Abstract
We evaluated the activities of quinupristin-dalfopristin (Q-D), alone or in combination with rifampin, against three strains of Staphylococcus aureus susceptible to rifampin (MIC, 0.06 μg/ml) and to Q-D (MICs, 0.5 to 1 μg/ml) but displaying various phenotypes of resistance to macrolide-lincosamide-streptogramin antibiotics: S. aureus HM1054 was susceptible to quinupristin and dalfopristin (MICs of 8 and 4 μg/ml, respectively); for S. aureus RP13, the MIC of dalfopristin was high (MICs of quinupristin and dalfopristin for strain RP13, 8 and 32 μg/ml, respectively); and S. aureus HM1054R was obtained after conjugative transfer of macrolide-lincosamide-streptogramin B constitutive resistance to HM1054, and the MIC of quinupristin for this strain was high (MICs of quinupristin and dalfopristin, 64 and 4 μg/ml, respectively). In vitro time-kill curve studies showed no difference between Q-D and rifampin, at a concentration of four times the MIC, against the three strains. Rabbits with aortic endocarditis were treated 4 days with Q-D, rifampin, or their combination. In vivo, the combination was highly bactericidal and synergistic against strains susceptible to quinupristin (HM1054 and RP13) and sterilized 94% of the animals. In contrast, the combination was neither synergistic nor bactericidal against the quinupristin-resistant strain (HM1054R) and did not prevent the emergence of mutants resistant to rifampin. We conclude that the in vivo synergistic and bactericidal activity of the combination of Q-D and rifampin against S. aureus is predicted by the absence of resistance to quinupristin but not by in vitro combination studies.
Staphylococcus aureus resistant to methicillin is a major cause of nosocomial infection. Most strains are now also resistant to fluoroquinolones, tetracyclines, and aminoglycosides (6, 18, 22). Glycopeptides remain the standard therapy of systemic infections due to such strains. However, recently several authors reported the emergence of strains of S. aureus with reduced susceptibility to vancomycin, increasing the need for therapeutic options (13, 21).
Quinupristin-dalfopristin (Q-D), a semisynthetic injectable streptogramin, is a combination of streptogramin A (dalfopristin) and streptogramin B (quinupristin) in a 70:30 ratio. Q-D is active in vitro against methicillin-resistant S. aureus, with MICs of <1 μg/ml for 99% of tested strains (1, 4, 5, 16).
A clinically relevant problem is the impact of resistance to quinupristin or dalfopristin on the in vivo activities of Q-D. Cross-resistance to macrolides, lincosamides, and streptogramin B-type antibiotics (MLSB) by methylation of the ribosomal target is the most common mechanism of resistance to these antibiotics in S. aureus (14, 17). The expression of this resistance may be inducible or constitutive in S. aureus. If inducible, quinupristin remains active, because it is not an inducer of the methylase (11). If constitutive, quinupristin is inactive and the bactericidal activity of Q-D is altered in vitro and in vivo, as previously reported (7, 11).
Resistance to a streptogramin A-type antibiotic is infrequent (2, 15, 17). Efflux and inactivation of the streptogramin A compound are the two reported mechanisms of resistance in S. aureus (2, 17). We recently reported that the bactericidal activity of Q-D is retained in vitro and in vivo against S. aureus susceptible to Q-D but resistant to dalfopristin (23). However, in infections with a large inoculum, mutants resistant to Q-D can be selected (23). Therefore, the combination of Q-D with another antibiotic might be of interest in the treatment of strains resistant to quinupristin or dalfopristin, in order to increase the bactericidal activity and/or to prevent the emergence of resistance in vivo.
The aim of the present study was to investigate the activities of Q-D and rifampin alone or in combination against strains of S. aureus susceptible to Q-D and rifampin and susceptible or resistant to quinupristin or dalfopristin, in terms of bactericidal activity, synergy, and emergence of resistance in vitro and in experimental endocarditis.
MATERIALS AND METHODS
In vitro studies. (i) Organisms.
Three strains of S. aureus susceptible to rifampin and Q-D were used (Table 1). They included S. aureus HM1054, a clinical strain susceptible to quinupristin and dalfopristin, and S. aureus RP13, a clinical strain susceptible to quinupristin but resistant to dalfopristin. The mechanism of resistance of S. aureus RP13 was not known: no vat, vatB, or vga sequences were detected by PCR, as previously reported (23). The third strain, S. aureus HM1054R, was a conjugant strain built by transconjugative transfer of constitutive MLSB resistance from a clinical strain to S. aureus HM1054 (11). In addition, a mutant of Q-D previously selected in experimental endocarditis in rabbits treated with Q-D and designated S. aureus RP 13-1 (23) was also used in vitro for the investigation of the interaction of Q-D with rifampin. The resistance phenotypes displayed by the bacteria were characterized by the agar diffusion technique, using disks of erythromycin, lincomycin, quinupristin, dalfopristin, and Q-D, as previously described (16).
TABLE 1.
Characteristics of the S. aureus strains used in the study
| Strain | Origin | Resistance phenotypea | MICs (μg/ml) ofb:
|
|||
|---|---|---|---|---|---|---|
| Q | D | Q-D | RIF | |||
| HM1054 | Blood culture | Oxa | 8 | 4 | 0.5 | 0.06 |
| RP13 | Blood culture | SgA | 8 | 32 | 1 | 0.06 |
| HM1054R | Transconjugant | Oxa SgB | 64 | 4 | 0.5 | 0.06 |
Oxa, oxacillin; SgA, streptogramin A; SgB, streptogramin B.
D, dalfopristin; Q, quinupristin; RIF, rifampin.
(ii) Media and antibiotics.
Trypticase soy agar was used for subcultures, Mueller-Hinton agar was used for MIC determination, brain heart agar was used for selection of resistant mutants, and brain heart infusion was used for overnight cultures. All media were bought from Difco (Detroit, Mich.). Quinupristin, dalfopristin, and Q-D were provided by Aventis, Vitry sur Seine, France, and rifampin was provided by Marion Merell Dow, Lille, France.
(iii) In vitro susceptibilities to antibiotics.
MICs of quinupristin, dalfopristin, Q-D, and rifampin were determined by the agar dilution method, as previously described (19). For each strain, five to six independant determinations were performed.
(iv) Study of antibiotic bactericidal activity.
Time-kill curves were used to test the bactericidal activities of Q-D, rifampin, and their combination against the three strains. For each strain, time-kill curves were determined at least twice. Overnight cultures were diluted in fresh Mueller-Hinton broth to yield an inoculum of 5 × 106 CFU/ml. Q-D and rifampin were used at a concentration of four times the MIC. After 0, 3, 6, and 24 h of incubation at 37°C, serial dilutions of 0.1-ml samples were subcultured onto Trypticase soy agar plates using a spiral plater (Spiral System Inc., Cincinnati, Ohio) and were incubated 24 h at 37°C before CFU were counted. CFU were counted for two different dilutions at which colonies were enumerable. The lower limit of detection of the assay using the spiral plater was 20 CFU/ml (1.3 log10 CFU/ml). In preliminary experiments, antibiotic carryover was ruled out by plating samples of bacterial suspension containing 101 to 103 CFU/ml in the presence or absence of antibiotics (10). Bactericidal activity was defined as a decrease of at least 3 log10 CFU/ml in the original inoculum. Synergy was defined as a decrease of at least 2 log10 CFU/ml, as compared with the more active antibiotic used alone, after 24 h of incubation.
Staphylococcal experimental aortic endocarditis.
Investigations were performed with New Zealand White female rabbits (2.2 to 2.5 kg). Aortic endocarditis was induced in groups of 9 to 12 rabbits by insertion of a polyethylene catheter through the right carotid artery into the left ventricle to induce the formation of vegetations (20). Twenty-four hours after catheter insertion, each rabbit was inoculated by an ear vein with 1 × 106 to 5 × 106 CFU of S. aureus in 1 ml of sterile saline. This inoculum produced endocarditis in all rabbits with proper placement of the catheter. The catheter was left in place throughout the experiment. Untreated rabbits were killed at the start of the therapy and served as control animals. For all strains but S. aureus HM1054R, the sacrifice of control animals and the start of therapy were performed 48 h after the bacterial inoculation. For strain HM1054R, this delay resulted in an extremely high concentration of bacteria in the vegetations and the death of almost 80% of the rabbits before therapy was given. Therefore, for this strain, the sacrifice of control animals and the start of therapy were performed 36 h after bacterial challenge. This resulted in comparable weights of vegetations and bacterial concentrations in vegetations for the three strains (Table 2). Animals were treated for 4 days with one of the following regimens: Q-D, 30 mg/kg intramuscularly (i.m.) every 8 h; rifampin, 10 mg/kg of body weight i.m. every 8 h; or their combination, at two different sites of injection. Control and Q-D regimens for strains HM1054 and RP13 were previously performed and recently published (23) and were not repeated here although one or two control animals were included in each new regimen performed with these strains. This dosing regimen of Q-D has previously been shown to provide areas under the curve for quinupristin and dalfopristin comparable to those achieved with humans (8). This rifampin regimen produced peak and trough serum levels that were close to those achieved with humans after a 10 mg/kg intravenous injection (3). Animals were killed by intravenous injection of phenobarbital 8 h after the last antibiotic injection. The heart was removed and all vegetations from each rabbit were excised, put on ice immediately after excision, rinsed in sterile saline, pooled, and weighed. They were homogenized in 1 ml of sterile saline. Vegetation homogenates were diluted 10-fold (up to 10−6) and were plated on Trypticase agar to count surviving bacteria after 24 h of incubation. An in vivo bactericidal activity was defined as a decrease of at least 3 log10 CFU/g of vegetation in treated animals as compared with controls. Vegetations were considered sterile when no colony grew after plating 100 μl of the undiluted vegetation homogenate. For the calculation of the mean CFU per gram of vegetation, sterile vegetation was considered to contain 1 CFU, corresponding to 1.6 to 2.7 log10 CFU/g of vegetation, according to their weights.
TABLE 2.
Activities of Q-D and rifampin against three strains of S. aureus with different phenotypes of resistance to MLS antibiotics after 4 days of treatment in rabbit endocarditis
| Regimen | Log10 CFU/g of vegetation (no. of sterile/no. of treated animals) with strain:
|
||
|---|---|---|---|
| HM1054 (Qs Ds) | RP13 (Qs Dr) | HM1054R (Qr Ds) | |
| Controls | 10.2 ± 0.6 (—/14)a | 9.1 ± 0.6 (—/8)a | 9.1 ± 1.1 (—/7) |
| Q-D | 4.3 ± 1.2 (1/9)ab | 5.6 ± 1.5 (1/12)ab | 7.3 ± 0.5 (0/5) |
| Rifampin | 5.1 ± 3.4 (2/4)b | 4.0 ± 2.2 (1/6)b | Not done |
| Q-D + rifampin | 2.0 ± 0.3 (6/6)c | 2.2 ± 1.0 (9/10)c | 7.6 ± 1.4 (0/13) |
Results previously published (23).
P < 0.01 versus controls.
P < 0.01 versus Q-D alone and rifampin alone.
In vivo selection of mutants.
Portions (0.1 ml) of each undiluted vegetation homogenate were also plated onto brain heart infusion agar containing Q-D, at 2 or 4 times the MIC, or rifampin, at 10 times the MIC, in order to detect mutants after 24 and 48 h of incubation at 37°C. After 48 h of incubation, CFU were counted and the MICs of Q-D and rifampin were determined.
Determination of plasma antibiotic concentrations. (i) Samples.
Peak and trough antibiotic plasma levels were determined for three uninfected rabbits 2 h (peak) and 8 h (trough) after an i.m. injection of 10 mg/kg for rifampin and 0.5 h (peak) and 8 h (trough) after an injection of 30 mg/kg for Q-D, as previously reported (23). For each sample of Q-D, 2 ml of blood was taken by an ear vein, placed in a sterile tube containing 0.5 ml of 0.25 N hydrochloric acid, gently stirred, and then centrifuged (10 min at 3,000 × g, at 4°C) (10). The upper phase was immediately stored at −80°C.
(ii) Assays.
Rifampin was measured by high performance liquid chromatography coupled with UV detection. The lower limit of detection was 0.1 μg/ml. Quinupristin and dalfopristin plasma concentrations were measured using two bacterial strains differentially susceptible to these compounds, as previously described (23). The assay of quinupristin was performed using the strain S. aureus HBD511, which was susceptible to quinupristin and resistant to dalfopristin. This strain harbors a gene belonging to the vat family encoding an acetylase. The assay was performed in Mueller-Hinton agar no. 2 in the presence of 20 μg of dalfopristin/ml. The assay of dalfopristin was performed with S. epidermidis HBD523, harboring an erm gene, which was constitutively resistant to quinupristin and susceptible to dalfopristin. The assay of dalfopristin was performed with the medium no. 5 from Difco in the presence of 20 μg of quinupristin/ml. The limits of detection of the assay were 0.07 μg/ml for quinupristin and 0.3 μg/ml for dalfopristin, and the coefficient of variation of the assay was consistently less than 10%.
Statistics.
All results were expressed as the mean ± standard deviation. Variance analysis followed by Scheffe's test for multiple comparisons was used to compare bacterial counts in vegetations from groups of animals infected with the same strain and treated with different regimens. The proportions of sterile rabbits in the Q-D-, rifampin-, or combination-treated groups were compared by the Fisher exact test. P < 0.05 was considered significant.
RESULTS
Susceptibility to antibiotics.
MICs of Q-D and rifampin for the three study strains are shown in Table 1. The three strains remained susceptible to Q-D (MICs, 0.5 to 1 μg/ml), whatever their resistance profile to MLS antibiotics. The three strains were also equally susceptible to rifampin (MIC, 0.06 μg/ml).
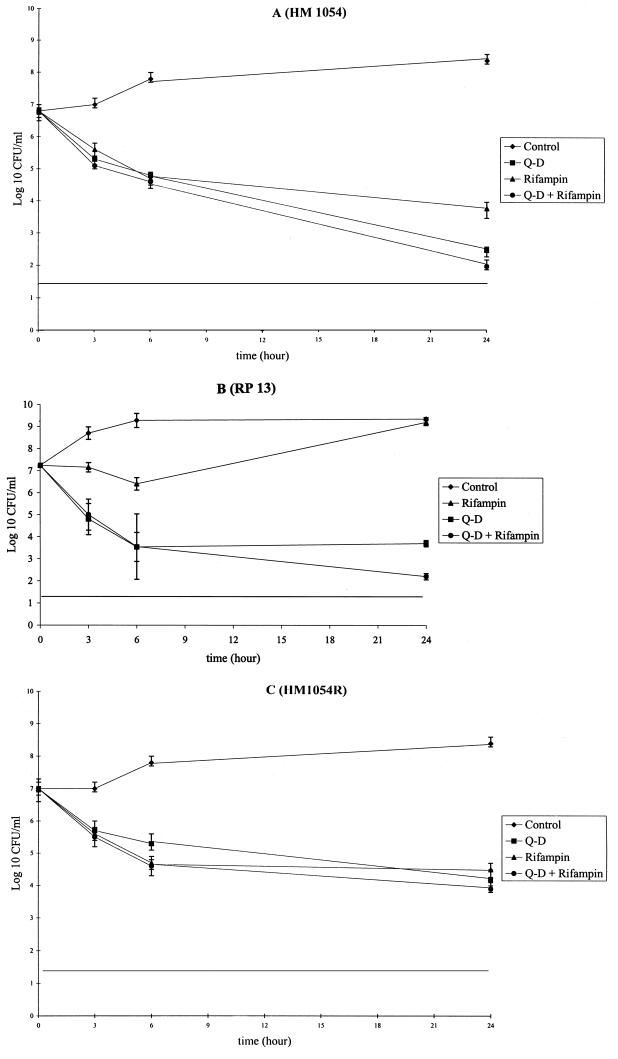
As previously reported, the bactericidal effect of Q-D was reduced against the quinupristin-resistant strain and was similar against the dalfopristin-resistant strain, compared to the susceptible strain (Fig. 1). After 24 h of incubation, Q-D at four times the MIC produced reductions of 4.3, 3.6, and 2.8 log10 CFU/ml against strains HM1054, RP13, and HM1054R, respectively. The Q-D and rifampin combination was bactericidal against the three strains, with a killing rate of 4.8, 4.8, and 3.1 log10 CFU/ml after 24 h of incubation, and there was no difference against the three strains.
FIG. 1.
Time-kill curves of Staphylococcus aureus HM1054 (A), RP13 (B), and HM1054R (C) grown in Mueller-Hinton broth in the absence (controls) or presence of Q-D and/or rifampin at concentrations of four times the MIC. Each point is the mean of at least two determinations. Vertical bars represent standard deviations.
In order to investigate the interaction of Q-D and rifampin against the mutant S. aureus RP13-1, resistant to Q-D, we determined an in vitro time-kill curve for the combination of Q-D and rifampin against this strain (data not shown). The combination was highly bactericidal and synergistic, producing a very rapid kill of the strain and without any regrowth such as that observed under exposure to rifampin alone.
Antibiotic concentrations in plasma.
After a single injection of 30 mg/kg of Q-D, peak concentrations in plasma of dalfopristin and quinupristin were 10.9 ± 4.5 μg/ml and 3.32 ± 0.97 μg/ml, respectively. At trough, concentrations of dalfopristin were not detectable and quinupristin concentrations were 0.22 ± 0.2 μg/ml (range, not detectable to 0.38 μg/ml), as previously reported (23). After a single injection of 10 mg of rifampin/kg, rifampin concentrations were 9.3 ± 0.5 μg/ml at peak and 0.2 ± 0.1 μg/ml at trough.
Activities of antimicrobial agents in experimental endocarditis.
The activities under the different antibiotic regimens are shown in Table 2. As previously reported, the activity of Q-D was similar against the susceptible strain and against the dalfopristin-resistant strain, but was reduced against the MLSB constitutively resistant strain, HM1054R. Rifampin alone was bactericidal against the two tested strains, with a level of activity that was comparable to that of Q-D. The combination of Q-D and rifampin was highly bactericidal against HM1054 and RP13, with 8.2 and 6.9 mean reductions, respectively, in log10 CFU per gram of vegetation compared with control animals, allowing a very high rate of sterilization of the vegetations among the treated rabbits (6 of 6 and 9 of 10, respectively). Thus, animals infected with quinupristin-susceptible strains (HM1054 and RP13) and treated with the combination of Q-D and rifampin had a 94% (15 of 16) rate of sterilization of cardiac vegetations. The combination was significantly more effective than any tested monotherapy against these two strains (P < 0.01). In contrast, the combination was not bactericidal against HM1054R and was not more active than Q-D alone. No animal had a sterile vegetation at the end of treatment with the combination therapy against the MLSB constitutively resistant strain (HM1054R).
In vivo selection of streptogramin- and rifampin-resistant mutants.
No vegetation isolated from animals infected with strain HM1054 or HM1054R and treated with Q-D alone contained mutants resistant to Q-D at the end of therapy (Table 3). However, 1 of 12 rabbits infected with S. aureus RP13 and treated with Q-D retained mutants resistant to Q-D, as previously reported (23). All rabbits treated with rifampin alone had either a sterile vegetation or a vegetation containing mutants resistant to rifampin. Those mutants had a high level of resistance to rifampin (MICs, >500 μg/ml). No resistant mutant was detected in animals infected with strain HM1054 or RP13 and treated with the combination of Q-D and rifampin. In contrast, 11 of 13 rabbits infected with S. aureus HM1054R and treated with the combination therapy contained mutants resistant to rifampin (Table 3).
TABLE 3.
Selection of mutants resistant to Q-D and rifampin in cardiac vegetations from rabbits with aortic endocarditis due to S. aureus after a 4-day treatment with Q-D and/or rifampin
| Regimen | No. of animals with Q-D or rifampin mutants/ no. of treated animals with straina:
|
|||||
|---|---|---|---|---|---|---|
| HM1054 (Qs Ds)
|
RP13 (Qs Dr)
|
HM1054R (Qr Ds)
|
||||
| Q-D mt | RIF mt | Q-D mt | RIF mt | Q-D mt | RIF mt | |
| Q-D | 0/9b | 1/12bc | 0/5 | |||
| Rifampin | 2/4d | 5/6d | Not done | |||
| Q-D + rifampin | 0/6 | 0/6 | 0/10 | 0/10 | 0/13 | 11/13d |
Mutants were selected on agar containing 2 times the MIC of Q-D and 10 times the MIC of rifampin. mt, mutants; RIF, rifampin.
Results were previously published (23).
MIC of Q-D, 8 μg/ml.
MICs of rifampin, >500 μg/ml.
DISCUSSION
We previously demonstrated that quinupristin and dalfopristin have a synergic and bactericidal effect in vitro and in experimental endocarditis due to S. aureus strains susceptible to quinupristin and dalfopristin (10). In contrast, when S. aureus harbors the MLSB constitutive phenotype, the bactericidal activity of the streptogramin complex may be altered in vitro and in vivo (11). More recently, we reported that Q-D remained bactericidal in vitro and in vivo against strains of S. aureus with reduced susceptibility to dalfopristin (MICs, 32 to 64 μg/ml) but remained susceptible to Q-D. (23). However, we demonstrated that mutants resistant to Q-D that were resistant not only to dalfopristin but also to the macrolide, lincosamide, and streptogramin B antibiotics could be selected in vivo under the selective pressure of Q-D (23). Therefore, in staphylococcal infections with a large inoculum due to such strains with reduced susceptibility to dalfopristin, the emergence of resistant mutants might give rise to clinical failure.
Thus, the combination of Q-D with another antibiotic might be of interest against strains of S. aureus that are resistant to quinupristin or dalfopristin, in order to increase the bactericidal activity and/or prevent the emergence of resistance. Rifampin was chosen because of its excellent in vitro activity against S. aureus, its good tissue penetration, and the beneficial effect of its combination with other antibiotics for the treatment of staphylococal endocarditis, despite the absence of in vitro synergism (9). In addition, the combination of oral streptogramins with rifampin has been used empirically for decades in France for the treatment of staphylococcal infections.
In this study, we demonstrated that the combination of Q-D and rifampin was highly bactericidal and synergistic in vivo against the two strains that were susceptible to quinupristin, whatever their susceptibility to dalfopristin. Fifteen of sixteen (94%) animals infected with quinupristin-susceptible strains and treated with this combination had sterile vegetations, a unique result in our model, which is characterized by a high mortality and a low rate of sterilization after an antibiotic treatment of 4 or 5 days. It is important to outline that this in vivo bactericidal synergy did not correlate with the results of in vitro study of the bactericidal activity of the Q-D and rifampin combination. Similar discrepancies between in vitro and in vivo results have already been reported with rifampin combined with other agents in staphylococcal endocarditis (3, 12).
The in vivo synergy could clearly be explained by the prevention of emergence of mutants resistant to rifampin or Q-D by the combination therapy (9). When used alone, rifampin was bactericidal in vivo against the two tested strains, but selected resistant mutants. Fifty to one hundred percent of the bacteria surviving in the vegetations at the end of treatment were resistant mutants, with rifampin MICs of >500 μg/ml. In contrast, combination therapy allowed the sterilization of the vegetations of 15 of 16 rabbits infected with a quinupristin-susceptible strain, indicating that it efficiently prevented the emergence of rifampin-resistant mutants. The single rabbit with vegetations that were not sterile at the end of therapy did not retain mutants resistant to rifampin.
The combination of Q-D and rifampin also prevented the emergence of resistance to Q-D previously observed when the RP13 strain was exposed in vivo to Q-D alone. In addition, this combination was synergistic and bactericidal against the mutant RP13-1 selected in vivo with Q-D alone (23). However, the combination of Q-D and rifampin was not bactericidal against the quinupristin-resistant strain harboring the constitutive MLSB phenotype and did not add any benefit to Q-D alone. This result may be explained by the decreased bactericidal activity of Q-D against such strains, leading to the failure to prevent the emergence of mutants resistant to rifampin. Indeed, 11 of 13 rabbits infected with the quinupristin-resistant strain and treated with the combination therapy retained mutants resistant to rifampin at the end of treatment, which represented 75 to 100% of the surviving bacteria.
In conclusion, the combination of Q-D and rifampin might be of clinical interest in the treatment of infections due to quinupristin-susceptible strains of S. aureus, in order to increase the bactericidal activity and the rate of sterilization and to prevent the emergence of resistance to these antibiotics in severe infections due to S. aureus. Resistance to quinupristin, but not in vitro combination studies, predicted the absence of benefit from the combination.
ACKNOWLEDGMENTS
Virginie Zarrouk was supported by a grant from la Fondation pour la Recherche Médicale. Part of this work was supported by a grant from Aventis.
We thank M. L. Ozoux from Aventis for the technical assistance for antibiotic assays.
REFERENCES
- 1.Aldridge K, Schiro D D, Varner L M. In vitro antistaphylococcal activity and testing of RP 59500, a new streptogramin, by two methods. Antimicrob Agents Chemother. 1992;36:854–855. doi: 10.1128/aac.36.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allignet J, Aubert S, Morvan A, El Sohl N. Distribution of genes encoding resistance to streptogramin A and related compounds among staphylococci resistant to these antibiotics. Antimicrob Agents Chemother. 1996;40:2523–2528. doi: 10.1128/aac.40.11.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer A S, Lam K. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlation. J Infect Dis. 1985;151:157–161. doi: 10.1093/infdis/151.1.157. [DOI] [PubMed] [Google Scholar]
- 4.Bouanchaud D. In-vitro and in-vivo synergic activity and fractional inhibitory concentration of the components of a semisynthetic streptogramin RP, 59500. J Antimicrob Chemother. 1992;30(Suppl. A):95–99. doi: 10.1093/jac/30.suppl_a.95. [DOI] [PubMed] [Google Scholar]
- 5.Brumfitt W, Hamilton Miller J M T, Shah S. In vitro activity of RP 59500, a new semisynthetic streptogramin against Gram-positive bacteria. J Antimicrob Chemother. 1992;30(Suppl. A):29–37. doi: 10.1093/jac/30.suppl_a.29. [DOI] [PubMed] [Google Scholar]
- 6.Brumfitt W, Hamilton-Miller J. Methicillin resistant Staphylococcus aureus. N Engl J Med. 1992;320:1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- 7.Entenza J M, Drugeon H, Glauser M P, Moreillon P. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob Agents Chemother. 1995;39:1419–1424. doi: 10.1128/aac.39.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etienne S D, Montay G, Le Liboux A, Frydman A, Garaud J J. A phase I, double-blind, placebo-controlled study of the tolerance and pharmacokinetic behaviour of RP 59500. J Antimicrob Chemother. 1992;30(Suppl. A):123–131. doi: 10.1093/jac/30.suppl_a.123. [DOI] [PubMed] [Google Scholar]
- 9.Fantin B, Carbon C. In vivo antibiotic synergism: contribution of animal models. Antimicrob Agents Chemother. 1992;36:123–131. doi: 10.1128/aac.36.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fantin B, Leclercq R, Ottaviani M, Vallois J M, Mazière B, Duval J, Pocidalo J J, Carbon C. In vivo activities and penetration of the two components of the streptogramin RP 59500 in cardiac vegetations of experimental endocarditis. Antimicrob Agents Chemother. 1994;38:432–437. doi: 10.1128/aac.38.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantin B, Leclercq R, Merlé Y, Saint-Julien L, Veyrat C, Duval J, Carbon C. Critical influence of resistance to streptogramin B-type antibiotics on activity of RP 59500 (quinupristin/dalfopristin) in experimental endocarditis due to Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:400–405. doi: 10.1128/aac.39.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galetto D W, Boscia J A, Kobasa W D, Keaye D. Teicoplanin compared with vancomycin for treatment of experimental endocarditis due to methicillin-resistant Staphylococcus epidermidis. J Infect Dis. 1986;154:69–73. doi: 10.1093/infdis/154.1.69. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu K, Akarita N, Hanaki H, Horoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leclercq R, Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991;35:1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leclercq R, Nantas L, Soussy C J, Duval J. Activity of RP 59500, a new parenteral semisynthetic streptogramin, against staphylococci with various mechanisms of resistance to macrolide-lincosamide-streptogramin antibiotics. J Antimicrob Chemother. 1992;30(Suppl. A):67–75. doi: 10.1093/jac/30.suppl_a.67. [DOI] [PubMed] [Google Scholar]
- 17.Lina G, Quaglia A, Reverdy M E, Leclercq R, Vandenesch F, Etienne J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43:1062–1066. doi: 10.1128/aac.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mapple P A C, Hamilton-Miller J M T, Brumfitt W. World-wide antibiotic resistance in methicillin resistant Staphylococcus aureus. Lancet. 1989;i:537–539. doi: 10.1016/s0140-6736(89)90076-7. [DOI] [PubMed] [Google Scholar]
- 19.Pearson R D, Steibigel R T, Davis H T, Chapman S W. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980;18:699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlman B B, Freedman L R. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971;44:206–213. [PMC free article] [PubMed] [Google Scholar]
- 21.Smith T L, Pearson M L, Wilcox K R, Cruz C, Lancaster M V, Robinson-Dunn B, Tenover F C, Zervos M T, Band J D, White E, Jarvis W R. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-intermediate Staphylococcus aureus working group. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 22.Voss, A. D., C. Milatovic, C. Wallrauch-Scwarz, V. T. Rosdahl, and I. Braveny. Methicillin-resistant Staphylococcus aureus in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 13:50–55. [DOI] [PubMed]
- 23.Zarrouk V, Bozdogan B, Leclercq R, Garry L, Carbon C, Fantin B. Influence of resistance to streptogramin A type antibiotics on the activity of quinupristin-dalfopristin in vitro and in experimental endocarditis due to Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1168–1173. doi: 10.1128/aac.44.5.1168-1173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]