Skinner OS, Stackowitz E, Haller RG, et al. Circulating markers of NADH-reductive stress correlate with mitochondrial disease severity. J Clin Investig. 2021;131(2). 10.1172/jci136055. [DOI] [PMC free article] [PubMed]
- 14.Beyrath J, Pellegrini M, Renkema H, Houben L, Pecheritsyna S, van Zandvoort P, et al. KH176 safeguards mitochondrial diseased cells from redox stress-induced cell death by interacting with the thioredoxin system/peroxiredoxin enzyme machinery. Sci Rep. 2018;8(1):6577. doi: 10.1038/s41598-018-24900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bottani E, Lamperti C, Prigione A, Tiranti V, Persico N, Brunetti D. Therapeutic approaches to treat mitochondrial diseases: "one-size-fits-all" and "precision medicine" strategies. Pharmaceutics. 2020;12(11):1083. doi: 10.3390/pharmaceutics12111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinelli D, Catteruccia M, Piemonte F, Pastore A, Tozzi G, Dionisi-Vici C, et al. EPI-743 reverses the progression of the pediatric mitochondrial disease--genetically defined Leigh Syndrome. Mol Genet Metab. 2012;107(3):383–388. doi: 10.1016/j.ymgme.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Frantz MC, Skoda EM, Sacher JR, Epperly MW, Goff JP, Greenberger JS, et al. Synthesis of analogs of the radiation mitigator JP4-039 and visualization of BODIPY derivatives in mitochondria. Org Biomol Chem. 2013;11(25):4147–4153. doi: 10.1039/c3ob40489g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shabalina IG, Vyssokikh MY, Gibanova N, Csikasz RI, Edgar D, Hallden-Waldemarson A, et al. Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1. Aging. 2017;9(2):315–339. doi: 10.18632/aging.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo KS, Kim JH, Min KN, Moon JA, Roh TC, Lee MJ, et al. KL1333, a novel NAD(+) modulator, improves energy metabolism and mitochondrial dysfunction in MELAS fibroblasts. Front Neurol. 2018;9:552. doi: 10.3389/fneur.2018.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leipnitz G, Mohsen AW, Karunanidhi A, Seminotti B, Roginskaya VY, Markantone DM, et al. Evaluation of mitochondrial bioenergetics, dynamics, endoplasmic reticulum-mitochondria crosstalk, and reactive oxygen species in fibroblasts from patients with complex I deficiency. Sci Rep. 2018;8(1):1165. doi: 10.1038/s41598-018-19543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seminotti B, Leipnitz G, Karunanidhi A, Kochersperger C, Roginskaya VY, Basu S, et al. Mitochondrial energetics is impaired in very long-chain acyl-CoA dehydrogenase deficiency and can be rescued by treatment with mitochondria-targeted electron scavengers. Hum Mol Genet. 2019;28(6):928–941. doi: 10.1093/hmg/ddy403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Renkema H, Pennings B, Pecheritsyna S, Schoeman JC, Hankemeier T, et al. Mechanism of action and potential applications of selective inhibition of microsomal prostaglandin E synthase-1-mediated PGE(2) biosynthesis by sonlicromanol's metabolite KH176m. Sci Rep. 2021;11(1):880. doi: 10.1038/s41598-020-79466-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koene S, Spaans E, Van Bortel L, Van Lancker G, Delafontaine B, Badilini F, et al. KH176 under development for rare mitochondrial disease: a first in man randomized controlled clinical trial in healthy male volunteers. Orphanet J Rare Dis. 2017;12(1):163. doi: 10.1186/s13023-017-0715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen MCH, Koene S, de Laat P, Hemelaar P, Pickkers P, Spaans E, et al. The KHENERGY study: safety and efficacy of KH176 in mitochondrial m.3243A>G spectrum disorders. Clin Pharmacol Ther. 2019;105(1):101–111. doi: 10.1002/cpt.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell D, Rosenbaum P, Avery L, Lane M. Gross motor function measure (GMFM-66 and GMFM-88) user's manual: clinics in developmental medicine. London: Mac Keith Press; 2002. [Google Scholar]
- 26.Smith YA, Hong E, Presson C. Normative and validation studies of the Nine-hole Peg Test with children. Percept Mot Skills. 2000;90(3 Pt 1):823–843. doi: 10.2466/pms.2000.90.3.823. [DOI] [PubMed] [Google Scholar]
- 27.Nagy S, Schmidt S, Hafner P, Klein A, Rubino-Nacht D, Gocheva V, et al. Measurements of motor function and other clinical outcome parameters in ambulant children with duchenne muscular dystrophy. J Vis Exp. 2019;143. 10.3791/58784. [DOI] [PubMed]
- 28.Tyson S, Connell L. The psychometric properties and clinical utility of measures of walking and mobility in neurological conditions: a systematic review. Clin Rehabil. 2009;23(11):1018–1033. doi: 10.1177/0269215509339004. [DOI] [PubMed] [Google Scholar]
- 29.Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. Eur J Neurol. 1999;6(S4):s23–s35. doi: 10.1111/j.1468-1331.1999.tb00031.x. [DOI] [Google Scholar]
- 30.Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. 1999;41(6):404–411. doi: 10.1017/s0012162299000870. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia. Neurology. 2006;66(11):1717. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 32.Dumas HM, Fragala-Pinkham MA, Haley SM, Ni P, Coster W, Kramer JM, et al. Computer adaptive test performance in children with and without disabilities: prospective field study of the PEDI-CAT. Disabil Rehabil. 2012;34(5):393–401. doi: 10.3109/09638288.2011.607217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koene S, Hendriks JCM, Dirks I, de Boer L, de Vries MC, Janssen MCH, et al. International Paediatric Mitochondrial Disease Scale. J Inherit Metab Dis. 2016;39(5):705–712. doi: 10.1007/s10545-016-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O'Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 35.Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karaa A, Haas R, Goldstein A, Vockley J, Cohen BH. A randomized crossover trial of elamipretide in adults with primary mitochondrial myopathy. J Cachexia Sarcopenia Muscle. 2020;11(4):909–918. doi: 10.1002/jcsm.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 38.Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manip Physiol Ther. 2004;27(1):26–35. doi: 10.1016/j.jmpt.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 39.EuroQol. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 40.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eiser C, Morse R. The measurement of quality of life in children: past and future perspectives. J Dev Behav Pediatr. 2001;22(4):248–256. doi: 10.1097/00004703-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson C, Lombardi D, Sjostedt P, Squires L. Best practice recommendations regarding the assessment of palatability and swallowability in the development of oral dosage forms for paediatric patients. Ther Innov Regul Sci. 2015;49(5):647–658. doi: 10.1177/2168479015573585. [DOI] [PubMed] [Google Scholar]
- 44.Lifesphere EDC system and Central Coding . Vendor: Arisglobal. 2022. [Google Scholar]
- 45.Fujii T, Nozaki F, Saito K, Hayashi A, Nishigaki Y, Murayama K, et al. Efficacy of pyruvate therapy in patients with mitochondrial disease: a semi-quantitative clinical evaluation study. Mol Genet Metab. 2014;112(2):133–138. doi: 10.1016/j.ymgme.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Koene S, van Bon L, Bertini E, Jimenez-Moreno C, van der Giessen L, de Groot I, et al. Outcome measures for children with mitochondrial disease: consensus recommendations for future studies from a Delphi-based international workshop. J Inherit Metab Dis. 2018;41(6):1267–1273. doi: 10.1007/s10545-018-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancuso M, McFarland R, Klopstock T, Hirano M. International Workshop: Outcome measures and clinical trial readiness in primary mitochondrial myopathies in children and adults. Consensus recommendations. 16–18 November 2016, Rome, Italy. Neuromuscul Disord. 2017;27(12):1126–1137. doi: 10.1016/j.nmd.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sage-Schwaede A, Engelstad K, Salazar R, Curcio A, Khandji A, Garvin JH, Jr, et al. Exploring mTOR inhibition as treatment for mitochondrial disease. Ann Clin Transl Neurol. 2019;6(9):1877–1881. doi: 10.1002/acn3.50846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liufu T, Wang Z. Treatment for mitochondrial diseases. Rev Neurosci. 2020; [published online ahead of print. 10.1515/revneuro-2020-0034. [DOI] [PubMed]
- 50.Lyseng-Williamson KA. Idebenone: a review in Leber's hereditary optic neuropathy. Drugs. 2016;76(7):805–813. doi: 10.1007/s40265-016-0574-3. [DOI] [PubMed] [Google Scholar]
- 51.Viscomi C, Zeviani M. Strategies for fighting mitochondrial diseases. J Intern Med. 2020;287(6):665–684. doi: 10.1111/joim.13046. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Hu LF, Zhou TJ, Qi LY, Xing L, Lee J, et al. Gene therapy strategies for rare monogenic disorders with nuclear or mitochondrial gene mutations. Biomaterials. 2021;277:121108. doi: 10.1016/j.biomaterials.2021.121108. [DOI] [PubMed] [Google Scholar]
- 53.Russell OM, Gorman GS, Lightowlers RN, Turnbull DM. Mitochondrial diseases: hope for the future. Cell. 2020;181(1):168–188. doi: 10.1016/j.cell.2020.02.051. [DOI] [PubMed] [Google Scholar]
- 54.de Haas R, Das D, Garanto A, Renkema HG, Greupink R, van den Broek P, et al. Therapeutic effects of the mitochondrial ROS-redox modulator KH176 in a mammalian model of Leigh Disease. Sci Rep. 2017;7(1):11733. doi: 10.1038/s41598-017-09417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study. However, the datasets generated from the current study will be made available from the corresponding author upon reasonable request and in agreement with the research collaboration and data transfer guidelines of the UMC Nijmegen.