ABSTRACT
A third vaccine dose against COVID-19 is already a reality in some countries around the world. In this study, we aimed to evaluate the effectiveness of the Brazilian immunization policy for COVID-19, which involves a booster shot. Participants (n = 210) provided serum samples, which were subjected to enzyme-linked immunosorbent assay (ELISA). Immunological profiles were defined as individuals with or without previous SARS-CoV-2 infection who received at least one vaccine dose in the immunization regimens of AstraZeneca, CoronaVac, or CoronaVac plus a booster shot with Pfizer. In addition, nonvaccinated/infected individuals were also included. As main results, we observed that the numbers of infected individuals were significantly reduced among those who were vaccinated, even with one dose. This result indicates that vaccines are highly protective against COVID-19. However, we observed a significant tendency of serum level decreases of specific antibodies over the time after the second dose. In contrast, the booster shot with the Pfizer vaccine after a CoronaVac immunization regimen showed a significant increase in the specific SARS-CoV-2 IgG serum levels. Moreover, we found that vaccination induced a significantly higher humoral immunological status than only the natural infection with SARS-CoV-2. Collectively, results presented here indicate that vaccines are necessary to induce a robust immunological status, which is maintained, restored, or even improved by booster shots.
IMPORTANCE COVID-19 continues to spread around the world despite significant progress in vaccine distribution and population immunity. The dynamics of the antiviral antibody response postvaccination is critical to evaluate vaccine effectiveness across different vaccine platforms and over time. In this study, we evaluate the serum levels of antiviral antibodies in patients from Brazil that received either the CoronaVac or the AstraZeneca vaccine. We found that antibody levels wane over time, vaccines induce protective immunity, and humoral immunity is enhanced with a third vaccine dose. This study reveals that the COVID-19 humoral immunological status induced by vaccines significantly benefits from a booster shot.
KEYWORDS: AstraZeneca, COVID-19, CoronaVac, booster shot, vaccine
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the coronavirus disease 2019 (COVID-19) (1, 2). It became a worldwide major public health problem in the last years. More than 500 million cases with nearly 6.8 million deaths have been attributed to COVID-19 since its initial report in late 2019 (3). Vaccines are being shown as essential in the control of COVID-19 worldwide (4, 5). However, it is necessary to follow up immunity longevity, specificity, and effectiveness in a real-time manner in order to guide the best possible immunization policies, especially in this scenario of frequent emergence of SARS-CoV-2 variants of concern (VOC) (6, 7).
A third vaccine against COVID-19 is already a reality in some countries around the world, such as the United States, Russia, Turkey, Chile, Uruguay, Israel, and Brazil (8–10). In this last country, the vaccination campaign started on January 2021, and AstraZeneca (AZD1222 or ChAdOx1-S) and CoronaVac (Sinovac-CoronaVac COVID-19 vaccine) vaccine formulations were the most widely used (11). CoronaVac is based on a purified inactivated virus (12), while AstraZeneca is based on an adenovirus-vector encoding the spike protein of SARS-CoV-2 (10, 13), the causative virus of COVID-19. Immunization regimens of AstraZeneca and CoronaVac are composed of two doses, 90 and 28 days apart, respectively (10, 12). In November 2021, the Brazilian Ministry of Health recommended a booster dose with the Pfizer (BNT126b2) COVID-19 vaccine (RNA-based vaccine) (14, 15) for people over 18 years old at least 5 months after the second dose (9). The effectiveness of this immunization policy for COVID-19 remains to be elucidated. In this study, we aimed to evaluate the effectiveness of the Brazilian immunization policy for COVID-19.
RESULTS
Vaccines are highly protective.
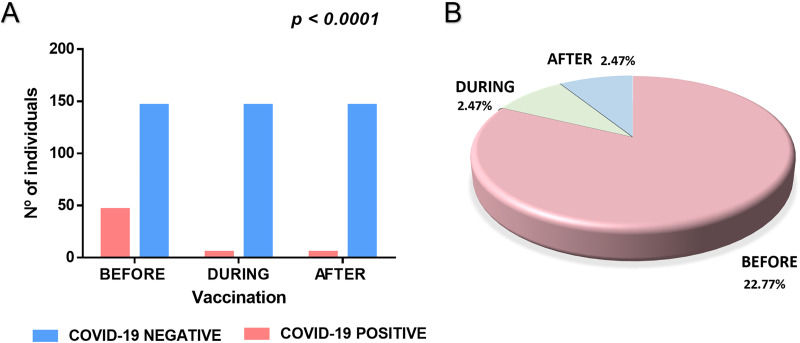
We first asked in our cross-sectional study if vaccines were, in fact, protective in the population studied. As shown in Fig. 1A, we observed that the numbers of infected individuals were significantly reduced among those who were vaccinated, even with one dose (Fig. 1A). In contrast, there was a much higher proportion of individuals infected who did not receive any dose of vaccine (Fig. 1B). We observed individuals with COVID-19 history after the first vaccine dose (during the immunization regimen) or after the second vaccine dose (after the immunization regimen). However, vaccines significantly reduced the proportions of COVID-19 history in the population studied. This result indicates that vaccines are highly protective against COVID-19.
FIG 1.
(A) Total numbers of infections with SARS-CoV-2 before, during, and after the immunization regimens were subjected to a Chi-square analysis. The numbers of infected individuals were significantly reduced (P ≤ 0.05). (B) Proportions of infections with SARS-CoV-2 before (22.77%), during (2.47%), and after the immunization (2.47%) among those who were vaccinated. The number of positive patients (n = 56) corresponds to 27.72% of the total of individuals who received at least one dose of vaccine (n = 202). Among these, 46 individuals (22.77%) had the infection before being vaccinated. In addition, five individuals (2.47%) had the disease between (after the first vaccine dose), and five individuals (2.47%) had COVID-19 after the second vaccine dose.
Antibody serum levels wane over time.
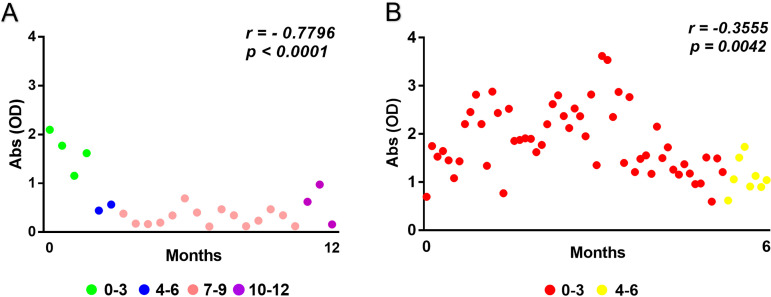
Although we found that vaccines are highly protective, we asked if the immunological status, represented here by specific antibody serum levels, would be maintained over time. We observed a significant tendency of decreasing antiviral antibody serum levels over the time after the second dose in our cross-sectional study population. Equivalent tendencies were observed for both the CoronaVac (Fig. 2A) and AstraZeneca (Fig. 2B) vaccine formulations. These results indicate that serum levels of specific antibodies to SARS-CoV-2 nucleoprotein and spike protein which were elicited by vaccines wane over time and are relevantly reduced 4 to 6 months after the second vaccine dose.
FIG 2.
Serum levels of antiviral antibodies wane over time after the second vaccine dose. (A and B) Fluctuations over time of specific antibody serum levels (shown as optical densities) elicited by CoronaVac (A) and AstraZeneca (B) vaccines were subjected to a correlation analysis. Significant tendencies of serum level decreases of specific antibodies over the time after the second dose (0 to 3, 4 to 6, 7 to 9, and 10 to 12 months) were observed (P ≤ 0.05; r < 0). The y axis shows absorbance as optical density (OD) values. The x axis shows the time after last dose in which the sample was obtained. Both r and P values are shown in the figure.
A booster shot with the Pfizer COVID-19 vaccine restores humoral immunological status.
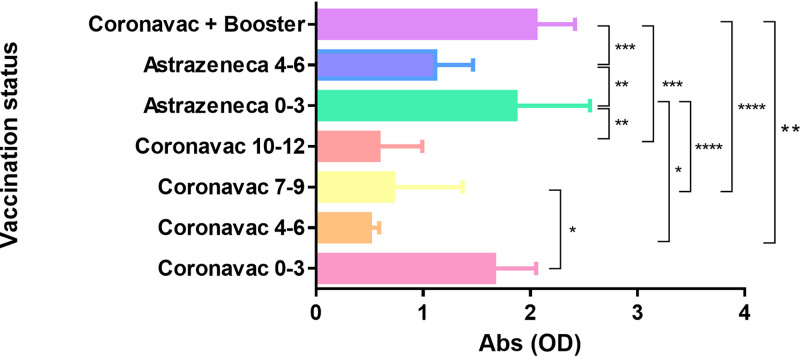
We aimed to understand if the immunization policy involving a third shot with the Pfizer vaccine would be plausible. As shown in Fig. 3, we found that the booster shot with the Pfizer vaccine after a CoronaVac immunization regimen significantly increased the SARS-CoV-2-specific IgG serum levels in comparison with those serum levels found 4 to 6 months or more after the second vaccine dose (see Table 1 for statistical details). The booster shot after the CoronaVac regimen also increased the antiviral antibody serum levels in comparison with those found 4 to 6 months after two doses of AstraZeneca vaccine (Fig. 3). These results indicate that the third vaccine dose recommended by Brazilian authorities restores a high antiviral antibody serum level after its decrease.
FIG 3.
Serum levels of specific antibodies (represented as optical densities [OD]) found after immunization regimens were compared by analysis of variance (ANOVA) followed by Bonferroni multiple-comparison test. The y axis shows immunization regimens, and the respective time after last dose is shown in months by numbers (0 to 3, 4 to 6, 7 to 9, and 10 to 12 months). The x axis shows absorbance as optical density values. Significance was set as P ≤ 0.05. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
TABLE 1.
Results and P values of analysis of variance (ANOVA) followed by Bonferroni multiple-comparison test to compare serum levels of antiviral antibodies found after immunization regimens at different time pointsa
| Immunization regimens | CoronaVac 0–3 | CoronaVac 4–6 | CoronaVac 7–9 | CoronaVac 10–12 | AstraZeneca 0–3 | AstraZeneca 4–6 |
|---|---|---|---|---|---|---|
| CoronaVac 4–6 | NS | |||||
| CoronaVac 7–9 | P < 0.05 | NS | ||||
| CoronaVac 10–12 | NS | NS | NS | |||
| AstraZeneca 0–3 | NS | P < 0.05 | P < 0.0001 | P < 0.001 | ||
| AstraZeneca 4–6 | NS | NS | NS | NS | P < 0.001 | |
| CoronaVac + booster | NS | P < 0.001 | P < 0.0001 | P < 0.0001 | NS | P < 0.0001 |
Numbers indicate the range of months after immunization regimen. NS, nonsignificant.
The immunological status conferred by vaccines is better than that elicited by SARS-CoV-2 infection.
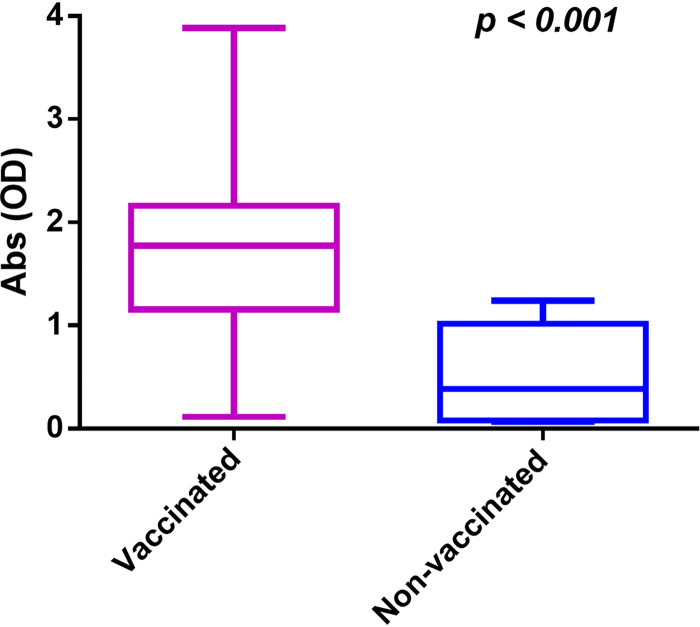
We finally asked which immunization confers the higher antiviral antibody serum level: vaccines or infection with SARS-CoV-2? As shown in Fig. 4, we found that vaccination induced a significantly higher antiviral antibody serum level (vaccinated) than only the natural infection with SARS-CoV-2 (nonvaccinated). This result indicates that vaccination is necessary to induce a higher immunological status. Infection with SARS-CoV-2 does not guarantee the best immunological status. Moreover, people should be vaccinated even after getting COVID-19. Collectively, results presented here indicate that vaccines are necessary to induce a robust immunological status, which is maintained, restored, or even improved by booster shots.
FIG 4.
Vaccination induces significantly higher serum levels of antiviral antibodies than only natural infection with SARS-CoV-2. Serum levels of specific antibodies in vaccinated and nonvaccinated individuals were compared by Student’s t test. We had a total of 202 vaccinated individuals and 8 nonvaccinated individuals in this study. Statistical significance was set as P ≤ 0.05. The y axis shows absorbance as optical density (OD) values.
DISCUSSION
Vaccines represent the most effective strategy of COVID-19 control worldwide (4, 5). However, in this scenario of frequent emergence of SARS-CoV-2 variants of concern (VOC) it is necessary to follow up immunity longevity and effectiveness in a real-time manner in order to guide the best possible immunization policies. In this study, we aimed to evaluate the effectiveness of the Brazilian immunization policy for COVID-19, which involves booster shots in order to keep a high serum level of antiviral antibodies. Here, we evaluated serum samples of individuals with or without previous SARS-CoV-2 infection who received at least one vaccine dose in the COVID-19 vaccination regimens of AstraZeneca, CoronaVac, or CoronaVac plus booster shot with Pfizer. In addition, nonvaccinated/infected individuals were also included.
The statistical significances shown in our results clearly indicate that (i) the vaccines used in Brazil are highly protective against COVID-19, (ii) there is a tendency toward a decrease of the serum levels of antiviral antibodies over time after conclusion of the immunization regimen, and (iii) the booster policy is right because the COVID-19 humoral immunological status significantly benefits from it. This is an expected outcome, as observed for other diseases which are preventable by vaccines. The surprise is that the immunological status is relevantly decreased with remarkable speed, only 4 to 6 months after the second dose with both the CoronaVac and AstraZeneca regimens. It is expected that vaccines based on inactivated viral particles, such as CoronaVac, induce immune responses with low longevity. However, the AstraZeneca COVID-19 vaccine also induced an immune response of low longevity regarding serum levels of antiviral antibodies. It was recently reported that COVID-19 vaccines based on mRNA and viral vectors carrying the spike protein gene induce immunological T cell memory able to cross-recognize variants from Alpha to Omicron (16). In contrast to our results involving humoral immunity, the authors found active memory T cells 6 months postvaccination.
It is important to highlight that at the time of the beginning of this study, the Brazilian policy for COVID-19 immunization was to recommend a booster shot only for people over 70 years old, immunocompromised people, and health care workers (8). We also highlight that at the time of this study, people who received two doses of the AstraZeneca vaccine formulation did not receive the booster dose. The group of nonvaccinated/infected individuals was composed of teenagers because most of the adults had received at least one vaccine dose at the time of this study. In addition, a cross-sectional survey may generate limited conclusions in comparison to a longitudinal one. Moreover, the sample size and the lack of evaluations regarding neutralizing antibodies and the T cell-mediated immune response are also limitations that must be informed. Nevertheless, we show with very clear statistical significance that vaccines are necessary to induce a robust immunological status, which is maintained, restored, or even improved by booster shots. Of course, further studies will certainly be necessary to better understand the longevity of immune responses induced by these vaccines. Moreover, further studies will be necessary to determine if booster shots will be required every 4 to 6 months, in order to avoid a possible decrease of the immune status of populations and an increase in the risk of new waves of COVID-19.
MATERIALS AND METHODS
Study design.
The cross-sectional study population consisted of 210 individuals (63 males and 147 females, ages ranging from 13 to 66 years old) from Barreiras (Bahia, Brazil), enrolled from September 2021 to November 2021. Immunological profiles were defined as individuals with (n = 46) or without (n = 146) previous SARS-CoV-2 infection who received at least one vaccine dose in the immunization regimens of AstraZeneca (one dose, n = 6; two doses, n = 63), CoronaVac (one dose, n = 4; two doses, n = 24) or CoronaVac plus booster shot with Pfizer (booster shot given at least 6 months after the second CoronaVac dose, n = 49). In addition, nonvaccinated/infected individuals were also included 15 to 22 days post-symptom onset. We computed the numbers of individuals who had COVID-19 between the first and second vaccine doses (n = 5) and after the second dose (n = 5). COVID-19 history was based on experimental validation by reverse transcriptase quantitative PCR (RT-qPCR), using a previously described CDC method (17), and on validated records of local public health authorities. Participants provided serum samples, which were subjected to enzyme-linked immunosorbent assay (ELISA). Participants who received two doses of vaccine were grouped according to time after the second dose at the moment of serum sampling—CoronaVac 0 to 3 months (n = 4); CoronaVac 4 to 6 months (n = 2); CoronaVac 7 to 9 months (n = 15); CoronaVac 10 to 12 months (n = 3); AstraZeneca 0 to 3 months (n = 55); AstraZeneca 4 to 6 months (n = 8). In addition, participants provided information validated by local health authorities regarding their immunization history and contact with SARS-CoV-2. All the research complied with all relevant ethical and biosafety guidelines. Ethics approval was obtained from the institutional ethics committee of the Federal University of Western Bahia (CAAE 40779420.6.0000.8060). All procedures and possible risks were explained to the volunteers.
ELISA.
Serum samples were analyzed using the EIE COVID-19 IgG N/S kit (Bio-Manguinhos, Fiocruz, Rio de Janeiro, Brazil), according to the manufacturer’s instructions. The serum levels of antibodies specific to SARS-CoV-2 were defined according to optical density values. In brief, enzyme-linked immunosorbent assay (ELISA) with solid-phase bound nucleoprotein (N) and spike (S) recombinant antigens was carried out with volunteers’ serum samples. Kit controls and samples were added to wells after dilution (1:101) with kit diluent. After 30 min at 37°C, plates were washed five times with kit washing buffer. Kit conjugated and previously diluted at 1:100 was added to wells. After 30 min at 37°C, plates were again washed five times, and reactions were developed by adding kit developing solution to the wells. After 10 min, reactions were stopped with 2 M H2SO4. Reactions were measured at A 450 nm.
Ethics statement.
Ethics approval was obtained from the institutional review board (ethics committee) (CAAE 40779420.6.0000.8060) of the Universidade Federal do Oeste da Bahia. Samples were collected only after volunteers gave written informed consents.
Statistical analyses.
The numbers of infection with SARS-CoV-2 before, during, and after the immunization regimens were analyzed by Chi-square. Fluctuations in values of specific antibody serum levels over time were subjected to correlation analyses. Serum levels of specific antibodies found after immunization regimens were compared by analysis of variance (ANOVA) followed by the Bonferroni multiple-comparison test. In addition, serum levels of specific antibodies in vaccinated and nonvaccinated individuals were compared by Student’s t test. Statistical significance was set as P ≤ 0.05.
Data availability.
Data will be provided upon request.
ACKNOWLEDGMENTS
Funding was provided by Instituto Serrapilheira/Serra-1708-15285, Consórcio Multifinalitário do Oeste da Bahia (CONSID-001), and 27968 FINEP/RTR/PRPq/REDE COVID-19 (9-UFOB). The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
We declare that we have no competing interests.
Contributor Information
Jaime Henrique Amorim, Email: jaime.amorim@ufob.edu.br.
Tom Gallagher, Loyola University Chicago.
REFERENCES
- 1.Zhou P, Yang X, Lou Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RDi, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. 2022. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/.
- 4.CDC . 2021. COVID-19 Vaccine Effectiveness. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/index.html. Accessed on November 29, 2021. [Google Scholar]
- 5.WHO . 2021. Vaccine efficacy, effectiveness and protection. https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection. Accessed on November 29, 2021.
- 6.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD, Graham BS, Mascola JR, Chang JY, Yin MT, Sobieszczyk M, Kyratsous CA, Shapiro L, Sheng Z, Huang Y, Ho DD. 2021. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature 593:130–135. 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 7.Cele S, Gazy I, Jackson L, Hwa SH, Tegally H, Lustig G, Giandhari J, Pillay S, Wilkinson E, Naidoo Y, Karim F, Ganga Y, Khan K, Bernstein M, Balazs AB, Gosnell BI, Hanekom W, Moosa MYS, Lessells RJ, de Oliveira T, Sigal A, COMMIT-KZN Team. 2021. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature 593:142–146. 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, Valluri SR, Pan K, Angulo FJ, Jodar L, McLaughlin JM. 2021. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 398:1407–1416. 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO . 2021. Vaccine efficacy, effectiveness and protection. WHO. https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection. Accessed on November 29, 2021. [Google Scholar]
- 10.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, et al. 2021. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99–111. 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerqueira-Silva T, Oliveira VDA, Boaventura VS, Pescarini JM, Júnior JB, Machado TM, Flores-Ortiz R, Penna GO, Ichihara MY, de Barros JV, Barreto ML, Werneck GL, Barral-Netto M. 2022. Influence of age on the effectiveness and duration of protection of Vaxzevria and CoronaVac vaccines: a population-based study. Lancet Reg Heal Am 6:100154. 10.1016/j.lana.2021.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, Pullukçu H, Batum Ö, Şimşek Yavuz S, Turhan Ö, Yıldırmak MT, Köksal İ, Taşova Y, Korten V, Yılmaz G, Çelen MK, Altın S, Çelik İ, Bayındır Y, Karaoğlan İ, Yılmaz A, Özkul A, Gür H, Unal S, Kayaaslan B, Hasanoğlu İ, Dalkıran A, Aydos Ö, Çınar G, Akdemir-Kalkan İ, İnkaya AÇ, Aydin M, Çakir H, Yıldız J, Kocabıyık Ö, Arslan S, Nallı B, Demir Ö, Singil S, Ataman-Hatipoğlu Ç, Tuncer-Ertem G, Kınıklı S, Önal U, Mete B, Dalgan G, Taşbakan M, et al. 2021. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 398:213–222. 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knoll MD, Wonodi C. 2021. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 397:72–74. 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group. 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chagla Z. 2021. The BNT162b2 (BioNTech/Pfizer) vaccine had 95% efficacy against COVID-19 ≥7 days after the 2nd dose. Ann Intern Med 174:JC15. 10.7326/ACPJ202102160-015. [DOI] [PubMed] [Google Scholar]
- 16.Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, Bloom NI, Goodwin B, Phillips E, Mallal S, Sidney J, Filaci G, Weiskopf D, Antunes R.dS, Crotty S, Grifoni A, Sette A. 2022. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 185:847–859.e11. 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha ALS, Pinheiro JR, Nakamura TC, da Silva JDS, Rocha BGS, Klein RC, Birbrair A, Amorim JH. 2021. Fomites and the environment did not have an important role in COVID-19 transmission in a Brazilian mid-sized city. Sci Rep 11:847–859.e11. 10.1038/s41598-021-95479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided upon request.