ABSTRACT
Hadal snailfishes are the deepest-living fishes in the ocean, inhabiting trenches from depths of ∼6,000 to 8,000 m. While the microbial communities in trench environments have begun to be characterized, the microbes associated with hadal megafauna remain relatively unknown. Here, we describe the gut microbiomes of two hadal snailfishes, Pseudoliparis swirei (Mariana Trench) and Notoliparis kermadecensis (Kermadec Trench), using 16S rRNA gene amplicon sequencing. We contextualize these microbiomes with comparisons to the abyssal macrourid Coryphaenoides yaquinae and the continental shelf-dwelling snailfish Careproctus melanurus. The microbial communities of the hadal snailfishes were distinct from their shallower counterparts and were dominated by the same sequences related to the Mycoplasmataceae and Desulfovibrionaceae. These shared taxa indicate that symbiont lineages have remained similar to the ancestral symbiont since their geographic separation or that they are dispersed between geographically distant trenches and subsequently colonize specific hosts. The abyssal and hadal fishes contained sequences related to known, cultured piezophiles, microbes that grow optimally under high hydrostatic pressure, including Psychromonas, Moritella, and Shewanella. These taxa are adept at colonizing nutrient-rich environments present in the deep ocean, such as on particles and in the guts of hosts, and we hypothesize they could make a dietary contribution to deep-sea fishes by degrading chitin and producing fatty acids. We characterize the gut microbiota within some of the deepest fishes to provide new insight into the diversity and distribution of host-associated microbial taxa and the potential of these animals, and the microbes they harbor, for understanding adaptation to deep-sea habitats.
IMPORTANCE Hadal trenches, characterized by high hydrostatic pressures and low temperatures, are one of the most extreme environments on our planet. By examining the microbiome of abyssal and hadal fishes, we provide insight into the diversity and distribution of host-associated life at great depth. Our findings show that there are similar microbial populations in fishes geographically separated by thousands of miles, reflecting strong selection for specific microbial lineages. Only a few psychropiezophilic taxa, which do not reflect the diversity of microbial life at great depth, have been successfully isolated in the laboratory. Our examination of deep-sea fish microbiomes shows that typical high-pressure culturing methodologies, which have largely remained unchanged since the pioneering work of Claude ZoBell in the 1950s, may simulate the chemical environment found in animal guts and helps explain why the same deep-sea genera are consistently isolated.
KEYWORDS: hadal, piezophile, snailfish, trench
INTRODUCTION
The gut microbiome plays an essential role in the physiology of fishes. Microbiota within fishes can help digest food by producing degradative enzymes, provide the host with vitamins and fatty acids, and competitively exclude pathogens (1–4). While the importance of gut microbiomes is recognized, few studies have explored the structure and function of microbiomes in deep-sea fishes. Cultivation of microorganisms from deep-sea animals has revealed the presence of piezophiles (5–8), microbes capable of optimal growth under in situ, deep-sea high hydrostatic pressure conditions. This includes members of the genera Colwellia, Psychromonas, Shewanella, Moritella, and Photobacterium, some of the only lineages which have been experimentally demonstrated in the laboratory to be piezophilic (9, 10). These microbes represent a small fraction of the broader water and sediment communities in the deep ocean, which are instead composed primarily of members of the Thaumarchaeota, Marinimicrobia (SAR406), and other members of the Proteobacteria (11–14). However, a description of the complete breadth of microbial diversity within the guts of deep-sea fishes is lacking.
Distinct fish communities have evolved to life in the deep sea, with pronounced compositional changes within different depth zones (15). The abyssal ocean (depths 4,000 to 6,000 m) is home to several major fish families with cosmopolitan distributions, including the rattails (Macrouridae), cusk eels (Ophidiidae), eelpouts (Zoarcidae), cutthroat eels (Synaphobranchidae), and tripodfishes (Ipnopidae). Rattails are attracted to bait and therefore have been the focus of much of the deep-sea demersal fish literature. Members of the rattail genus Coryphaenoides, which includes Coryphaenoides yaquinae Iwamoto and Stein 1974 (16) and Coryphaenoides armatus Hector 1875 (17), are among the most widespread fishes in abyssal ecosystems (18). Coryphaenoides species are known scavengers (19–22), and their predominant food source is deep-sea carrion, although stomach contents and stable isotope analyses show that rattails also feed on fishes, squid, and crustaceans (18). Culture-based analyses of the microbiota associated with Coryphaenoides have found piezophilic members related to the lineages Moritella and Shewanella (5, 6). However, whether these lineages are representative of the entire microbiota within the gut of Coryphaenoides, one of the most widespread fishes in the ocean, is unknown.
In hadal trenches, sites deeper than 6,000 m, which are typically formed at subduction zones, the fish community differs from that of the surrounding abyssal plain. Snailfishes (family Liparidae) are the dominant fishes below 6,000 m, with at least 12 species found in nine trenches worldwide (23). The Liparidae include the planet’s deepest-dwelling known vertebrates, such as Pseudoliparis swirei Gerringer & Linley 2017 (24) (depth range, 6,198 to 8,078 m) and Notoliparis kermadecensis Nielsen 1964 (24, 25, 26) (depth range, 5,879 to 7,669 m). Many hadal snailfish species have been found in only one trench and are likely endemic, confined to one specific hadal environment (23, 25–27). These fishes have evolved adaptations to high pressure, including intrinsic enzyme adaptations (28, 29) and the accumulation of protein-stabilizing osmolytes such as trimethylamine n-oxide (TMAO) (30, 31). No fishes have been found deeper than ∼8,200 m, a putative physiological depth limit for vertebrates arising from the osmotic constraints of this TMAO pressure adaptation strategy. Stomach contents, stable isotope analyses, and observed feeding behavior indicate that snailfishes are one of the top predators at hadal depths, consuming highly abundant amphipods in trench habitats (26, 32, 33). Recently, a member of the Mycoplasmataceae was identified in Pseudoliparis swirei that may provide the host with riboflavin (34). However, it is unclear how differences in fish species, diet, and environmental conditions may influence the composition of gut microbiomes of abyssal and hadal fishes or how these microbial associates impact the physiology of the host.
Here, we describe the gut microbiota of four representative, ecologically important deep-sea fishes using 16S rRNA gene amplicon sequencing. This includes two of the deepest-living hadal snailfishes, Pseudoliparis swirei from the Mariana Trench and Notoliparis kermadecensis from the Kermadec Trench. These fishes and the trenches they inhabit are geographically separated, residing approximately 6,000 km apart within the Pacific Ocean. The Mariana Trench is located in the Northern Hemisphere and extends to a depth in excess of 10,900 m (35). The Kermadec Trench is in the Southern Hemisphere off the coast of New Zealand and reaches a depth exceeding 10,000 m (36). We compared the microbiota of the snailfishes with two shallower-dwelling fishes, the abyssal macrourid Coryphaenoides yaquinae, which inhabits depths of ∼3,000 to 7,000 m (26), and Careproctus melanurus Gilbert 1892 (37), a demersal snailfish typically found at depths of 200 to 1,600 m (38). Our findings inform new understanding of host-symbiont interactions in the abyssal and hadal ocean, the ecology of piezophilic microbes, and the biology of the planet’s deepest-living vertebrates.
RESULTS
The gut microbial communities within snailfish from the Mariana Trench (Pseudoliparis swirei; n = 18, collection depths of 6,898 to 7,966 m) and Kermadec Trench (Notoliparis kermadecensis; n = 7, collection depths of 6,456 to 7,515 m) were compared against those in a continental shelf-dwelling snailfish (Careproctus melanurus; n = 11, collection depths of 381 to 834 m) and an abyssal rattail (Coryphaenoides yaquinae; n = 4, collection depths of 4,441 to 6,081 m) (Fig. 1; see also Table S1 in the supplemental material). We identified a total of 2,034 amplified sequence variants (ASVs) across these four species with final amplicon libraries ranging from 2,545 to 106,059 reads per sample (average of ∼46,500 reads per sample).
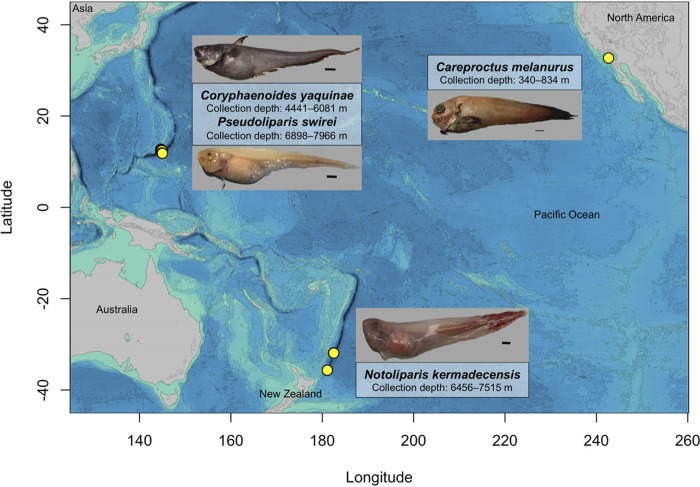
FIG 1.
Map of the Pacific Ocean showing the locations and depths of collection of the four fish species described in this study. (a) Pseudoliparis swirei (n = 18), Mariana Trench, 7,626 m, no. 200133; scale bar, 1 cm. (b) Notoliparis kermadecensis (n = 7), Kermadec Trench, 7,515 m, no. 100171; scale bar, 1 cm. (c) Coryphaenoides yaquinae (n = 4), abyssal plain, 5,255 m, no. 200152; scale bar, 5 cm. (d) Careproctus melanurus (n = 11), continental slope, representative image; scale bar, 1 cm.
Sample information for each specimen described in this study. Download Table S1, XLSX file, 0.01 MB (14.8KB, xlsx) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Fish microbiome comparative analyses.
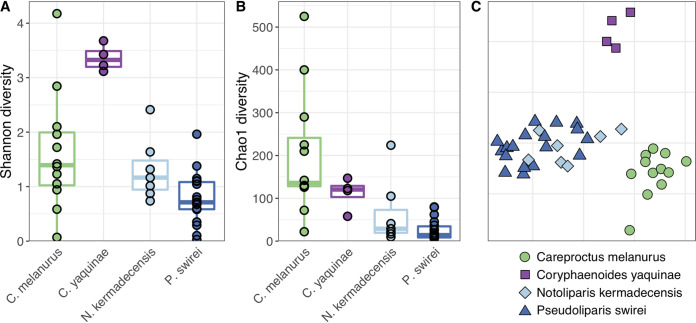
Gut microbial communities were distinct between the hadal snailfishes, the rattail C. yaquinae, and the slope-dwelling C. melanurus. The hadal fishes had lower alpha diversity than the shallower fishes, with the gut microbiome of C. yaquinae appearing more even (Fig. 2 and Fig. S1). Nonmetric multidimensional scaling (NMDS) ordination analysis of Bray-Curtis dissimilarity demonstrated that microbial gut communities of each fish species were distinct from one another, where species type accounted for 37% of the variability (Fig. 2C; permutational analysis of variance [PERMANOVA], R2 = 0.37, F = 7.22, df = 3, P < 0.001). Pairwise comparisons showed that while the microbiome of Pseudoliparis swirei differed from that in Notoliparis kermadecensis (R2 = 0.09, P < 0.013, F = 2.52), these differences were small compared to those in the other fishes. For further context, the gut microbiomes of the four species of interest were compared to those from a diverse collection of fishes. This data set included 16 marine fish hosts (Iacuaniello CM, Blanton JM, Allen EE, unpublished data) spanning a range of depths (all shallower than 1,000 m) and feeding strategies (15, 39). The abyssal and hadal microbial communities were also distinct from those within the broader fish gut data set, while bathydemersal Careproctus melanurus gut communities were interspersed with samples from other shallower fishes (Fig. S2; species type, R2 = 0.46, F = 4.25, df = 16, P < 0.001).
FIG 2.
Alpha (A, Shannon; B, Chao1) and beta (C, NMDS ordination based on Bray-Curtis dissimilarity; stress = 0.12) diversity comparisons of the four fishes in this study show that their gut microbiomes are unique. Snailfishes from the continental slope (Careproctus melanurus) and hadal trenches (Notoliparis kermadecensis and Pseudoliparis swirei) are compared to an abyssal rattail (Coryphaenoides yaquinae). Colors are the same in all panels, with each species in panel C also reflected by a different shape.
Chao1 (A) and Shannon (B) alpha diversity of the different fishes sequenced in this study, including the wider dataset. The diversity of the wider dataset will be described elsewhere. Download FIG S1, TIF file, 1.0 MB (1MB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
NMDS ordination based on Bray-Curtis beta diversity of the four study fishes and a wider dataset as described in the text (stress = 20.7). The diversity of the wider dataset will be described elsewhere, and those samples are shown as empty circles. Download FIG S2, TIF file, 0.3 MB (336.3KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The hadal snailfish microbiome.
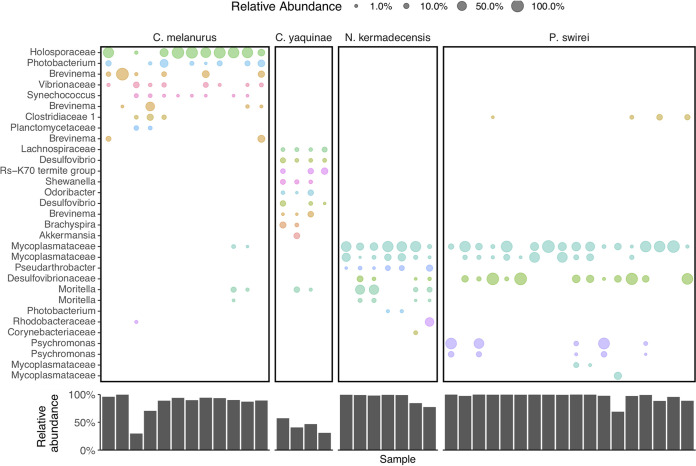
The microbiome of Notoliparis kermadecensis was dominated by only a few ASVs such that the top 10 most abundant sequences made up more than 75% of the communities of each fish (Fig. 3). Two of the most abundant ASVs were related to the Mycoplasmataceae and composed ∼60% (range, ∼5 to 99%) of the gut community. One of these Mycoplasmataceae ASVs was identical to the 16S rRNA gene reported from a hadal fish from the Mariana Trench (34) and was distantly related (<95% similar) to sequences found within other fishes (40, 41, 42) (Fig. S3). The second ASV was also similar to those found in other cold-water fish, including notothenioids from Antarctica (43) and grayling from Siberia (44). Two sequences related to Moritella together made up ∼19% of each community (range, ∼0 to 60%) and were more than 97% similar to 16S rRNA genes from both piezophilic (45) and nonpiezophilic taxa. An ASV related to the Desulfovibrionaceae was present in all seven N. kermadecensis specimens (mean, ∼3%; range, ∼0.005 to 14%). This ASV was more than 97% similar to sequences from notothenioid fish from Antarctic waters (43) and freshwater grayling from Lake Baikal but less than 95% similar to other sequences (Fig. S4). We also identified an ASV related to the Rhodobacteraceae (range, ∼0 to 43%) that was present in one sample in high abundance and was identical to sequences from the deep ocean, including the Japan Trench at 7,000 m (46). Other abundant ASVs included those classified as members of the Pseudarthrobacter (Micrococcaceae; mean, ∼5%; range, ∼0 to 17%; identified in every specimen), Corynebacteriaceae (mean, ∼0.5%; range, ∼0 to 3%), and Photobacterium (average, ∼0.5%; range, ∼0 to 1.5%).
FIG 3.
(Top) The most abundant ASVs present within each fish species, colored and labeled by their lowest identifiable taxonomic rank. ASVs are shown only if they reach relative abundances greater than 0.5% in a given sample. (Bottom) The total, summed relative abundance of the taxa shown above within each sample.
Phylogenetic tree of Mycoplasmataceae ASVs within the Mariana and Kermadec snailfish. The sequences from this study are shown in blue. Download FIG S3, TIF file, 0.9 MB (896.1KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogenetic tree of an ASV related to the Desulfovibrionaceae found in both the Mariana and Kermadec snailfish. The sequence from this study is shown in blue. Download FIG S4, TIF file, 0.9 MB (942.1KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Like N. kermadecensis, the microbiome of Pseudoliparis swirei was primarily composed of only a few taxa (Fig. 3). Members of the Mycoplasmataceae were some of the most abundant (combined mean abundance of four ASVs, 53%; range, ∼2 to 99%). Two of these Mycoplasmataceae ASVs were the same as those present in N. kermadecensis. A third ASV was present in only one fish but made up ∼27% of that community. The fourth Mycoplasmataceae ASV, closely related to sequences from stone flounder and turbot, was detected in five P. swirei specimens with a mean abundance of ∼0.5% (range, ∼0 to 9%). The Desulfovibrionaceae ASV found in N. kermadecensis was also present at high abundances in P. swirei (mean, ∼27%; range, ∼0 to 98%). Other taxa included two ASVs related to the genus Psychromonas (combined mean abundance, ∼15%; range, ∼0 to 93%). These sequences were similar to known piezophilic microbes obtained from deep-sea amphipod material (Fig. S5) (7, 47, 48).
Phylogenetic tree of two Psychromonas ASVs abundant in P. swirei. Sequences in this study are shown in blue, while those in black boldface are either known piezophiles or obtained from abyssal or hadal environments. Download FIG S5, TIF file, 0.7 MB (716.3KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
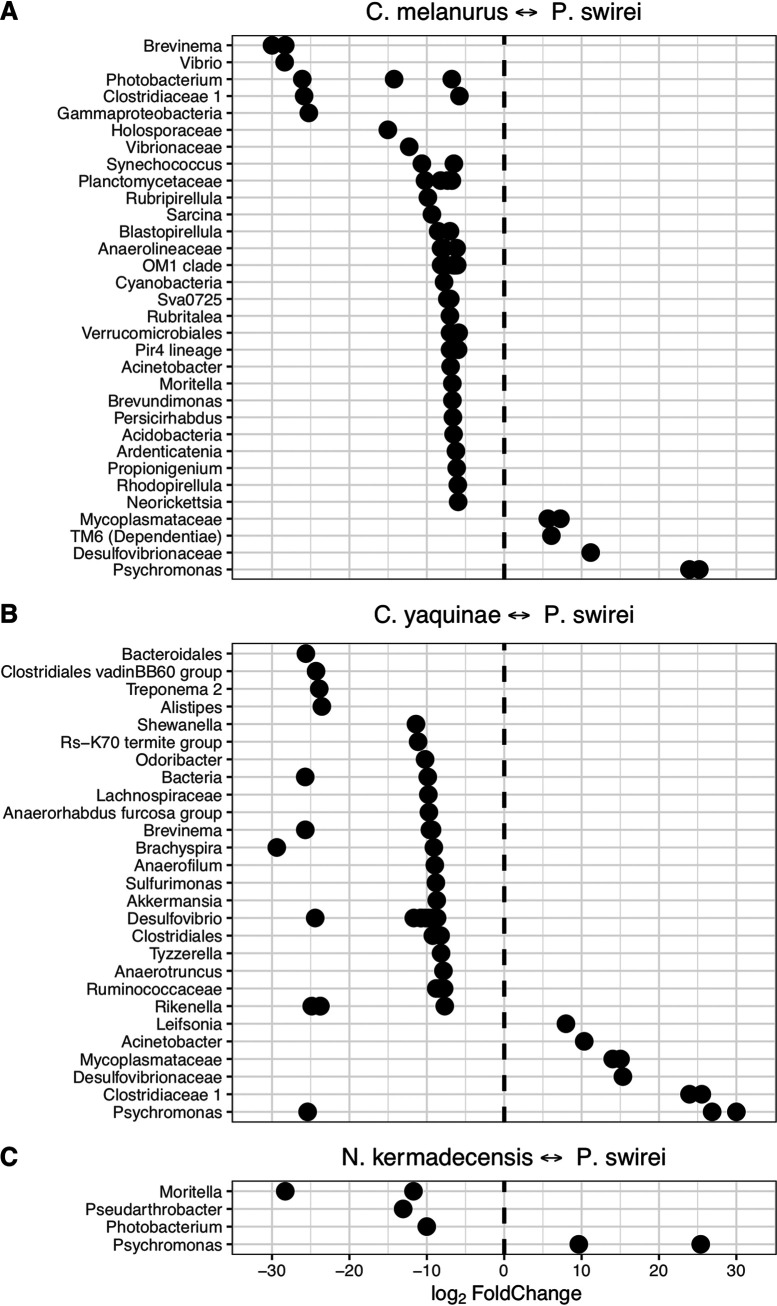
We compared the microbial communities in the two hadal fishes against one another. Psychromonas was more abundant in the Mariana snailfish, while Moritella, Pseudarthrobacter, and Photobacterium were enriched in the Kermadec snailfish (Fig. 4C). Sequences related to the Mycoplasmataceae and Desulfovibrionaceae were not differentially enriched within either fish.
FIG 4.
ASVs identified as differentially abundant when comparing fish species against one other. Communities from the Mariana snailfish, Pseudoliparis swirei, compared to (A) the snailfish Careproctus melanurus from the continental slope, (B) the abyssal rattail Coryphaenoides yaquinae, and (C) a hadal snailfish from the Kermadec Trench, Notoliparis kermadecensis. ASVs are labeled based on their lowest identifiable taxonomic rank.
Bathyal, abyssal, and hadal fish gut microbiome comparisons.
We also analyzed the microbiota of the shallower-living snailfish Careproctus melanurus collected from ∼300- to 800-m depth. Although this fish had higher alpha diversity than the hadal snailfishes (Fig. 2), the gut-associated microbial community was still dominated by only a few sequences (Fig. 3). The most abundant ASV was related to the Holosporaceae (mean, ∼50%; range, ∼0 to 92%) and showed >97% sequence similarity to taxa identified within other host-associated systems, including marine shrimp (49). Other ASVs included three relatives of the genus Brevinema (combined mean abundance, ∼20%; range, ∼0 to 100%), which were similar to those found in graylings from Lake Baikal, mudsuckers, and unicornfish. We note the presence of two ASVs related to the genera Moritella (combined mean, ∼1%; range, 0 to 11%) and Photobacterium (combined mean, ∼7%; range, ∼0 to 33%). These ASVs were similar (>99%) to both known piezophilic and piezosensitive species. Other abundant ASVs included taxa in the Vibrionaceae (mean, ∼3%; range, ∼0 to 13%), Clostridiaceae (mean, ∼2%; range, ∼0 to 17%), and Synechococcus (mean, ∼1%; range, ∼0.005 to 4%). The Clostridiaceae ASV was present in both N. kermadecensis and P. swirei at low abundances.
When comparing C. melanurus against P. swirei, many ASVs were more abundant in the continental slope-dwelling fish, reflecting the overall lower alpha diversity of the hadal snailfish (Fig. 4). Among the Gammaproteobacteria, sequences related to the genera Vibrio, Moritella, and Photobacterium were more abundant in C. melanurus, while Psychromonas was more abundant in P. swirei. Other taxa of note included the enrichment of Synechococcus and other Cyanobacteria within the shallower fish and the enrichment of ASVs related to the Mycoplasmataceae, Desulfovibrionaceae, and the phylum TM6 within P. swirei. The sequence belonging to the phylum TM6 was similar to those collected from deep-ocean sediments (50, 51). Comparisons between C. melanurus and N. kermadecensis revealed trends in differentially abundant taxa similar to those with P. swirei.
In contrast to the snailfishes, microbial community composition within the gut of the rattail Coryphaenoides yaquinae, collected from 4,000- to 6,000-m depth, was much more even. The 10 most abundant ASVs represented 31 to 57% of the community (Fig. 3). Four of the top 10 most abundant ASVs, related to the Desulfovibrio, Deltaproteobacteria group Rs-K70, Brachyspira, and family Lachnospiraceae (combined mean, ∼22%; range 6 to 37%), were most closely related to sequences from various host-associated and low-oxygen environments (e.g., see references 52, 53, and 54). A further four ASVs had highest identity to sequences from fish samples and were classified as belonging to the genera Akkermansia, Brevinema, Desulfovibrio, and Odoribacter (combined mean, ∼15%; range, 3 to 27%). We also identified sequences similar to Shewanella (mean, ∼4%; range, ∼0.2 to 8%) and Moritella (mean, ∼3.5%; range, ∼0.3 to 12%) in all Coryphaenoides yaquinae specimens. The ASV related to Shewanella was most similar to the piezophiles S. benthica KT99 and S. violacea (55, 56) and to sequences previously identified from Coryphaenoides yaquinae (Fig. S6) (6). The Moritella ASV was the same as that within N. kermadecensis and C. melanurus and was highly similar (>99%) to both piezophilic and piezosensitive strains (45). Because the communities of C. yaquinae and P. swirei were so distinct from one another, comparisons between the two fishes showed that many of the differentially abundant taxa were also the dominant members of the respective communities (Fig. 4).
Phylogenetic tree of an ASV related to Shewanella present within Coryphaenoides yaquinae. Sequences in this study are shown in blue, while those in black boldface are known piezophiles. Download FIG S6, TIF file, 0.8 MB (873.7KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
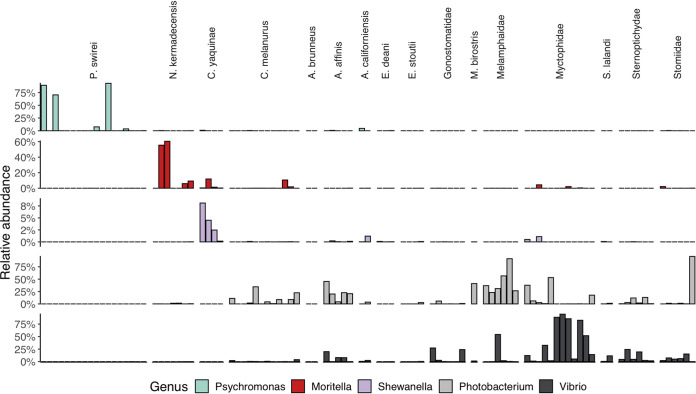
Finally, we leveraged a broader data set of fishes and environmental samples to investigate microbial lineages specific to the hadal fishes. We first screened these samples for specific ASVs that were abundant in the hadal fishes, including those related to the Mycoplasmataceae, Desulfovibrionaceae, Psychromonas, Moritella, and Shewanella. While these lineages dominated the hadal samples, they were not found at high abundances in any other fish (Fig. S7). These ASVs represented a miniscule fraction of Mariana Trench sediment and water samples, reflecting on average only 0.007% of the community (identified in 4 of 16 samples; maximum abundance, 0.036%). We broadened our search to include any ASV related to the Mycoplasmataceae and found that many of the shallower fish gut microbiomes contained this family (Fig. S8). While we did not find high abundances of sequences related to known piezophilic lineages in the comparison fishes, we found that almost all of the shallower-living fish gut communities included Photobacterium and Vibrio (Fig. 5).
FIG 5.
Abundances of gammaproteobacterial genera known to contain cultured piezophilic and/or piezosensitive members within the four comparison species and a wider data set of fishes. Top row, Psychromonas; second row, Moritella; third row; Shewanella; fourth row, Photobacterium; bottom row; Vibrio.
Presence of seven hadal-abundant ASVs within the four comparison species and a wider dataset of fishes. ASVs are labeled based on their lowest identifiable taxonomic rank. Download FIG S7, TIF file, 1.0 MB (1MB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances of ASVs related to the family Mycoplasmataceae within a broad dataset of fish species. Download FIG S8, TIF file, 1.0 MB (1.1MB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
We describe the gut-associated microbial communities within the hadal snailfishes Pseudoliparis swirei and Notoliparis kermadecensis, the abyssal rattail Coryphaenoides yaquinae, and the continental slope-dwelling snailfish Careproctus melanurus. These fishes include some of the dominant vertebrates at abyssal and hadal depths. The microbial communities within these four species were distinct from one another. Our findings show that while the shallow and deep-water snailfishes belong to the same family, they have large differences in their gut microbiota. The communities were also different between the abyssal and hadal teleosts, indicating fishes that experience similar environmental conditions at great depth do not necessarily have similar microbial gut flora. In contrast, many of the most abundant lineages were shared between both hadal snailfishes. It has been proposed that Pseudoliparis and Notoliparis should be synonymized as one genus (24, 57), suggesting these fish have similar host physicochemical variables that could influence their microbiome (e.g., pH and O2) (58, 59, 60). One explanation for the observed differences in the fish gut microbiomes could be diet. Host trophic strategy influences the diversity of microbial communities within the gut (59 to ,62) and may be one reason for the shift in fishes at the abyssal-hadal boundary (63). Hadal snailfishes primarily eat amphipods and occasionally polychaetes and decapod shrimp, reflecting a restrictive diet. The diet of Careproctus melanurus also consists of crustaceans, including amphipods, shrimp, mysids, and tanaids, but can also include bivalves, polychaetes, and fish (64, 65). In contrast, the diet of C. yaquinae is composed primarily of carrion, along with squid, crustaceans, and other fish (18, 33). The relatively narrow dietary choices of hadal fishes may shape their gut microbiomes in relation to shallower fishes. Future work should investigate how host physiology, diet, and environmental factors, such as differences in water mass or organic matter input (32, 66, 67), impact deep-sea fish gut microbiota.
The hadal fish gut microbiomes were composed of only a few ASVs and largely dominated by members of the Mycoplasmataceae, a family common in the digestive tracts of fishes (41, 68). While Mycoplasma can be pathogenic (69–71), it was recently suggested that Mycoplasmataceae in P. swirei supply the host with the cofactor riboflavin (34). Based on our analyses, there are multiple strains of Mycoplasmataceae present within hadal snailfishes. This family was present in nearly all fishes in the broader data set but was not as abundant in our two comparison species. Instead, C. melanurus appears to have high abundances of the Holosporaceae, a group known to infect shrimp and ciliates (49, 72). We show that known host-associated, potentially pathogenic lineages such as Mycoplasmataceae are common in fishes from the surface ocean to hadal depths, although with apparent differences at the ASV level. The Mycoplasmataceae represent interesting targets for identifying adaptations to high pressure because of their exceptionally reduced genome sizes and limited metabolic functionality (73).
Trenches are typically isolated by large expanses where seafloor depths are shallower than 6,000 m. If we assume that hadal species are obligately adapted to in situ pressures, trenches would have high rates of biogeographic isolation. Indeed, many megafaunal species found in trenches appear to be endemic (32, 74, 75), including hadal snailfishes, which appear genetically isolated from one another (24). Despite the geographic and genetic separation of the hosts, several identical ASVs were found within both hadal snailfishes, including those related to the Mycoplasmataceae and Desulfovibrionaceae. Neither the Mycoplasmatacaeae nor Desulfovibrionaceae sequences were present in high abundances in any of the other fishes analyzed in this study. One explanation is that the extant Mycoplasmataceae have not undergone appreciable genomic evolution to diverge from the ancestral symbiont present within snailfishes prior to their radiation into separate trenches approximately 20 to 40 mya (57). An alternative explanation is the dispersal of very closely related microbial taxa between two trenches 6,000 km apart and subsequent host selection for these lineages. Certain microbial symbionts are highly specific within deep-sea anglerfishes and may be dispersed horizontally through the water column (76, 77). The possibility of dispersal of water and sediment microorganisms between trenches has been previously highlighted (12, 13, 78). Whole-genome sequencing, e.g., metagenome-assembled genomes, will be required to determine if these strains are similar beyond their 16S rRNA gene and to understand their dispersal and evolution in hadal habitats.
We show that abyssal and hadal fishes have high abundances of sequences related to known piezophilic taxa, including Psychromonas, Moritella, and Shewanella. This finding is consistent with the observation that guts of deep-sea animals can show high levels of piezophily (5, 79–81) and that piezophiles are most successfully cultivated from deep-sea hosts (6–8, 55, 82, 83). This is in contrast to hadal water and sediment communities where sequences associated with previously cultured piezophiles represent relative abundances of less than 1% (11–13, 84). We therefore add to a growing body of evidence that known, isolated piezophilic genera are associated with deep-sea animals. However, different piezophilic lineages were abundant in each species of fish: Psychromonas in P. swirei, Moritella in N. kermadecensis, and Shewanella in C. yaquinae. None of the Mariana snailfish reported in a different study had high abundances of Psychromonas (n = 2) (34). One hypothesis is that these piezophilic taxa represent more transient members of the fish gut, for example, acquired through the consumption of amphipods (e.g., Psychromonas) (48, 85, 86, 87). If there was a strong signal from transient taxa, we might expect to see Pseudoalteromonas or Psychrobacter, which can reach abundances of >20% of the gut-associated microbiota of amphipods in the Mariana Trench (86, 87), within the guts of amphipod-feeding hadal fish. However, we did not find these genera in appreciable abundances in any of the abyssal or hadal fish (maximum of 0.13% for Pseudoalteromonas and 0.36% for Psychrobacter). It is therefore likely that the piezophilic microbes are present at least in part because of host-microbe specificity. Indeed, piezophilic Colwellia with >99% average genomic nucleotide identity have been isolated from deep-sea amphipods collected over 30 years apart (88), highlighting strong selection temporally in some hadal organisms. Representative piezophiles can contain genes encoding chitinase, including isolates belonging to Psychromonas, Moritella, Shewanella, and Colwellia (56, 83, 88, 89), and sediments amended with chitin also showed a response of known piezophilic taxa (90). Moreover, a recent metagenomic analysis of salmonid fishes revealed gut-associated Mycoplasma harbor genes putatively involved in the degradation of long-chain polymers such as chitin (91). Amendments of fish guts with chitin, coupled to metagenomic and metatranscriptomic sequencing, may reveal catabolic functions that benefit the host via the processing of recalcitrant dietary compounds.
One unifying characteristic of these piezophilic Gammaproteobacteria is the synthesis of long-chain omega-3 polyunsaturated fatty acids (LC-PUFAs), with Psychromonas, Moritella, and Colwellia species producing docosahexaenoic acid (DHA; 22:6n-3) and Shewanella species producing eicosapentaenoic acid (EPA; 20:5n-3) (92). LC-PUFAs are essential fatty acids required for proper development and growth of all metazoans, yet most vertebrates are unable to synthesize them de novo; thus, they need to be obtained from the diet. In shallow marine habitats, phytoplankton are the primary producers of LC-PUFAs; however, the quality and quantity of these essential fatty acids that reach abyssal and hadal zones is likely minimal. Thus, it is compelling to hypothesize that the enrichment of LC-PUFA producing taxa in hadal metazoan microbiomes represent the primary source for delivery of these essential fatty acid nutrients to their hosts.
While members of piezophilic genera were not present in the broader fish data set analyzed here, we found high abundances of the gammaproteobacterial genera Photobacterium and Vibrio (Fig. 5). Photobacterium are common within microbiomes of marine fishes (93–95) and can be moderate piezophiles, with some strains showing growth up to 70 MPa (96, 97). To our knowledge, no member of the genus Photobacterium has been isolated at in situ pressures from hadal depths. Although the Photobacterium ASVs in C. melanurus and N. kermadecensis are distinct, the high similarity of the 16S rRNA genes of piezophilic and nonpiezophilic ecotypes (98) precludes an analysis here of their putative pressure sensitivity. We present the hypothesis that there is a change in the dominant heterotrophic Gammaproteobacteria within the microbiomes of animals as a result of the selective pressure of increasing water depth. At shallower depths (e.g., 0 to 2,000 m), taxa such as Photobacterium and Vibrio may be abundant, but with increasing depth the gut community may shift toward hyperpiezophiles, including members of the genera Psychromonas, Moritella, and Shewanella. An analysis of fish gut microbial communities along a more comprehensive depth gradient, for example, targeting depths between 1,000 and 4,000 m, will be needed to assess this hypothesis.
The observation that representatives of known, isolated piezophilic taxa are abundant within deep-ocean animals reveals two important insights into the lack of high-pressure-adapted isolate diversity in the literature. First, nearly all attempts to isolate microbes from abyssal and hadal samples have used nutrient-rich media, which ultimately select for copiotrophic lineages. The gut of a host would similarly select for taxa capable of taking advantage of a high-nutrient environment, unlike the carbon-limited niches in deep-ocean water or sediments. Second, pressure vessels are generally static incubation chambers, requiring organisms to cope with variable waste, oxygen, and nutrient concentrations. Similar conditions might be expected in the guts of an abyssal or hadal fish undergoing various events of feast and starvation. Piezophilic taxa are capable of responding to variable environmental conditions. A nonexhaustive list includes enrichment of these groups on detritus (90), particles (99, 100), oil and dispersant (101–105), methane (106), low-oxygen conditions (107), in eukaryotic mesocosms (108), in pressure-retaining samplers (109), and in hadal sediments after long-term, static, and unamended conditions (13). Therefore, genera such as Psychromonas, Shewanella, Moritella, and Colwellia are likely isolated because of their ability to adapt to the variable nutrient and oxygen conditions found within the guts of deep-sea megafauna and the pressure vessels used for cultivation in the laboratory. The implications of this observation are that static mesocosms performed in the lab using current methods will almost always select for a distinct group of microorganisms that are not representative of environmental deep-sea communities at large but that nonetheless fill a specific niche in the deep ocean on particles and in the guts of megafauna.
In addition to clarifying the role of recognized, lab-characterized piezophilic lineages, the examination of microbial diversity associated with extreme deep-sea animals reveals new taxa that likely possess pressure-adapted lifestyles. These taxa represent broad phylogenetic groups that significantly extend the hyperpiezophile ranks beyond the Gammaproteobacteria, including Mycoplasmataceae, Desulfobacterota, and Actinobacteria (Pseudarthrobacter). For example, the presence of Desulfovibrionaceae ASVs within both abyssal and hadal fishes indicates the presence of sulfate reduction occurring within the guts of deep-ocean fishes at high hydrostatic pressure. Future studies that integrate metagenomic profiling combined with novel cultivation approaches that mimic the in vivo fish gut microbial ecosystem will be required to more fully define the breadth of metabolic activities that support the success of the microbes and the fish they inhabit within the deep sea.
MATERIALS AND METHODS
Sample collection.
Abyssal and hadal fishes were collected from the Kermadec and Mariana trenches aboard the R/V Thomas G. Thompson and R/V Falkor during April to May 2014 and November to December 2014, respectively. Fishes were caught using free-vehicle lander systems equipped with acoustic releases (26). The traps were baited with mackerel and squid wrapped in nylon mesh to limit bait ingestion by sampled taxa. Once fish specimens were on board they were immediately placed on ice and processed. Gut material was carefully extracted from the hindgut, flash-frozen in liquid nitrogen either dry or in RNA Later, and stored at −80°C. One Notoliparis specimen was reported as belonging to a different species, Notoliparis stewarti, based on morphological characteristics (NK100329) (110). Because of the similarity of these two potentially different species, their presentation as the same species in previous publications, and the apparent similarity of their microbiomes, we report this one specimen as N. kermadecensis throughout the manuscript but acknowledge future work is needed to fully characterize the taxonomy of hadal fishes. Specimens of Careproctus melanurus were collected by trawl aboard the F/V Noah’s Ark and F/V Last Straw during the summer 2014 NOAA NWFSC Groundfish Bottom Trawl Survey. One specimen was also collected from a 2015 UC Ship Funds-supported student cruise aboard the R/V Sproul. Following the storage of whole fishes at −20°C, specimens were defrosted and the hindgut dissected. Gut contents from C. melanurus were submerged in Chaos lysis buffer (5 M guanidine thiocyanate, 2% sarkosyl, 50 mM EDTA, 40 μg/mL proteinase K, and 15% beta-mercaptoethanol) and stored at −80°C prior to analysis. We acknowledge that these slightly different methods of sample processing and preservation, given the constraints of shipboard sample collection, may influence microbial community composition downstream.
DNA extraction and 16S rRNA gene amplicon sequencing.
DNA was extracted from gut samples using an organic extraction method. Intestinal contents were defrosted and resuspended in Chaos buffer. After a 30-min incubation at 55°C, samples were homogenized by bead-beating with silica beads. Lysate was then treated with one volume of phenol-chloroform-isoamyl alcohol (25:24:1). DNA in the resulting aqueous layer was cleaned with the Zymo Research Quick-gDNA MiniPrep kit (Irvine, CA). Negative-control extractions were performed with each set of fish samples.
After extraction, the V4 region (∼290 bp) of the 16S rRNA gene was amplified using a two-step PCR protocol to create dual-barcoded amplicons. The first reaction used primers 515F-Y and 806rb with overhangs for attachment of Illumina-compatible indexes in the second reaction (111). The initial reaction was performed in triplicate using Q5 polymerase (NEB, Ipswitch, MA) with the following steps: initial denaturation of 30 s at 98°C; 25 cycles of 10 s at 98°C, 20 s at 50°C, 30 s at 72°C; final extension of 2 min at 72°C. Triplicate reaction mixtures were combined, and 5 μL of each sample pool was used as the template in a second reaction to attach unique indexing primer pairs. The second reaction was performed as described above except using only 8 cycles and an annealing temperature of 56°C. Barcoded amplicons were cleaned using AMPure XP beads (Beckman Coulter, Brea, CA), pooled at equimolar concentrations, and sequenced on Illumina's MiSeq platform (2 × 300 bp) at the UC San Diego Institute for Genomic Medicine and the UC Davis Genome Center.
Sequence processing and analysis.
Paired raw reads were trimmed with Trimmomatic v0.35 (112) and filtered to sequences of ≥100 bp. Trimmed reads were imported into the QIIME 2 platform v2018.6 (113), where the Dada2 workflow plugin v2018.6 (114) was used to trim primer regions, denoise, and merge sequences to generate ASVs. Chimeras were removed using the consensus method. Nonribosomal sequences were excluded and taxonomy was assigned to ASVs using the scikit-learn naive Bayes machine-learning classifier (115) in QIIME 2 trained on the SILVA v128 small subunit database (116). Further filtering and all downstream analyses were performed in R (117). Singletons, sequences classified as eukaryotic, or those unassigned at the domain level were removed. To ensure a conservative analysis, potential contaminants were identified by cooccurrence network of all ASVs using the R package ccrepe v1.24.0 (118). Twelve ASVs that belonged to the genera Acinetobacter and Pseudomonas and the families Comamonadaceae, Caulobacteraceae, and Methylophilaceae were identified as both cooccurring and representative of common contaminants (119). These ASVs were filtered from all samples (see Fig. S9 in the supplemental material). Alpha and beta diversity of communities were estimated using the R package phyloseq v1.32.0 (120). Differentially abundant taxa between the different fishes were identified using DESeq2 v1.28.1 (121) with ASVs of less than 10 reads excluded. We statistically tested the importance of host species on structuring Bray-Curtis dissimilarity by permutational analysis of variance (PERMANOVA) using the adonis and pairwiseAdonis functions (122, 123). For phylogenetic analyses, representative 16S rRNA gene sequences were aligned using SINA Aligner (124) and trees built using FastTree using default settings (125). Trees were visualized using the Interactive Tree of Life (iTOL) (126).
Cooccurrence map highlighting clusters of taxa common in low-biomass samples, potentially representing contamination sequences. Analysis was performed using CCREPE, with 12 taxa identified and filtered from libraries before downstream analysis. Download FIG S9, TIF file, 0.4 MB (441.3KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
For further context, the gut microbiomes of the four fish of interest were compared to microbial data sets from a diverse collection of fishes from depths shallower than 1,000 m and water and sediments from the Mariana Trench. The comparative fish data set included catshark (Apristurus brunneus), hatchetfish (Argyropelecus affinis and other Sternoptichydae), smelt (Atherinopsis californiensis), hagfish (Eptatretus deani, Eptatretus stoutii), bristlemouths (Gonostomatidae), ridgehead (Melamphaidae), manta ray feces (Mobula birostris), lanternfish (Myctophidae), California yellowtail (Seriola lalandi), and dragonfishes (Stomiidae). These fishes were typically frozen at −20°C prior to hindgut dissection and then processed in the same manner as that described above. The complete microbial communities of these fish will be described elsewhere (Iacuniello et al., unpublished). The water (RG02, RG07, RG08, RG16, RG18; 3.0-, 0.2-, and 0.1-μm size-fractionated samples) and sediment samples (FVCR02, FVCR03, FVCR04; 0- to 1-cm depth fraction) were collected from depths exceeding 5,000 m in the Mariana Trench. A full description of their collection and extraction has been previously published (12, 13). PCR amplification and all further downstream analyses were performed as described above.
Data availability.
Raw sequencing data for the fish species in this study have been submitted to the NCBI Short Read Archive under BioProject no. PRJNA720542.
ACKNOWLEDGMENTS
We thank the crews of the R/V Falkor, R/V Thompson, R/V Sproul, F/V Noah’s Ark, and F/V Last Straw for help at sea. We are grateful to all members of the HADal Ecosystem Studies (HADES) team for scientific advice.
We express our appreciation for the financial support provided by the National Science Foundation (1130712 to J.C.D., 1536776 to D.H.B., MCB-114552, and OCE-1837116 to E.E.A.), the Schmidt Ocean Institute (cruise FK141109), and the Prince Albert II Foundation (Project 1265 to D.H.B.).
Contributor Information
Eric E. Allen, Email: eallen@ucsd.edu.
Barbara J. Campbell, Clemson University
REFERENCES
- 1.Llewellyn MS, Boutin S, Hossein HS, Derome N. 2014. Teleost microbiomes: the state of the art in their characterization, manipulation, and importance in aquaculture and fisheries. Front Microbiol 5:207. doi: 10.3389/fmicb.2014.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayak SK. 2010. Role of gastrointestinal microbiota in fish. Aquaculture Res 41:1553–1573. doi: 10.1111/j.1365-2109.2010.02546.x. [DOI] [Google Scholar]
- 3.Ray AK, Ghosh K, Ringø E. 2012. Enzyme-producing bacteria isolated from fish gut: a review. Aquaculture Nutr 18:465–492. doi: 10.1111/j.1365-2095.2012.00943.x. [DOI] [Google Scholar]
- 4.Tarnecki AM, Burgos FA, Ray CL, Arias CR. 2017. Fish intestinal microbiome: diversity and symbiosis unravelled by metagenomics. J Appl Microbiol 123:2–17. doi: 10.1111/jam.13415. [DOI] [PubMed] [Google Scholar]
- 5.Yano Y, Nakayama A, Yoshida K. 1995. Population sizes and growth pressure responses of intestinal microfloras of deep-sea fish retrieved from the abyssal zone. Appl Environ Microbiol 61:4480–4483. doi: 10.1128/aem.61.12.4480-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama A, Saito R, Matsuzaki M, Yano Y, Yoshida K. 2005. Phylogenetic analysis based on 16S rRNA gene sequences of deep-sea bacteria isolated from intestinal contents of deep-sea fishes retrieved from the abyssal zone. J Gen Appl Microbiol 51:385–394. doi: 10.2323/jgam.51.385. [DOI] [PubMed] [Google Scholar]
- 7.Yayanos AA, Dietz AS, Van Boxtel R. 1979. Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science 205:808–810. doi: 10.1126/science.205.4408.808. [DOI] [PubMed] [Google Scholar]
- 8.Kusube M, Kyaw TS, Tanikawa K, Chastain RA, Hardy KM, Cameron J, Bartlett DH. 2017. Colwellia marinimaniae sp. nov., a hyperpiezophilic species isolated from an amphipod within the Challenger Deep, Mariana Trench. Int J Sys Evol Microbiol 67:824–831. doi: 10.1099/ijsem.0.001671. [DOI] [PubMed] [Google Scholar]
- 9.Jebbar M, Franzetti B, Girard E, Oger P. 2015. Microbial diversity and adaptation to high hydrostatic pressure in deep-sea hydrothermal vents prokaryotes. Extremophiles 19:721–740. doi: 10.1007/s00792-015-0760-3. [DOI] [PubMed] [Google Scholar]
- 10.Nogi Y. 2017. Microbial life in the deep sea: psychropiezophiles. In Psychrophiles: from biodiversity to biotechnology, p 133–152. Springer, Cham, Switzerland. [Google Scholar]
- 11.Tarn J, Peoples LM, Hardy K, Cameron J, Bartlett DH. 2016. Identification of free-living and particle-associated microbial communities present in hadal regions of the Mariana Trench. Front Microbiol 7:665. doi: 10.3389/fmicb.2016.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peoples LM, Donaldson S, Osuntokun O, Xia Q, Nelson A, Blanton J, Allen EE, Church MJ, Bartlett DH. 2018. Vertically distinct microbial communities in the Mariana and Kermadec trenches. PLoS One 13:e0195102. doi: 10.1371/journal.pone.0195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peoples LM, Grammatopoulou E, Pombrol M, Xu X, Osuntokun O, Blanton JM, Allen EE, Nunnally CC, Drazen J, Mayor DJ, Bartlett DH. 2019. Microbial community diversity within sediments from two geographically separated hadal trenches. Front Microbiol 10:347. doi: 10.3389/fmicb.2019.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauberger C, Glud RN, Hausmann B, Trouch B, Maignien L, Poulain J, Wincker P, Arnaud-Haond S, Wenzhöfer F, Thamdrup B. 2021. Microbial community structure in hadal sediments: high similarity along trench axes and strong changes along redox gradients. ISME J 15:3455–3467. doi: 10.1038/s41396-021-01021-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priede IG. 2017. Deep-sea fishes: biology, diversity, ecology, and fisheries. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 16.Iwamoto T, Stein DL. 1974. Systematic review of the rattail fishes (Macrouridae: Gadiformes) from Oregon and adjacent waters. No. RLO-2227-T-12-35. California Academy of Sciences, San Francisco, CA. doi: 10.5962/bhl.part.15932. [DOI] [Google Scholar]
- 17.Hector J. 1875. Descriptions of five new species of fishes obtained in the New Zealand seas by H.M.S Challenger expedition, July 1874. Ann Magazine Nat Hist 15:78–82. doi: 10.1080/00222937508681027. [DOI] [Google Scholar]
- 18.Drazen JC, Popp BN, Choy CA, Clemente T, De Forest L, Smith JK. 2008. Bypassing the abyssal benthic food web: macrourid diet in the eastern Pacific inferred from stomach content and stable isotopes analyses. Limnol Oceanogr 53:2644–2654. doi: 10.4319/lo.2008.53.6.2644. [DOI] [Google Scholar]
- 19.Wilson RR, Jr, Waples RS. 1983. Distribution, morphology, and biochemical genetics of Coryphaenoides armatus and C. yaquinae (Pisces:Macrouridae) in the central and eastern North Pacific. Deep Sea Res Pt A 30:1127–1145. doi: 10.1016/0198-0149(83)90092-4. [DOI] [Google Scholar]
- 20.Wilson RR, Smith KL. 1984. Effect of near-bottom currents on detection of bait by the abyssal grenadier fishes Coryphaenoides spp., recorded in situ with a video camera on a free vehicle. Mar Biol 84:83–91. doi: 10.1007/BF00394530. [DOI] [Google Scholar]
- 21.Priede IG, Smith JK. 1986. Behaviour of the abyssal grenadier, Coryphaenoides yaquinae, monitored using ingestible acoustic transmitters in the Pacific Ocean. J Fish Biology 29:199–206. doi: 10.1111/j.1095-8649.1986.tb05011.x. [DOI] [Google Scholar]
- 22.Jamieson AJ, Priede IG, Craig J. 2012. Distinguishing between abyssal macrourids Coryphaenoides yaquinae and C. armatus from in situ photography. Deep Sea Res Pt I 64:78–85. doi: 10.1016/j.dsr.2012.02.001. [DOI] [Google Scholar]
- 23.Gerringer ME. 2019. On the success of the hadal snailfishes. Integr Org Biol 1:obz004. doi: 10.1093/iob/obz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerringer ME, Linley TD, Jamieson AJ, Goetze E, Drazen JC. 2017. Pseudoliparis swirei sp. Nov.: a newly-discovered hadal snailfish (Scorpaeniformes: Liparidae) from the Mariana Trench. Zootaxa 4358:161–177. doi: 10.11646/zootaxa.4358.1.7. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen JG. 1964. Fishes from depths exceeding 6000 meters. Galathea Rep 7:113–124. [Google Scholar]
- 26.Linley TD, Gerringer ME, Yancey PH, Drazen JC, Weinstock CL, Jamieson J. 2016. Fishes of the hadal zone including new species, in situ observations and depth records of Liparidae. Deep Sea Res Pt I 114:99–110. doi: 10.1016/j.dsr.2016.05.003. [DOI] [Google Scholar]
- 27.Fujii T, Jamieson AJ, Solan M, Bagley PM, Priede IG. 2010. A large aggregation of Liparids at 7703 meters and a reappraisal of the abundance and diversity of hadal fish. Bioscience 60:506–515. doi: 10.1525/bio.2010.60.7.6. [DOI] [Google Scholar]
- 28.Gerringer ME, Drazen JC, Yancey PH. 2017. Metabolic enzyme activities of abyssal and hadal fishes: pressure effects and a re-evaluation of depth-related changes. Deep Sea Res Pt I 125:135–146. doi: 10.1016/j.dsr.2017.05.010. [DOI] [Google Scholar]
- 29.Gerringer ME, Yancey PH, Tikhonova OV, Vavilov NE, Zgoda VG, Davydov DR. 2020. Pressure tolerance of deep-sea enzymes can be evolved through increasing volume changes in protein transitions: a study with lactate dehydrogenases from abyssal and hadal fishes. FEBS J 287:5394–5410. doi: 10.1111/febs.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamieson AJ, Yancey PH. 2012. On the validity of the Trieste Flatfish: dispelling the myth. Biol Bull 222:171–175. doi: 10.1086/BBLv222n3p171. [DOI] [PubMed] [Google Scholar]
- 31.Yancey PH, Gerringer ME, Drazen JC, Rowden AA, Jamieson A. 2014. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc Natl Acad Sci USA 111:4461–4465. doi: 10.1073/pnas.1322003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamieson A. 2015. The hadal zone: life in the deepest oceans. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 33.Gerringer ME, Popp BN, Linley TD, Jamieson AJ, Drazen JC. 2017. Comparative feeding ecology of abyssal and hadal fishes through stomach content and amino acid isotope analysis. Deep Sea Res Pt I 121:110–120. doi: 10.1016/j.dsr.2017.01.003. [DOI] [Google Scholar]
- 34.Lian CA, Yan GY, Huang JM, Danchin A, Wang Y, He LS. 2019. Genomic characterization of a novel gut symbiont from the hadal snailfish. Front Microbiol 10:2978. doi: 10.3389/fmicb.2019.02978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamieson AJ, Stewart HA. 2021. Hadal zones of the Northwest Pacific Ocean. Progress in Oceanography 190:102477. doi: 10.1016/j.pocean.2020.102477. [DOI] [Google Scholar]
- 36.Angel MV. 1982. Ocean trench conservation. Environmentalist 2:1–17. doi: 10.1007/BF02340472. [DOI] [Google Scholar]
- 37.Gilbert CH. 1892. Descriptions of thirty-four new species of fishes collected in 1889, principally among the Santa Barbara Islands and in the Gulf of California. Proc US Natl Museum 14:539–566. doi: 10.5479/si.00963801.14-880.539. [DOI] [Google Scholar]
- 38.Chernova NV, Stein DL, Andriashev AP. 2004. Family Liparidae Scopoli 1777. Calif Acad Sci Annot Checklists Fishes 31, 1–72. https://www.calacademy.org/sites/default/files/assets/docs/liparidae.pdf. [Google Scholar]
- 39.Drazen JC, Sutton TT. 2017. Dining in the deep: the feeding ecology of deep-sea fishes. Annu Rev Mar Sci 9:337–366. doi: 10.1146/annurev-marine-010816-060543. [DOI] [PubMed] [Google Scholar]
- 40.Holben WE, Williams P, Gilbert M, Saarinen M, Särkilahti LK, Apajalahti JH. 2002. Phylogenetic analysis of intestinal microflora indicates a novel Mycoplasma phylotype in farmed and wild salmon. Microb Ecol 44:178–185. doi: 10.1007/s00248-002-1011-6. [DOI] [PubMed] [Google Scholar]
- 41.Bano N, Smith AD, Bennett W, Vasquez L, Hollibaugh JT. 2007. Dominance of Mycoplasma in the guts of the Long-Jawed Mudsucker, Gillichthys mirabilis, from five California salt marshes. Environ Microbiol 9:2636–2641. doi: 10.1111/j.1462-2920.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 42.Green TJ, Smullen R, Barnes AC. 2013. Dietary soybean protein concentrate-induced intestinal disorder in marine farmed Atlantic salmon, Salmo salar is associated with alterations in gut microbiota. Vet Microbiol 166:286–292. doi: 10.1016/j.vetmic.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Ward NL, Steven B, Penn K, Methé BA, Detrich WH, III.. 2009. Characterization of the intestinal microbiota of two Antarctic notothenioid fish species. Extremophiles 13:679–685. doi: 10.1007/s00792-009-0252-4. [DOI] [PubMed] [Google Scholar]
- 44.Sukhanova EV, Denikina NN, Triboy TI, Dzyuba EV, Belkova NL. 2014. Molecular and phylogenetic studies of a Mycoplasma from the intestine of Siberian fish. Bio-Genetics J 2:37–41. [Google Scholar]
- 45.Nogi Y, Kato C. 1999. Taxonomic studies of extremely barophilic bacteria isolated from the Mariana Trench and description of Moritella yayanosii sp. nov., a new barophilic bacterial isolate. Extremophiles 3:71–77. doi: 10.1007/s007920050101. [DOI] [PubMed] [Google Scholar]
- 46.Nunoura T, Hirai M, Yoshida-Takashima Y, Nishizawa M, Kawagucci S, Yokokawa T. 2016. Distribution and niche separation of planktonic microbial communities in the water columns from the surface to the hadal waters of the Japan Trench under the eutrophic ocean. Front Microbiol 7:1261. doi: 10.3389/fmicb.2016.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauro FM, Stratton TK, Chastain RA, Ferriera S, Johnson J, Goldberg SMD, Yayanos AA, Bartlett DH. 2013. Complete genome sequence of the deep-sea bacterium Psychromonas strain CNPT3. Genome Announc 1:e00304-13. doi: 10.1128/genomeA.00304-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.León-Zayas R, Novotny M, Podell S, Shepard CM, Berkenpas E, Nikolenko S, Pevzner P, Lasken RS, Bartlett DH. 2015. Single cells within the Puerto Rico Trench suggest hadal adaptation of microbial lineages. Appl Environ Microbiol 81:8265–8276. doi: 10.1128/AEM.01659-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunan LM, Pantoja CR, Gomez-Jimenez S, Lightner DV. 2013. Candidatus Hepatobacter penaei, an intracellular pathogenic enteric bacterium in the Hepatopancreas of the marine shrimp Penaeus vannamei (Crustacea: Decapoda). Appl Environ Microbiol 79:1407–1409. doi: 10.1128/AEM.02425-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santelli CM, Orcutt BN, Banning E, Bach W, Moyer CL, Sogin ML, Staudigel H, Edwards KJ. 2008. Abundance and diversity of microbial life in ocean crust. Nature 453:653–656. doi: 10.1038/nature06899. [DOI] [PubMed] [Google Scholar]
- 51.Wu YH, Liao L, Wang CS, Ma WL, Meng FX, Wu M, Xu XW. 2013. A comparison of microbial communities in deep-sea polymetallic nodules and the surrounding sediments in the Pacific Ocean. Deep Sea Res Pt I 79:40–40. doi: 10.1016/j.dsr.2013.05.004. [DOI] [Google Scholar]
- 52.Huber JA, Johnson HP, Butterfield DA, Baross JA. 2006. Microbial life in ridge flank crustal fluids. Environ Microbiol 8:88–99. doi: 10.1111/j.1462-2920.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- 53.Andeta AF, Vandeweyer D, Teffera EF, Woldesenbet F, Verreth C, Crauwels S, Lievens B, Vancampenhout K, Van Campenhout L. 2019. Effect of fermentation system on the physicochemical and microbial community dynamics during enset (Ensete ventricosum) fermentation. J Appl Microbiol 126:842–853. doi: 10.1111/jam.14173. [DOI] [PubMed] [Google Scholar]
- 54.Vandeweyer D, Wynants E, Crauwels S, Verreth C, Viaene N, Claes J, Lievens B, Van Campenhout L. 2018. Microbial dynamics during industrial reaering, processing, and storage of tropical house crickets (Gryllodes sigillatus) for human consumption. Appl Environ Microbiol 84:e00255-18. doi: 10.1128/AEM.00255-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lauro FM, Chastain RA, Ferriera S, Johnson J, Yayanos AA, Bartlett DH. 2013. Draft genome sequence of the deep-sea bacterium Shewanella benthica KT99. Genome Announc 1:e00210-13. doi: 10.1128/genomeA.00210-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nogi Y, Kato C, Horikoshi K. 1998. Taxonomic studies of deep-sea barophilic Shewanella strains and description of Shewanella violacea sp. nov. Arch Microbiol 170:331–338. doi: 10.1007/s002030050650. [DOI] [PubMed] [Google Scholar]
- 57.Orr JW, Spies I, Stevenson DE, Longo GC, Kai Y, Ghods S, Hollowed M. 2019. Molecular phylogenetics of snailfishes (Cottoidei: Liparidae) based on MtDNA and RADseq genomic analyses, with comments on selected morphological characters. Zootaxa 4642:zootaxa.4642.1.1-079. doi: 10.11646/zootaxa.4642.1.1. [DOI] [PubMed] [Google Scholar]
- 58.Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. 2011. Evidence for a core gut microbiota in the zebrafish. ISME J 5:1595–1608. doi: 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullam KE, Essinger SD, Lozupone CA, O'Connor MP, Rosen GL, Knight R, Kilham SS, Russell JA. 2012. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol Ecol 21:3363–3378. doi: 10.1111/j.1365-294X.2012.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith CCR, Snowberg LK, Caporaso JG, Knight R, Bolnick DI. 2015. Dietary input of microbes and host genetic variation shape among-population differences in stickleback gut microbiota. ISME J 9:2515–2526. doi: 10.1038/ismej.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolnick DI, Snowberg LK, Hirsch P, Lauber CL, Knight R, Caporaso JG, Svanbäck R. 2014. Individuals’ diet diversity influences gut microbial diversity in two freshwater fish (threespine stickleback and Eurasian perch). Ecol Lett 17:979–987. doi: 10.1111/ele.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu H, Guo X, Gooneratne R, Lai R, Zeng C, Zhan F, Wang W. 2016. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci Rep 6:24340. doi: 10.1038/srep24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linley TD, Stewart AL, McMillan PJ, Clark MR, Gerringer ME, Drazen JC, Fujii T, Jamieson AJ. 2017. Bait attending fishes of the abyssal zone and hadal boundary: community structure, functional groups and species distribution in the Kermadec, New Hebrides and Mariana trenches. Deep Sea Res Pt I 121:38–53. doi: 10.1016/j.dsr.2016.12.009. [DOI] [Google Scholar]
- 64.Love ML. 2011. Certainly more than you want to know about the fishes of the Pacific Coast: a postmodern experience. Really Big Press, Santa Barbara, CA. [Google Scholar]
- 65.Gallo ND. 2018. Influence of ocean deoxygenation on demersal fish communities: lessons from upwelling margins and oxygen minimum zones. UC San Diego, San Diego, CA. https://escholarship.org/uc/item/6bb6v4z8. [Google Scholar]
- 66.Longhurst A, Sathyendranath S, Platt T, Caverhill C. 1995. An estimate of global primary production in the ocean from satellite radiometere data. J Plankton Res 17:1245–1271. doi: 10.1093/plankt/17.6.1245. [DOI] [Google Scholar]
- 67.Glud RN, Berg P, Thamdrup B, Larsen M, Stewart HA, Jamieson AJ, Glud A, Oguri K, Sanei H, Rowden AA, Wenzhöfer F. 2021. Hadal trenches are dynamic hotspots for early diagenesis in the deep sea. Commun Earth Environ 2:21. doi: 10.1038/s43247-020-00087-2. [DOI] [Google Scholar]
- 68.Llewellyn MS, McGinnity P, Dionne M, Letourneau J, Thonier F, Carvalho GR, Creer S, Derome N. 2016. The biogeography of the atlantic salmon (Salmo salar) gut microbiome. ISME J 10:1280–1284. doi: 10.1038/ismej.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stadtländer C, Kirchhoff H. 1990. Surface parasitism of the fish mycoplasma Mycoplasma mobile 163 K on tracheal epithelial cells. Vet Microbiol 21:339–343. doi: 10.1016/0378-1135(90)90005-G. [DOI] [PubMed] [Google Scholar]
- 70.Stadtländer CTKH, Lotz W, Körting W, Kirchhoff H. 1995. Piscine gill epithelial cell necrosis due to Mycoplasma mobile strain 163 K: comparison of in-vivo and in-vitro infection. J Comp Pathol 112:351–359. doi: 10.1016/S0021-9975(05)80016-7. [DOI] [PubMed] [Google Scholar]
- 71.Razin S, Yogev D, Naot Y. 1998. Molecular biology and pathogenicity of Mycoplasmas. Microbiol Mol Biol Rev 62:1094–1156. doi: 10.1128/MMBR.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Görtz HD, Schmidt HJ. 2015. Holosporaceae fam. nov. In Whitman WB, Goodfellow M, Kämpfer P, Busse H-J, Trujillo ME, Ludwig W, Suzuki K-I, Parte A (ed), Bergey’s manual of systematic bacteriology, 2nd ed. Springer-Verlag, New York, NY. [Google Scholar]
- 73.Moran NA. 2002. Microbial minimalism: genome reduction in bacterial pathogens. Cell 108:583–586. doi: 10.1016/s0092-8674(02)00665-7. [DOI] [PubMed] [Google Scholar]
- 74.Wolff T. 1970. The concept of the hadal or ultra-abyssal fauna. Deep Sea Res Oceanogr Abstr 17:983–1003. doi: 10.1016/0011-7471(70)90049-5. [DOI] [Google Scholar]
- 75.Beliaev GM. 1989. Deep sea ocean trenches and their fauna. Nauka Publishing House, Moscow, Russia. [Google Scholar]
- 76.Baker LJ, Freed LL, Easson CG, Lopez JV, Fenolio D, Sutton TT, Nyholm SV, Hendry TA. 2019. Diverse deep-sea anglerfishes share a genetically reduced luminous symbiont that is acquired from the environment. Elife 8:e47606. doi: 10.7554/eLife.47606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freed LL, Easson C, Baker LJ, Fenolio D, Sutton TT, Khan Y. 2019. Characterization of the microbiome and bioluminescent symbionts across life stages of Ceratioid Anglerfishes of the Gulf of Mexico. FEMS Microbiol Ecol 95:fiz146. doi: 10.1093/femsec/fiz146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lauro FM, Chastain RA, Blankenship LE, Yayanos AA, Bartlett DH. 2007. The unique 16S rRNA genes of piezophiles reflect both phylogeny and adaptation. Appl Environ Microbiol 73:838–845. doi: 10.1128/AEM.01726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deming JW, Colwell RR. 1981. Barophilic bacteria associated with deep-sea animals. Bioscience 31:507–511. doi: 10.2307/1308493. [DOI] [Google Scholar]
- 80.Deming JW, Tabor PS, Colwell RR. 1981. Barophilic growth of bacteria from intestinal tracts of deep-sea invertebrates. Microb Ecol 7:85–94. doi: 10.1007/BF02010480. [DOI] [PubMed] [Google Scholar]
- 81.Ohwada K, Tabor PS, Colwell RR. 1980. Species composition and barotolerance of gut microflora of deep-sea benthic macrofauna collected at various depths in the Atlantic Ocean. Appl Environ Microbiol 40:746–755. doi: 10.1128/aem.40.4.746-755.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yayanos AA, Dietz AS, Van Boxtel R. 1981. Obligately barophilic bacterium from the Mariana Trench. Proc Natl Acad Sci USA 78:5212–5215. doi: 10.1073/pnas.78.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yayanos AA. 1986. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc Natl Acad Sci USA 83:9542–9546. doi: 10.1073/pnas.83.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eloe EA, Fadrosh DW, Novotny M, Zeigler Allen L, Kim M, Lombardo M-J, Yee-Greenbaum J, Yooseph S, Allen EE, Lasken R, Williamson SJ, Bartlett DH. 2011. Going deeper: metagenome of a hadopelagic microbial community. PLoS One 6:e20388. doi: 10.1371/journal.pone.0020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang W, Tian R-M, Sun J, Bougouffa S, Ding W, Cai L, Lan Y, Tong H, Li Y, Jamieson AJ, Bajic VB, Drazen JC, Bartlett D, Qian P-Y. 2018. Genome reduction in Psychromonas species within the gut of an amphipod from the ocean’s deepest point. mSystems 3:e00009-18. doi: 10.1128/mSystems.00009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang W, Watanabe HK, Ding W, Lan Y, Tian R-M, Sun J, Chen C, Cai L, Li Y, Oguri K, Toyofuku T, Kitazato H, Drazen JC, Bartlett D, Qian P-Y. 2019. Gut microbial divergence between two populations of the hadal amphipod Hirondellea gigas. Appl Environ Microbiol 85:e02032-18. doi: 10.1128/AEM.02032-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng X, Wang Y, Li J, Yan G, He L. 2019. Comparative analysis of the gut microbial communities between two dominant amphipods from the Challenger Deep, Mariana Trench. Deep Sea Res Pt I 151:103081. doi: 10.1016/j.dsr.2019.103081. [DOI] [Google Scholar]
- 88.Peoples LM, Kyaw TS, Ugalde JA, Mullane KK, Chastain RA, Yayanos AA, Kusube M, Methé BA, Bartlett DH. 2020. Distinctive gene and protein characteristics of extremely piezophilic Colwellia. BMC Genomics 21:1–18. doi: 10.1186/s12864-020-07102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nogi Y, Hosoya S, Kato C, Horikoshi K. 2007. Psychromonas hadalis sp. Nov., a novel piezophilic bacterium isolated from the bottom of the Japan Trench. Int J Syst Evol Microbiol 57:1360–1364. doi: 10.1099/ijs.0.64933-0. [DOI] [PubMed] [Google Scholar]
- 90.Hoffmann K, Hassenrück C, Salman-Carvalho V, Holtappels M, Bienhold C. 2017. Response of bacterial communities to different detritus compositions in Arctic deep-sea sediments. Front Microbiol 8:e266. doi: 10.3389/fmicb.2017.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rasmussen JA, Villumsen KR, Duchêne DA, Puetz LC, Delmont TO, Sveier H, Jørgensen LG, Præbel K, Martin MD, Bojesen AM, Gilbert MTP, Kristiansen K, Limborg MT. 2021. Genome-resolved metagenomics suggests a mutualistic relationship between Mycoplasma and salmonid hosts. Commun Biol 4:579. doi: 10.1038/s42003-021-02105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allemann MN, Allen AE. 2018. Characterization and application of marine microbial omega-3 polyunsaturated fatty acid synthesis. Methods Enzymol 605:3–32. doi: 10.1016/bs.mie.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 93.Ast JC, Dunlap PV. 2005. Phylogenetic resolution and habitat specificity of members of the Photobacterium phosphoreum species group. Environ Microbiol 7:1641–1654. doi: 10.1111/j.1462-2920.2005.00859.x. [DOI] [PubMed] [Google Scholar]
- 94.Rivas AJ, Lemos ML, Osorio CR. 2013. Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front Microbiol 4:283. doi: 10.3389/fmicb.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Egerton S, Culloty S, Whooley J, Stanton C, Ross RP. 2018. The gut microbiota of marine fish. Front Microbiol 9:873. doi: 10.3389/fmicb.2018.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeLong EF, Franks DG, Yayanos AA. 1997. Evolutionary relationships of cultivated psychrophilic and barophilic deep-sea bacteria. Appl Environ Microbiol 63:2105–2108. doi: 10.1128/aem.63.5.2105-2108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nogi Y, Masui N, Kato C. 1998. Photobacterium profundum sp. nov., a new, moderately barophilic bacterial species isolated from a deep-sea sediment. Extremophiles 2:1–7. doi: 10.1007/s007920050036. [DOI] [PubMed] [Google Scholar]
- 98.Lauro FM, Eloe-Fadrosh EA, Richter TKS, Vitulo N, Ferriera S, Johnson JH, Bartlett DH. 2014. Ecotype diversity and conversion in Photobacterium profundum strains. PLoS One 9:e96953. doi: 10.1371/journal.pone.0096953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boeuf D, Edwards BR, Eppley JM, Hu SK, Poff KE, Romano AE, Caron DA, Karl DM, DeLong EF. 2019. Biological composition and microbial dynamics of sinking particulate organic matter at abyssal depths in the oligotrophic open ocean. Proc Natl Acad Sci USA 116:11824–11832. doi: 10.1073/pnas.1903080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Preston CM, Durkin CA, Yamahara KM. 2020. DNA metabarcoding reveals organisms contributing to particulate matter flux to abyssal depths in the North East Pacific Ocean. Deep Sea Res Pt II 173:104708. doi: 10.1016/j.dsr2.2019.104708. [DOI] [Google Scholar]
- 101.Baelum J, Borglin S, Chakraborty R, Fortney JL, Lamendella R, Mason OU, Auer M, Zemla M, Bill M, Conrad ME, Malfatti SA, Tringe SG, Holman H-Y, Hazen TC, Jansson JK. 2012. Deep-sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environ Microbiol 14:2405–2416. doi: 10.1111/j.1462-2920.2012.02780.x. [DOI] [PubMed] [Google Scholar]
- 102.Redmond MC, Valentine DL. 2012. Natural gas and temperature structured a microbial community response to the Deepwater Horizon oil spill. Proc Natl Acad Sci USA 109:20292–20297. doi: 10.1073/pnas.1108756108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mason OU, Han J, Woyke T, Jansson JK. 2014. Single-cell genomics reveals features of a Colwellia species that was dominant during the Deepwater Horizon oil spill. Front Microbiol 5:332. doi: 10.3389/fmicb.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kleindienst S, Seidel M, Ziervogel K, Grim S, Loftis K, Harrison S, Malkin SY, Perkins MJ, Field J, Sogin ML, Dittmar T, Passow U, Medeiros PM, Joye SB. 2015. Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. Proc Natl Acad Sci USA 112:14900–14905. doi: 10.1073/pnas.1507380112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noirungsee N, Hackbusch S, Viamonte J, Bubenheim P, Liese A, Müller R. 2020. Influence of oil, dispersant, and pressure on microbial communities from the Gulf of Mexico. Sci Rep 10:7079. doi: 10.1038/s41598-020-63190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goffredi SK, Tilic E, Mullin SW, Dawson KS, Keller A, Lee RW, Wu F, Levin LA, Rouse GW, Cordes EE, Orphan VJ. 2020. Methanotrophic bacterial symbionts fuel dense populations of deep-sea feather duster worms (Sabellida, Annelida) and extend the spatial influence of methane seepage. Sci Adv 6:eaay8562. doi: 10.1126/sciadv.aay8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stewart FJ, Dalsgaard T, Young CR, Thamdrup B, Revsbech NP, Ulloa O, Canfield DE, DeLong EF. 2012. Experimental incubations elicit profound changes in community transcription in OMZ bacterioplankton. PLoS One 7:e37118. doi: 10.1371/journal.pone.0037118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsagaraki TM, Pree B, Leiknes Ø, Larsen A, Bratbak G, Øvreås L, Egge JK, Spanek R, Paulsen ML, Olsen Y, Vadstein O, Thingstad TF. 2018. Bacterial community composition responds to changes in copepod abundance and alters ecosystem function in an Arctic mesocosm study. ISME J 12:2694–2705. doi: 10.1038/s41396-018-0217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garel M, Bonin P, Martini S, Guasco S, Roumagnac M, Bhairy N, Armougom F, Tamburini C. 2019. Pressure-retaining sampler and high-pressure systems to study deep-sea microbes under in situ conditions. Front Microbiol 10:453. doi: 10.3389/fmicb.2019.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stein DL. 2016. Description of a new hadal Notoliparis from the Kermadec Trench, New Zealand, and redescription of Notoliparis kermadecensis (Nielsen) (Liparidae, Scorpaeniformes). Copeia 104:907–920. doi: 10.1643/CI-16-451. [DOI] [Google Scholar]
- 111.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. 2011. Scikit-learn: machine learning in Python. J Machine Learning Res 2011:2825–2830. [Google Scholar]
- 116.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.R Core Team. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- 118.Schwager E, Weingart G, Bielski C, Huttenhower C. 2014. CCREPE: compositionality corrected by permutation and renormalization. R/Bioconductor doi: 10.18129/B9.bioc.ccrepe. [DOI] [Google Scholar]
- 119.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW. 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McMurdie PJ, Holmes S. 2013. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, et al. 2008. The vegan Package. Community Ecology Package 10:631–637. [Google Scholar]
- 123.Martinez Arbizu P. 2020. pairwiseAdonis: pairwise multilevel comparison using adonis. R package version 0.4.
- 124.Pruesse E, Peplies J, Glöckner FO. 2012. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Letunic I, Bork P. 2007. Interactive Tree of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample information for each specimen described in this study. Download Table S1, XLSX file, 0.01 MB (14.8KB, xlsx) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Chao1 (A) and Shannon (B) alpha diversity of the different fishes sequenced in this study, including the wider dataset. The diversity of the wider dataset will be described elsewhere. Download FIG S1, TIF file, 1.0 MB (1MB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
NMDS ordination based on Bray-Curtis beta diversity of the four study fishes and a wider dataset as described in the text (stress = 20.7). The diversity of the wider dataset will be described elsewhere, and those samples are shown as empty circles. Download FIG S2, TIF file, 0.3 MB (336.3KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogenetic tree of Mycoplasmataceae ASVs within the Mariana and Kermadec snailfish. The sequences from this study are shown in blue. Download FIG S3, TIF file, 0.9 MB (896.1KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogenetic tree of an ASV related to the Desulfovibrionaceae found in both the Mariana and Kermadec snailfish. The sequence from this study is shown in blue. Download FIG S4, TIF file, 0.9 MB (942.1KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogenetic tree of two Psychromonas ASVs abundant in P. swirei. Sequences in this study are shown in blue, while those in black boldface are either known piezophiles or obtained from abyssal or hadal environments. Download FIG S5, TIF file, 0.7 MB (716.3KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogenetic tree of an ASV related to Shewanella present within Coryphaenoides yaquinae. Sequences in this study are shown in blue, while those in black boldface are known piezophiles. Download FIG S6, TIF file, 0.8 MB (873.7KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Presence of seven hadal-abundant ASVs within the four comparison species and a wider dataset of fishes. ASVs are labeled based on their lowest identifiable taxonomic rank. Download FIG S7, TIF file, 1.0 MB (1MB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances of ASVs related to the family Mycoplasmataceae within a broad dataset of fish species. Download FIG S8, TIF file, 1.0 MB (1.1MB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cooccurrence map highlighting clusters of taxa common in low-biomass samples, potentially representing contamination sequences. Analysis was performed using CCREPE, with 12 taxa identified and filtered from libraries before downstream analysis. Download FIG S9, TIF file, 0.4 MB (441.3KB, tif) .
Copyright © 2022 Blanton et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Raw sequencing data for the fish species in this study have been submitted to the NCBI Short Read Archive under BioProject no. PRJNA720542.