ABSTRACT
E-cigarettes (e-cigs) have drastically increased in popularity during the last decade, especially among teenagers. While recent studies have started to explore the effect of e-cigs in the oral cavity, little is known about their effects on the oral microbiota and how they could affect oral health and potentially lead to disease, including periodontitis and head and neck cancers. To explore the impact of e-cigs on oral bacteria, we selected members of the genus Streptococcus, which are abundant in the oral cavity. We exposed the commensals Streptococcus sanguinis and Streptococcus gordonii and the opportunistic pathogen Streptococcus mutans, best known for causing dental caries, to e-liquids and e-cig aerosols with and without nicotine and with and without menthol flavoring and measured changes in growth patterns and biofilm formation. Our results demonstrate that e-cig aerosols hindered the growth of S. sanguinis and S. gordonii, while they did not affect the growth of S. mutans. We also show that e-cig aerosols significantly increased biofilm formation by S. mutans but did not affect the biofilm formation of the two commensals. We found that S. mutans exhibits higher hydrophobicity and coaggregation abilities along with higher attachment to OKF6 cells than S. sanguinis and S. gordonii. Therefore, our data suggest that e-cig aerosols have the potential to dysregulate oral bacterial homeostasis by suppressing the growth of commensals while enhancing the biofilm formation of the opportunistic pathogen S. mutans. This study highlights the importance of understanding the consequences of e-cig aerosol exposure on selected commensals and pathogenic species. Future studies modeling more complex communities will provide more insight into how e-cig aerosols and vaping affect the oral microbiota.
IMPORTANCE Our study shows that e-cigarette aerosol exposure of selected bacteria known to be residents of the oral cavity hinders the growth of two streptococcal commensals while enhancing biofilm formation, hydrophobicity, and attachment for the pathogen S. mutans. These results indicate that e-cigarette vaping could open a niche for opportunistic bacteria such as S. mutans to colonize the oral cavity and affect oral health.
KEYWORDS: e-cigarette, S. mutans, streptococci, e-cigarette aerosols, oral streptococci, oral health
INTRODUCTION
E-cigarette (e-cig) use has increased drastically in the last decade, with 4.7% of middle-school students and 19.6% of high-school students currently using an e-cig device (1–3). The base components of e-liquids are propylene glycol (PG) and vegetable glycerin (VG), used in various ratios. Additional components include nicotine and flavorings that can be added by the e-cig user (4). Among adolescents who vape any type of flavored e-cigarettes, the most commonly used flavors are fruit (73.1%; 1.83 million users), mint (55.8%; 1.39 million), and menthol (37.0%; 920,000) according to the U.S. Department of Health and Human Services (2). These e-liquids are heated into an aerosol using an electronic nicotine delivery system (ENDS) and are orally inhaled by the user (so-called “vaping”). While e-cigs are advertised as a safer alternative to conventional cigarettes (5), there is a lack of long-term epidemiological data available to support this claim (4). It has been reported that ecigs generate reactive oxidative species (ROS) and other carcinogenic components, such as formaldehyde, nitrosamines, and toxic carbonyls (6–8), similar to those present in traditional cigarette smoke condensate (9–11). These ecig metabolites have been shown to induce DNA damage, thereby enhancing mutational susceptibility and tumorigenic transformation (12, 13).
Oral bacteria and oral epithelial cells are directly exposed to e-cig aerosols during a vaping session. E-cig vaping has been found to affect oral health through the induction of inflammatory-cytokine release and oxidative damage (14). A metagenomic study showed that e-cig aerosol exposure increased the abundance of bacterial genes encoding quorum sensing, biofilm formation, stress response, and virulence factors (15). However, current data regarding the effects of e-cigs on the oral microbiome are limited and controversial. While one report showed that e-cigs did not affect oral microbiome diversity (16), others agreed on changes in the oral microbial diversity and differences in the relative abundance of several bacterial taxa, including Porphyromonas, Veillonella (1), and Haemophilus, in e-cig users compared to healthy individuals (17).
The oral microbiome is dominated by oral commensal Streptococcus spp. (80%) (18, 19), leading to our interest in investigating the effect of e-cig aerosols in the modification of these oral resident bacteria. At homeostasis, Streptococcus sanguinis and S. gordonii dominate the niche. Two recent studies showed that e-liquid and e-cig aerosols did not cause changes in the exponential growth phase of Streptococcus commensals, including S. gordonii, S. mitis, and S. oralis (20, 21). In contrast, another recent study demonstrated that flavored e-cig aerosols hindered the exponential growth of S. gordonii, S. intermedius, S. mitis, and S. oralis more strongly than unflavored e-cig aerosols (22). Streptococcus mutans is an opportunistic pathogen known for its cariogenic role in the oral cavity (18) and its association with severe periodontitis (23, 24). Typically, S. mutans is present in the oral cavity as part of a mature dental biofilm. However, it can become dominant over other species due to environmental changes, including density, nutritional availability, and pH, causing dysregulation in oral bacterial homeostasis (25). Kreth et al. studied competition and coexistence between different species occupying the same ecological niche using S. mutans and S. sanguinis as a model (25). Based on their data, occupation of a niche by one species prevents colonization by the other, yet simultaneous colonization by both species results in coexistence (25). Currently, there are few data available regarding the effect of e-cig aerosols on the colonization of the oral cavity by Streptococcus spp. To better understand how e-cigs affect these oral interspecies interactions, we focused on the effect e-cig aerosols had on growth patterns and biofilm formation of two known early colonizers, the commensals S. sanguinis and S. gordonii, as well as the opportunistic pathogen and recognized cariogenic bacterium S. mutans. Our results show that e-cig aerosols reduce the biomass accumulation of S. sanguinis and S. gordonii without affecting the biomass accumulation of S. mutans. E-cig aerosols promoted S. mutans biofilm formation but had no effect on S. sanguinis and S. gordonii. When we assessed bacterial hydrophobicity and growth rate, we showed dominance of S. mutans over the commensal family members, demonstrating the potential role of e-cig vaping to shift the bacterial population by altering growth and colonization of every species differently.
RESULTS
E-cigarette aerosols suppress the growth of S. sanguinis and S. gordonii but not S. mutans.
In this study, we explored the effect of the common propylene glycol-vegetable glycerin (PG/VG; 50/50) e-cig base without any additives and with 3 mg/mL nicotine. Since menthol is one of the most popular flavorings, we also included treatments with nicotine-free menthol flavoring and menthol containing 3 mg/mL nicotine. These e-liquids were heated into aerosols using an electronic nicotine delivery system (ENDS). For this study, we focused on closely related commensals from the Streptococcus sanguinis group, S. sanguinis and S. gordonii, which are reported to be early colonizers and able to adhere to all oral cavity surfaces (18). We also selected S. mutans, which is a known opportunistic pathogen that under certain condition can become dominant over other species and cause caries and periodontal disease (25). To assess competition between S. mutans and S. gordonii or S. sanguinis, we cultured each of the commensals, adding S. mutans in an equal volume for 24 h. S. mutans growth increased by 32-fold (P < 0.01) by the end of the coculture, while S. sanguinis viability in the presence of S. mutans was below the limit of detection (see Fig. S1 in the supplemental material). Similarly, when equal volumes of S. mutans and S. gordonii were incubated for 24 h, S. mutans showed a 39-fold increase in growth (P < 0.001), while S. gordonii exhibited only a 3-fold increase (Fig. S1). Interestingly, when grown in medium supplemented with the supernatant from S. mutans cultures, the viability of S. sanguinis and S. gordonii was equally impaired. These results indicate that the inhibition of viability or growth is mediated by secreted factors present in the medium (Fig. S1) and support the idea that the opportunistic growth of S. mutans is not due to a growth kinetic advantage, since the commensals grew when cultured alone in the same time frame. Overall, these data suggest that S. mutans can outcompete both S. sanguinis and S. gordonii, consistent with previous research on the competition between S. sanguinis and S. mutans in the oral cavity (25–28).
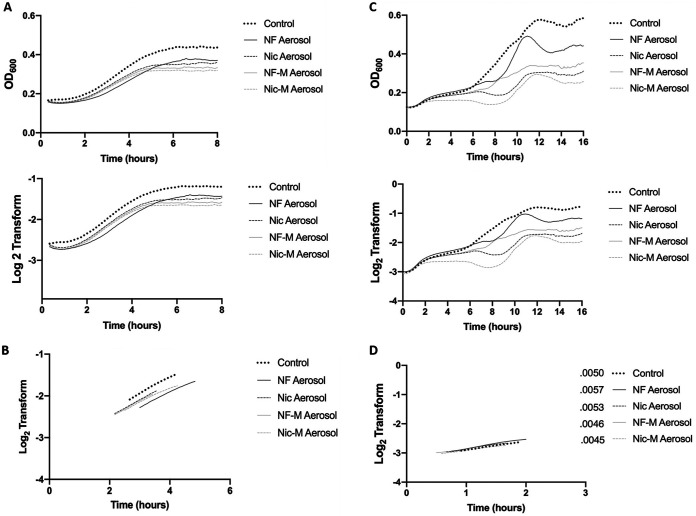
To understand the effect of e-cig aerosols on S. sanguinis and S. gordonii growth pattern, we grew the bacteria in TSB medium pre-exposed to e-cig aerosol overnight and recorded bacterial turbidity every 5 min as the optical density at 600 nm (OD600). We observed that upon exposure to e-cig aerosol-pretreated medium, the growth of S. sanguinis was negatively affected, reaching a lower plateau than the untreated control (Fig. 1A). The most significant reduction of biomass accumulation was observed under conditions containing the combination of nicotine and menthol flavoring, as seen by the lowest plateau curve. Nicotine-free aerosol overall affected growth compared to the untreated control but was less harmful than the aforementioned condition. The bacterial growth rates were plotted as the linear regression of the exponential phase (Fig. 1B; Fig. S2). While the growth rates remained the same when these commensals were exposed to aerosol, the overall biomass accumulation was lower for all the e-cig aerosol treatments than the control. Overall, e-cig aerosols with and without nicotine and with and without menthol flavor all significantly hindered the bacterial biomass accumulation of S. sanguinis at 4, 6, and 8 h, compared to the control (Table 1).
FIG 1.
E-cigarette aerosols reduce the growth of S. sanguinis and S. gordonii. (A) Overnight cultures of S. sanguinis were diluted 1:10 into e-cig aerosol-pretreated medium. OD600 was measured every 5 min over the course of 8 h. The average was calculated and plotted for OD600 (top) and log2 transform (bottom). (B) Slopes for linear regression of S. sanguinis are presented based on the derivative of the log2 transform. (C) Overnight cultures of S. gordonii were diluted 1:10 into e-cig aerosol-pretreated medium. Optical density was measured every 5 min over the course of 16 h. The average was calculated and plotted OD600 (top) and log2 transform (bottom). (D) Slopes for linear regression of S. gordonii are presented based on the derivative of the log2 transform. Data are representative of one biological (n = 3) replicate repeated at least twice. OD600 of treatment groups was compared to control OD600, using one-way ANOVA (Dunnett’s correction). All conditions were compared to the control; for a complete breakdown of significance, refer to Table 1.
TABLE 1.
Biomass analysis for the different vape conditionsa
| Organism | Condition |
P at time (h) |
||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | 14 | 16 | ||
| S. sanguinis | NF | <0.0001 | 0.0011 | 0.0002 | ||||
| Nic | 0.0002 | 0.0005 | 0.0003 | |||||
| NF-M | <0.0001 | 0.0002 | <0.0001 | |||||
| Nic-M | <0.0001 | <0.0001 | <0.0001 | |||||
| S. gordonii | NF | NS | NS | 0.0342 | NS | 0.0056 | 0.0228 | 0.0107 |
| Nic | NS | 0.0169 | 0.0029 | 0.0036 | <0.0001 | 0.0006 | 0.0003 | |
| NF-M | NS | 0.0404 | 0.0277 | 0.0123 | 0.0002 | 0.0010 | 0.0007 | |
| Nic-M | <0.0001 | <0.0001 | 0.0002 | 0.0008 | <0.0001 | 0.0001 | <0.0001 | |
| S. mutans | NF | NS | NS | NS | ||||
| Nic | NS | NS | NS | |||||
| NF-M | NS | NS | NS | |||||
| Nic-M | NS | NS | NS | |||||
OD600 values at each time point were compared to the control OD600 using a one-way ANOVA (Dunnett’s correction). Significance identifies OD600 values that are lower than the control, which represents a lower biomass. NS, not significant.
Similarly, exposure of S. gordonii to e-cig aerosol-pretreated medium resulted in reduced biomass accumulation under all conditions. Growth in nicotine-containing aerosol and, more so, the combination of nicotine and menthol flavoring reached a lower plateau than the other conditions (Fig. 1C). S. gordonii shows a biphasic growth curve characterized by two exponential growth phases that are separated by a lag phase. We plotted the linear regression of the first exponential phase, which did not show significant changes between the treatment groups and the untreated control (Fig. 1D; Fig. S2). However, all four treatments hindered the growth of S. gordonii, as observed by the disorganized growth patterns and the lower growth plateau under these conditions. All the aerosol treatments significantly decreased the biomass accumulation of S. gordonii, observed by the lower OD600 at 8, 12, 14, and 16 h than that of the control (Table 1).
When e-liquid was added to the bacterial growth medium without heating and aerosolization, only a minor reduction in bacterial growth for both S. sanguinis and S. gordonii was observed (Fig. S3). This indicates that the heat generated by the atomizer heating coil results in the emission and inhalation of harmful and toxic vape by-products, as seen in Fig. 1. These results support previous reports that nonaerosolized e-liquid does not affect the growth of Streptococcus spp. tested, including S. gordonii (20, 22). Oxidation and decomposition upon heating of the universal carrier components, glycerol and propylene glycol (4), can result in the formation of formaldehyde and acetaldehyde (29). It has been reported that these chemicals are bactericidal and have a negative effect on the oral microbiota (30).
Next, we explored the effect e-cig aerosols have on the known oral pathogen S. mutans. The experiment was performed the same way as described for S. sanguinis and S. gordonii. S. mutans growth in the different conditions was recorded. The growth curve for S. mutans shows no changes or OD600 reduction compared to the control (Fig. 2A), suggesting resistance to e-cig aerosols compared to the streptococcal commensals. Furthermore, the linear regression of the exponential phase is almost identical, as seen from the overlapping slopes (Fig. 2B; Fig. S2). No changes were observed when S. mutans was exposed to e-liquid alone (Fig. S3). Overall, we can conclude that S. mutans growth remains unaffected when exposed to e-cig aerosols regardless of nicotine content or flavoring.
FIG 2.
E-cigarette aerosols did not affect the growth patterns of S. mutans. (A) Overnight cultures of S. mutans were diluted 1:10 into e-cig aerosol-pretreated medium. Optical density was measured every 5 min over the course of 8 h. The average was calculated and plotted for OD600 (top) and log2 transform (bottom). (B) Slopes for linear regression of S. mutans are presented based on the derivative of the log2 transform. Data are representative of one biological replicate (n = 3) repeated at least twice.
Additionally, we compared the linear regression of the three species when they were exposed to the different e-cig aerosols. We observed significantly higher growth rates for S. mutans than S. sanguinis and S. gordonii under all conditions (Fig. 3). These results highlight how the impact of e-cig aerosols greatly differs between bacterial strains and could ultimately lead to an imbalance by suppressing the growth of the commensals, thereby providing a growth advantage to S. mutans. We speculate that these opposing effects could lead to changes in oral bacterial homeostasis of e-cig users.
FIG 3.
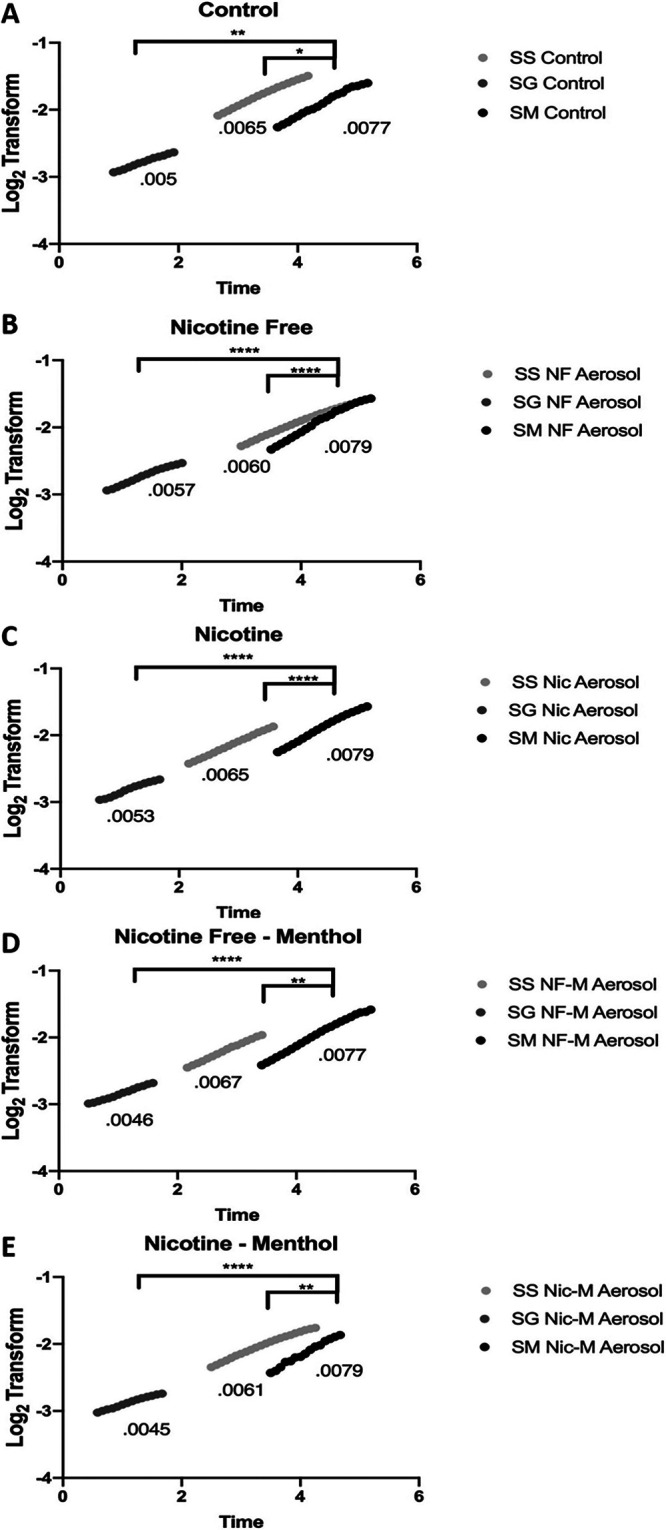
S. mutans has a significantly higher growth rate than the commensal streptococcal species. Overnight cultures of S. sanguinis (SS), S. gordonii (SG), and S. mutans (SM) were diluted 1:10 in TSB (A) or e-cig aerosol that was nicotine free (B), nicotine containing (C), nicotine free with menthol (D), or nicotine containing with menthol (E). Optical density was measured every 5 min, and growth curves were analyzed. Slopes for linear regression are presented based on the derivative of the averaged log2 transform. Data are representative of one biological (n = 3) replicate repeated at least twice. For each condition, the commensal’s growth rate was compared to that of S. mutans using one-way ANOVA (Dunnett’s correction). *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
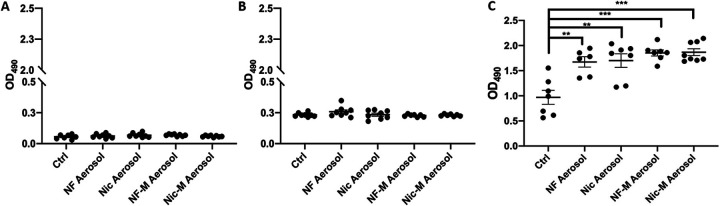
E-cigarette aerosol exposure results in changes in hydrophobicity and promotes biofilm formation of S. mutans compared to S. sanguinis and S. gordonii.
Bacterial biofilms are found on most surfaces in the oral cavity and are often formed as a response to an environmental challenge. As such, they ensure that bacteria can survive in a protected environment, resisting eradication (31, 32). Therefore, changes in biofilm formation upon e-cig aerosols could affect the composition of the oral streptococcal community. We measured biofilm formation of S. sanguinis (Fig. 4A), S. gordonii (Fig. 4B), and S. mutans (Fig. 4C) when grown in e-cig aerosol-pretreated medium without physical disruptions for 24 h. The biofilm was stained with safranin and quantified by measuring OD490. Our results show that S. sanguinis and S. gordonii biofilm formation was unaffected when the bacteria were exposed to e-cig aerosol treatments regardless of the presence of nicotine or flavorings (Fig. 4A and B). As reported in the literature, S. sanguinis and S. gordonii utilize sucrose as a carbon source to synthesize glucan, which is an important component of their biofilms (33). We therefore performed biofilm assays for these commensals in medium supplemented with 1% sucrose along with e-cig exposure to determine if the more physiological conditions would enhance the resistance to e-cig aerosols. Our results showed that while the overall capacity for biofilm formation was enhanced in the presence of sucrose, bacterial biofilm formation in response to e-cig vape was not affected compared to the sucrose-supplemented control (Fig. S4).
FIG 4.
E-cigarette aerosols promote biofilm the formation of S. mutans but not S. sanguinis and S. gordonii. Overnight cultures of S. sanguinis (A), S. gordonii (B), and S. mutans (C) were diluted 1:10 in e-cig-pretreated medium and grown for 24 h. Biofilms were stained with safranin, and OD490 was measured. The data are means and SEM (n = 7) for one biological replicate of 3. Groups were compared to the control using one-way ANOVA (Dunnett’s correction). **, P < 0.01; ***, P < 0.001.
We observed a significant increase in biofilm formation when S. mutans was exposed to nicotine-free aerosol, nicotine aerosol, nicotine-free menthol aerosol, and nicotine menthol aerosol, all compared to untreated control (Fig. 4C). E-liquid exposure for 24 h showed only minimal effects on S. mutans biofilm formation (Fig. S5), again stressing the importance of heating the e-liquid and aerosolizing the components. We surmise that the observed increase in biofilm formation upon e-cig exposure could potentially confer an advantage to S. mutans by allowing protection and subsequently further colonization of the oral cavity.
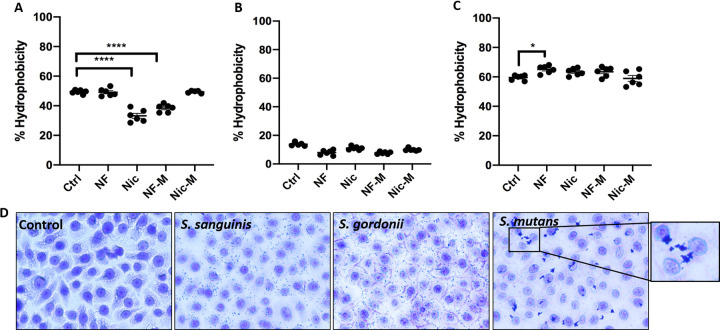
Several bacterial properties can be an indication for the ability to adhere to inert surfaces, one of them being hydrophobicity (34). Additionally, bacterial hydrophobicity has been correlated with changes in biofilm formation (35). As bacteria can switch their membrane phenotype between hydrophilic and hydrophobic due to environmental changes (36), we wanted to explore the effect of e-cig aerosol exposure on the hydrophobicity of these streptococcal strains. S. sanguinis had a significant decrease in hydrophobicity when exposed to nicotine and nicotine-free menthol (Fig. 5A). S. gordonii (Fig. 5B) displayed a lower hydrophobicity overall. Interestingly, the hydrophobicity of S. mutans was overall higher than that of the commensals and remained around 65% when the organisms were exposed to e-cig aerosol (Fig. 5C) with a significant increase in nicotine-free conditions. To visualize how the hydrophobicity could affect growth patterns of the streptococcal strains in the presence of a biotic surface, we cultured immortalized oral epithelial OKF6 cells with each of the streptococci at a multiplicity of infection (MOI) of 10 for 1 h. At the end of the coculture, we used crystal violet to visualize the attached bacteria by bright-field microscopy (Fig. 5D). All three strains were able to adhere to OKF6 cells, yet S. mutans had the highest propensity to coaggregate, which relates back to its highest hydrophobicity.
FIG 5.
S. mutans shows a higher hydrophobicity and coaggregation capacity than S. sanguinis and S. gordonii. Overnight cultures of S. sanguinis (A), S. gordonii (B), and S. mutans (C) were diluted 1:10 in e-cig-pretreated medium and grown for 24 h. Chloroform was added to each condition, and after incubation, the aqueous layer was collected and OD600 was measured. The data are means and SEM) (n = 3) for three biological replicates. Groups were compared to controls using one-way ANOVA (Dunnett’s correction). *, P < 0.05; ****, P < 0.0001. (D) Bright-field microscopy of crystal violet-stained 1-h cocultures (MOI, 10) of oral epithelial cells (OKF6) with S. sanguinis, S. gordonii, and S. mutans. Magnification, ×40.
S. mutans exhibits higher attachment to oral epithelial cells even when challenged with E-cig aerosols compared to S. sanguinis and S. gordonii.
Attachment to epithelial cells is an essential aspect of bacterial colonization in the oral cavity (36). We therefore aimed to identify the differences in attachment capabilities of the three model strains after exposure to e-cig aerosol. To quantify bacterial attachment to oral epithelial cells, we cultured OKF6 cells with each of the streptococci at an MOI of 1 for 3 h and enumerated CFU for control and e-cig aerosol-exposed cocultures. Although we did not observe significant differences between the treated and control groups for each species, we consistently observed that S. mutans exhibits a higher attachment capacity than the two commensal strains (Fig. 6). Bacterial attachment to the mucosal membrane can lead to inflammation and other systemic diseases (18, 37). In many instances, microbial adhesion, aggregation, and biofilm formation cause serious damage and disease (36). We have demonstrated that S. mutans exhibits all these characteristics.
FIG 6.
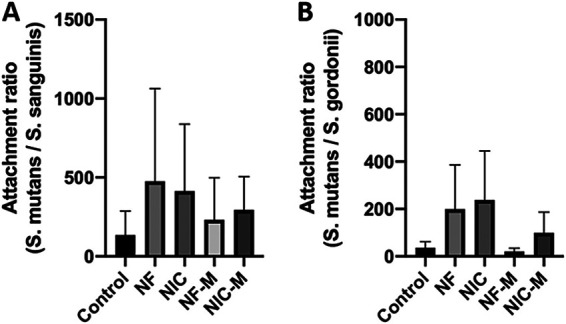
S. mutans has a higher capacity to adhere to oral epithelial cells than S. sanguinis and S. gordonii. OKF6 cells were cocultured with S. sanguinis, S. gordonii, and S. mutans for 3 h at an MOI of 1. OFK6 cells with possible internalized and attached bacteria were permeabilized and serial dilutions plated for CFU/mL counts. For each condition, the attachment of S. mutans was compared to that of S. sanguinis (A) or S. gordonii (B) by determining the ratio of S. mutans to the commensal. (SEM; n = 3). Groups were compared to control using one-way ANOVA (Dunnett’s correction).
DISCUSSION
E-cigs were introduced to the United States and Europe 15 years ago, but their effects on oral health have not been well established yet (4). Tobacco smoking results in significant shifts in the oral microbiome, as observed in patients with oral cancer (38). Similar to conventional cigarette smoke, e-cigs are known to cause dysbiosis by affecting the growth of oral commensals, allowing opportunistic pathogens to grow (1). However, the role e-cigs play in the behavior and survival of specific bacterial species in the oral cavity remains unclear. Our study explored the effect of e-cigs on three Streptococcus spp. commonly found in the oral cavity. We demonstrated how e-cig aerosol exposure induces differential responses depending on the strain of oral bacterium, potentially contributing to changes in oral bacterial composition.
To better understand how e-cig aerosols affect the oral resident bacteria, we exposed three Streptococcus spp. to e-cig aerosols with and without nicotine or flavoring and measured growth patterns, biofilm formation, hydrophobicity, and attachment to oral epithelial cells. Our results show that e-cig aerosols disrupt the growth patterns of S. sanguinis and S. gordonii, without affecting the growth of S. mutans. E-cig devices utilize e-liquid, which generally contains propylene glycol and vegetable oil to generate vapor and acts as a carrier for additives such as nicotine and flavors (39). Propylene glycol itself has been shown to have a bactericidal effect and can therefore modulate the oral microbiota (30). These reports support our finding of overall lower biomass accumulation in even the nicotine-free and flavorless condition. Interestingly, few pathogens have been shown to be resistant to propylene glycol, one of them being Staphylococcus aureus (30); we report here that S. mutans is resistant as well.
Nicotine has been shown to support S. mutans oral colonization by increasing biofilm formation and viability of S. mutans (40). More so, nicotine concentrations at 1 to 4 mg/mL upregulate the expression of virulence receptor proteins and extracellular polysaccharides, ultimately stimulating glycolytic pathway intermediates (41, 42). Overall, it has been reported that e-cig aerosols enhance adherence of S. mutans to the teeth and the ability to form biofilms (43). Mechanistically, e-cig aerosols regulate the expression of biofilm-associated genes in S. mutans. Specifically, comC, -D, and -E, which are related to quorum sensing genes, are central to cell viability and biofilm formation in response to environmental conditions (43). Our data further support the idea that S. mutans may be at an advantage when environmental stressors such as e-cig aerosol exposure arise in the oral cavity.
Bacteria contain cell surface hydrophobicity (CSH) features, including hydrophobic amino acid residues, outer membrane proteins and lipids, and lipoteichoic acid (36). Interestingly, bacteria can switch their membrane phenotypes between hydrophilic and hydrophobic due to environmental changes (36). The importance of hydrophobic measurements is supported by the findings that patients with a high risk for dental caries have a higher prevalence of hydrophobic bacteria than patients with low dental caries risk (44). We assessed hydrophobicity, which has also been correlated with adhesion (36) but is only one factor associated with adhesion and attachment to surfaces in the context of e-cig aerosol exposure. We observed that e-cig aerosol exposure reduced the hydrophobicity of S. sanguinis, while the hydrophobicity of S. gordonii remained low. On the other hand, e-cig aerosols did not affect the higher hydrophobicity of S. mutans. More interestingly, we observed a tendency in S. mutans to coaggregate. Overall, the higher hydrophobicity and coaggregation capacities observed for S. mutans could be correlated with tissue invasion, inflammation, and disease (36).
The same oral epithelial cells (OKF6) we used in this study were subjected to biofilm and planktonic S. gordonii (45) challenge by Ebersole et al. (46). Their findings elucidated major gene differences of epithelial cells in the presence of biofilm versus planktonic S. gordonii. The highest upregulation of immune-related genes was found with the biofilm group and included important cytokines involved in the innate and adaptive immune response, including interleukin 8 (IL-8; CXCL8). S. mutans, in a biofilm challenge experiment using gingival cells, stimulated the expression of IL-8 without other bacteria present but did not induce antimicrobial peptides such as DEFB4B. In contrast, coculture with S. mitis, which is involved in early plaque formation, resulted in the expression of antimicrobials, including DEFB4B, but did not influence inflammatory gene expression (46). This study highlights another mechanism of S. mutans oral colonization: while bacteria involved in early plaque formation can induce the synthesis of antimicrobial peptides, bacteria like S. mutans that are secondary colonizers are not recognized as pathogenic by epithelial cells and subsequently evade the immune response, which may allow opportunistic colonization (46).
We evaluated the interaction of the selected streptococcal strains with OKF6 cells measuring attachment only and observed that S. mutans overall had a higher capacity to adhere to OKF6 cells than the commensals. While the results from the attachment assay correlated with biofilm formation and hydrophobicity, the mechanisms for differences in adhesions need to be further determined. Specific surface proteins called adhesins can be involved in the binding of bacteria to human cells (47). For example, S. gordonii expresses the surface proteins of the antigen I/II adhesin family, SspA and SspB. These adhesins bind directly to β1 integrin and mediate internalization (48). It has also been reported that Streptococcus gordonii, Streptococcus sanguinis, and Streptococcus oralis (49) have surface appendages that play a role in the adhesion of streptococci to host cells but also in interbacterial aggregation (50, 51). These findings raise the questions of whether surface marker expression is distinct in the three model bacteria we selected and whether e-cig vape could modify the cell membrane composition of the bacteria. Our investigation stops short of identifying mechanisms of interbacterial binding and adhesion to epithelial cells, but it highlights the ideas that ligand-binding specificities of surface proteins may account for species-specific adherence within the human oral cavity and that the effects of e-cig aerosols can differ between strains. This could be the reason behind differences seen between the three species and their attachment.
Given that 80% of the oral cavity microbiota is composed of Streptococcus spp., our study is proof of concept for how e-cig exposure can alter diverse behaviors in the three model organisms we analyzed, affecting commensals differently from opportunistic pathogens. By extension, we propose that e-cig vaping can modify the oral microbiome differentially. In homeostasis, S. sanguinis and S. gordonii are early colonizers (18). As such, they protect the oral cavity from colonization by pathogens like S. mutans (18). In addition, S. mutans is a known opportunistic pathogen, and its role in dental caries and periodontitis has been well established (23, 24). When environmental changes such as e-cig vapor exposure disrupt the oral microbial homeostasis, the decrease in commensals can allow opportunistic pathogens to colonize and cause disease (52). The repetitive exposure to anthropogenic stressors, like e-cigs, can exert an evolutionary pressure that can lead to the dysregulation of the oral bacterial homeostasis. This homeostasis is important because prevalent oral diseases, including dental caries, gingivitis, and periodontitis, are driven by bacteria and their capacity to form dental plaque (51). A major limitation of this study is the restriction to three organisms, when the oral cavity is colonized with complex communities. To expand on that and to provide a more physiological model of Streptococci spp. in the oral cavity, more in vivo and in vitro multispecies studies will be required in the future. In addition, more research needs to be conducted regarding interspecies interactions and the long-term consequences e-cig aerosols could have on host-bacterial interactions with further analysis of host responses.
Based on our data, we can merely speculate that e-cig aerosol exposure can shift the abundance of bacteria present in the oral cavity, as seen with three key species, and result in significant consequences for oral health.
MATERIALS AND METHODS
Cell lines.
Human oral epithelial cells (OKF6) (53) were cultured in keratinocyte serum-free medium (10724-011; Life Technologies Co., Grand Island, NY, USA), supplemented with epithelial growth factor (1 ng/mL), bovine pituitary extract (0.05 mg/mL), and 1% penicillin-streptomycin (15140-122; Gibco Life Technologies Co., Carlsbad, CA, USA). Cells were incubated at 37°C with 5% CO2.
Bacterial cultures.
Streptococcus mutans (ATCC 25175), Streptococcus sanguinis (ATCC 10556), and Streptococcus gordonii (ATCC 51656) were all grown in tryptic soy broth (TSB; 211825; Becton Dickinson, Sparks, MD, USA) at 37°C and 5% CO2. Bacteria were grown in tryptic soy agar (TSA) plates for standard serial dilution plating. Bacteria were subcultured in TSB from an independent colony, snap frozen in liquid nitrogen for 30 min during the exponential growth phase, and stored at −80°C. To determine the number of CFU per milliliter, bacteria were serially diluted in phosphate-buffered saline (PBS) and spot plated (20 μL) in TSA.
Competition assay.
Overnight cultures were diluted 1:10 in TSB. The controls contained only one species, while the competitive growth tubes contained 1 part S. mutans and 1 part commensal (either S. sanguinis or S. gordonii). At 0 h and 24 h, each tube was serially diluted and plated on blood agar medium via spot inoculations, and the number of CFU/mL was calculated for each time point. Hemolysis patterns were analyzed to differentiate between the two Streptococcus spp. S. mutans was identified on the basis of beta hemolysis, while S. sanguinis and S. gordonii were identified via alpha hemolysis. Competitive growth data were shown as fold change by calculating the ratio between the 24-h and the 0-h time points. Regarding the supernatant exposure assay, S. sanguinis and S. gordonii were grown in TBS supplemented with 1 mL PBS (control group) or 1 mL of S. mutans supernatant. After 24 h, numbers of CFU were determined. Fold changes was determined based on the CFU/mL data.
E-cigarette aerosol exposure.
Commercially available e-liquid was purchased from Vapor Vapes, all prepared with a base of 50% propylene glycol and 50% vegetable glycerol (50/50 PG/VG). In this study, we had four experimental groups: the control PG/VG without nicotine or flavoring (NF), the nicotine control with PG/VG and nicotine (3 mg/mL) (Nic), the control flavored PG/VG (menthol) without nicotine (NF-M), and the PG/VG with menthol and nicotine (3 mg/mL) (Nic-M). These four e-liquid combinations were heated by a fourth-generation e-cig (G-Priv Baby kit) and vaporized (one 10-s puff, 5-min exposure) into TSB medium (10 mL in a 10-cm dish) before every experiment.
Growth curve.
TSB was pretreated with e-cigarette aerosols (one 10-s puff, 5-min exposure) before every experiment. Overnight cultures were diluted 1:10 in pretreated medium and loaded in a 96-well plate (100 μL per well) incubated for 24 h at 37°C in a Biotek Synergy plate reader. During this incubation, optical density (600 nm) readings were done every 5 min. Growth curve data were graphed and analyzed on GraphPad (Prism 8).
Biofilm formation.
TSB was pretreated with e-cigarette aerosols (10-s puff, 5-min exposure), and overnight cultures were diluted 1:10 in pretreated medium and incubated for 24 h in a 96-well plate (100 μL per well). After 24 h of incubation, plates were washed with deionized water and air dried at 37°C for 20 min. To stain the biofilm, safranin (65092B-95; Millipore Sigma, Darmstadt, Germany; 125 μL containing 6.04g/L safranin, 19% ethanol, and 1% methanol, diluted in water) was added to the wells and incubated at room temperature for 20 min. Safranin was discarded, and the wells were washed with deionized water and air dried at 37°C. The biofilm was dissociated by resuspending in ethanol-acetone mix (80:20). The absorbance was read at OD490. Results were normalized to blanks, graphed, and analyzed on GraphPad Prism (Prism 8).
Hydrophobicity assay.
To measure bacterial surface hydrophobicity, we carried out a hydrophobicity assay (54), as previously described by Serebryakova et al. (54) with slight modifications. Bacterial cultures at stationary phase (2 mL) were mixed with chloroform (500 μL), vortexed for 2 min, and incubated at 37°C for 15 min to allow separation between the aqueous and hydrophobic phases. During this separation, the optical density of the aqueous (hydrophilic) phase decreases, as the hydrophobic bacteria moves into the chloroform (hydrophobic) phase. The aqueous layer (Aa) was collected, and OD600 was measured. The OD600 of the overnight culture (At) was measured to account for the total bacteria before the phase separation. The percent hydrophobicity was calculated as follows: [(At − Aa)/At] × 100.
Bacterial attachment.
To visualize bacterial attachment to oral human cells, cells were seeded at a confluence of 300,000 cells/well in a 4-chamber slide (154526; Nalge Nunc International, Rochester, NY, USA). Bacterial cells were added to the slide at an MOI of 10. After the 3-h coculture, the slide was rinsed extensively with PBS to remove any unattached bacterial cells and then fixed with methanol and acetone (1:1) for 10 min. Cells were then stained with crystal violet and visualized with a bright-field microscope. For quantification of attachment and internalization, oral human cells were seeded at a confluence of 2.5 × 105 cells/mL in 24-well plates and cocultured with e-cig-pretreated streptococcal strains at an MOI of 1 for 3 h. At the end of the incubation time, OKF6 cells were washed with PBS, and the remaining cells with attached and potentially internalized bacteria were permeabilized with 1% Triton X-100 for 10 min followed by a 1:10 dilution with sterile PBS. Next, the lysates were serially diluted and plated to enumerate CFU/mL. The final attachment counts were normalized to the initial number of input bacteria (both in CFU/mL) per well. For each experimental condition, counts of attached S. mutans bacteria were compared to those of both commensals by calculating the ratios of S. mutans to S. sanguinis and of S. mutans to S. gordonii. This ratio indicates the fold change between the attachment capacities of the commensal and pathogenic strains.
Analysis and statistics.
Experiments were performed in biological replicates, each consisting of at least three technical replicates. The data are presented as means and standard errors of the means (SEM; n = 3). When comparing only two conditions, we used Student’s t test (Welch’s correction) to analyze statistically significant differences. When comparing multiple conditions to the untreated control, we used one-way analysis of variance (ANOVA) (Dunnett's correction) to analyze statistical significance differences.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a COM grant to C.D.A. and by F31DE029102 to A.C.-V.
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Claudia D. Andl, Email: claudia.andl@ucf.edu.
Justin R. Kaspar, Ohio State University
REFERENCES
- 1.Pushalkar S, Paul B, Li Q, Yang J, Vasconcelos R, Makwana S, González JM, Shah S, Xie C, Janal MN, Queiroz E, Bederoff M, Leinwand J, Solarewicz J, Xu F, Aboseria E, Guo Y, Aguallo D, Gomez C, Kamer A, Shelley D, Aphinyanaphongs Y, Barber C, Gordon T, Corby P, Li X, Saxena D. 2020. Electronic cigarette aerosol modulates the oral microbiome and increases risk of infection. iScience 23:100884. doi: 10.1016/j.isci.2020.100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, King BA. 2020. E-cigarette use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep 69:1310–1312. doi: 10.15585/mmwr.mm6937e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Wang X, Bertrand D, Kern F, Zhang T, Duleba M, Srivastava S, Khor CC, Hu Y, Wilson LH, Blaszyk H, Rolshud D, Teh M, Liu J, Howitt BE, Vincent M, Crum CP, Nagarajan N, Ho KY, McKeon F, Xian W. 2016. Mutational spectrum of Barrett’s stem cells suggests paths to initiation of a precancerous lesion. Nat Commun 7:10380. doi: 10.1038/ncomms10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang I, Sandeep S, Rodriguez J. 2020. The oral health impact of electronic cigarette use: a systematic review. Crit Rev Toxicol 50:97–127. doi: 10.1080/10408444.2020.1713726. [DOI] [PubMed] [Google Scholar]
- 5.Grana RA, Ling PM. 2014. “Smoking revolution.” A content analysis of electronic cigarette retail websites. Am J Prev Med 46:395–403. doi: 10.1016/j.amepre.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustamante G, Ma B, Yakovlev G, Yershova K, Le C, Jensen J, Hatsukami DK, Stepanov I. 2018. Presence of the carcinogen N′-nitrosonornicotine in saliva of e-cigarette users. Chem Res Toxicol 31:731–738. doi: 10.1021/acs.chemrestox.8b00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. 2014. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health 11:11. doi: 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caponnetto P, Maglia M, Prosperini G, Busà B, Polosa R. 2018. Carbon monoxide levels after inhalation from new generation heated tobacco products. Respir Res 19:164. doi: 10.1186/s12931-018-0867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grana RA, Ling PM, Benowitz N, Glantz S. 2014. Electronic cigarettes. Circulation 129:e490–e492. doi: 10.1161/CIRCULATIONAHA.114.008545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, Benowitz N. 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. 2015. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 372:392–394. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 12.Lee H-W, Park S-H, Weng M-W, Wang H-T, Huang WC, Lepor H, Wu X-R, Chen L-C, Tang M-S. 2018. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA 115:E1560–E1569. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH, Breen EC. 2018. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol 314:R834–R847. doi: 10.1152/ajpregu.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atuegwu NC, Perez MF, Oncken C, Thacker S, Mead EL, Mortensen EM. 2019. Association between regular electronic nicotine product use and self-reported periodontal disease status: population assessment of tobacco and health survey. Int J Environ Res Public Health 16:1263. doi: 10.3390/ijerph16071263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganesan SM, Dabdoub SM, Nagaraja HN, Scott ML, Pamulapati S, Berman ML, Shields PG, Wewers ME, Kumar PS. 2020. Adverse effects of electronic cigarettes on the disease-naive oral microbiome. Sci Adv 6:eaaz0108. doi: 10.1126/sciadv.aaz0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart CJ, Auchtung TA, Ajami NJ, Velasquez K, Smith DP, De La Garza R, II, Salas R, Petrosino JF. 2018. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: a pilot study. PeerJ 2018:e4693. doi: 10.7717/peerj.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopyk J, Bojanowski CM, Shin J, Moshensky A, Fuentes AL, Bonde SS, Chuki D, Pride DT, Crotty Alexander LE. 2021. Compositional differences in the oral microbiome of e-cigarette users. Front Microbiol 12:0–1250. doi: 10.3389/fmicb.2021.599664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreth J, Merritt J, Qi F. 2009. Bacterial and host interactions of oral streptococci. DNA Cell Biol 28:397–403. doi: 10.1089/dna.2009.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson JM, Cuadra GA, Palazzolo DL. 2019. A comparison of flavorless electronic cigarette-generated aerosol and conventional cigarette smoke on the planktonic growth of common oral commensal streptococci. Int J Environ Res Public Health 16:5004. doi: 10.3390/ijerph16245004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuadra GA, Smith MT, Nelson JM, Loh EK, Palazzolo DL. 2019. A comparison of flavorless electronic cigarette-generated aerosol and conventional cigarette smoke on the survival and growth of common oral commensal streptococci. Int J Environ Res Public Health 16:1669. doi: 10.3390/ijerph16101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischman JS, Sista S, Lee DK, Cuadra GA, Palazzolo DL. 2020. Flavorless vs. flavored electronic cigarette-generated aerosol and e-liquid on the growth of common oral commensal streptococci. Front Physiol 11:585416. doi: 10.3389/fphys.2020.585416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contardo MS, Díaz N, Lobos O, Padilla C, Giacaman RA. 2011. Oral colonization by Streptococcus mutans and its association with the severity of periodontal disease in adults. Rev Clín Periodon Implantol Rehabil Oral 4:9–12. doi: 10.1016/S0718-5391(11)70058-1. [DOI] [Google Scholar]
- 24.Dani S, Prabhu A, Chaitra K, Desai N, Patil S, Rajeev R. 2016. Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: a clinico-microbiological study. Contemp Clin Dent 7:529–534. doi: 10.4103/0976-237X.194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreth J, Merritt J, Shi W, Qi F. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol 187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowden GH, Hamilton IR. 1987. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and “S. mitior” growing in continuous culture. Can J Microbiol 33:824–827. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- 27.Valdebenito B, Tullume-Vergara PO, González W, Kreth J, Giacaman RA. 2018. In silico analysis of the competition between Streptococcus sanguinis and Streptococcus mutans in the dental biofilm. Mol Oral Microbiol 33:168–180. doi: 10.1111/omi.12209. [DOI] [PubMed] [Google Scholar]
- 28.Díaz-Garrido N, Lozano CP, Kreth J, Giacaman RA. 2020. Competition and caries on enamel of a dual-species biofilm model with Streptococcus mutans and Streptococcus sanguinis. Appl Environ Microbiol 86:e01262-20. doi: 10.1128/AEM.01262-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaegers NR, Hu W, Weber TJ, Zhi Hu J. 2021. Low-temperature (< 200°C) degradation of electronic nicotine delivery system liquids generates toxic aldehydes. Sci Rep 11:7800. doi: 10.1038/s41598-021-87044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nalawade TM, Bhat K, Sogi PSH. 2015. Bactericidal activity of propylene glycol, glycerine, polyethylene glycol 400, and polyethylene glycol 1000 against selected microorganisms. J Int Soc Prev Community Dent 5:114. doi: 10.4103/2231-0762.155736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gambino M, Cappitelli F. 2016. Biofilm responses to oxidative stress. Biofouling 32:167–178. doi: 10.1080/08927014.2015.1134515. [DOI] [PubMed] [Google Scholar]
- 32.Jefferson KK. 2004. What drives bacteria to produce a biofilm? FEMS Microbiol Lett 236:163–173. doi: 10.1016/j.femsle.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Zhu B, Song L, Kong X, Macleod LC, Xu P. 2018. A novel regulator modulates glucan production, cell aggregation and biofilm formation in Streptococcus sanguinis SK36. Front Microbiol 9. doi: 10.3389/fmicb.2018.01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krausova G, Hyrslova I, Hynstova I. 2019. In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation 5:100. doi: 10.3390/fermentation5040100. [DOI] [Google Scholar]
- 35.Courtney HS, Ofek I, Penfound T, Nizet V, Pence MA, Kreikemeyer B, Podbielski A, Podbielbski A, Hasty DL, Dale JB. 2009. Relationship between expression of the family of M proteins and lipoteichoic acid to hydrophobicity and biofilm formation in Streptococcus pyogenes. PLoS One 4:e4166. doi: 10.1371/journal.pone.0004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krasowska A, Sigler K. 2014. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol 4:112. doi: 10.3389/fcimb.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yumoto H, Hirota K, Hirao K, Ninomiya M, Murakami K, Fujii H, Miyake Y. 2019. The pathogenic factors from oral streptococci for systemic diseases. Int J Mol Sci 20:4571. doi: 10.3390/ijms20184571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanioka T, Morita M, Yamamoto T, Inagaki K, Wang P-L, Ito H, Morozumi T, Takeshita T, Suzuki N, Shigeishi H, Sugiyama M, Ohta K, Nagao T, Hanada N, Ojima M, Ogawa H. 2019. Smoking and periodontal microorganisms. Jpn Dent Sci Rev 55:88–94. doi: 10.1016/j.jdsr.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodall M, Jacob J, Kalsi KK, Schroeder V, Davis E, Kenyon B, Khan I, Garnett JP, Tarran R, Baines DL. 2020. E-cigarette constituents propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 319:L957–L967. doi: 10.1152/ajplung.00123.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang R, Li M, Gregory RL. 2015. Nicotine promotes Streptococcus mutans extracellular polysaccharide synthesis, cell aggregation and overall lactate dehydrogenase activity. Arch Oral Biol 60:1083–1090. doi: 10.1016/j.archoralbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Huang R, Zhou X, Qiu W, Xu X, Gregory RL. 2016. Effect of nicotine on cariogenic virulence of Streptococcus mutans. Folia Microbiol (Praha) 61:505–512. doi: 10.1007/s12223-016-0465-8. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Wu T, Zhou X, Zhang B, Huo S, Yang Y, Zhang K, Cheng L, Xu X, Li M. 2018. Nicotine is a risk factor for dental caries: an in vivo study. J Dent Sci 13:30–36. doi: 10.1016/j.jds.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rouabhia M, Semlali A. 2021. Electronic cigarette vapor increases Streptococcus mutans growth, adhesion, biofilm formation, and expression of the biofilm-associated genes. Oral Dis 27:639–647. doi: 10.1111/odi.13564. [DOI] [PubMed] [Google Scholar]
- 44.Lucena PH, Biondi L, de Torres R. 2010. Hydrophobicity test in mutans streptococci. Acta Odontol Latinoam 23:210–215. [PubMed] [Google Scholar]
- 45.Ebersole DJL, Peyyala R, Gonzalez OA. 2019. Biofilm induced profiles of immune response gene expression by oral epithelial cells. Mol Oral Microbiol 34:12251. doi: 10.1111/omi.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eberhard J, Pietschmann R, Falk W, Jepsen S, Dommisch H. 2009. The immune response of oral epithelial cells induced by single-species and complex naturally formed biofilms. Oral Microbiol Immunol 24:325–330. doi: 10.1111/j.1399-302X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 47.Desvaux M, Dumas E, Chafsey I, Hébraud M. 2006. Protein cell surface display in Gram-positive bacteria: from single protein to macromolecular protein structure. FEMS Microbiol Lett 256:1–15. doi: 10.1111/j.1574-6968.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 48.Nobbs AH, Lamont RJ, Jenkinson HF. 2009. Streptococcus adherence and colonization. Microbiol Mol Biol Rev 73:407–450. doi: 10.1128/MMBR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elliott D, Harrison E, Handley PS, Ford SK, Jaffray E, Mordan N, McNab R. 2003. Prevalence of Csh-like fibrillar surface proteins among mitis group oral streptococci. Oral Microbiol Immunol 18:114–120. doi: 10.1034/j.1399-302x.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 50.Handley PS, Harty DWS, Wyatt JE, Brown CR, Doran JP, Gibbs ACC. 1987. A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. J Gen Microbiol 133:3207–3217. doi: 10.1099/00221287-133-11-3207. [DOI] [PubMed] [Google Scholar]
- 51.Handley PS, Carter PL, Fielding J. 1984. Streptococcus salivarius strains carry either fibrils or fimbriae on the cell surface. J Bacteriol 157:64–72. doi: 10.1128/jb.157.1.64-72.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wade WG. 2013. The oral microbiome in health and disease. Pharmacol Res 69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 20:1436–1447. doi: 10.1128/MCB.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serebryakova EV, Darmov IV, Medvedev NP, Alekseev SM, Rybak SI. 2002. Evaluation of the hydrophobicity of bacterial cells by measuring their adherence to chloroform drops. Mikrobiologiia 71:237–239. (In Russian.). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM02421-21_Supp_1_seq9.pdf, PDF file, 0.8 MB (863.4KB, pdf)