Abstract
The blaFEZ-1 gene coding for the metallo-β-lactamase of Legionella (Fluoribacter) gormanii ATCC 33297T was overexpressed via a T7 expression system in Escherichia coli BL21(DE3)(pLysS). The product was purified to homogeneity in two steps with a yield of 53%. The FEZ-1 metallo-β-lactamase exhibited a broad-spectrum activity profile, with a preference for cephalosporins such as cephalothin, cefuroxime, and cefotaxime. Monobactams were not hydrolyzed. The β-lactamase was inhibited by metal chelators. FEZ-1 is a monomeric enzyme with a molecular mass of 29,440 Da which possesses two zinc-binding sites. Its zinc content did not vary in the pH range of 5 to 9, but the presence of zinc ions modified the catalytic efficiency of the enzyme. A model of the FEZ-1 three-dimensional structure was built.
Metallo-β-lactamases (class B of the molecular classification of Ambler [1] or group 3 according to the functional classification of Bush et al. [6]) constitute a very heterogeneous family. Although their primary structures exhibit low degrees of sequence isology (38) (generally less than 43%), their three-dimensional structures show high degrees of similarity (7, 8, 9, 12, 35). All class B β-lactamases share five main characteristics (38): (i) they hydrolyze carbapenem compounds, (ii) they do not interact with monobactams, (iii) they are inhibited by chelating agents such as dipicolinic acid and 1,10-o-phenanthroline, (iv) they contain zinc ions as the naturally occurring cation, and (v) they exhibit two metal-binding sites.
On the basis of structural analyses, these enzymes cluster into three different groups: subclass B1 contains most known zinc β-lactamases (for example, BcII from Bacillus cereus 569H [18], CcrA [also named CfiA] from Bacteroides fragilis [29], and the plasmid-encoded enzyme IMP-1 found in some isolates of Pseudomonas aeruginosa, Serratia marcescens, and other gram-negative bacteria [19, 24]), subclass B2 includes the Aeromonas enzymes (CphA [21], ImiS [37], and CphA2 [28]), and subclass B3 contains the tetrameric L1 enzyme produced by Stenotrophomonas maltophilia (33, 36) and the Chryseobacterium meningosepticum GOB-1 (2) and the Legionella (Fluoribacter) gormanii FEZ-1 (4) metallo-β-lactamases.
In the known crystal structures of metallo-β-lactamases, Zn-1 is tetrahedrally coordinated to three histidines and a water molecule. When present, Zn-2 interacts with three residues (a cysteine, an aspartic acid, and a histidine in the case of BcII, IMP-1, and CcrA or an aspartic acid and two histidines for L1) and two water molecules in a trigonal pyramid. In the di-zinc form, one water molecule is bridged between the metal ions.
The enzymes of subclass B1 are monomeric proteins. They possess a broad-spectrum activity profile (10, 13, 14, 20, 26, 40) and are inhibited by thiol compounds such as SB25566 (9, 16, 34). Interestingly, the mono- and di-zinc form of the BcII and CcrA enzymes are nearly equally active. Kinetic and spectroscopic studies indicated that for both forms a transient noncovalent intermediate is formed during the hydrolysis of the substrate.
To date, no structure of a subclass B2 enzyme is available. For these β-lactamases, the optimal activity is observed with the mono-zinc form. The second zinc ion behaves as a noncompetitive inhibitor (17). Only carbapenems are efficiently hydrolyzed by these enzymes (13), while all other β-lactams are poor substrates. In addition, cephamycins and oxacephems behave as poor inactivators of CphA (14, 27).
The enzymes belonging to subclass B3 can be either monomeric (GOB-1) or multimeric (L1). Detailed kinetic studies performed on the L1 and GOB-1 metallo-β-lactamases showed that the enzymes exhibit broad-spectrum activity profiles (2, 10). In FEZ-1, all the residues which interact with the zinc ions in L1 are conserved (4), while in GOB-1 the first histidine is replaced by a glutamine residue (2). Interestingly, preliminary biochemical studies of the L. gormanii FEZ-1 metallo-β-lactamase reveal a broad-spectrum activity profile, but with a striking preference for cephalosporin compounds (4, 15).
In the work described here we produced the FEZ-1 metallo-β-lactamase of L. gormanii ATCC 32197T in Escherichia coli. The enzyme was purified to homogeneity, and a detailed kinetic study was performed. The effects of various chelating agents, pH, and zinc ion concentration on enzyme activity were tested. In addition, a molecular model of the enzyme structure was built by knowledge-based modeling methods.
(The results described here were presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17 to 20 September 2000.)
MATERIALS AND METHODS
Antibiotics and other chemicals.
Choramphenicol, ampicillin (Δɛ235 = −820 M−1 cm−1), cephalothin (Δɛ260 = −6,500 M−1 cm−1), cephaloridine (Δɛ260 = −100,000 M−1 cm−1), cefoxitin (Δɛ260 = −7,000 M−1 cm−1), cefotaxime (Δɛ260 = −7,500 M−1 cm−1), methicillin (Δɛ260 = −100 M−1 cm−1), carbenicillin (Δɛ235 = −780 M−1 cm−1), cloxacillin (Δɛ260 = +140 M−1 cm−1), EDTA, pyridine-2,6-dicarboxylic acid (dipicolinic acid), and 1,10-o-phenanthroline were purchased from Sigma Chemical Co. (St. Louis, Mo.). Kanamycin was purchased from Merck (Darmstadt, Germany). Isopropyl-β-d-thiogalactopyranoside (IPTG) was purchased from Eurogentech (Liège, Belgium). Imipenem (Δɛ300 = −9,000 M−1 cm−1) was a gift from Merck Sharp & Dohme Research Laboratories (Rahway, N.J.). Meropenem (Δɛ298 = −6,500 M−1 cm−1) was a gift from ICI Pharmaceuticals (Macclesfield, England). Biapenem (Δɛ294 = −9,960 M−1 cm−1) and piperacillin (Δɛ235 = −820 M−1 cm−1) were gifts from Wyeth Lederle (Tokyo, Japan). Nitrocefin (Δɛ482 = +15,000 M−1 cm−1) was purchased from Unipath Oxoid (Basingstoke, United Kingdom). Benzylpenicillin (Δɛ235 = −775 M−1 cm−1) was a gift from Rhône-Poulenc (Paris, France). Cefuroxime (Δɛ260 = −7,600 M−1 cm−1) and ceftazidime (Δɛ260 = −9,000 M−1 cm−1) were from Glaxo Group Research (Greenford, United Kingdom). Temocillin (Δɛ235 = −660 M−1 cm−1), ticarcillin (Δɛ235 = −660 M−1 cm−1), 6-aminopenicillanic acid (6-APA; Δɛ235 = −690 M−1 cm−1), and clavulanic acid were gifts from SmithKline Beecham Pharmaceuticals (Brentford, United Kingdom). Cefepime (Δɛ260 = −10,000 M−1 cm−1) and aztreonam (Δɛ320 = −700 M−1 cm−1) were gifts from S.A. Bristol-Meyers Squibb (Brussels, Belgium). Tazobactam (Δɛ235 = −1,970 M−1 cm−1) was a gift from Wyeth-Ayerst Laboratories (West Chester, Pa.). Moxalactam (Δɛ260 = −4,000 M−1 cm−1) and cefpodoxime (Δɛ260 = −10,000 M−1 cm−1) were gifts from Sankyo Pharmaceuticals (Tokyo, Japan).
Bacterial strains and vectors.
Plamids pBLL/FEZ-1 and pET24/FEZ-1 have been described previously (4). E. coli XL1-Blue (Stratagene Inc., La Jolla, Calif.) was used as the host for recombinant plasmids during construction of the expression vectors. E. coli BL21(DE3)(pLysS) (Novagen Inc., Madison, Wis.) was used as the host for the T7-based expression vectors in overexpression experiments. Plasmid pCR 2.1 (TA Cloning Kit; Invitrogen BU, NV Leek, The Netherlands) was used to clone the PCR products. The expression vector pET26b(+) (Novagen Inc.) was used for the construction of the T7-based expression vector.
Construction of expression vector and preliminary expression experiments.
The putative position of the signal peptidase cleavage site in the FEZ-1 pre-β-lactamase was calculated with the help of SignalP program (23), available under the server page of the Centre for Biological sequence analysis (http://www.cbs.dtu.dk).
To allow the removal of the predicted signal peptide amino acid sequence, the NdeI or NcoI restriction site was introduced into blaFEZ-1 after the signal peptide nucleotide sequence. A BamHI restriction site was created after the stop codon of the metallo-β-lactamase gene in order to eliminate all unwanted DNA sequence. All these sites were generated by PCR. Primers NdeILegi (5′-TCACATATGGCATATCCAATGCCAAATCCTTTTCCC-3′) and BamHILegi (5′-CTGGGATCCTGAACAATTAGGCAGTTTCTTCTT-3′) or primers NcoILegi (5′-CAACCATGGCATATCCAATGCCAAATCCTTTTCCC-3′) and BamHILegiwere the two oligonucleotide primer pairs used for this purpose (newly introduced restriction sites are underlined).
PCR conditions were as follows: incubation at 95°C for 5 min and 30 cycles of amplification (denaturation at 95°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min). The tth DNA polymerase (Eurogentec, Seraing, Belgium) was used for PCR. The PCR products (790 bp) were cloned into the pCR 2.1 vector to obtain recombinant plasmids named pDML1807 (NdeI restriction site) and pDML1808 (NcoI restriction site). These plasmids were used to transform E. coli XL1-Blue competent cells. The nucleotide sequences of the PCR-generated fragments were verified in order to rule out the presence of any unwanted mutations. pDML1807 was digested with the NdeI and BamHI restriction enzymes and pDML1808 was digested with the NcoI and BamHI restriction enzymes, and then the digested fragments were subcloned into the pET26b(+) vector. The corresponding plasmids, named pDML1809 and pDML1810, respectively, were introduced into E. coli BL21(DE3)(pLysS) competent cells. In pDML1810 the gene coding for the mature form of FEZ-1 was fused in frame with the nucleotide sequence of the PelB signal peptide present in the pET26b(+) vector. Since the amino acid sequence of the N-terminal part of FEZ-1 possesses a peculiar proline-rich motif (PMPNPFPP), a third expression vector based on pET26b(+) was constructed in which this proline-rich sequence was removed by introducing by PCR an NcoI restriction site after the nucleotide sequence coding for the polyproline motif. The primers used in this case were NcoILegi2 (5′-CCCATGGCTGGAAACTTGTACTATGTAGGCACTGAT-3′; newly introduced restriction sites are underlined) and BamHILegi. This PCR product was cloned into the pCR 2.1 vector to yield recombinant plasmid pDML1811. The NcoI-BamHI fragment isolated after digestion of pDML1811 was cloned in pET26b(+) to yield recombinant plasmid pDML1812. Also, in this case, the gene coding for the truncated β-lactamase was fused to the PelB signal peptide sequence.
In preliminary expression experiments, single colonies of E. coli BL21(DE3)(pLysS) with pDML1809, pDML1810, pDML1812, or pET24/FEZ-1 were used to inoculate 6 ml of Super broth (SB) (32) supplemented with kanamycin (50 μg/ml) and chloramphenicol (30 μg/ml). The cultures were incubated overnight at 37 or 28°C with orbital shaking at 250 rpm. A total of 2.5 ml of the different cultures was added to 100 ml of SB that had been preincubated at 37 or 28°C. The bacteria were grown to an optical density at 600 nm of 0.6, and IPTG was added at a final concentration of 0.1 or 0.5 mM. Aliquots (1 ml) of the different cultures were withdrawn at 0, 2, 4, 6, and 10 h after induction. After centrifugation at 5,000 × g for 10 min, the bacterial pellet was resuspended in 15 mM sodium cacodylate buffer (pH 6.0) and sonicated (three times for 30 s each time at 12 W). The cells debris was eliminated by centrifugation at 13,000 × g for 30 min. A total of 20 μl of the different solutions was loaded onto a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel. The run was performed at a constant voltage (120 V). The protein concentration of each crude extract was measured with the help of the BCA (Pierce, Rockford, Ill.) kit. The β-lactamase activity of each preparation was determined by measuring the initial rate of hydrolysis of 100 μM cefuroxime.
Production and purification of the zinc β-lactamase.
The FEZ-1 enzyme was produced by E. coli BL21(DE3)(pLysS) carrying pDML1810 in SB medium containing kanamycin and choramphenicol as the selecting agents during growth of the bacteria at 28°C under orbital shaking. A total of 40 ml of an overnight preculture in SB was used to inoculate 1 liter of fresh SB supplemented as described above with kanamycin and choramphenicol. IPTG (final concentration, 0.5 mM) was added at an absorbance value of 0.6 at 600 nm, and the culture was continued for 6 h. Cells were harvested by centrifugation (5,000 × g for 10 min at 4°C), and the pellet was resuspended in 100 ml of 30 mM sodium cacodylate buffer (pH 6.0; buffer A). The bacteria were disrupted with the help of cell disrupter equipment (Basic Z model; Constant Systems Ltd., Warwick, United Kingdom). Cell debris was removed by centrifugation (30,000 × g for 45 min at 4°C), and the supernatant was dialyzed overnight against buffer A at 4°C. Thereafter, the crude extract was loaded onto an S-Sepharose FF column (2.6 by 34 cm; Pharmacia, Uppsala, Sweden) equilibrated in buffer A. The column was washed with buffer A until the A280 of the effluent was <0.1, and the enzyme was eluted with a linear salt gradient (0 to 0.5 M) in five column volumes. The active fractions were collected and concentrated on a YM-10 membrane (Amicon, Beverly, Mass.) to a final volume of 5 ml. The sample was loaded in a molecular sieve Sephacryl-100 (1.5 by 56 cm) column equilibrated in 15 mM sodium cacodylate buffer (pH 6.0) containing 0.2 M NaCl. The fractions that exhibited β-lactamase activity were collected and their specific activities were monitored by measuring the rate of hydrolysis of cefuroxime. The purity was also checked by SDS-polyacrylamide gel electrophoresis (PAGE). The fractions that exhibited constant specific activities were pooled (volume, 26 ml) and concentrated to a final concentration of 1 mg/ml. The enzyme preparation was stored at −20°C in 30 mM sodium cacodylate buffer (pH 6.0).
Determination of quaternary structure of native metallo-β-lactamase.
A total of 100 μl of FEZ-1 (1 mg/ml) was loaded onto a molecular sieve (Superdex HR75; 1 by 30 cm; Pharmacia) column equilibrated in 30 mM sodium cacodylate buffer (pH 6.0)–0.2 M NaCl. The sample was eluted with the same buffer at a flow rate of 0.5 ml/min. The column was calibrated with lysozyme (14,400 Da), carbonic anhydrase (31,000 Da), ovalbumin (43,000 Da), and bovine serum albumin (BSA; 66,200 Da) as standard proteins. The volume of the fractions was 1 ml. The retention volumes were measured by monitoring the A280, and the retention volumes for the sample containing β-lactamase were measured by determining the enzyme activity.
Determination of N-terminal sequence and molecular mass.
The N-terminal sequence was determined with the help of a gas-phase sequencer (Prosite 492 protein sequencer; Applied Biosystems, Foster City, Calif.) The Mr of the enzyme was estimated with an electrospray mass spectrometer (VG Bio-Q) upgraded with a Platform source (Micromass, Altrincham, United Kingdom). The samples (100 pmole) were suspended in 0.05% formic acid–50% acetonitrile in water and were injected into the source of the mass spectrometer with a syringe pump (Harvard Instruments, South Natick, Mass.) at a flow rate of 6 μl/min. The capillary was held at 2.7 kV, and the cone voltage was set at 40 V. Fifteen scans covering 600 to 1,500 amu were accumulated for 135 s and processed with the Masslynx software delivered with the instrument. Calibration was performed with horse heart myoglobin.
Determination of kinetic parameters.
Hydrolysis of the antibiotics by FEZ-1 was followed by monitoring the variation in the absorbance of the β-lactam solution in 15 mM sodium cacodylate buffer (pH 6.0). All the measurements were made on a Uvikon 940 spectrophotometer connected to a personal computer via an RS232C interface. The reactions were performed in a total volume of 500 μl at 30°C. BSA (20 μg/ml) was added to diluted solutions of β-lactamase in order to prevent enzyme denaturation. The steady-state kinetic parameters (Km and kcat) were determined by analyzing the complete hydrolysis time courses as described by De Meester et al. (11) or by using the Hanes linearization of the Michaelis-Menten equation.
Inactivation by chelating agents.
The loss of β-lactamase activity was monitored in the presence of different concentrations of EDTA, dipicolinic acid, and 1,10-o-phenanthroline. The progressive inactivation of the enzyme was monitored by analyzing the complete hydrolysis time course of 100 μM cefuroxime in 15 mM sodium cacodylate (pH 6.0), used as a reporter substrate. The dependence of the pseudo-first-order inactivation rate constant (ki) upon the chelating agent concentration was determined.
Zn2+ dependence of FEZ-1 metallo-β-lactamase activity.
Enzyme activity was measured by monitoring the initial rates of hydrolysis of 100 μM cefuroxime, cefotaxime, imipenem, nitrocefin, benzylpenicillin, and moxalactam in 15 mM sodium cacodylate buffer (pH 6.0) containing 100 μM Zn2+. Kinetic parameters were compared with those obtained under the same conditions but without Zn2+.
pH dependence of FEZ-1 metallo-β-lactamase activity.
The Km and kcat values were calculated for cefuroxime, a good substrate of FEZ-1, in the following buffers: 15 mM sodium acetate (pH 5.0), 15 mM sodium cacodylate (pH 6.0), 15 mM HEPES (pH 7.0), and 15 mM HEPES (pH 8.0).
Determination of Zn2+ content of FEZ-1 at different pH values.
The pH dependence of the metal content of the metallo-β-lactamase was measured by inductively coupled mass spectrometry (ICPMS). One milliliter samples of FEZ-1 (0.88 mg/ml) were dialyzed overnight at 4°C against 1 liter of the following buffers: 15 mM sodium acetate (pH 5.0), 15 mM sodium cacodylate (pH 6.0), 15 mM HEPES (pH 7.0), and 15 mM HEPES (pH 8.0). The zinc concentration was determined in the protein sample and the dialyzing buffer by ICPMS, and the Zn/enzyme molar ratio was calculated.
Molecular modeling.
The FEZ-1 β-lactamase structural model was based on the three-dimensional structure of the L1 enzyme from S. maltophilia (35). The molecular model was built with the Homology module of the Insight program (Molecular Simulations, San Diego, Calif.) running on a Silicon Graphics workstation. After model building, energy minimization was achieved with the Discover module of the same package to avoid bad molecular contacts. Finally, the geometric features were analyzed with the Insight II program. The newly proposed BBL numbering of the class B β-lactamases was used (15a).
RESULTS AND DISCUSSION
Overexpression of FEZ-1 β-lactamase in E. coli.
The production of FEZ-1 in E. coli BL21(DE3)(pLysS) was tested with three different expression vectors (pDML1809, pDML1810, and pDML1812). pET24/FEZ-1, which was constructed previously (4), was also included in these experiments for comparison.
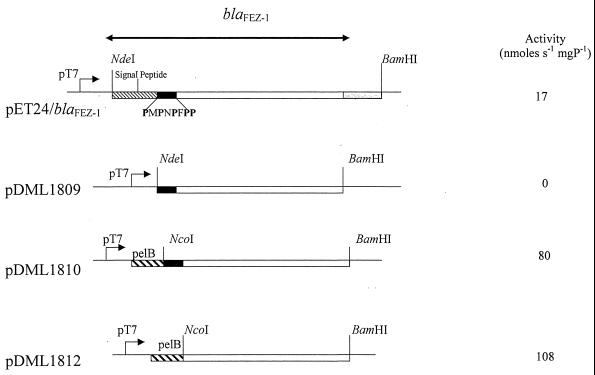
With pDML1809, which encodes a FEZ-1 derivative from which the signal peptide is deleted, no detectable production of metallo-β-lactamase was found when the cultures were grown at 37 or 28°C in the presence or the absence of 500 μM IPTG. With the other constructs, which encode FEZ-1 forms that contain either the original signal peptide (pET24/FEZ-1) or a PelB heterogeneous signal peptide fused with two different protein amino termini (pDML1810 and pDML1812), β-lactamase activity was not detectable when the cultures were grown at 37°C (in the absence or the presence of IPTG) but was detectable in cultures grown at 28°C in the presence of 100 or 500 μM IPTG. In these cases, the production of the FEZ-1 enzyme seemed to be optimal in the presence of 500 μM IPTG. With pDML1810 and pDML1812, the maximum activity was reached after 6 h of induction, when the specific activities of the crude extracts against 100 μM cefuroxime were 80 and 108 nmol · s−1 · mg of protein−1, respectively (Fig. 1).
FIG. 1.
Physical map of the different overproducing constructs. The double-headed arrow delineates the nucleotide sequence of the blaFEZ-1 gene. The nucleotide sequences corresponding to the FEZ-1 signal peptide, the pelB sequence, and the PMPNPFPP peptide are represented by boxes with light dashes, boxes with heavy dashes, and black boxes, respectively. mgP, milligrams of protein.
A different behavior was found with pET24/FEZ-1 which, as reported previously (4), yielded the maximum cell-associated β-lactamase activity 2 h after induction, with a specific activity against cefuroxime of 17 nmol · s−1 · mg of protein−1.
Replacement of the FEZ-1 signal peptide by the PelB leader sequence therefore allowed the production of FEZ-1 in a soluble form and in larger amounts than those obtained with the endogenous signal peptide. Moreover, the highest level of enzyme production was obtained when the proline-rich sequence (PMPNPFPPF) close to the FEZ-1 amino terminus was also deleted. Fractionation of the different cultures in the soluble fraction (periplasm plus cytoplasm) and the insoluble fraction (membrane plus insoluble material) showed that 30% of the FEZ-1 was produced as insoluble material. Nevertheless, in order to use the more physiologically relevant preparation of enzyme, it was decided that strain E. coli BL21(DE3)(pLysS)/pDML1810 would be used for large-scale production.
Purification of FEZ-1 β-lactamase.
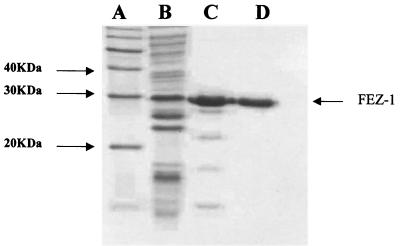
FEZ-1 overproduced in E. coli BL21(DE3)(pLysS) was purified in two chromatography steps as described in Materials and Methods. In the first purification step, the enzyme was eluted from an S-Sepharose column at a salt concentration of 0.2 M. At that stage, the purification yield of FEZ-1 was about 66% (Table 1) and other proteins with a lower molecular masses were revealed by SDS-PAGE (Fig. 2). To remove these contaminants the enzyme preparation was loaded onto a Sephacryl-100 molecular sieve column equilibrated with 30 mM sodium cacodylate (pH 6.0) containing 0.2 M NaCl. The active fractions were pooled and concentrated on an Amicon YM-10 membrane to a final concentration of 1 mg/ml. Above this concentration, a decrease in the specific activity of the preparation was observed, a phenomenon that may be due to partial aggregation of the β-lactamase. The final yield of the purification was 53%. Under these conditions it was not necessary to perform the additional cation-exchange chromatography step described by Fujii et al. (15). The ability to overproduce the zinc β-lactamase in E. coli facilitated the purification process and allowed simplification of the protocol. The N-terminal sequence of the purified FEZ-1 enzyme was AYPMPNPFPPF, as expected. Mass spectrometry confirmed the homogeneity of the protein preparation (data not shown). The Mr value was estimated to be 29,447 ± 7, which is very close to that deduced from the amino acid sequence (Mr = 29,440).
TABLE 1.
Purification of the FEZ-1 metallo-β-lactamase of L. gormanii produced by a 1-liter culture of E. coli BL21(DE3)(pLysS)/pDML1810
| Step | Vol (ml) | Total activity (μmol · min−1) | Total amt of protein (mg) | Sp act (μmol · min−1 · mg−1) | % Yield |
|---|---|---|---|---|---|
| Crude extract | 120 | 292 | 379 | 0.77 | 100 |
| S-Sepharose elution | 10 | 195 | 3.98 | 49 | 66 |
| Sephacryl-100 | 5 | 154 | 2.7 | 57 | 53 |
FIG. 2.
SDS-PAGE after the different purification steps. Lane A, molecular mass standards; lane B, crude extract of E. coli BL21(DE3)(pLysS)/pDML1810; lane C, protein pattern after passage through an S-Sepharose column; lane D, protein pattern after passage through a Sephacryl-100 molecular sieve. The arrow indicates the Mr of FEZ-1.
The subclass B3 FEZ-1 β-lactamase is a monomeric enzyme.
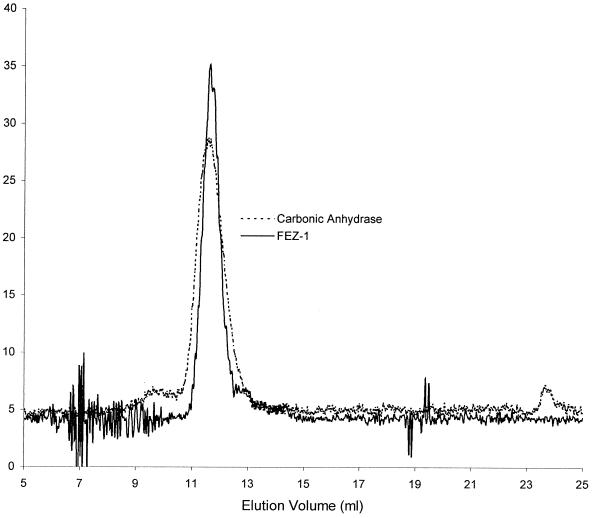
Calibration of the Superdex HR75 column yielded the following retention volumes: lysozyme, 17.38 ml; carbonic anhydrase, 11.42 ml; ovalbumin, 10.08 ml; and BSA, 9.1 ml. The retention volume of FEZ-1 was 11.50 ml, close to that of carbonic anhydrase (Fig. 3). Its molecular mass, determined by molecular sieve chromatography on Superdex HR75, was thus approximately 30,000 Da, indicating that the native enzyme is a monomer, in agreement with data previously obtained with crude extracts (4). Therefore, metallo-β-lactamases of subclass B3 can be either monomeric (FEZ-1, GOB-1) or multimeric (L1), a phenomenon which was not observed for the metallo-β-lactamases of other subclasses.
FIG. 3.
Elution pattern of the FEZ-1 β-lactamase and carbonic anhydrase on a Superdex HR75 molecular sieve column. The values on the y axis represent the A280.
Kinetic parameters of the FEZ-1 enzyme.
The kcat and Km values were determined for a representative set of β-lactam antibiotics (Table 2). The results showed that the β-lactamase exhibited a broad-spectrum activity profile (Table 2), although with notable differences for different substrates. It did not significantly hydrolyze monobactams such as aztreonam and carumonam (kcat/Km <0.0001 μM−1 s−1). The activity of the β-lactamase was not affected by a prolonged incubation (1 h at room temperature) of the enzyme in the presence of both monobactams at a concentration of 1 mM each. In addition, the rate of hydrolysis of nitrocefin was not modified by the presence of a high concentration (1 mM) of aztreonam. Among the suicide substrates designed against active-site serine β-lactamase tested, clavulanic acid (final concentration, 1 mM) was not recognized and tazobactam behaved as a substrate (Table 2).
TABLE 2.
Kinetic parameters of the L. gormanii metallo-β-lactamase produced in E. coli BL21(DE3)(pLysS)
| Substrate | kcat (s−1) | Km (μM) | kcat/Km (μM−1 s−1) |
|---|---|---|---|
| Ampicillin | >5.5 | >5,000 | 0.011 ± 0.003 |
| 6-APA | 30 ± 6 | 1,600 ± 200 | 0.018 ± 0.002 |
| Benzylpenicillin | 70 ± 5 | 590 ± 70 | 0.11 ± 0.02 |
| Carbenicillin | 35 ± 1 | 1,600 ± 100 | 0.023 ± 0.001 |
| Cloxacillin | 90 ± 5 | 700 ± 100 | 0.12 ± 0.02 |
| Piperacillin | 50 ± 2 | 4,200 ± 100 | 0.012 ± 0.01 |
| Temocillin | >65 | >5,000 | 0.013 ± 0.005 |
| Ticarcillin | >65 | >5,000 | 0.013 ± 0.003 |
| Cefepime | >6 | >1,000 | 0.006 ± 0.002 |
| Cefotaxime | 165 ± 15 | 70 ± 8 | 2.4 ± 0.1 |
| Cefpodoxime | 36 ± 6 | 70 ± 7 | 0.51 ± 0.03 |
| Ceftazidime | >4 | >1,000 | 0.004 ± 0.001 |
| Cefuroxime | 320 ± 30 | 50 ± 3 | 6.6 ± 0.4 |
| Cephaloridine | 16 ± 2 | 1,000 ± 150 | 0.016 ± 0.002 |
| Cephalothin | 300 ± 30 | 120 ± 10 | 2.5 ± 0.2 |
| Nitrocefin | 90 ± 9 | 100 ± 10 | 0.9 ± 0.1 |
| Cefoxitin | 3 ± 0.5 | 11 ± 1 | 0.27 ± 0.05 |
| Moxalactam | 3 ± 0.3 | 18 ± 2 | 0.17 ± 0.02 |
| Biapenem | 70 | >1,000 | 0.07 ± 0.02 |
| Imipenem | >200 | >1,000 | 0.2 ± 0.03 |
| Meropenem | 45 ± 2 | 85 ± 3 | 0.5 ± 0.02 |
| Clavulanic acidb | <10−2 | >1,000 | <0.00001 |
| Tazobactam | 40 ± 5 | 700 ± 100 | 0.06 ± 0.01 |
| Aztreonamb | <10−2 | >1,000 | <0.00001 |
| Carumonamb | <10−2 | >1,000 | <0.00001 |
The measurements were performed in 10 mM sodium cacodylate buffer (pH 6) at 30°C.
No hydrolysis was detectable under our experimental conditions.
FEZ-1 exhibited a higher level of activity against some cephalosporins than against penicillins and carbapenems. kcat/Km values for cephalosporins varied from 0.004 to 6.6 μM−1 s−1. The best substrates for the L. gormanii metallo-β-lactamase are cephalothin, cefuroxime, and cefotaxime (an oxyiminocephalosporin). The catalytic efficiency of FEZ-1 against these drugs was higher than 2 μM−1 s−1. On the other hand, ceftazidime and cefepime were poorly hydrolyzed (kcat/Km, <0.006 μM−1 s−1). The comparison of the kinetic constants for the hydrolysis of cephalothin and cefoxitin indicates that the presence of an α-methoxy group at position C-7 negatively affects the activity of the metallo-β-lactamase. The decreased catalytic efficiency versus cefoxitin compared to the catalytic efficiency of cephalothin was accompanied by a 10-fold decrease in the Km values, suggesting that the α-methoxy group at position C-7 has a remarkable detrimental effect on hydrolysis but also enhances recognition of the cephalosporin substrate. This was consistent with the high affinity exhibited by the enzyme for moxalactam. In fact, the Km values for the oxacephamycins were significantly lower than those measured for all other substrates.
All the penicillin derivatives tested were poorly recognized by the FEZ-1 metallo-β-lactamase. The measured Km values were always high (>700 μM). Benzylpenicillin and cloxacillin were the best substrates. The presence of a small lateral chain (6-APA) or the presence of a bulky substituent (as in piperacillin) at position C-6 somewhat decreased the catalytic efficiency. In addition, the presence of a charged C-6 substituent (as in ampicillin, carbenicillin, and ticarcillin) did not significantly modify the enzyme efficiency.
As observed for all the other class B β-lactamases, carbapenems were well hydrolyzed by the L. gormanii enzyme (kcat/Km, >0.07 μM−1 s−1), with meropenem being the best substrate due to its low Km value.
The ratio between the kcat/Km values for different antibiotics and the kcat/Km value for imipenem calculated for different class B β-lactamases underlines the peculiar activity profile of FEZ-1 (Table 3). CphA only hydrolyzed carbapenems efficiently. IMP-1, IMP-2, VIM-2, and L1 exhibited similar activities toward the different β-lactam families. CcrA and BcII had higher levels of activity against penicillins and cephalosporins than against imipenem, while benzylpenicillin was the best substrate of BlaB. Finally, some cephalosporin compounds were the best substrates of FEZ-1. It is interesting that cefoxitin behaved as a rather good substrate for FEZ-1, CcrA, ULA511, IMP-1, IMP-2, VIM-2, and BlaB, a poor substrate for BcII, and an inactivator for CphA.
TABLE 3.
Values of (kcat/Km for an antibiotic)/(kcat/Km for imipenem) for FEZ-1, BcII, CcrA, ULA511, IMP-1, IMP-2, VIM-2, BlaB, and CphAa
| Antibiotic | (kcat/Km for antibiotic)/(kcat/Km for imipenem) for the following enzyme:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| FEZ-1 | BcII | CcrA | ULA511 | IMP-1 | IMP-2 | VIM-2 | BlaB | CphA | |
| Benzylpenicillin | 0.55 | 3.75 | 6.6 | 0.27 | 0.52 | 0.23 | 1.15 | 9.2 | <0.001 |
| Ampicillin | 0.05 | 6 | 2 | 6 | 4 | 4 | ND | ND | <0.001 |
| Ticarcillin | 0.06 | 2 | 1.7 | 3.6 | 0.001 | 0.7 | ND | ND | <0.001 |
| Cephaloridine | 0.08 | 0.16 | 1 | 0.13 | 2 | 0.29 | ND | 0.5 | <0.001 |
| Cephalothin | 13 | ND | 12 | 0.13 | 2 | 4 | 1.3 | ND | <0.001 |
| Cefuroxime | 33 | 6.5 | 9.3 | 3.75 | 0.2 | ND | 0.55 | ND | <0.001 |
| Cefotaxime | 12 | 5.6 | 6 | 3.6 | 0.3 | ND | 0.86 | 0.23 | <0.001 |
| Ceftazidime | 0.02 | ND | 2.6 | ND | 0.15 | 0.2 | 0.9 | ND | <0.001 |
| Cefoxitin | 1.35 | 0.08 | 0.13 | 0.73 | 1.7 | 1 | 0.12 | 0.26 | Inact |
The steady-state kinetic parameters were affected at pH 6 when increasing Zn2+ ion concentrations were added to the enzyme (Table 4). The catalytic efficiencies for cefotaxime, imipenem, and nitrocefin increased in the presence of zinc ions, and the kcat values for nitrocefin and cefotaxime were modified. The corresponding Km values were not influenced by the presence of zinc ions. Even at a high zinc concentration (100 μM), the individual kinetic constant for imipenem could not be determined. In this case, the Km value was larger than 1 mM. A slight decrease in the Km value was found for cefuroxime. Nevertheless, the catalytic efficiency of FEZ-1 against this antibiotic was not significantly modified. The catalytic efficiency of the FEZ-1 β-lactamase against benzylpenicillin and moxalactam was not affected by the zinc concentration. In the case of moxalactam, the kcat and Km value increased by approximately the same amounts, yielding unmodified values of the catalytic efficiency in the presence of an excess of zinc ions.
TABLE 4.
Dependence of the steady-state kinetic parameters kcat, Km, and kcat/Km upon zinc ion concentrationa
| Antibiotic | Zn2+ concn of 100 μM
|
Zn2+ concn of ≤0.4 μM
|
||||
|---|---|---|---|---|---|---|
| kcat (s−1) | Km (μM) | kcat/Km (μM−1 s−1) | kcat (s−1) | Km (μM) | kcat/Km (μM−1 s−1) | |
| Cefuroxime | 330 ± 40 | 35 ± 5 | 9.4 ± 1.3 | 320 ± 30 | 50 ± 3 | 6.6 ± 0.4 |
| Cefotaxime | 430 ± 80 | 70 ± 10 | 6.1 ± 1 | 170 ± 20 | 70 ± 8 | 2.36 ± 0.08 |
| Imipenem | >2,000 | >1,000 | 2 | >200 | >1,000 | 0.2 |
| Nitrocefin | 600 ± 60 | 190 ± 15 | 3.16 ± 0.25 | 90 ± 9 | 100 ± 10 | 0.9 ± 0.1 |
| Benzylpenicillin | 50 ± 5 | 280 ± 35 | 0.18 ± 0.03 | 70 ± 5 | 590 ± 70 | 0.11 ± 0.02 |
| Moxalactam | 10 ± 2 | 82 ± 6 | 0.12 ± 0.01 | 3 ± 0.3 | 18 ± 2 | 0.17 ± 0.02 |
The experiments were done at 30°C and pH 6.
pH dependence of activity and zinc content of FEZ-1.
The pH dependence of the zinc content of the metallo-β-lactamase of L. gormanii was measured by ICPMS the (Table 5). Other metallic ions such as Co(II), Cd(II), Ca(II), Cu(II), Mn(II), Ni(II), and Fe(II) and Fe(II) or Fe(III) were not found. In the absence of excess metal, between pH 6 and 8, two zinc ions were found per enzyme molecule. A similar zinc content was found for the L1 enzyme (10). No measurements of the zinc/enzyme molar ratios were made with the GOB-1 enzyme (2). The presence of a glutamine residue as a putative metal ligand may influence the zinc content of the latter enzyme. All the metallo-β-lactamases studied have contained two zinc-binding sites (8, 17, 20, 25). However, with the exception of metallo-β-lactamases isolated from Aeromonas species and when the mono-zinc species can be prepared, the presence of the second metal ion does not strongly modify the catalytic efficiency of the enzyme (17). The study of the interaction between nitrocefin and the L1 enzyme showed that the second zinc ion might participate in the stabilization of an intermediate (22). The same phenomenon was observed for CcrA (38). In the mono-zinc form, the zinc ion participates in the formation of the nucleophilic hydroxide and provides Lewis acid catalysis by polarization of the carbonyl of the β-lactam ring (5). We can hypothesize that the mechanism of the FEZ-1 enzyme is similar to that of L1. Finally, the pH dependence of the steady-state kinetic parameters (kcat and Km) of FEZ-1 with cefuroxime in the absence of added zinc was measured. A time-dependent inactivation of the enzyme by cefuroxime was observed at pH 5. The ki value was 3 × 10−3 s−1 with 100 μM cefuroxime. The same phenomenon was observed even when the reaction was performed in the presence of 100 μM Zn2+. The Km and kcat values were not strongly modified between pH 6 and 8, with maximum activity detected at pH 6 (kcat = 320 s−1; Km = 48 μM). These data are in good agreement with the results obtained for other metallo-β-lactamases. For example, the maximum activities of the B. cereus 569H (4) and IMP-1 (20) enzymes were observed in the same pH range.
TABLE 5.
Metal/enzyme ratio and catalytic efficiencies of the FEZ-1 zinc β-lactamase as a function of pHa
| pH | Buffer | kcat (s−1) | Km (μM) | kcat/Km (μM−1 s−1) | Zn/enzyme |
|---|---|---|---|---|---|
| 5 | Sodium acetate | Inactivation | Inactivation | Inactivation | 1.5 |
| 6 | Sodium cacodylate | 320 | 50 | 6.4 | 1.8 |
| 7 | HEPES | 250 | 60 | 4.1 | 1.8 |
| 8 | HEPES | 310 | 64 | 4.8 | 1.5 |
The substrate used for this experiment was cefuroxime. Standard deviations were between 10 and 20%.
Inactivation by Zn-chelating agents.
All the chelating agents tested behaved as strong inactivators. In all cases, a time-dependent ki could be measured. The ki values were found to be independent of the chelator concentration and were similar for all three compounds (Table 6). These data indicated that at pH 6 the chelators probably act by scavenging the free metal, and the ki value might represent the rate of dissociation of the protein-zinc ion complex. Interestingly, and in contrast to all the subclass B1 and B2 enzymes (BlaB [31], IMP-1 [20], BcII [unpublished data], CfiA [unpublished data], and CphA [17]), in which inactivation occurs via the formation of a transient enzyme-metal-chelator ternary complex, a similar behavior is observed with the L1 subclass B3 enzyme (3) and the ki values are strikingly similar (from 1.7 × 10−2 to 3.8 × 10−2 s−1). In addition, the concentration needed to completely inactivate FEZ-1 is far lower than the concentration needed to completely inactivate the other enzymes tested (17, 20). For example, EDTA and dipicolinic acid behaved as poor inactivators of the IMP-1 β-lactamase. IMP-1 could be inactivated by behaved the enzyme in the presence of 10 mM EDTA or 300 μM dipicolinic acid (20). It would be interesting to further analyze the mechanisms of action of chelating agents against the other subclass B3 enzymes.
TABLE 6.
Inactivation of the L. gormanii metallo β-lactamase by chelating agents
| Chelating agent | Concn range (μM) | ki (s−1) |
|---|---|---|
| EDTA | 0.5–10 | (2.5 ± 0.3) × 10−2 |
| 1,10-o-Phenanthroline | 2.5–50 | (2.5 ± 0.2) × 10−2 |
| Dipicolinic acid | 1–15 | (3.8 ± 0.5) × 10−2 |
Structural features of the FEZ-1 enzyme.
Molecular modeling of the FEZ-1 enzyme on the basis of the known structure of the L1 metallo-β-lactamase revealed some interesting features.
(i) Overall fold.
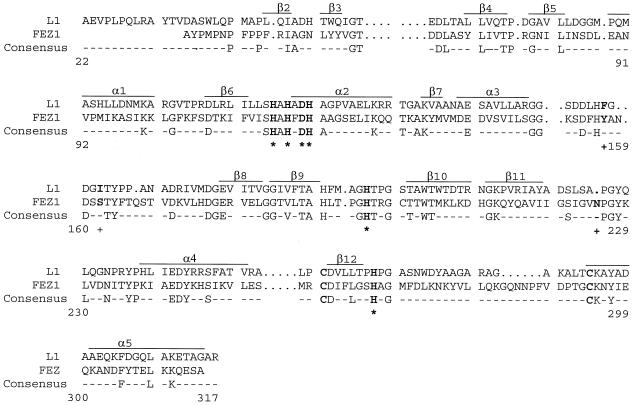
As already pointed out by Boschi et al. (4), FEZ-1 and L1 could be aligned over the entire sequence without the introduction of major gaps (Fig. 4). The major differences between L1 and FEZ-1 are the absence of the N-terminal 310 helix region, a two-residue insertion in the loop between α3 and β8, and a six-residue insertion in the loop between β12 and the C-terminal α5 helix. The latter loop is already considerably longer in L1 than in the subclass B1 enzymes, and it is constrained by an intramolecular disulfide bridge that links the cysteine residues at positions 256 and 290 (35). Such a disulfide bridge between cysteine residues can be predicted in FEZ-1 (Fig. 4).
FIG. 4.
Comparison of the amino acid sequence of the mature zinc β-lactamase of L. gormanii (FEZ-1) with that of the S. maltophilia zinc β-lactamase (L1). The consensus sequence indicates the identical amino acid residues. Residues involved in zinc coordination are in boldface and are marked by asterisks. The residues in boldface and labeled with a plus sign are residues which may interact with the C-7 side chain of cefuroxime. Cysteine residues in boldface are presumably involved in a disulfide bridge in the FEZ-1 metallo-β-lactamase.
(ii) Zinc ligands.
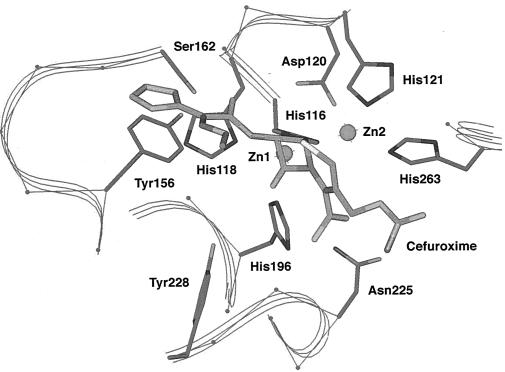
The six residues known to be involved in Zn2+ binding in the L1 β-lactamase are conserved in the FEZ-1 enzyme, i.e., His-116, His-118, and His-196 for the first Zn2+ binding site and Asp-120, His-121, and His-263 for the second one. The orientations of the zinc ligands are maintained by an extensive hydrogen bond network which is also very similar in the two enzymes. However, the group that orients the first His is the Asp-220 side chain in L1 and the Ser-262 side chain via a water molecule in FEZ-1, as in most subclass B1 enzymes.
(iii) Substrate binding site.
In the case of subclass B1 β-lactamases (7, 8, 9), most investigators postulate that the carbonyl oxygen of the β-lactam substrate lies in an oxyanion hole formed by Zn-1 and the side chain of a conserved Asn (Asn-233), while the substrate carboxylate moiety interacts with the ammonium group of a conserved lysine residue (Lys 224). In L1, the side chain of the Asn is 14 Å away from the substrate carbonyl oxygen, and it is proposed that in this case the oxyanion is stabilized by interaction with the side chain of Tyr-228 (35). This tyrosine is conserved and could play the same role in FEZ-1. In turn, the lysine that interacts with the β-lactam carboxylate in subclass B1 β-lactamases is replaced by Ser-223 in L1 and by a Gly residue in FEZ-1. However, just after this Gly, an Asn residue is inserted in the loop that connects β11 and α4. The side chain of this Asn-233 is ideally placed to interact with the substrate carboxylate, especially those of cephalosporins (Fig. 5). The hydrophobic β substituent of the β-lactam substrate generally fits in a hydrophobic pocket formed by the “flap” that connects β3 and β4 and by the loop between α3 and β8, which is considerably longer in subclass B3 enzymes than in subclass B1 enzymes. In this pocket, the hydrophobic residues Phe-156 and Ile-162 in L1 are replaced by a Tyr and a Ser residue in FEZ-1, respectively. These substitutions should influence the substrate specificity, with a facilitated interaction between FEZ-1 and β-lactams that bear a less hydrophobic β side chain.
FIG. 5.
Active site of the modeled structure of the FEZ-1 enzyme showing the side chains of the amino acids discussed in the text. A molecule of cefuroxime is docked in the catalytic cavity.
Conclusions.
The study of the FEZ-1 metallo-β-lactamase underlines the heterogeneity of the biochemical properties of the class B β-lactamases. The enzyme is monomeric and contains two zinc ions. As shown for other metalloproteins, the enzyme was inactivated by Zn chelators.
The FEZ-1 enzyme exhibits a broad-spectrum activity profile and was at least 1 order of magnitude more active against cephalosporins than against penicillins and carbapenems. Molecular modeling studies suggested that the general conformation of the zinc-binding sites are very similar in the FEZ-1 and L1 β-lactamases. However, the side chains of the Asn-225, Tyr-156, and Ser-162 residues, which protrude in the FEZ-1 active site, could probably be responsible for the specific substrate profile of the enzyme. Detailed structural and mechanistic studies are in progress in order to demonstrate the functions of these residues.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the European Union (grant ERB3512-IC15-CT98-0914) as part of the Training and Mobility of Researchers Program and by the Belgian Program Pôles d'Attraction Interuniversitaire initiated by the Belgian State, Prime Minister's Office, Services Fédéraux des Affaires Economiques, Techniques et Culturelles (PAI P4/03). B.D. is a postdoctoral researcher of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
The ICPMS measurements were performed by the Laboratoire de la Santé et de l'Environnement, Institut Malvoz de la Province de Liège, Liège, Belgium.
REFERENCES
- 1.Ambler R P. The structure of beta-lactamase. Philos Trans R Soc B Ser B Biol Sci. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Bellais S, Aubert D, Naas T, Nordmann P. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing β-lactamases in Chryseobacterium meningosepticum. Antimicrob Agents Chemother. 2000;44:1878–1886. doi: 10.1128/aac.44.7.1878-1886.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bicknell R, Emanuel E L, Gagnon J, Waley S G. The production and molecular properties of the zinc beta-lactamase of Pseudomonas maltophilia IID 1275. Biochem J. 1985;229:791–797. doi: 10.1042/bj2290791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boschi L, Mercuri P S, Riccio M L, Amicosante G, Galleni M, Frère J M, Rossolini G M. The Legionella (Fluoribacter) gormanii metallo-β-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 β-lactamases. Antimicrob Agents Chemother. 2000;44:1538–1543. doi: 10.1128/aac.44.6.1538-1543.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bounaga S, Laws A P, Galleni M, Page M I. Unsual pH dependence of the class B β-lactamase-catalysed hydrolysis of substrates and their inhibition by thiols. Biochem J. 1998;331:703–707. doi: 10.1042/bj3310703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush K, Jacoby G, Medeiros A A. A functional classification for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfi A, Pares S, Duée E, Galleni M, Duez C, Frère J M, Dideberg O. The 3D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Concha N O, Rasmussen B A, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 9.Concha N O, Janson C A, Rowling P, Pearson S, Cheever C A, Clarke B P, Lewis C, Galleni M, Frère J M, Payne D J, Bateson J H, Abdel-Meguid S S. Crystal structure of the IMP-1 metallo beta-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent broad-spectrum inhibitor. Biochemistry. 2000;39:4288–4298. doi: 10.1021/bi992569m. [DOI] [PubMed] [Google Scholar]
- 10.Crowder M W, Walsh T R, Banovic W, Pettit M, Spencer J. Overexpression, purification, and characterization of the cloned metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1998;42:921–926. doi: 10.1128/aac.42.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Meester F, Joris B, Reckinger G, Bellefroid- Bourguignon C, Frère J M, Waley S G. Automated analysis of enzyme inactivator phenomena. Biochem Pharmacol. 1987;36:2393–2403. doi: 10.1016/0006-2952(87)90609-5. [DOI] [PubMed] [Google Scholar]
- 12.Fabiane S M, Sohi M K, Wan T, Payne D J, Bateson J H, Mitchell T, Sutton B J. Crystal structure of the zinc-dependent beta-lactamase from Bacillus cereus at 1.9 Å resolution: binuclear active site with features of a mononuclear enzyme. Biochemistry. 1998;37:12404–12411. doi: 10.1021/bi980506i. [DOI] [PubMed] [Google Scholar]
- 13.Felici A, Amicosante G, Oratore A, Strom R, Ledent P, Joris B, Fanuael L, Frère J M. An overwiew of the kinetic parameter of class B β-lactamases. Biochem J. 1993;291:151–155. doi: 10.1042/bj2910151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felici A, Amicosante G. Kinetic analysis of extension of substrate specificity with Xanthomonas maltophilia, Aeromonas hydrophila, and Bacillus cereus metallo-β-lactamases. Antimicrob Agents Chemother. 1995;39:192–199. doi: 10.1128/aac.39.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii T, Sato K, Miyata K, Inoue M, Mitsuhashi S. Biochemical properties of β-lactamase produced by Legionella gormanii. Antimicrob Agents Chemother. 1996;29:925–926. doi: 10.1128/aac.29.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Galleni M, Lamotte-Brasseur J, Rossolini Gian Maria, Spencer Jim, Dideberg Otto, Frère J-M The Metallo-β-Lactamase Working Group. Standard numbering scheme for class B β-lactamases. Antimicrob Agents Chemother. 2001;45:660–663. doi: 10.1128/AAC.45.3.660-663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenlee M L, Laub J B, Balkovee J M, Hammond M L, Hammond G G, Pompliano D L, Epstein-Toney J H. Synthesis and SAR of thioester and thiol inhibitors of IMP-1 metallo-beta-lactamase. Bioorg Med Chem Lett. 1999;9:2549–2554. doi: 10.1016/s0960-894x(99)00425-4. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Valladares M, Felici A, Weber G, Adolph H-W, Zeppezauer M, Rossolini G M, Amicosante G, Frère J M, Galleni M. Zn(II) dependence of the Aeromonas hydrophilia AE036 metallo β-lactamase activity and stability. Biochemistry. 1997;36:11534–11541. doi: 10.1021/bi971056h. [DOI] [PubMed] [Google Scholar]
- 18.Hussain M, Carlino A, Madonna M J, Lampen O. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569H in Escherichia coli. J Bacteriol. 1985;164:223–229. doi: 10.1128/jb.164.1.223-229.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laraki N, Galleni M, Thamm I, Riccio M L, Amicosante G, Frère J M, Rossolini G M. Structure of In31, a blalMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laraki N, Franceschini N, Rossolini G M, Santucci P, Meunier C, dePauw E, Amicosante G, Frère J M, Galleni M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo β-lactamase IMP-1 produced by Escherichia coli. Antimicrob Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massida O, Rossolini G M, Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among class B metallo-β-lactamases. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McManus-Munoz S, Crowder M W. Kinetic mechanism of metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Biochemistry. 1999;38:1547–1553. doi: 10.1021/bi9826512. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen H, Brunak S, von Heijne G. Machine learning approaches for the prediction of signal peptides and other sorting signals. Protein Eng. 1999;12:3–9. doi: 10.1093/protein/12.1.3. [DOI] [PubMed] [Google Scholar]
- 24.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul-Soto R, Bauer R, Frère J M, Galleni M, Meyer-Klaucke W, Nolting H, Rossolini G M, de Seny D, Hernandez-Valladares M, Zeppezauer M, Adolph H-W. Mono- and binuclear Zn2+-β-lactamase: role of the conserved cysteine in the catalytic mechanism. J Biol Chem. 1999;274:13242–13249. doi: 10.1074/jbc.274.19.13242. [DOI] [PubMed] [Google Scholar]
- 26.Poirel L, Naas T, Nicolas D, Collet L, Bellais S, Cavallo J D, Nordmann P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother. 2000;44:891–897. doi: 10.1128/aac.44.4.891-897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quiroga M I, Franceschini N, Rossolini G M, Gutkind G, Bonfiglio G, Franchino L, Amicosante G. Interaction of cefotetan and the metallo beta-lactamases produced in Aeromonas spp. and in vitro activity. Chemotherapy (Basel) 2000;46:177–183. doi: 10.1159/000007275. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen B A, Gluzman Y, Tally F P. Cloning and sequencing of the class B β-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990;34:1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riccio M L, Franceschini N, Boschi L, Caravelli B, Cornaglia G, Fontana R, Amicosante G, Rossolini G M. Characterization of the metallo β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allele variants carried by gene cassettes of different phylogeny. Antimicrob Agents Chemother. 2000;44:1229–1235. doi: 10.1128/aac.44.5.1229-1235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossolini G M, Franceschini N, Riccio M L, Mercuri P S, Perilli M, Galleni M, Frère J M, Amicosante G. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B beta-lactamase showing a broad substrate profile. Biochem J. 1998;332:145–152. doi: 10.1042/bj3320145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Sanchagrin F, Dufresne J, Levesque R C. Molecular heterogeneity of the L-1 metallo β-lactamase family from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1998;42:1245–1248. doi: 10.1128/aac.42.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scrofani S D, Chung J, Huntley J J, Benkovic S J, Wright P E, Dyson H J. NMR characterization of the metallo-beta-lactamase from Bacteroides fragilis and its interaction with a tight-binding inhibitor: role of an active-site loop. Biochemistry. 1999;38:14507–14514. doi: 10.1021/bi990986t. [DOI] [PubMed] [Google Scholar]
- 35.Ullah J H, Walsh T R, Taylor I A, Emery D C, Verma C S, Gamblin S J, Spencer J. The crystal structure of the L1 metallo β-lactamase from Stenotromonas maltophilia at 1.7 Å resolution. J Mol Biol. 1998;284:125–136. doi: 10.1006/jmbi.1998.2148. [DOI] [PubMed] [Google Scholar]
- 36.Walsh T R, Hall L, Assinda S J, Nichols W W, Cartwright S J, MacGowan A P, Bennett P M. Sequence and analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochem Biophys Acta. 1994;1219:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 37.Walsh T R, Neville W A, Haran M H, Tolson D, Payne D J, Bateson J H, Mac Gowan A P, Bennett P M. Nucleotide and amino acid sequences of the metallo-beta-lactamase, ImiS, from Aeromonas veronii bv. sobria. Antimicrob Agents Chemother. 1998;42:436–439. doi: 10.1128/aac.42.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Fast W, Valentine A M, Benkovic S J. Metallo β-lactamase: structure and mechanism. Curr: Opin. Chem Biol. 1999;3:614–622. doi: 10.1016/s1367-5931(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Fast W, Benkovic S J. On the mechanism of the metallo beta-lactamase from Bacteroides fragilis. Biochemistry. 1999;38:10013–10023. doi: 10.1021/bi990356r. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Rasmussen B A, Bush K. Biochemical characterization of an imipenem-hydrolyzing metallo-β-lactamase from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1992;36:1155–1157. doi: 10.1128/aac.36.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]