ABSTRACT
Engagement of host receptors is essential for viruses to enter target cells and initiate infection. Expression patterns of receptors in turn dictate host range, tissue tropism, and disease pathogenesis during infection. Mammalian orthoreovirus (reovirus) displays serotype-dependent patterns of tropism in the murine central nervous system (CNS) that are dictated by the viral attachment protein σ1. However, the receptor that mediates reovirus CNS tropism is unknown. Two proteinaceous receptors have been identified for reovirus, junctional adhesion molecule A (JAM-A) and Nogo-66 receptor 1 (NgR1). Engagement of JAM-A is required for reovirus hematogenous dissemination but is dispensable for neural spread and infection of the CNS. To determine whether NgR1 functions in reovirus neuropathogenesis, we compared virus replication and disease in wild-type (WT) and NgR1−/− mice. Genetic ablation of NgR1 did not alter reovirus replication in the intestine or transmission to the brain following peroral inoculation. Viral titers in neural tissues following intramuscular inoculation, which provides access to neural dissemination routes, also were comparable in WT and NgR1−/− mice, suggesting that NgR1 is dispensable for reovirus neural spread to the CNS. The absence of NgR1 also did not alter reovirus replication, neural tropism, and virulence following direct intracranial inoculation. In agreement with these findings, we found that the human but not the murine homolog of NgR1 functions as a receptor and confers efficient reovirus binding and infection of nonsusceptible cells in vitro. Thus, neither JAM-A nor NgR1 is required for reovirus CNS tropism in mice, suggesting that other unidentified receptors support this function.
IMPORTANCE Viruses engage diverse molecules on host cell surfaces to navigate barriers, gain cell entry, and establish infection. Despite discovery of several reovirus receptors, host factors responsible for reovirus neurotropism are unknown. Human NgR1 functions as a reovirus receptor in vitro and is expressed in CNS neurons in a pattern overlapping reovirus tropism. We used mice lacking NgR1 to test whether NgR1 functions as a reovirus neural receptor. Following different routes of inoculation, we found that murine NgR1 is dispensable for reovirus dissemination to the CNS, tropism and replication in the brain, and resultant disease. Concordantly, expression of human but not murine NgR1 confers reovirus binding and infection of nonsusceptible cells in vitro. These results highlight species-specific use of alternate receptors by reovirus. A detailed understanding of species- and tissue-specific factors that dictate viral tropism will inform development of antiviral interventions and targeted gene delivery and therapeutic viral vectors.
KEYWORDS: reovirus, NgR1, JAM-A, viral tropism, host range, neuropathogenesis
INTRODUCTION
Binding to host receptors is the first step in virus entry into host cells. While virus-receptor interactions are highly specific, several viruses engage more than a single receptor for cell entry (1, 2). The capacity to bind multiple receptors may be required to mobilize the cellular internalization machinery or invade specific tissues in the host. Low-affinity interactions with attachment factors that are abundantly expressed at the cell surface also can promote high-affinity interactions with receptors that mediate cell entry (3, 4). Tissue-specific patterns of receptor expression often dictate dissemination routes and tissue tropism and shape disease outcomes during viral infection. Moreover, host-specific receptor expression and receptor polymorphisms can influence infection and transmissibility of viruses between species (5, 6). Understanding receptor requirements is essential to identify targets to disrupt virus cell entry and design highly selective viral vectors for therapeutic applications. However, for many viruses, the identity of receptors mediating cell entry and functions of known receptors in pathogenesis remain poorly understood.
Mammalian orthoreovirus (reovirus) displays a broad host range and causes serotype-dependent disease in many young mammals (7). While reovirus infects most humans prior to adolescence (8), severe disease outcomes are rare (9, 10). Studies of reovirus infection using mice have established that the routes of transmission following inoculation and tissue tropism in the central nervous system (CNS) are serotype dependent. Following peroral inoculation, reovirus first replicates in gut-associated lymphoid tissue and intestinal epithelium (11–13). Subsequently, serotype 1 (T1) reovirus spreads through hematogenous routes, infects ependymal cells, and causes nonlethal hydrocephalus (14, 15), whereas serotype 3 (T3) reovirus spreads through both hematogenous and neural routes, infects distinct neuronal populations in the CNS, and causes lethal encephalitis (13, 15–17). The distinct patterns of CNS tropism displayed by T1 and T3 reovirus are dictated by viral attachment protein σ1 (16, 18), suggesting that engagement of specific host receptors by σ1 dictates tropism. While multiple reovirus receptors have been identified, their function in serotype-dependent tropism remains elusive.
Reovirus binds sialylated glycans, junctional adhesion molecule A (JAM-A), and the human homolog of Nogo-66 receptor 1 (hNgR1) expressed on cells. While reovirus serotypes engage distinct sialylated glycans (19, 20), and glycan interactions influence the severity of disease following inoculation, glycan engagement is dispensable for reovirus CNS tropism (21–23). JAM-A is an immunoglobulin superfamily receptor expressed primarily at tight junctions and by hematopoietic cells. Both human and murine homologs of JAM-A are bound by the σ1 protein of strains representing all three reovirus serotypes (24, 25). JAM-A is required for reovirus to access the bloodstream and disseminate hematogenously from the intestine to sites of secondary replication in the host (13). However, if hematogenous dissemination is bypassed by intramuscular or intracranial inoculation, reovirus is capable of transmission through neural routes and can infect the brains of mice lacking JAM-A expression (13). Thus, both sialic acid and JAM-A are dispensable for CNS tropism and disease, suggesting that alternative neural receptors must exist for reovirus.
Human NgR1 was identified as a reovirus receptor in a genome-wide RNA interference screen using HeLa cells (26). NgR1 is a leucine-rich repeat protein expressed primarily on neurons (27). Binding to NgR1 by a variety of structurally dissimilar myelin-associated ligands acts to regulate axonal growth and remodeling (28). The pattern of murine NgR1 (mNgR1) expression in the CNS (29) overlaps with sites of T3 reovirus tropism (13), including neuronal populations in the cerebral cortex, hippocampus, and thalamus. Soluble hNgR1 binds reovirus virions, and expression of hNgR1 in nonsusceptible Chinese hamster ovary (CHO) cells confers reovirus binding and infection (26). Importantly, infection by T3 reovirus is diminished in primary cultures of neurons derived from cerebral cortices of embryonic NgR1−/− mice (26), suggesting that mNgR1 functions as a neural receptor for reovirus. However, the function of mNgR1 in reovirus pathogenesis and tropism remained unexplored.
Here, we investigated a function for mNgR1 in reovirus neuropathogenesis using mice genetically lacking NgR1 expression. Mice deficient in expression of both JAM-A and NgR1 were used to dissect potential redundant functions of these receptors in reovirus infection. Peroral inoculation was used to mimic the natural fecal-oral route of reovirus transmission, whereas intramuscular and intracranial inoculations were used to provide direct access to neural routes of dissemination. Regardless of the route of inoculation, we found that mNgR1 was dispensable for T3 reovirus spread through both hematogenous and neural routes as well as tropism and replication in the CNS. mNgR1 also was not required for T1 reovirus infection of the brain following intracranial inoculation. Concordant with these in vivo findings, expression of mNgR1 did not allow efficient reovirus binding or infection of CHO cells, whereas hNgR1 supported both binding and infection. These findings exclude a function for known murine reovirus receptors in CNS tropism and suggest that alternate receptors must exist to mediate reovirus encephalitis in mice.
RESULTS
Murine NgR1 is dispensable for reovirus replication in the intestine and spread to the brain following peroral inoculation.
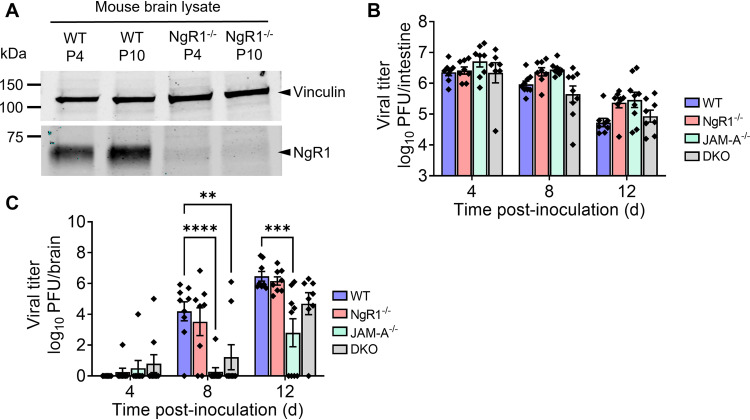
To determine whether mNgR1 functions in reovirus pathogenesis, we monitored reovirus spread in mice lacking NgR1 expression (NgR1−/−) following peroral inoculation, which mimics the natural fecal-oral transmission route of reovirus. The absence of mNgR1 expression in knockout animals was confirmed by immunoblot analysis of brain homogenates from newborn mice (Fig. 1A). Mice deficient in JAM-A (JAM-A−/−) or both JAM-A and NgR1 (double knockout [DKO]) were included as controls to exclude the possibility that one receptor might compensate for loss of the other. Neurovirulent reovirus strain T3SA−, which does not bind sialic acid (30), was used in these studies to eliminate the potential confounding variable of sialic acid binding in reovirus pathogenesis (22). Newborn wild-type (WT), NgR1−/−, JAM-A−/−, and DKO mice were inoculated perorally with 104 plaque-forming units (PFU) of reovirus T3SA−. Mice were euthanized at 4, 8, and 12 days post-inoculation (dpi), intestines and brains were removed, and titers in homogenates of these tissues were determined using plaque assays. The absence of JAM-A, NgR1, or both receptors did not alter titers in the intestine relative to those in WT mice at any time point examined (Fig. 1B). This result suggests that both JAM-A and mNgR1 are dispensable for reovirus replication in the intestine. Titers in the intestine decreased over the experimental time course, likely due to clearance of the virus from this site. Titers in brains of animals lacking JAM-A were lower than those in WT or NgR1−/− mice (Fig. 1C). These results are consistent with a previous study establishing a requirement for JAM-A in hematogenous reovirus dissemination to the brain following peroral inoculation (13). However, titers in the brains of NgR1−/− mice were comparable to those in WT mice at all time points tested. These results suggest that mNgR1 is dispensable for reovirus dissemination to and replication in the brain following peroral inoculation.
FIG 1.
NgR1 is dispensable for reovirus spread to the brain and replication at that site following peroral inoculation. (A) NgR1 expression in brain-tissue lysates of WT and NgR1−/− mice at postnatal days 4 (P4) and 10 (P10) were determined by immunoblotting using an antibody specific for mouse NgR1 (AF1440). Vinculin was used as a loading control. (B and C) WT, JAM-A−/−, NgR1−/−, and DKO mice were inoculated perorally with 104 PFU of reovirus T3SA−. Titers in the (B) intestine and (C) brain at the indicated intervals are shown. n = 8 or 9 animals per group for each time point. Error bars indicate standard errors of the means (SEM). Titers in tissues from knockout animals were compared with those in WT mice at each time point. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (two-way analysis of variance [ANOVA] with Dunnett’s multiple-comparison test).
Murine NgR1 is not required for reovirus replication in the brain and neurovirulence following intracranial inoculation.
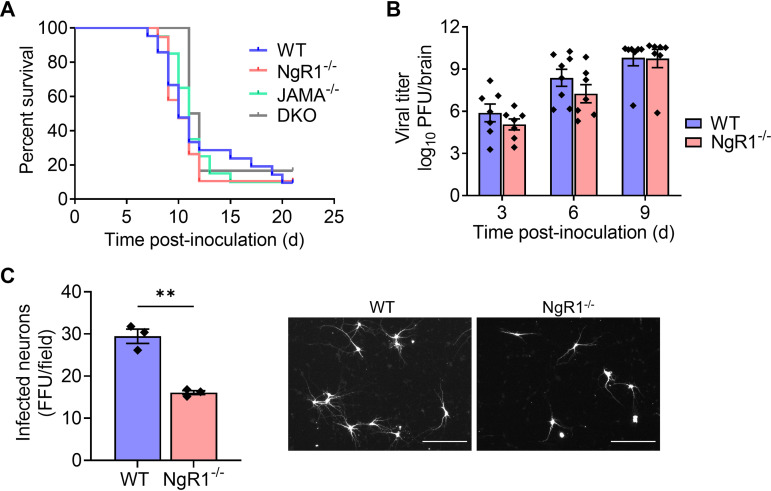
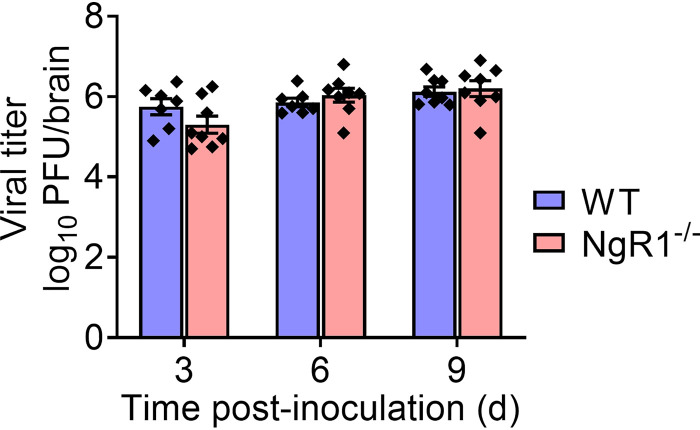
We next tested whether mNgR1 is required for reovirus neurovirulence. For these experiments, we introduced the virus by intracranial inoculation to bypass any function for mNgR1 in mediating spread to the brain following inoculation by other routes. Newborn WT, JAM-A−/−, NgR1−/−, and DKO mice were inoculated intracranially with 25 PFU of T3SA− and monitored for encephalitic symptoms and survival for 21 dpi. Signs of neurological disease (data not shown) and survival times of mice lacking NgR1 (Fig. 2A) were comparable to those of WT, JAM-A−/−, and DKO animals, suggesting that both NgR1 and JAM-A are dispensable for reovirus virulence following intracranial inoculation.
FIG 2.
NgR1 is not required for reovirus neuropathogenesis following intracranial inoculation. (A and B) Newborn WT, JAM-A−/−, NgR1−/−, and DKO mice were inoculated intracranially with 25 PFU of reovirus T3SA−. (A) Mice were monitored for disease signs and survival for 21 days and euthanized when moribund. n = 6 to 21 mice per group. Differences in survival relative to WT mice were not significant, as determined by log-rank test. (B) At 3, 6, and 9 days after inoculation, mice were euthanized, brains were removed and hemisected, and viral titers in homogenates of the inoculated half of the brain were determined by plaque assay. Results are expressed as mean viral titers. Error bars indicate SEM. n = 8 to 11 mice per group for each time point. Differences in titer between WT and NgR1−/− mice at each time point were not significant (P > 0.05), as determined by two-way ANOVA with Dunnett’s multiple-comparison test. (C) Cortical neurons isolated from E15.5 WT or NgR1−/− mice were adsorbed with T3SA+ virions at an MOI of 500 PFU/cell. Cells were fixed at 24 h postadsorption and stained using reovirus-specific antiserum. Results are expressed as the mean number of infected neurons, identified based on morphology, per field-of-view. Error bars indicate SEM for triplicate samples from one representative experiment of two conducted. **, P < 0.01 (t test). Representative micrographs on the right show infected neurons in an area approximately one-fourth of the field-of-view examined. Bars, 150 μm.
Despite the lack of overt differences in disease phenotypes, we hypothesized that the absence of NgR1 might diminish reovirus replication and dissemination in the brain. To test this hypothesis, we inoculated newborn WT and NgR1−/− mice intracranially with 25 PFU of T3SA−. Mice were euthanized at 3, 6, and 9 dpi, brains were removed and hemisected, and titers from the inoculated half of the brain (right hemisphere) were determined. Viral titers in brain tissue of WT mice were comparable to those in mice lacking NgR1 (Fig. 2B), suggesting that mNgR1 is not required for reovirus replication in the brain following intracranial inoculation. These results are in contrast with the significant reduction in reovirus infectivity of cortical neurons cultured from NgR1−/− mice observed previously (26) and here (Fig. 2C).
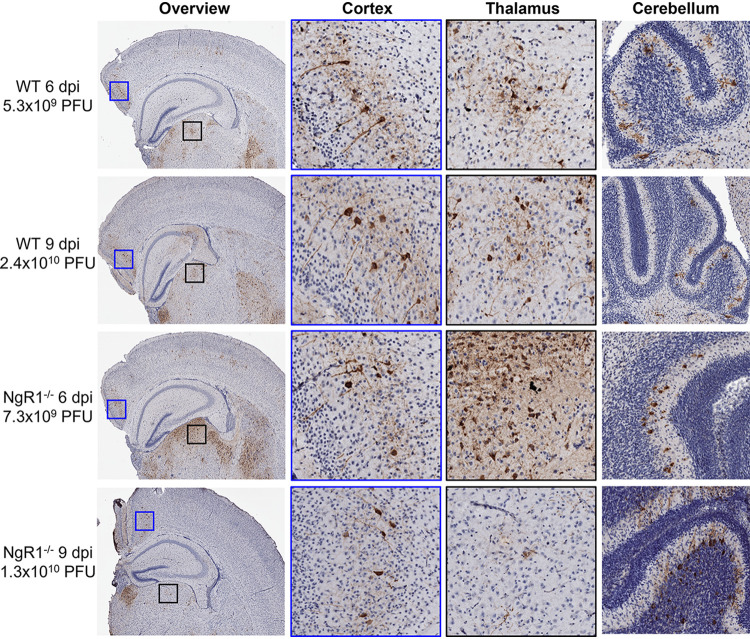
We thought it possible that mNgR1 might mediate spread to and infection of specific neuronal subsets, without affecting overall titers in the brain and neurovirulence. To test this hypothesis, we examined the distribution of reovirus antigen in the brain hemisphere opposing the inoculated hemisphere. Coronal sections of formalin-fixed and paraffin-embedded left-brain hemispheres from the experiments to determine brain viral titers (Fig. 2B) were stained with reovirus-specific antiserum. The intensity of reovirus antigen staining mostly correlated with viral titers from the matched right-brain hemispheres in both WT and NgR1−/− mice (Fig. 3). Reovirus-infected neurons, identified based on morphology and antigen staining, were distributed across the middle layers of the cortex, CA3 region of the hippocampus, thalamus, and cerebellar Purkinje neurons in both WT and NgR1−/− mouse brains. Based on histological sections from four mice examined for each genotype, the thalamus appeared to be more severely infected in NgR1−/− mice than in WT mice. Overall, reovirus antigen staining and distribution were similar independent of mouse genotype and consistent with previous reports of reovirus neurotropism (13, 31). Together, these results suggest that NgR1 is dispensable for reovirus replication, tropism in the brain, and neurovirulence.
FIG 3.
Reovirus tropism in the brain is unaltered in the absence of NgR1. Newborn WT and NgR1−/− mice were inoculated intracranially in the right brain hemisphere with 25 PFU of reovirus T3SA−. Mice were euthanized 6 or 9 dpi, and brains were removed and hemisected. Left-brain hemispheres were fixed in formalin and embedded in paraffin. Coronal sections of the left-brain hemisphere were stained with reovirus-specific antiserum and hematoxylin. Representative sections show reovirus antigen in cortex, thalamus, and cerebellum. Enlarged images of areas boxed in the overview show reovirus infection of neurons in the cortex (blue boxes) and thalamus (black boxes). Viral titers from the paired right-brain hemispheres are reported adjacent to the micrographs.
Murine NgR1 is not required for reovirus transmission along neural routes following intramuscular inoculation.
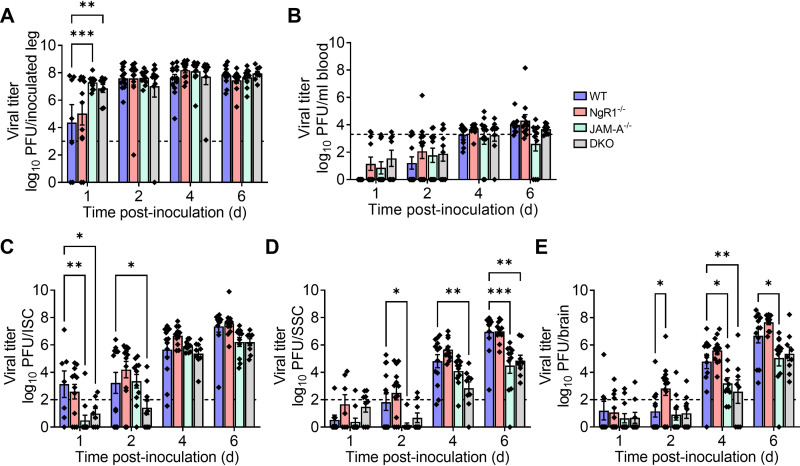
We hypothesized that mNgR1 might function to mediate reovirus spread through neural routes to the brain. To test this hypothesis, we inoculated newborn WT, JAM-A−/−, NgR1−/−, and DKO mice intramuscularly in the hind limb with 5 × 106 PFU of T3SA−. At 1, 2, 4, and 6 dpi, mice were euthanized, and viral titers in the inoculated limb, inferior and superior spinal cord (ISC and SSC, respectively), brain, and blood were determined. Titers in the inoculated limb increased rapidly and reached significantly higher levels by 1 dpi in mice lacking JAM-A (JAM-A−/− and DKO mice) relative to WT and NgR1−/− mice (Fig. 4A). Titers in the limb were comparable in all genotypes by 2 dpi and remained comparable during the experimental time course (Fig. 4A). Viral titers in the blood were initially undetectable and reached the detection limit by 6 to 8 dpi in all genotypes (Fig. 4B). Titers in the ISC, SSC, and brain also continued to increase over the time course in all genotypes (Fig. 4C to E). Interestingly, titers in the ISC, SSC, and brain of mice lacking JAM-A were lower than those in WT and NgR1−/− mice at multiple time points (Fig. 4C to E). Importantly, titers in neural tissue of mice lacking NgR1 were comparable to those in mice expressing NgR1, suggesting that mNgR1 is dispensable for reovirus neural spread.
FIG 4.
NgR1 is dispensable for reovirus neural transmission following intramuscular inoculation. WT, JAM-A−/−, NgR1−/−, and DKO mice were inoculated intramuscularly in the hind limb with 5 × 106 PFU of reovirus T3SA−. Titers in the (A) inoculated hind limb, (B) blood, (C) ISC, (D) SSC, and (E) brain at the indicated intervals are shown. Dotted lines indicate the limit of detection. n = 8 to 13 animals per group for each time point. Error bars indicate SEM. Titers in tissues from knockout animals were compared with those in WT mice at each time point. *, P < 0.05; **, P < 0.01; ***; P < 0.001 (two-way ANOVA with Dunnett’s multiple-comparison test).
Serotype 1 reovirus does not require murine NgR1 for replication in the brain.
Expression of hNgR1 in nonsusceptible cells allows infection by both T1 and T3 reovirus strains (26). Therefore, we tested whether NgR1 is required for reovirus infection of the brain by T1 reovirus strain T1L. WT and NgR1−/− mice were inoculated intracranially with 100 PFU of T1L. Mice were euthanized at 3, 6, and 9 dpi, brains were removed and hemisected, and titers from the inoculated half of the brain were determined. Viral titers in brain tissue of WT mice were comparable to those in mice lacking NgR1 (Fig. 5), suggesting that mNgR1 is dispensable for T1 reovirus replication in the brain following intracranial inoculation.
FIG 5.
NgR1 is not required for serotype 1 reovirus replication in the murine brain. Newborn WT and NgR1−/− mice were inoculated intracranially with 100 PFU of reovirus T1L. At 3, 6, and 9 days after inoculation, mice were euthanized, brains were removed and hemisected, and viral titers in homogenates of the inoculated half of the brain were determined by plaque assay. Results are expressed as mean viral titers. Error bars indicate SEM. n = 7 to 8 mice per group for each time point. Differences in titer between WT and NgR1−/− mice at each time point were not significant (P > 0.05), as determined by two-way ANOVA with Dunnett’s multiple-comparison test.
Human but not murine NgR1 functions as a reovirus receptor.
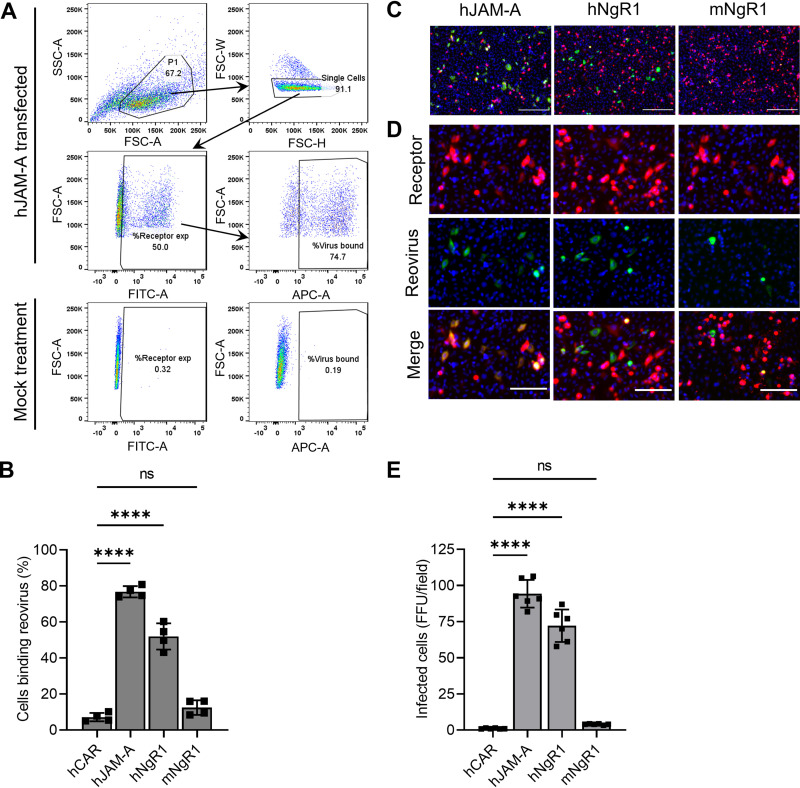
hNgR1 directly engages reovirus virions and allows infection of nonsusceptible cells (26). Given the function of hNgR1 as a reovirus receptor, the dispensability of mNgR1 in reovirus pathogenesis was puzzling and suggested that mNgR1 is incapable of engaging reovirus and mediating infection. Therefore, we tested the capacity of CHO cells expressing mNgR1 or hNgR1 to bind and allow infection by reovirus. Human JAM-A (hJAM-A) was used as a positive control, and the human coxsackievirus and adenovirus receptor (hCAR), which does not engage reovirus (24, 32), was used as a negative control. A significant fraction (>50%) of hJAM-A- or hNgR1-expressing cells bound reovirus, whereas hCAR- or mNgR1-expressing cells bound reovirus poorly (Fig. 6A and B). Differences in reovirus binding to cells expressing hCAR or mNgR1 were not statistically significant. The observed differences in binding are not attributable to differences in transfection or receptor expression efficiencies, as >75% of transfected cells expressed hNgR1 or mNgR1 on the surface, and only receptor-expressing cells were included in the determination of the percentage of cells bound by reovirus. Differences in binding paralleled differences in infectivity. hJAM-A or hNgR1 expression led to infection of CHO cells, but hCAR or mNgR1 expression did not (Fig. 6C-E). These results confirm that mNgR1 does not engage reovirus efficiently and correspondingly does not allow infection of nonsusceptible CHO cells.
FIG 6.
Murine NgR1 does not serve as an efficient reovirus receptor. (A and B) CHO cells were transfected with plasmids encoding the receptors shown and incubated with 105 particles/cell of fluorescently labeled reovirus T3SA− at 4°C for 1 h. Unbound virus was removed, and cells were fixed using paraformaldehyde and stained with receptor-specific antibodies to determine expression. The percentage of receptor-expressing cells bound by reovirus was quantified using flow cytometry. (A) Representative gating strategy for flow cytometry data analysis is shown for hJAM-A-transfected cells. Single cells identified based on scatter profiles were gated for receptor expression. Receptor-expressing cells were then gated for reovirus binding. Gates were defined based on mock-treated controls (bottom panels). Mock-treated samples were not treated with receptor-specific antibodies or reovirus. (B) Results are expressed as the mean percentage of reovirus-bound cells from duplicate samples of two independent experiments. The mean percentage of transfected cells expressing cell-surface receptors was 91% for hCAR, 54% for hJAM-A, 80% for hNgR1, and 85% for mNgR1. Error bars indicate SEM. ns, not significant (P > 0.05); ****, P < 0.0001 (one-way ANOVA with Dunnett’s multiple-comparison test). (C to E) Transfected CHO cells were adsorbed with 30 PFU/cell of T3SA− at 4°C for 1 h. Infectivity was quantified at 24 h post-adsorption. (C) Representative micrographs display nuclei stained with DAPI (blue), receptor expression (red), and reovirus antigen staining (green). Bars, 200 μm. (D) Enlarged versions of images in panel C show individual channels stained for receptor expression or reovirus antigen along with DAPI. Bars, 100 μm. (E) Mean numbers of infected cells per field-of-view from three independent experiments, each including duplicate samples. For each sample, FFU in 8 to 9 fields-of-view were averaged and presented as a single data point. Error bars indicate SEM. ns, not significant (P > 0.05); ****, P < 0.0001 (one-way ANOVA with Dunnett’s multiple-comparison test).
DISCUSSION
Serotype-specific receptor engagement dictates reovirus tropism for distinct CNS regions (15, 16, 18). However, host receptors that mediate reovirus neurotropism are unknown. Human NgR1 functions as a receptor for reovirus in vitro and is expressed primarily in neural tissues, suggesting that NgR1 mediates reovirus neurotropism. We tested this hypothesis by examining reovirus dissemination and replication in NgR1−/− mice. The absence of mNgR1 did not alter replication of neurotropic reovirus strain T3SA− in the intestine or its dissemination to the brain following peroral inoculation. Following intramuscular inoculation, which provides access to neural routes, T3SA− titers increased in the spinal cord and brain of WT and NgR1−/− mice comparably, suggesting that neural dissemination routes are intact in NgR1−/− mice. Viral titers in DKO mouse tissue homogenates were lower than those in WT mice and comparable to those in JAM-A−/− mice, indicating that the differences in viral titers are attributable to the lack of JAM-A expression and concomitant diminished hematogenous viral spread (13, 33). Viral titers in the inoculated limb increased more rapidly in the absence of JAM-A, perhaps due to more efficient viral entry using other receptors or increased tissue permeability in the absence of JAM-A (34), which might allow more efficient infectivity. Reovirus T3SA− replication, tissue tropism, and virulence in the CNS were comparable in WT and NgR1−/− mice, excluding an autonomous function for mNgR1 in reovirus neuropathogenesis. These results were reconciled by our finding that human but not mNgR1 efficiently binds reovirus and functions as a receptor in vitro.
The poor capacity of mNgR1 to allow reovirus binding and infection of nonsusceptible CHO cells is in contrast with its requirement for infection of murine cortical neuron cultures reported previously (26). Receptor blockade with NgR1-specific antibodies or absence of mNgR1 expression diminishes reovirus infection of primary murine cortical neurons in culture (26). Our findings recapitulate the diminished infectivity observed for T3 reovirus in cortical neurons prepared from embryonic NgR1−/− mice relative to those from WT mice (Fig. 2C), although the magnitude of the infectivity difference was not as striking as previously observed (26). These differences could be due to subtle variations in the culture conditions that alter expression of mNgR1 or an alternative neural receptor for reovirus that allows infection in the absence of mNgR1.
Our results demonstrating that mNgR1 is dispensable for reovirus neuropathogenesis raise two important questions. First, how does mNgR1, despite its inability to efficiently bind reovirus, promote infection of cortical neurons in culture? Second, what is the basis for the mNgR1 requirement for infection of cortical neurons in culture but not for in vivo infection? If the binding of reovirus to mNgR1 is of low affinity, then reovirus bound to mNgR1-expressing CHO cells at steady state may not be detectable by flow cytometry and may not allow efficient infection. However, neurons may express additional factors that contribute to higher-affinity binding and allow reovirus infection. In support of this idea, while hNgR1 expression in CHO cells allows infection by both T1 and T3 reovirus (26), neuronal cultures allow infection by only T3 reovirus (13, 18, 35), suggesting that neuronal factors other than NgR1 determine reovirus tropism both in vitro and in vivo. These alternative receptors may not be expressed efficiently in neurons cultured from embryonic mice, thereby shifting the dependency to mNgR1 for infection. Murine NgR1 also may act as a receptor for reovirus in other specific host tissues that we did not monitor in this study. However, our results show that mNgR1 is dispensable for reovirus neural infection.
There is precedent for the failure of viral receptors that function in cell culture to serve as receptors in vivo. For example, Axl, Mertk, and Tyro3 function as Zika virus entry receptors and allow infection of nonsusceptible cells in vitro but are dispensable for Zika virus infection and tropism in mice (36). Similarly, CD46 is ubiquitously expressed by nucleated cells and acts as an entry receptor for measles virus vaccine strains in vitro (37). However, tropism of measles virus vaccine strains in macaques is limited to specific tissues and does not correlate with CD46 expression (38). Therefore, in some cases, in vitro viral receptors are neither necessary nor sufficient for in vivo infection.
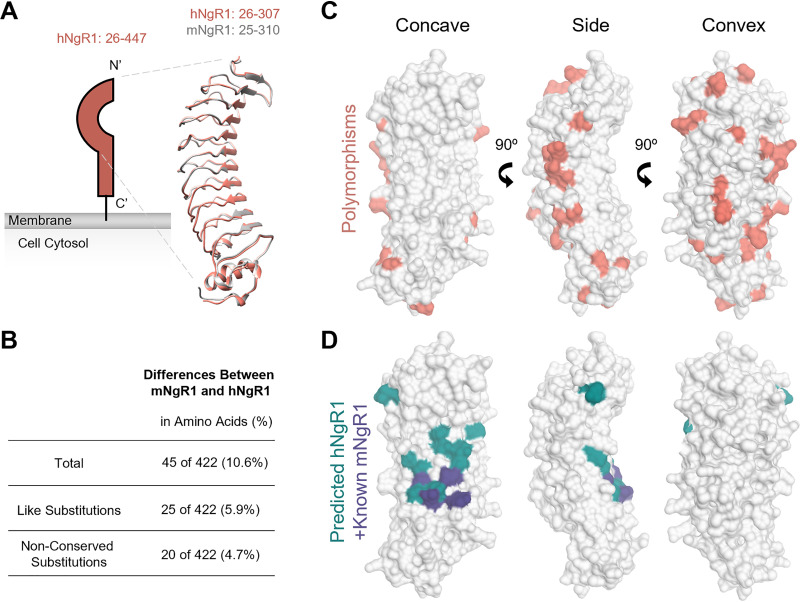
Species-specific polymorphisms in viral receptors can influence the capacity of a virus to establish infection in the host. Human and mouse NgR1 share structural and functional homology (Fig. 7A and B). Both are crescent-shaped, leucine-rich-repeat proteins that engage ligands in the CNS using conserved residues on the concave surface (27, 39). However, differences in key contact residues may allow hNgR1 to function efficiently as a reovirus receptor but impede reovirus engagement of mNgR1. The mature hNgR1 and mNgR1 proteins share nearly 90% amino acid identity (Fig. 7B), with the concave surface of the leucine-rich-repeat domains being highly conserved (Fig. 7C). This conserved surface has been implicated as a binding region for structurally dissimilar NgR1 ligands (e.g., Lingo-1, myelin-associated glycoprotein, Nogo-66, and oligodendrocyte myelin glycoprotein) and is the confirmed site for BAI1 binding to mNgR1 (39, 40). However, residues on the side and convex surface of NgR1 also influence binding to some NgR1 ligands (39). Therefore, it is possible that reovirus binding to NgR1 requires conserved sequences on the concave surface and polymorphic sequences elsewhere on the molecule.
FIG 7.
Comparison of human and mouse NgR1 structures and binding surfaces. (A) Schematic of NgR1 alongside overlaid ribbon tracings of hNgR1 (PDB ID 1OZN) (52) and mNgR1 (PDB ID 5O0K) (53). Amino acids included in the model are indicated. N and C termini are labeled. (B) Comparison of hNgR1 and mNgR1 amino acid sequences. (C) Surface representations of hNgR1 amino acids 26 to 307 (PDB ID 1OZN), with mNgR1 polymorphisms shown in coral. (D) Surface representations of hNgR1, with residues identified by alanine mutagenesis predicted to be required for the binding of hNgR1 by neural ligands (39) shown in teal. Residues required for the binding of mNgR1 by BAI1 (40) are shown in purple. Structure representations were made using Chimera UCSF (54).
There are many examples of engagement of host-specific receptors by viruses. Cedar virus uses murine but not human ephrin-A1 as an entry receptor, and the specificity is determined by a single contact residue in the receptor-ligand interface (41). Similarly, while the murine homolog of the hepatitis B virus receptor does not support virus entry, a chimeric murine receptor with a four-amino-acid replacement from the human receptor is sufficient to allow virus entry and replication (5, 42). Further structural insights are required to understand differences in reovirus interactions with mNgR1 and hNgR1.
Our findings clarify functions for known reovirus receptors in pathogenesis and open doors for identification of alternative receptors that dictate reovirus CNS tropism. While JAM-A is required for establishment of viremia following reovirus replication in the intestine (13, 33), both JAM-A and mNgR1 are dispensable for neural spread and infection of the brain. Thus, a reovirus neural receptor that dictates tropism and neuropathogenesis remains to be identified. Importantly, our results do not exclude a function for NgR1 in reovirus infections of humans. While reovirus does not commonly cause severe acute disease in humans, reovirus occasionally infects the CNS in children (9, 10) and is implicated in the loss of immunological tolerance to dietary gluten and development of celiac disease (43). Receptors mediating reovirus infection in humans are unknown, and engagement of species-specific receptors merits evaluation in this context. Reovirus has been used in clinical trials as an oncolytic agent (44), and defining the human receptors bound and their functions at distinct sites, including NgR1, is essential to precisely target oncolytic reovirus vectors. Although virus-receptor interactions often are described as specific, akin to a lock-and-key mechanism, some viruses use multiple keys to open several locks. Understanding tissue- and host-specific factors required by viruses for cell entry will inform development of specific interventions, engineering of effective vaccines, and use of viruses for targeted gene delivery.
MATERIALS AND METHODS
Cells and viruses.
L929 cells were grown in either suspension or monolayer cultures in Joklik's modified Eagle's minimal essential medium (U.S. Biological) supplemented to contain 5% fetal bovine serum (FBS; VWR, 97068-085). CHO cells were grown in Ham’s F-12 medium (Gibco) supplemented to contain 10% FBS. Both L929 and CHO cell media were supplemented to contain 2 mM l-glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B. Primary cortical neurons were isolated and cultivated in parallel from brains of embryonic day 15.5 WT or NgR1−/− mice, as described elsewhere (18). Neurons were cultivated for 5 to 7 days in vitro before inoculation with reovirus.
Reovirus strains T1L, T3SA+, and T3SA− were prepared from laboratory stocks, which were recovered using reverse genetics. Viruses were isolated using plaque purification followed by passaging in L929 cells. Virions were purified from infected L929 cell lysates using cesium chloride density gradients, as described elsewhere (45). Viral titers were determined by plaque assay using L929 cells (46). Purified virion particle concentrations were determined from the optical density at 260 nm (an OD260 of 1 = 2.1 × 1012 particles/mL) (47).
Reovirus virions were fluorescently labeled by incubating 6 × 1012 particles/mL in freshly prepared 50 mM sodium bicarbonate buffer with 20 μM Alexa Fluor 647 succinimidyl ester dye (Invitrogen) at room temperature for 90 min. Unreacted dye was removed by dialysis against phosphate-buffered saline (PBS) overnight at 4°C.
Mice.
C57BL/6J (WT) mice were obtained from The Jackson Laboratory. JAM-A−/− mice (48), provided by Thomas Sato (Cornell University, NY), and NgR1−/− mice (49), provided by Stephen Strittmatter (Yale University, CT), were backcrossed with background strain C57BL/6J mice. JAM-A−/− and NgR1−/− mice were interbred to recover DKO mice. Disruption of the JAM-A- and NgR1-encoding genes was confirmed by PCR.
Inoculation of mice.
Newborn mice (2 to 3 days old) weighing 1.4 to 2.3 g were inoculated with purified reovirus suspended in PBS. Mice were inoculated intracranially in the right cerebral hemisphere (18), intramuscularly in the left hind limb, or perorally (13). Viral titers in the inocula were confirmed using plaque assays. For analyses of virulence, inoculated mice were monitored daily for weight loss and disease signs for 21 days and euthanized when moribund. Criteria for moribundity included immobility, seizures, paralysis, or 25% body weight loss. For determination of viral titers, mice were euthanized at various intervals postinoculation, and organs were harvested into 1 mL of PBS and stored at −80°C. Samples were frozen and thawed twice and homogenized using a TissueLyser LT (Qiagen) instrument prior to quantification of viral titers by plaque assay using L929 cells. All animal experiments were conducted in accordance with Public Health Service policy and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Histology.
Mice were euthanized at various intervals following intracranial inoculation, and brains were removed and hemisected longitudinally. The right (inoculated) brain hemispheres were used to determine viral titers, and the left hemispheres were fixed using 10% neutral buffered formalin for at least 24 h, embedded in paraffin wax, and cut into 5-μm-thick sections. Tissue sections were stained with reovirus polyclonal antiserum to visualize reovirus antigen, as described elsewhere (18).
Plasmid transfection and reovirus binding.
Plasmids encoding human homologs of JAM-A, CAR, and NgR1 have been described (24, 26). Murine NgR1 cDNA, provided by S. Strittmatter, was subcloned into the pcDNA3.1+ expression plasmid using sticky-end mutagenesis and custom primers. CHO cells (5 × 104 seeded per sample) were transfected with 1 μg of receptor-encoding plasmid using TransIT-LT1 transfection reagent (Mirusbio). Cells were used to assess reovirus binding or infection 48 h post-transfection.
For assessment of reovirus binding, transfected CHO cells were detached from cell culture dishes using Cellstripper (Corning) treatment at 37°C for 15 min, quenched with culture medium, and washed once with ice-cold PBS. Cells in suspension were adsorbed with 2 × 1010 reovirus virions (Alexa Fluor 647 conjugated) at 4°C for 1 h. Cells were washed twice with fluorescence-activated cell sorting (FACS) buffer (PBS containing 2% FBS) to remove unbound virions, and cell-bound virus was fixed using 1% paraformaldehyde. Cells were incubated on ice for 20 min and washed twice with 10 mM glycine-containing PBS to quench the fixative. Cells were further incubated with the following primary antibodies to determine cell-surface expression of receptors: J10.4, mouse monoclonal antibody against JAM-A (provided by Charles Parkos, Emory University) (50); rabbit monoclonal antibody against CAR (Sinobiological, 10799-R271); goat polyclonal antibody against hNgR1 (R&D Systems, AF1208); and goat polyclonal antibody against mNgR1 (R&D Systems, AF1440). Secondary antibodies conjugated to Alexa Fluor 488 were used to detect receptor expression. Cells were analyzed using an LSRII flow cytometer (BD Bioscience), and intensity of reovirus bound to receptor-expressing cells was quantified using FlowJo software.
Reovirus infectivity assay.
CHO cells transfected with receptor-encoding plasmids were adsorbed with reovirus T3SA− virions diluted in PBS at a multiplicity of infection (MOI) of 30 PFU/cell. Cortical neurons in culture were adsorbed with reovirus T3SA+ virions diluted in PBS at an MOI of 500 PFU/cell. Following incubation at 37°C for 1 h, the inoculum was removed, and cells were incubated at 37°C for an additional 24 h. Reovirus antigen was visualized in fixed cells by indirect immunofluorescence using polyclonal reovirus antiserum (51) and a fluorophore-conjugated secondary antibody. DAPI (4′,6-diamidino-2-phenylindole) was used to stain nuclei. Cells were imaged using a LionHeart FX imager. Reovirus antigen-positive cells (fluorescence focus units [FFU]) were enumerated using Gen5 software for CHO cells and manually for neurons.
Statistical analysis.
All statistical tests were conducted using Prism 8 (GraphPad Software). P values of less than 0.05 were considered to be statistically significant. Descriptions of the specific tests used are provided in the figure legends.
ACKNOWLEDGMENTS
We are grateful to members of the Dermody lab for insightful discussions. We thank Hannah Hartman for expert technical assistance with immunoblotting. Tissues were processed for histology and immunohistochemistry at the histology core at the Rangos Research Center at UPMC Children’s Hospital of Pittsburgh. Histological slides were imaged by the University of Pittsburgh Biospecimen Core.
This work was supported by U.S. Public Health Service award R01 AI038296 (T.S.D.). Additional support was provided by UPMC Children’s Hospital of Pittsburgh (P.A.) and the Heinz Endowments (T.S.D.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare that no conflicts of interest exist.
P.A. designed and conducted experiments, analyzed results, and wrote the manuscript. C.G.-C., K.U., O.L.W., J.L.K.-A., and D.M.S. designed and conducted experiments, analyzed results, and reviewed the manuscript. T.S.D. designed experiments, analyzed results, and wrote the manuscript.
Contributor Information
Terence S. Dermody, Email: terence.dermody@chp.edu.
Susana López, Instituto de Biotecnologia/UNAM.
REFERENCES
- 1.Delpeut S, Noyce RS, Siu RW, Richardson CD. 2012. Host factors and measles virus replication. Curr Opin Virol 2:773–783. 10.1016/j.coviro.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Lindenbach BD, Rice CM. 2013. The ins and outs of hepatitis C virus entry and assembly. Nat Rev Microbiol 11:688–700. 10.1038/nrmicro3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolly CL, Sattentau QJ. 2013. Attachment factors, p 1–23. In Poehlmann S, Simmons G (ed), Viral entry into host cells. Springer Science+Business Media, New York, NY. [Google Scholar]
- 4.Koehler M, Aravamudhan P, Guzman-Cardozo C, Dumitru AC, Yang J, Gargiulo S, Soumillion P, Dermody TS, Alsteens D. 2019. Glycan-mediated enhancement of reovirus receptor binding. Nat Commun 10:4460. 10.1038/s41467-019-12411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan H, Peng B, He W, Zhong G, Qi Y, Ren B, Gao Z, Jing Z, Song M, Xu G, Sui J, Li W. 2013. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J Virol 87:7977–7991. 10.1128/JVI.03540-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. 2014. The evolution and genetics of virus host shifts. PLoS Pathog 10:e1004395. 10.1371/journal.ppat.1004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dermody TS, Parker JS, Sherry B. 2013. Orthoreoviruses, p 1304–1346. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 8.Tai JH, Williams JV, Edwards KM, Wright PF, Crowe JE, Jr, Dermody TS. 2005. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J Infect Dis 191:1221–1224. 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouattara LA, Barin F, Barthez MA, Bonnaud B, Roingeard P, Goudeau A, Castelnau P, Vernet G, Paranhos-Baccala G, Komurian-Pradel F. 2011. Novel human reovirus isolated from children with acute necrotizing encephalopathy. Emerg Infect Dis 17:1436–1444. 10.3201/eid1708.101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyler KL, Barton ES, Ibach ML, Robinson C, Campbell JA, O'Donnell SM, Valyi-Nagy T, Clarke P, Wetzel JD, Dermody TS. 2004. Isolation and molecular characterization of a novel type 3 reovirus from a child with meningitis. J Infect Dis 189:1664–1675. 10.1086/383129. [DOI] [PubMed] [Google Scholar]
- 11.Wolf JL, Rubin DH, Finberg R, Kauffman RS, Sharpe AH, Trier JS, Fields BN. 1981. Intestinal M cells: a pathway of entry for reovirus into the host. Science 212:471–472. 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- 12.Bass DM, Trier JS, Dambrauskas R, Wolf JL. 1988. Reovirus type I infection of small intestinal epithelium in suckling mice and its effect on M cells. Lab Invest 58:226–235. [PubMed] [Google Scholar]
- 13.Antar AAR, Konopka JL, Campbell JA, Henry RA, Perdigoto AL, Carter BD, Pozzi A, Abel TW, Dermody TS. 2009. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 5:59–71. 10.1016/j.chom.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masters C, Alpers M, Kakulas B. 1977. Pathogenesis of reovirus type 1 hydrocephalus in mice: significance of aqueductal changes. Arch Neurol 34:18–28. 10.1001/archneur.1977.00500130038008. [DOI] [PubMed] [Google Scholar]
- 15.Weiner HL, Drayna D, Averill DR, Jr, Fields BN. 1977. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci USA 74:5744–5748. 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner HL, Powers ML, Fields BN. 1980. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis 141:609–616. 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- 17.Boehme KW, Frierson JM, Konopka JL, Kobayashi T, Dermody TS. 2011. The reovirus σ1s protein is a determinant of hematogenous but not neural virus dissemination in mice. J Virol 85:11781–11790. 10.1128/JVI.02289-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland DM, Aravamudhan P, Dietrich MH, Stehle T, Dermody TS. 2018. Reovirus neurotropism and virulence are dictated by sequences in the head domain of the viral attachment protein. J Virol 92:e00974-18. 10.1128/JVI.00974-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter DM, Frierson JM, Halvorson EE, Kobayashi T, Dermody TS, Stehle T. 2011. Crystal structure of reovirus attachment protein σ1 in complex with sialylated oligosaccharides. PLoS Pathog 7:e1002166. 10.1371/journal.ppat.1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiss K, Stencel JE, Liu Y, Blaum BS, Reiter DM, Feizi T, Dermody TS, Stehle T. 2012. The GM2 glycan serves as a functional co-receptor for serotype 1 reovirus. PLoS Pathog 8:e1003078. 10.1371/journal.ppat.1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton ES, Youree BE, Ebert DH, Forrest JC, Connolly JL, Valyi-Nagy T, Washington K, Wetzel JD, Dermody TS. 2003. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J Clin Invest 111:1823–1833. 10.1172/JCI16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frierson JM, Pruijssers AJ, Konopka JL, Reiter DM, Abel TW, Stehle T, Dermody TS. 2012. Utilization of sialylated glycans as coreceptors enhances the neurovirulence of serotype 3 reovirus. J Virol 86:13164–13173. 10.1128/JVI.01822-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stencel-Baerenwald J, Reiss K, Blaum BS, Colvin D, Li XN, Abel T, Boyd K, Stehle T, Dermody TS. 2015. Glycan engagement dictates hydrocephalus induction by serotype 1 reovirus. mBio 6:e02356-14. 10.1128/mBio.02356-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441–451. 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 25.Campbell JA, Schelling P, Wetzel JD, Johnson EM, Forrest JC, Wilson GAR, Aurrand-Lions M, Imhof BA, Stehle T, Dermody TS. 2005. Junctional adhesion molecule A serves as a receptor for prototype and field-isolate strains of mammalian reovirus. J Virol 79:7967–7978. 10.1128/JVI.79.13.7967-7978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konopka-Anstadt JL, Mainou BA, Sutherland DM, Sekine Y, Strittmatter SM, Dermody TS. 2014. The Nogo receptor NgR1 mediates infection by mammalian reovirus. Cell Host Microbe 15:681–691. 10.1016/j.chom.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, Cate R, Strittmatter SM, Nikolov DB. 2003. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J 22:3291–3302. 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGee AW, Strittmatter SM. 2003. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci 26:193–198. 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 29.Barrette B, Vallieres N, Dube M, Lacroix S. 2007. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci 34:519–538. 10.1016/j.mcn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Barton ES, Connolly JL, Forrest JC, Chappell JD, Dermody TS. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J Biol Chem 276:2200–2211. 10.1074/jbc.M004680200. [DOI] [PubMed] [Google Scholar]
- 31.Richardson-Burns SM, Tyler KL. 2004. Regional differences in viral growth and central nervous system injury correlate with apoptosis. J Virol 78:5466–5475. 10.1128/jvi.78.10.5466-5475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forrest JC, Campbell JA, Schelling P, Stehle T, Dermody TS. 2003. Structure-function analysis of reovirus binding to junctional adhesion molecule 1. Implications for the mechanism of reovirus attachment. J Biol Chem 278:48434–48444. 10.1074/jbc.M305649200. [DOI] [PubMed] [Google Scholar]
- 33.Lai CM, Boehme KW, Pruijssers AJ, Parekh VV, Van Kaer L, Parkos CA, Dermody TS. 2015. Endothelial JAM-A promotes reovirus viremia and bloodstream dissemination. J Infect Dis 211:383–393. 10.1093/infdis/jiu476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, Dermody TS, Nusrat A, Parkos CA. 2007. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med 204:3067–3076. 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dichter MA, Weiner HL. 1984. Infection of neuronal cell cultures with reovirus mimics in vitro patterns of neurotropism. Ann Neurol 16:603–610. 10.1002/ana.410160512. [DOI] [PubMed] [Google Scholar]
- 36.Hastings A, Yockey L, Jagger B, Hwang J, Uraki R, Gaitsch H, Parnell L, Cao B, Mysorekar I, Rothlin C, Fikrig E, Diamond M, Iwasaki A. 2017. TAM receptors are not required for Zika virus infection in mice. Cell Rep 19:558–568. 10.1016/j.celrep.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorig RE, Marcil A, Chopra A, Richardson CD. 1993. The human Cd46 molecule is a receptor for measles-virus (Edmonston strain). Cell 75:295–305. 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi K, Nagata N, Kato SI, Ami Y, Suzaki Y, Suzuki T, Sato Y, Tsunetsugu-Yokota Y, Mori K, Van Nguyen N, Kimura H, Nagata K. 2012. Wild-type measles virus with the hemagglutinin protein of the Edmonston vaccine strain retains wild-type tropism in macaques. J Virol 86:3027–3037. 10.1128/JVI.06517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauren J, Hu F, Chin J, Liao J, Airaksinen MS, Strittmatter SM. 2007. Characterization of myelin ligand complexes with neuronal Nogo-66 receptor family members. J Biol Chem 282:5715–5725. 10.1074/jbc.M609797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Miao Y, Wicklein R, Sun Z, Wang J, Jude KM, Fernandes RA, Merrill SA, Wernig M, Garcia KC, Südhof TC. 2021. RTN4/NoGo-receptor binding to BAI adhesion-GPCRs regulates neuronal development. Cell 184:5869–5885.E25. 10.1016/j.cell.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laing ED, Navaratnarajah CK, Cheliout Da Silva S, Petzing SR, Xu Y, Sterling SL, Marsh GA, Wang LF, Amaya M, Nikolov DB, Cattaneo R, Broder CC, Xu K. 2019. Structural and functional analyses reveal promiscuous and species specific use of ephrin receptors by Cedar virus. Proc Natl Acad Sci USA 116:20707–20715. 10.1073/pnas.1911773116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guidotti LG, Matzke B, Schaller H, Chisari FV. 1995. High-level hepatitis B virus replication in transgenic mice. J Virol 69:6158–6169. 10.1128/JVI.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, Meisel M, Kim SM, Discepolo V, Pruijssers AJ, Ernest JD, Iskarpatyoti JA, Costes LM, Lawrence I, Palanski BA, Varma M, Zurenski MA, Khomandiak S, McAllister N, Aravamudhan P, Boehme KW, Hu F, Samsom JN, Reinecker HC, Kupfer SS, Guandalini S, Semrad CE, Abadie V, Khosla C, Barreiro LB, Xavier RJ, Ng A, Dermody TS, Jabri B. 2017. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356:44–50. 10.1126/science.aah5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakrabarty R, Tran H, Selvaggi G, Hagerman A, Thompson B, Coffey M. 2015. The oncolytic virus, Pelareorep, as a novel anticancer agent: a review. Invest New Drugs 33:761–774. 10.1007/s10637-015-0216-8. [DOI] [PubMed] [Google Scholar]
- 45.Furlong DB, Nibert ML, Fields BN. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol 62:246–256. 10.1128/JVI.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virgin HW, Bassel-Duby R, Fields BN, Tyler KL. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol 62:4594–4604. 10.1128/JVI.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith RE, Zweerink HJ, Joklik WK. 1969. Polypeptide components of virions, top component, and cores of reovirus type 3. Virology 39:791–810. 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- 48.Cera MR, Del Prete A, Vecchi A, Corada M, Martin-Padura I, Motoike T, Tonetti P, Bazzoni G, Vermi W, Gentili F, Bernasconi S, Sato TN, Mantovani A, Dejana E. 2004. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J Clin Invest 114:729–738. 10.1172/JCI21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JE, Liu BP, Park JH, Strittmatter SM. 2004. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 44:439–451. 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. 2000. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci 113:2363–2374. 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 51.Wetzel JD, Chappell JD, Fogo AB, Dermody TS. 1997. Efficiency of viral entry determines the capacity of murine erythroleukemia cells to support persistent infections by mammalian reoviruses. J Virol 71:299–306. 10.1128/JVI.71.1.299-306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He XL, Bazan JF, McDermott G, Park JB, Wang K, Tessier-Lavigne M, He Z, Garcia KC. 2003. Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron 38:177–185. 10.1016/s0896-6273(03)00232-0. [DOI] [PubMed] [Google Scholar]
- 53.Pronker MF, Tas RP, Vlieg HC, Janssen BJC. 2017. Nogo receptor crystal structures with a native disulfide pattern suggest a novel mode of self-interaction. Acta Crystallogr D Struct Biol 73:860–876. 10.1107/S2059798317013791. [DOI] [PubMed] [Google Scholar]
- 54.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]