ABSTRACT
Many bacterial and fungal pathogens cause disease across mucosal surfaces, and to a lesser extent through skin surfaces. Pathogens that potentially cause disease vaginally across epithelial cells include Staphylococcus aureus, group A and B streptococci, Escherichia coli, Neisseria gonorrhoeae, and Candida albicans. We have previously shown that staphylococcal and streptococcal superantigens induce inflammatory chemokines from vaginal epithelial cells through the immune costimulatory molecule CD40 through use of a CRISPR cas9 knockout mutant and complemented epithelial cell line. In this study, we show that the potential vaginal pathogens S. aureus, group A and B streptococci, E. coli, an Enterococcus faecalis strain, and C. albicans in part use CD40 to stimulate interleukin-8 (IL-8) production from human vaginal epithelial cells. In contrast, N. gonorrhoeae does not appear to use CD40 to signal IL-8 production. Normal flora Lactobacillus crispatus and an Enterococcus faecalis strain that produces reutericyclin do not induce IL-8. These data indicate that many potential pathogens, but no normal commensals, induce IL-8 to help disrupt the human vaginal epithelial barrier through CD40, thus providing a potential therapeutic target for drug development.
IMPORTANCE Most bacterial and fungal pathogens cause disease across mucosal, and to a lesser extent, skin barriers with the help of induced chemokines from epithelial cells. In this study, we showed that potential vaginal pathogens Staphylococcus aureus, group A and B streptococci, some Enterococcus faecalis strains, Escherichia coli, and Candida albicans use the immune costimulatory molecule CD40 to induce the chemokine interleukin-8 production. In contrast, Neisseria gonorrhoeae does not use CD40 to stimulate interleukin-8. Normal flora lactobacilli and at least one E. faecalis strain do not induce interleukin-8.
KEYWORDS: CD40, Candida, Escherichia coli, Neisseria gonorrhoeae, Staphylococcus, Streptococcus, chemokines, epithelial cells, superantigens
INTRODUCTION
Staphylococcus aureus, Streptococcus pyogenes (group A), Streptococcus agalactiae (group B), Escherichia coli, and Neisseria gonorrhoeae are all potential vaginal bacterial pathogens. Additionally, Candida albicans is a common vaginal fungal pathogen. In contrast, bacteria such as various lactobacilli are normal flora (1, 2). In one instance at least, a woman was identified with Enterococcus faecalis as the only vaginal microbe (3). This suggests this organism, in some individuals, is part of the normal vaginal microbiome.
Many of these pathogenic organisms often colonize mucous membranes and may colonize skin to initiate infections through these same surfaces (4, 5). The organisms are important vaginal mucosal pathogens but also cause infections across other mucosal surfaces. The Gram-positive pathogens (S. aureus, group A and B streptococci, and some strains of Enterococcus faecalis) also are causes of toxic shock syndrome (TSS) and many other kinds of infections through production of many virulence factors, including known or suspected superantigens (SAgs) (5–10). E. coli is the cause of 80% of urinary tract infections and a major cause of sepsis (11). Neisseria gonorrhoeae is the cause of gonorrhea, due to inflammation on mucosal surfaces including the vagina (12). C. albicans is the major cause of mucocutaneous candidiasis and is becoming a more common cause of sepsis (13).
All of these potential vaginal pathogens have been shown previously to be associated with elevated epithelial cell interleukin-8 (IL-8), a proinflammatory chemokine that attracts neutrophils to sites of infection (14–19). However, some of these studies evaluated epithelial cells not from the vagina but, instead, from the urinary tract (17), tonsils (16), or lungs (14). Other than through Toll-like receptor 2 (TLR2), other receptors for production of IL-8 were not determined. In one study, it was shown that some Streptococcus pyogenes strains (M1, for example) produce an IL-8 protease, leading to dampened IL-8 (16). One goal of the current studies was to assess possible additional receptors other than TLR2 used by potential vaginal pathogens to stimulate IL-8 production by human vaginal epithelial cells (HVECs). In prior studies, we evaluated the effect of S. aureus and its SAgs on both IL-8 and MIP-3α production (20). However, we later showed that MIP-3α is cleaved by S. aureus proteases (21). Thus, despite the 80-fold upregulation of IL-8 mRNA versus 400-fold upregulation of MIP-3α mRNA by S. aureus (20), the amount of MIP-3α protein was much reduced compared to IL-8; IL-8 was stable to S. aureus proteases (21). For this reason, the current studies focused on the effect of vaginal pathogens on IL-8 production.
We have shown that S. aureus SAgs dysregulate HVECs, causing thousands of genes to be up- or downregulated (20). The SAg TSS toxin-1 (TSST-1) altered by >2-fold nearly 2,400 genes in HVECs after 6 h of treatment (20). Many of the most highly upregulated genes were those encoding chemokines, such as IL-8 and MIP-3α, with MIP-3α expression, for example, being upregulated over 400-fold (20). The effects of TSST-1 and another SAg, staphylococcal enterotoxin B (SEB), on HVECs were mediated through SAg interaction with CD40, as demonstrated by CRISPR cas9 knockout studies of CD40 on these cells (22). There were estimated to be 5 × 104 TSST-1 receptors per HVEC (20).
We have also examined the effect of TSST-1, SEB, and two streptococcal pyrogenic exotoxins (SPEs A and C) on primary human keratinocytes (23). Both TSST-1 and SEB caused significant up- and downregulation of nearly 5,800 genes for TSST-1 and 4,300 genes for SEB when tested against human keratinocytes (23). As expected, there was considerable overlap in genes affected by the two SAgs, including the upregulation of expression of many chemokine genes (23). With use of immortalized human keratinocytes, we have shown multiple chemokine genes were upregulated by these same two SAgs (23); among the upregulated genes were those that encoded chemokines, such as IL-8 and MIP-3α. For SPEs A and C, we examined only IL-8 production from human keratinocytes and showed that both SPEs stimulate production of the chemokine. Because of the high relatedness of SPE A to SEB, we have not examined the effect of this SAg on HVECs.
The purpose of the present study was to assess the potential use of CD40 as a receptor for vaginal mucosal pathogens. Our studies show that the majority of these organisms in part stimulate HVECs to produce proinflammatory IL-8 through CD40.
RESULTS
All experiments in this study used replicates of three wells per treatment, and all experiments were performed a minimum of two times.
Stimulation of IL-8 production by Gram-positive bacterial strains.
We utilized the following Staphylococcus aureus strains in the current studies: S. aureus MN8, a TSST-1 gene (tst) knockout mutant of MN8, and MNPE (24). S. aureus MN8 is a typical USA200 (CC30) menstrual TSS isolate with a mutation in the alpha-toxin structural gene, significantly downregulating production of alpha-toxin (25). S. aureus MNPE is also a USA200 (CC30) strain from a patient with postinfluenza TSS (24), but like other skin isolates, this organism has a wild-type alpha-toxin gene and produces typical amounts of alpha-toxin (approximately 50 μg/mL) in standard growth medium (Todd-Hewitt broth [THB]). Both S. aureus strains produce TSST-1 at approximately 10 to 20 μg/mL in THB under high aeration. The strains also produce β-toxin (approximately 100 μg/mL), which is characteristic of USA200 (CC30) strains, and the strains contain the enterotoxin gene cluster (EGC) of 6 SAgs (SEG, SE-like I, M, N, O, and U) that are usually produced in small amounts (26, 27). The EGC SAgs are encoded on an operon, and these SAgs are thought to be more important for colonization than production of TSS, based on their low level of production (26). The MN8Δtst mutant is a clean mutant of MN8 in which the gene for TSST-1 production only was deleted. All three strains had stationary phases of approximately 7.0 × 109/mL in Todd-Hewitt medium after growth for 24 h, at 37°C, with shaking (200 rpm).
We performed dose-response experiments to assess the ability of dilutions of all three S. aureus strains (MN8, MN8Δtst, and MNPE) to cause interleukin-8 (IL-8) production from immortalized HVECs (Fig. 1). It should be noted that even though the HVECs are immortalized, the cell phenotype is that of nonimmortalized HVECs (20, 22). All organisms induced the production of IL-8 (chemoattractant of polymorphonuclear leukocytes [PMNs]) as a function of CFU per milliliter of bacteria. Only data for IL-8 production, as representative of chemokine production by HVECs, are shown in this and all subsequent figures. We did key experiments previously to show that pathogens stimulated production of MIP-3α (20, 23), a chemokine that attracts both innate and adaptive immune cells to areas of damage.
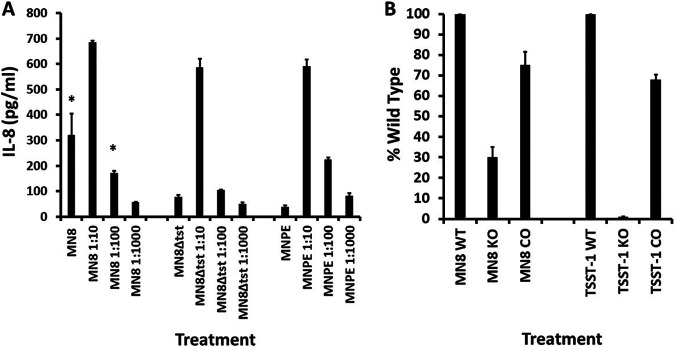
FIG 1.
Wild-type (WT) human vaginal epithelial cells (HVECs), CD40 knockout (KO) HVECs, and CD40-complemented (CO) HVECs treated for 6 h in triplicate with various dilutions of S. aureus MN8, MN8Δtst, and MNPE. (A) IL-8 ± standard deviations. *, significant mean differences between MN8 and MN8Δtst with P < 0.0001. (B) Percent wild type of HVEC CD40 knockout cells (KO) and CD40-complemented HVEC knockout cells (CO) treated with the dose of MN8 (MN8 1:10) that gave the peak IL-8 response by wild-type cells. TSST-1 was used at 50 μg/mL as a control.
All three S. aureus strains inhibited IL-8 production (Fig. 1A) at the highest bacterial concentration (7 × 109 CFU/mL), likely due to killing of HVECs though production of cytotoxins (28). However, at 10-fold-lower doses of all three S. aureus strains, IL-8 was maximally produced. The amount of IL-8 was reduced by treatment with lower doses of all strains. At the three higher doses of MN8, more IL-8 production was observed compared to MN8Δtst. This is consistent with TSST-1 contributing to IL-8 induction in HVECs as reported previously (20, 22).
We next evaluated the role of the immune costimulatory molecule CD40 in IL-8 production by S. aureus MN8, with TSST-1 as a positive control. We tested IL-8 production from wild-type HVECs, CRISPR cas9 CD40 knockout HVECs, and CD40-complemented cells in the CD40 knockout background. For purposes of ease of comparison, we show data as the percent stimulation compared to wild-type HVECs treated with 7 × 108 CFU/mL, the maximum CFU for stimulation, and set at 100%. We also set the response of wild-type HVECs to 50 μg/mL of TSST-1 at 100%. All data are reported after 6 h of exposure to S. aureus or TSST-1 in keratinocyte serum-free medium (KSFM) at 37°C in the presence of 5% CO2. These HVEC incubation conditions were also used for all subsequent experiments.
For S. aureus MN8, 70% of the IL-8 production depended on the presence of CD40 (100% for wild-type HVECs versus 30% in CD40 knockout cells) as shown in Fig. 1B. In contrast, as expected and previously published (22), TSST-1 induction of IL-8 production depended 100% on CD40. For both S. aureus MN8 and TSST-1, there was significant restoration of IL-8 production in CD40-complemented cells, suggesting a lack of off-target CRISPR cas9 effects.
Group A streptococcal strain 594 (29), T253(cured)T12, and its phage-free counterpart T253cured (30, 31) were evaluated in the same way as S. aureus, both in determination of dose response with HVECs for 6 h in production of IL-8 and in determination of percentage of the response due to interaction with CD40 (Fig. 2). Group A streptococcal strains 594 (29) and T253(cured)T12 are typical beta-hemolytic group A strains that encode the superantigen streptococcal pyrogenic exotoxin A (SPE A), a SAg related to SEB, encoded by a gene on the bacteriophage T12 (30, 31). The T253(cured)T12 strain, as opposed to the bacteriophage-free and thus SPE A-free strain T253, causes TSS in a rabbit model (32). Both of the SPE A-positive strains produce approximately 5 μg/mL of the SAg in THB.
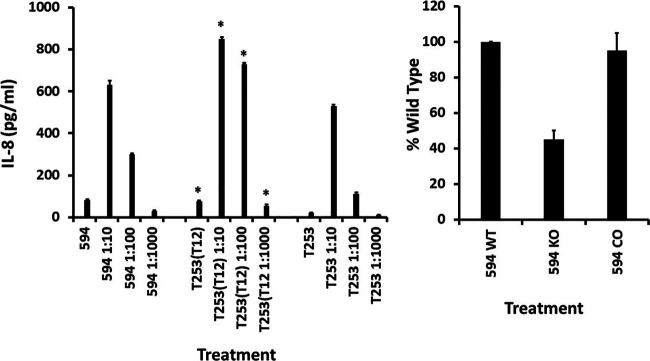
FIG 2.
Wild-type (WT) human vaginal epithelial cells (HVECs), CD40 knockout (KO) HVECs, and CD40-complemented (CO) HVECs treated for 6 h in triplicate with various dilutions of group A streptococci 594, T253(cured)T12, and T253(cured). Left panel shows IL-8 ± standard deviations. *, mean differences between T253(cured)T12 and T253(cured) with P < 0.0001. Right panel shows percent wild type of HVEC CD40 knockout cells (KO) and CD40-complemented HVEC knockout cells (CO) treated with the dose of strain 594 (1:10) that gave the peak IL-8 response by wild-type cells.
All three of the group A streptococcal strains grew to stationary phases of approximately 3 × 108 to 4 × 108 CFU/mL after overnight growth when incubated stationarily in a 5% CO2 incubator, at 37°C with a starting inoculum of approximately 107 CFU/mL. The three strains, when added to HVECs in KSFM, inhibited IL-8 production at the 3 × 108- to 4 × 108-CFU/mL dose (Fig. 2, left panel), likely due to killing through production of cytotoxins (28, 33). When strains were diluted 10-fold, maximum IL-8 production was observed. Incubation with lower doses of all three organisms resulted in less IL-8 production by HVECs. Strain T253(cured)T12 at all doses induced production of more IL-8 than was induced by incubation with T253(cured). This may have been the result of production of SPE A, a SAg with known ability to induce IL-8 from human keratinocytes (23), by T253(cured)T12 versus T253(cured). However, both strains induced significant IL-8 production. When HVECs were treated with 100 μg/mL of SPE A alone, the SAg led to an average ± standard deviation of production of 255 ± 7.3 pg/mL of IL-8. This amount is similar to the response of wild-type HVECs to the SPE A-related SAg SEB (22). The IL-8 response to SPE A was eliminated in CD40 knockout cells (1.7 ± 1.7 pg/mL) and restored to 184 ± 4.8 pg/mL in CD40-complemented cells.
Also shown in Fig. 2 are the results of incubation of strain 594 with wild-type HVECs, CD40 knockout HVECs, and CD40 complemented in the knockout strain. The data were reported as percentage of IL-8 produced by wild-type HVECs in response to 3.0 × 107 CFU/mL after 6 h of incubation, noting this is the dose of strain 594 that induced the greatest production of IL-8. The data showed that 60% of the IL-8 response of HVECs was due to direct or indirect interaction with CD40, as seen by a 60% reduction by the CD40 knockout strain. The CD40-complemented strains responded with nearly wild-type IL-8 production, indicating the reduced IL-8 response in the CD40 knockout strain was not the result of an off-target effect in the CRISPR cas9 knockout.
The same type of dose-response assay to a group B streptococcal strain associated with a neonatal TSS-like syndrome (9) (Fig. 3) was also performed. Also tested (Fig. 3) were a normal skin flora organism, Staphylococcus epidermidis (8 × 109 CFU/mL) (a single dose since no cytolysin was produced by the strain), a human vaginal Enterococcus faecalis strain from a woman colonized with a pure culture of the organism (normal vaginal flora [NVF]; single dose of 5 × 108 CFU/mL since no cytolysin was produced) (3), an E. faecalis strain from a patient with nonmenstrual TSS (single dose of 6 × 108/mL since no cytolysin was produced), and a Lactobacillus crispatus strain isolated from the vagina of a healthy woman (single dose of 2 × 109 CFU/mL since no cytolysin was produced).
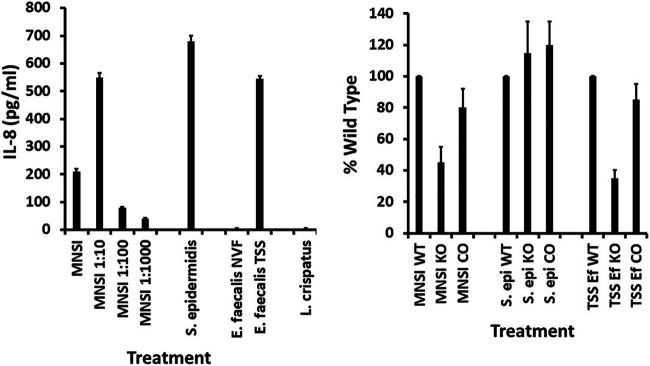
FIG 3.
Wild-type (WT) human vaginal epithelial cells (HVECs), CD40 knockout (KO) HVECs, and CD40-complemented (CO) HVECs treated for 6 h in triplicate with various dilutions of group B streptococci (MNSI) and a single dose of S. epidermidis, two Enterococcus faecalis strains (NVF, normal vaginal flora; TSS, toxic shock syndrome), and a strain of L. crispatus. Left panel shows IL-8 ± standard deviations. Right panel shows percent wild type of HVEC CD40 knockout cells (KO) and CD40-complemented HVEC knockout cells (CO) treated with the dose of optimal strain that gave the peak IL-8 response by wild-type cells.
The stationary phase of the group B streptococcal strain tested (MNSI) was 3.0 × 108 CFU/mL after 24 h of growth at 37°C, without shaking, in a 5% CO2 incubator; the starting inoculum was approximately 107 CFU/mL. Like both S. aureus and group A streptococci, the MNSI group B streptococcal strain caused high-dose inhibition of IL-8 production (Fig. 3, left panel), likely due to killing effects from cytotoxin production (28, 33). The peak for IL-8 production in HVECs was 3.0 × 107 CFU/mL. Just over 50% of the response of HVECs to MNSI depended on CD40, as shown by the 50% reduction in IL-8 in the CD40 knockout strain (Fig. 3, right panel). The CD40-complemented strain showed significant restoration of the CD40 response.
The normal flora microbe S. epidermidis also caused significant induction of IL-8 production by wild-type HVECs (Fig. 3). There was no reason to evaluate the complemented HVECs for this organism since the CD40 knockout HVECs showed similar IL-8 induction as wild-type HVECs.
The normal vaginal flora strains of E. faecalis (3) and L. crispatus (2) did not induce IL-8 production by wild-type HVECs (Fig. 3, left panel). At least for the E. faecalis strain, we have provided evidence that this lack of IL-8 production likely resulted from reutericyclin production by the organism (3).
We also tested an E. faecalis strain from a male patient with nonmenstrual TSS for IL-8 production by HVECs. This male had TSS associated with a break in the skin. SAgs are the known causes of TSS. This E. faecalis strain has not thus far been tested for SAgs, but we know E. faecalis has the ability to produce SAg-like molecules (34). Additionally, we have shown that SAgs induce IL-8 from HVECs and human keratinocytes with induction of the CD40 pathway (20, 22, 23). The TSS E. faecalis strain induced IL-8 production by wild-type HVECs (Fig. 3, left panel). This induction depended approximately 70% on the presence of CD40, as the CD40 HVECs showed a 70% reduction in IL-8 compared to wild type (Fig. 3, right panel). The response was restored in the CD40-complemented HVECs. The NVF Enterococcus faecalis and L. crispatus were not tested in either the CD40 knockout strain or the CD40-complemented strain.
Stimulation of IL-8 production by Gram-negative bacterial strains and Candida albicans.
We tested two Gram-negative bacteria and Candida albicans for stimulation of IL-8 production by HVECs and the percent use of CD40 as a receptor (Fig. 4). Both Gram-negative pathogens stimulated HVECs to produce IL-8, with Neisseria gonorrhoeae (5 × 109 CFU/mL) stimulating to a much greater extent (Fig. 4, left panel). Since our HVEC line lacks TLR-4 and major histocompatibility complex II molecules, the stimulation was not due to interaction with these molecules. The other known SAg receptors are T cell receptors; it is unlikely that HVECs have these receptors. Candida albicans (4 × 108 CFU/mL) stimulated HVECs to produce IL-8 to the same extent as Escherichia coli (2 × 109 CFU/mL) (Fig. 4, left panel). For Escherichia coli, 80% of the response depended on interaction with CD40, as the response of CD40 knockout cells was only 20% of wild-type HVECs (Fig. 4, right panel). This response was restored by complementation of the knockout HVECs with CD40.
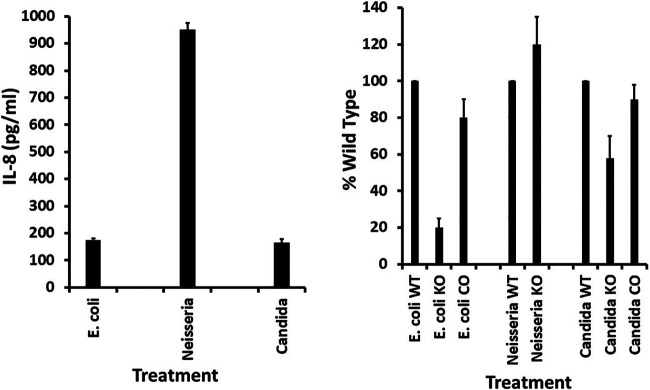
FIG 4.
Wild-type (WT) human vaginal epithelial cells (HVECs), CD40 knockout (KO) HVECs, and CD40-complemented (CO) HVECs treated for 6 h in triplicate with various amounts of Escherichia coli, Neisseria gonorrhoeae, and Candida albicans. Left panel shows IL-8 ± standard deviations. Right panel shows percent wild type of HVEC CD40 knockout cells (KO) and CD40-complemented HVEC knockout cells (CO) treated with the optimal dose of strains that gave the peak IL-8 response by wild-type cells.
The IL-8 response of HVECs to Neisseria gonorrhoeae did not depend on CD40, as knockout cells showed the same response as wild-type cells (Fig. 4, right panel). Thus, there was no reason to evaluate the complemented HVECs for this organism since the CD40 knockout HVECs showed similar IL-8 induction as wild-type HVECs.
The response of HVECs to Candida albicans depended 40% on the presence of CD40, as shown by the 40% reduction in IL-8 in the knockout cells (Fig. 4, right panel). The response was complemented by addition of CD40 into the knockout cells.
DISCUSSION
Many bacterial and Candida pathogens cause harmful chemokine production on mucosal surfaces, such as the vaginal mucosa (20, 22, 35). We have previously referred to this as outside-in signaling to disrupt the mucosal barrier, leading to downstream infection (35–37). While in the present paper we focused on IL-8 production by HVECs, the response to pathogens more broadly affects chemokine production by these human cells, including that of IL-8, MIP-3α, and IL-33 (20, 22, 23).
S. aureus and Streptococcus pyogenes are the two common causes of TSS (38, 39). S. aureus strains, usually USA200 (CC30), which produce the SAg TSST-1 cause 100% of menstrual TSS associated with vaginal infection (38–40). Streptococcus pyogenes strains are the major cause of streptococcal TSS, most often associated with infections through breaks in the skin (7, 8). However, highly fatal cases have been associated also with vaginal infections with Streptococcus pyogenes, usually in the late second and early third trimesters (41). Approximately 85% of streptococcal TSS cases are associated with the SAg SPE A (38, 42). These pathogens also produce cytolysins which can kill HVECs when present in high concentrations, contributing to SAg transport across barriers.
Previously, we have shown that the SAgs, TSST-1 and SEB, use CD40 as their sole receptor on HVECs for production of chemokines (22). We confirmed the findings for TSST-1-induced IL-8 in this paper and also showed that SPE A stimulates HVECs to produce IL-8, dependent on CD40. In contrast, the inflammatory receptor for the TSST-1, SEB, and SPE A SAgs on keratinocytes appears to involve at least one other receptor as well as CD40, this other receptor involving the glycoprotein gp130 (23).
It has also been shown that group B streptococci, viridans streptococci, and enterococci occasionally cause TSS and severe invasive diseases (9, 10, 39, 43). SAg characterization has not been thoroughly, or at all, investigated for these strains. However, our prior studies have shown that Enterococcus faecalis strain OG1ssp(pINY1801) produces cell-associated factors that mimic the actions of SAgs (34). Additionally, we partially characterized a SAg associated with a group B streptococcal strain associated with TSS (10). It is possible that uncharacterized SAgs or SAg-like molecules produced by these strains accounted for some of the IL-8 induction in HVECs in this study.
However, it is clear that HVECs are stimulated significantly through CD40 by the various Gram-positive, Gram-negative, and Candida pathogens studied in this work. However, since these pathogens showed variable stimulation of IL-8 production in CD40 knockout cells, from only 20% to 100%, there are other receptors engaged. For example, the SAg TSST-1 dysregulates only approximately 600 genes in HVECs, including those for many chemokines, whereas TSS S. aureus strains dysregulate the same genes but to a total of several thousand genes (20). We know that HVECs lack TLR-4, but the cells do contain TLR-2, and thus, some stimulation by many of the pathogens we studied could be through lipoteichoic acid-peptidoglycan engagement with TLR-2. Several studies have shown direct engagement of group B streptococci with lung epithelial cells through cytolysins (14) and with innate immune cells through TLRs (15, 44). The staphylococcal cytolysin, alpha-toxin, and Streptococcus pyogenes streptolysin O are cytotoxic to HVECs at high concentrations, as we observed in these studies. However, at reduced concentrations, as would be present in dilutions of S. aureus MNPE cultures or Streptococcus pyogenes strains, these cytotoxins are likely to stimulate IL-8 production (28, 33).
We observed that two normal vaginal flora microbes, an Enterococcus faecalis strain producing reutericyclin (3) and L. crispatus, did not stimulate IL-8 production. Reutericyclin has previously been shown to be anti-inflammatory as well as broadly antimicrobial (45, 46). These data collectively show that not all bacteria on mucosal surfaces induce harmful chemokine responses. It should be noted that we have also observed that 108 latex beads/mL do not stimulate chemokine production (3).
It is potentially of greatest importance that many vaginal pathogens, in part or entirely, use CD40 as the receptor on HVECs. Epithelial cells dominate the cell types present vaginally, as opposed to more-recognized types of immune cells (21). This raises the possibility that a small-molecule inhibitor of pathogen interaction with CD40 could be found. In this way, it is possible that reductions in vaginal infections and concomitant pathogenesis could be achieved. CD40 is a trimeric immune costimulatory molecule that is important systemically for immune responses (22, 47). However, there is little evidence that vaginal mucosal cell CD40 is critical for protective immune responses. Indeed, our studies suggest that interaction of pathogens with CD40 is more likely to be harmful (22). Thus, we think investigations should be initiated to identify such inhibitors that block pathogen-CD40 interactions. This of course depends on the small molecules having inhibitor activity and generally in the nanomolar range. We have already tested a monoclonal antibody that was designed to block CD40 interaction with its T lymphocyte ligand. Rather than blocking inflammation due to SAg interaction with CD40, the monoclonal antibody enhanced inflammation due to SAg, most likely due to stimulation of CD40 (22).
As a final critical point to be made, it is well recognized that TSST-1, and this TSST-1-producing S. aureus, causes menstrual TSS (40). It is also well known that group B streptococci cause neonatal sepsis and meningitis (9). However, it is not nearly as well known that approximately 2% of women have Streptococcus pyogenes vaginally. This makes these women potentially susceptible to streptococcal TSS associated with such vaginal colonization, often occurring during pregnancy or at delivery (41).
MATERIALS AND METHODS
Microbes.
S. aureus MN8 was obtained from the eighth menstrual TSS patient in Minnesota in 1980. The strain is a commonly used USA200 (CC30) organism (20, 22, 23, 48, 49). The organism produces TSST-1, has the enterotoxin gene cluster of SAgs (26), and has a mutation in the alpha-toxin gene, causing the strain to produce at least 50-fold less alpha-toxin (25). S. aureus MNPE was obtained from a fatal case of postinfluenza TSS as described previously (24). The organism is a USA200 (CC30 strain) that produces TSST-1, has the wild-type alpha-toxin gene (typical of skin strains), and contains the enterotoxin gene cluster of SAgs (26). A knockout strain of S. aureus MN8 in the TSST-1 gene was prepared as described previously (26). This strain has growth characteristics typical of S. aureus MN8, except the strain does not produce TSST-1. S. epidermidis was a skin isolate from a healthy individual (50). All strains of low passage number are maintained at −80°C in the Schlievert laboratory.
Group A streptococcal strain T253 was provided originally by Dennis Watson, University of Minnesota (now deceased), as a lyophilized stock culture. The Schlievert laboratory cured the strain of an endogenous bacteriophage, giving rise to strain T253(cured) (30, 31). The bacteriophage T12, which carries the gene for streptococcal pyrogenic exotoxin A (SPE A), was inserted into strain T253(cured) to give rise to T253(cured)T12 (30, 31). Strain 594 (SPE A positive) was obtained from Nauciel et al. (29). All strains are maintained as low-passage-number −80°C stocks in the Schlievert laboratory.
Group B streptococcal strain MNSI was from an infant with neonatal sepsis and meningitis (9). Two Enterococcus faecalis strains were used in this study. One was a vaginal isolate from a woman with a pure culture of this organism. The organism produces the broadly antimicrobial and anti-inflammatory molecule reutericyclin (3). The second isolate was submitted to the Schlievert laboratory as a strain from a patient with nonmenstrual TSS.
Escherichia coli (Watson) was originally a clinical isolate from a urinary tract infection, as generously provided by Dennis Watson, University of Minnesota (now deceased) (51). Neisseria gonorrhoeae was a clinical isolate kindly provided by Michael Apicella, University of Iowa (now retired). Candida albicans was a generous gift from Daniel Diekema, University of Iowa. All strains are maintained as low-passage-number cultures at −80°C.
Superantigens.
TSST-1 and SPE A, used as positive controls for interaction with CD40, were prepared from clones in S. aureus strain RN4220, a strain which does not appear to secrete SAgs (22, 52–54). In the 1980s, the United States Recombinant DNA Advisory Committee (RAC) gave approval for the Schlievert laboratory to clone SPE A in S. aureus RN4220 based on the relatedness of SPE A to both SEs B and C. The RAC agreed with Schlievert that SPE A and SEs B and C shared a common ancestor in S. aureus, with the SPE A gene most likely transferred to S. pyogenes by bacteriophages.
Microbial culture.
All S. aureus strains, Escherichia coli, and Candida albicans were cultured to stationary phase in Todd-Hewitt broth (THB; Difco, Detroit, MI) at 37°C with 200-rpm shaking. Streptococci and enterococci were cultured to stationary phase in THB at 37°C in the presence of 5% CO2 as stationary cultures. The stationary-phase organisms were used directly in experimentation. For those microbes that produced cytotoxins, dilutions of the stationary-phase cultures were made in THB. Neisseria gonorrhoeae was cultured on chocolate agar plates for 24 h at 37°C in the presence of 5% CO2. The organisms were gently scraped off the plates and used directly for experimentation.
Human vaginal epithelial cells (HVECs).
The wild-type, CD40 knockout, and CD40-complemented knockout cells have been described previously (22). The cells were cultured at 37°C in the presence of 5% CO2 in keratinocyte serum-free medium until nearly confluent. The cells were then split and cultured until confluent in 96-well microtiter plates.
Experimentation.
All experiments used triplicate replicates, and experiments were independently repeated at least one additional time. Microbes were cultured as given above. The HVECs were cultured in 180 μL to confluence, and medium was changed in 5% CO2 at 37°C. Then, 20 μL of each bacterial culture was added to each well, and wells were incubated for 6 h. Subsequently, the samples were frozen at −20°C for 24 h to release any intracellular chemokine. IL-8 was measured with use of Quantikine kits as purchased from R&D Systems, Minneapolis, MN.
Statistics.
Means ± standard deviations were determined. For data presented in Fig. 1 and 2, statistical significance of means of IL-8 between S. aureus MN8 wild type versus MN8Δtst and S. pyogenes T253(cured)T12 versus T253(cured), the analyses were performed on a fee-for-service basis by the Director of the Biostatistics Core in the University of Iowa, Carver College of Medicine, Clinical and Translational Science Award Program, Patrick Ten Eyck. The analyses included the following. We utilized the generalized linear modeling (GLM) framework to test for between-group differences in the outcome measure at different dilutions of microbes. Main effect predictors included group, dilution, and the interaction effect (group × dilution). To account for the right-skewed distribution in the outcome measure, we specified a log-link function and assessed the between-group mean ratio. Tests with P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENT
This work was supported by a grant from the University of Iowa, Carver College of Medicine.
Contributor Information
Patrick M. Schlievert, Email: patrick-schlievert@uiowa.edu.
Christopher N. LaRock, Emory University School of Medicine
REFERENCES
- 1.Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, Holmes KK. 1996. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 174:1058–1063. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 2.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosnahan AJ, Merriman JA, Salgado-Pabón W, Ford B, Schlievert PM. 2013. Enterococcus faecalis inhibits superantigen toxic shock syndrome toxin-1-induced interleukin-8 from human vaginal epithelial cells through tetramic acids. PLoS One 8:e61255. doi: 10.1371/journal.pone.0061255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 5.Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34. doi: 10.1128/CMR.13.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlievert PM, Davis CC. 2020. Device-associated menstrual toxic shock syndrome. Clin Microbiol Rev 33:e00032-19. doi: 10.1128/CMR.00032-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cone LA, Woodard DR, Schlievert PM, Tomory GS. 1987. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med 317:146–149. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- 8.Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, Kaplan E. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 9.Schlievert PM, Varner MW, Galask RP. 1983. Endotoxin enhancement as a possible etiology of early-onset group B beta-hemolytic streptococcal sepsis in the newborn. Obstet Gynecol 61:588–592. [PubMed] [Google Scholar]
- 10.Schlievert PM, Gocke JE, Deringer JR. 1993. Group B streptococcal toxic shock-like syndrome: report of a case and purification of an associated pyrogenic toxin. Clin Infect Dis 17:26–31. doi: 10.1093/clinids/17.1.26. [DOI] [PubMed] [Google Scholar]
- 11.Vila J, Saez-Lopez E, Johnson JR, Romling U, Dobrindt U, Canton R, Giske CG, Naas T, Carattoli A, Martinez-Medina M, Bosch J, Retamar P, Rodriguez-Bano J, Baquero F, Soto SM. 2016. Escherichia coli: an old friend with new tidings. FEMS Microbiol Rev 40:437–463. doi: 10.1093/femsre/fuw005. [DOI] [PubMed] [Google Scholar]
- 12.Unemo M, Seifert HS, Hook EW, III, Hawkes S, Ndowa F, Dillon JR. 2019. Gonorrhoea. Nat Rev Dis Primers 5:79. doi: 10.1038/s41572-019-0128-6. [DOI] [PubMed] [Google Scholar]
- 13.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. 2011. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doran KS, Chang JC, Benoit VM, Eckmann L, Nizet V. 2002. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J Infect Dis 185:196–203. doi: 10.1086/338475. [DOI] [PubMed] [Google Scholar]
- 15.Upadhyay K, Park JE, Yoon TW, Halder P, Kim YI, Metcalfe V, Talati AJ, English BK, Yi AK. 2017. Group B streptococci induce proinflammatory responses via a protein kinase D1-dependent pathway. J Immunol 198:4448–4457. doi: 10.4049/jimmunol.1601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soderholm AT, Barnett TC, Korn O, Rivera-Hernandez T, Seymour LM, Schulz BL, Nizet V, Wells CA, Sweet MJ, Walker MJ. 2018. Group A streptococcus M1T1 intracellular infection of primary tonsil epithelial cells dampens levels of secreted IL-8 through the action of SpyCEP. Front Cell Infect Microbiol 8:160. doi: 10.3389/fcimb.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agace WW, Hedges SR, Ceska M, Svanborg C. 1993. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest 92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fichorova RN, Desai PJ, Gibson FC, III, Genco CA. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spear GT, Zariffard MR, Cohen MH, Sha BE. 2008. Vaginal IL-8 levels are positively associated with Candida albicans and inversely with lactobacilli in HIV-infected women. J Reprod Immunol 78:76–79. doi: 10.1016/j.jri.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson ML, Ault K, Kremer MJ, Klingelhutz AJ, Davis CC, Squier CA, Schlievert PM. 2005. The innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin 1. Infect Immun 73:2164–2174. doi: 10.1128/IAI.73.4.2164-2174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlievert PM, Nemeth KA, Davis CC, Peterson ML, Jones BE. 2010. Staphylococcus aureus exotoxins are present in vivo in tampons. Clin Vaccine Immunol 17:722–727. doi: 10.1128/CVI.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlievert PM, Cahill MP, Hostager BS, Brosnahan AJ, Klingelhutz AJ, Gourronc FA, Bishop GA, Leung DYM. 2019. Staphylococcal superantigens stimulate epithelial cells through CD40 to produce chemokines. mBio 10:e00214-19. doi: 10.1128/mBio.00214-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlievert PM, Gourronc FA, Leung DYM, Klingelhutz AJ. 2020. Human keratinocyte response to superantigens. mSphere 5:e00803-20. doi: 10.1128/mSphere.00803-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald KL, Osterholm MT, Hedberg CW, Schrock CG, Peterson GF, Jentzen JM, Leonard SA, Schlievert PM. 1987. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. JAMA 257:1053–1058. doi: 10.1001/jama.257.8.1053. [DOI] [PubMed] [Google Scholar]
- 25.Lin YC, Anderson MJ, Kohler PL, Strandberg KL, Olson ME, Horswill AR, Schlievert PM, Peterson ML. 2011. Proinflammatory exoprotein characterization of toxic shock syndrome Staphylococcus aureus. Biochemistry 50:7157–7167. doi: 10.1021/bi200435n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stach CS, Vu BG, Merriman JA, Herrera A, Cahill MP, Schlievert PM, Salgado-Pabón W. 2016. Novel tissue level effects of the Staphylococcus aureus enterotoxin gene cluster are essential for infective endocarditis. PLoS One 11:e0154762. doi: 10.1371/journal.pone.0154762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer AJ, Kilgore SH, Singh SB, Allen PD, Hansen AR, Limoli DH, Schlievert PM. 2019. High prevalence of Staphylococcus aureus enterotoxin gene cluster superantigens in cystic fibrosis clinical isolates. Genes (Basel) 10:1036. doi: 10.3390/genes10121036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breshears LM, Gillman AN, Stach CS, Schlievert PM, Peterson ML. 2016. Local epidermal growth factor receptor signaling mediates the systemic pathogenic effects of Staphylococcus aureus toxic shock syndrome. PLoS One 11:e0158969. doi: 10.1371/journal.pone.0158969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nauciel C, Blass J, Mangalo R, Raynaud M. 1969. Evidence for two molecular forms of streptococcal erythrogenic toxin. Conversion to a single form by 2-mercaptoethanol. Eur J Biochem 11:160–164. doi: 10.1111/j.1432-1033.1969.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 30.Johnson LP, Schlievert PM. 1983. A physical map of the group A streptococcal pyrogenic exotoxin bacteriophage T12 genome. Mol Gen Genet 189:251–255. doi: 10.1007/BF00337813. [DOI] [PubMed] [Google Scholar]
- 31.Johnson LP, Schlievert PM. 1984. Group A streptococcal phage T12 carries the structural gene for pyrogenic exotoxin type A. Mol Gen Genet 194:52–56. doi: 10.1007/BF00383496. [DOI] [PubMed] [Google Scholar]
- 32.Schlievert PM, Assimacopoulos AP, Cleary PP. 1996. Severe invasive group A streptococcal disease: clinical description and mechanisms of pathogenesis. J Lab Clin Med 127:13–22. doi: 10.1016/S0022-2143(96)90161-4. [DOI] [PubMed] [Google Scholar]
- 33.Brosnahan AJ, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. 2009. Cytolysins augment superantigen penetration of stratified mucosa. J Immunol 182:2364–2373. doi: 10.4049/jimmunol.0803283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlievert PM, Gahr PJ, Assimacopoulos AP, Dinges MM, Stoehr JA, Harmala JW, Hirt H, Dunny GM. 1998. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun 66:218–223. doi: 10.1128/IAI.66.1.218-223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brosnahan AJ, Schlievert PM. 2011. Gram-positive bacterial superantigen outside-in signaling causes toxic shock syndrome. FEBS J 278:4649–4667. doi: 10.1111/j.1742-4658.2011.08151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase AT, Rakasz E, Schultz-Darken N, Nephew K, Weisgrau KL, Reilly CS, Li Q, Southern PJ, Rothenberger M, Peterson ML, Schlievert PM. 2015. Glycerol monolaurate microbicide protection against repeat high-dose SIV vaginal challenge. PLoS One 10:e0129465. doi: 10.1371/journal.pone.0129465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick JK, Yarwood JM, Schlievert PM. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol 55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 39.Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. 2013. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis 143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 41.Nyberg SL, Schlievert PM, Saul W, Johnson JR, Ferrieri P, Watkins VS. 1995. Successful management of a serious group A streptococcal infection during the third trimester of pregnancy. Clin Infect Dis 21:1058–1059. doi: 10.1093/clinids/21.4.1058. [DOI] [PubMed] [Google Scholar]
- 42.Hauser AR, Stevens DL, Kaplan EL, Schlievert PM. 1991. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J Clin Microbiol 29:1562–1567. doi: 10.1128/jcm.29.8.1562-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steiner M, Villablanca J, Kersey J, Ramsay N, Haake R, Ferrieri P, Weisdorf D. 1993. Viridans streptococcal shock in bone marrow transplantation patients. Am J Hematol 42:354–358. doi: 10.1002/ajh.2830420405. [DOI] [PubMed] [Google Scholar]
- 44.Mohammadi N, Midiri A, Mancuso G, Patane F, Venza M, Venza I, Passantino A, Galbo R, Teti G, Beninati C, Biondo C. 2016. Neutrophils directly recognize Group B streptococci and contribute to interleukin-1beta production during infection. PLoS One 11:e0160249. doi: 10.1371/journal.pone.0160249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowery CA, Park J, Gloeckner C, Meijler MM, Mueller RS, Boshoff HI, Ulrich RL, Barry CE, III, Bartlett DH, Kravchenko VV, Kaufmann GF, Janda KD. 2009. Defining the mode of action of tetramic acid antibacterials derived from Pseudomonas aeruginosa quorum sensing signals. J Am Chem Soc 131:14473–14479. doi: 10.1021/ja9056079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogan AP, Dickerson TJ, Janda KD. 2005. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci USA 102:309–314. doi: 10.1073/pnas.0408639102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hostager BS, Bishop GA. 2013. CD40-mediated activation of the NF-kappaB2 pathway. Front Immunol 4:376. doi: 10.3389/fimmu.2013.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlievert PM, Osterholm MT, Kelly JA, Nishimura RD. 1982. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann Intern Med 96:937–940. doi: 10.7326/0003-4819-96-6-937. [DOI] [PubMed] [Google Scholar]
- 49.Schlievert PM, Kelly JA. 1984. Clindamycin-induced suppression of toxic-shock syndrome-associated exotoxin production. J Infect Dis 149:471. doi: 10.1093/infdis/149.3.471. [DOI] [PubMed] [Google Scholar]
- 50.Stach CS, Vu BG, Schlievert PM. 2015. Determining the presence of superantigens in coagulase negative staphylococci from humans. PLoS One 10:e0143341. doi: 10.1371/journal.pone.0143341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlievert PM, Peterson ML. 2012. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One 7:e40350. doi: 10.1371/journal.pone.0040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasad GS, Earhart CA, Murray DL, Novick RP, Schlievert PM, Ohlendorf DH. 1993. Structure of toxic shock syndrome toxin 1. Biochemistry 32:13761–13766. doi: 10.1021/bi00213a001. [DOI] [PubMed] [Google Scholar]
- 53.Earhart CA, Vath GM, Roggiani M, Schlievert PM, Ohlendorf DH. 2000. Structure of streptococcal pyrogenic exotoxin A reveals a novel metal cluster. Protein Sci 9:1847–1851. doi: 10.1110/ps.9.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blomster-Hautamaa DA, Schlievert PM. 1988. Preparation of toxic shock syndrome toxin-1. Methods Enzymol 165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]