ABSTRACT
Coccidioidomycosis (Valley fever) is a disease caused by the fungal pathogens Coccidioides immitis and Coccidioides posadasii that are endemic to the southwestern United States and parts of Mexico and South America. Throughout the range where the pathogens are endemic, there are seasonal patterns of infection rates that are associated with certain climatic variables. Previous studies that looked at annual and monthly relationships of coccidioidomycosis and climate suggest that infection numbers are linked with precipitation and temperature fluctuations; however, these analytic methods may miss important nonlinear, nonmonotonic seasonal relationships between the response (Valley fever cases) and explanatory variables (climate) influencing disease outbreaks. To improve our current knowledge and to retest relationships, we used case data from three counties of high endemicity in southern Arizona paired with climate data to construct a generalized additive statistical model that explores which meteorological parameters are most useful in predicting Valley fever incidence throughout the year. We then use our model to forecast the pattern of Valley fever cases by month. Our model shows that maximum monthly temperature, average PM10, and total precipitation 1 month prior to reported cases (lagged model) were all significant in predicting Valley fever cases. Our model fits Valley fever case data in the region of endemicity of southern Arizona and captures the seasonal relationships that predict when the public is at higher risk of being infected. This study builds on and retests relationships described by previous studies regarding climate variables that are important for predicting risk of infection and understanding this fungal pathogen.
IMPORTANCE The inhalation of environmental infectious propagules from the fungal pathogens Coccidioides immitis and Coccidioides posadasii by susceptible mammals can result in coccidioidomycosis (Valley fever). Arizona is known to be a region where the pathogen is hyperendemic, and reported cases are increasing throughout the western United States. Coccidioides spp. are naturally occurring fungi in arid soils. Little is known about ecological factors that influence the growth of these fungi, and a higher environmental burden may result in increases in human exposure and therefore case rates. By examining case and climate data from Arizona and using generalized additive statistical models, we were able to examine the relationship between disease outbreaks and climatic variables and predict seasonal time points of increased infection risk.
KEYWORDS: Valley fever, human fungal pathogen, GAM, climate, coccidioidomycosis, Coccidioides, disease ecology, generalized additive model, mycology
INTRODUCTION
Over the past 40 years, the incidence of invasive fungal infections has dramatically increased, and the number of at-risk populations, such as those who are immunocompromised, has risen concurrently (1). Medically relevant fungal infections are emerging across the globe, with a wide range of disease outcomes from asymptomatic infections to systemic mycoses requiring lifelong treatment. One medically relevant group of organisms are the dimorphic fungi in the order Onygenales (2). This group includes human and animal pathogens such as Emergomyces, Emmonsiellopsis, Emmonsia, Blastomyces, and Histoplasma species complexes and the two species in the genus Coccidioides. The organisms in this order are known to cause subclinical to clinical diseases in humans as well as other mammals (2). Most fungal infections are environmentally acquired, yet few studies have assessed the ecological factors that may influence fungal transmission dynamics.
Coccidioidomycosis, commonly called Valley fever, is a fungal respiratory disease caused by Coccidioides immitis and Coccidioides posadasii that is known to infect mammals throughout the southwestern United States and semiarid to arid areas of Mexico, Central America, and South America (3, 4). Coccidioides immitis in found primarily in California and Washington state, and Coccidioides posadasii is found in all other regions of endemicity. These fungi have two distinct life cycles, an environmental life stage growing as vegetative mycelia in soil and a parasitic stage where the fungus undergoes a dramatic morphological shift into a spherule within the host (5). Infection occurs when a susceptible host inhales airborne spores. The pulmonary infection may result in symptoms such as fever, cough, night sweats, chest distress, and fatigue and can disseminate from the lungs to cause more severe disease outcomes such as meningitis (6). In areas of endemicity, Valley fever presents a significant public health risk. From 2011 to 2017 there were approximately 95,000 cases of Valley fever reported to the Centers for Disease Control and Prevention (CDC) in 26 different states with an average of 200 deaths every year, although due to inconsistent reporting, it is estimated that the actual number of cases is 6 to 14 times greater (7, 8).
The biotic and abiotic ecological factors that influence the distribution and environmental burden of Coccidioides spp. are not well understood due to difficulties in detecting and isolating the fungus from soil (9–12). Previous studies have shown weak correlations between soil properties such as alkaline pH and salinity and the presence of the fungus (9, 10, 13, 14). Certain meteorological parameters, such as precipitation and temperature, likely influence the fluctuation of fungal concentrations in the soil and air, which would lead to increases in infections at certain times of the year. Comrie showed evidence of bimodal seasonality of Valley fever cases that coincided with the precipitation regime of the Sonoran Desert and has assessed the role of dust storms (haboobs)—which appear to play no direct role in the incidence of Valley fever (15, 16). Talamantes et al. found no to weak statistical evidence of climatic factors driving Valley fever cases and attributed fluctuations to human activity (17). A case report in Arizona showed that linear statistics may not be the best approach to link climatic variables as well as PM10 to fluctuations in Valley fever cases (18). These previous approaches to examine the impact of climate on Valley fever case variabilities have been informative; however, with the growing number of cases we must examine if these patterns hold and develop methods that can define climate variables that predict seasonal or yearly outbreaks of Valley fever.
Accurately predicting an increase in Valley fever cases, and determining which months are likely to have high fungal burdens in the environment, will help health officials promote public safety in regions of endemicity. Here, we build statistical models that explain how climate variables are associated with seasonally recurrent human disease. Specifically, we fit generalized additive models (GAMs) to reported human case data to examine infection patterns of Valley fever in the areas of hyperendemicity with the greatest numbers of reported cases in the United States and a known prevalence of Coccidioides posadasii in the soil, namely, Pima, Pinal, and Maricopa counties in Arizona, using climatic variables as covariates.
Our goal is to understand the seasonal dynamics of human infections and how interannual variation in climate will affect the risk of increased cases by retesting what others have previously observed with new data and analytical methods. We chose our climatic covariates based on previous studies, as well as our own hypotheses. Temperature and precipitation are likely biologically important to the life cycle of the fungus (15, 19–21).
Inclement weather, such as dust storms (haboobs), has been proposed to increase the risk of infection by Coccidioides (22, 23). Although recent analysis shows no such connection (16), we still expect wind speed may be important for explaining human disease because of the dispersal of spores that establish infection (16, 17, 24, 25). Moreover, we hypothesize that the concentration of inhalable particulate matter, quantified as PM10, is important because infectious spores could be dispersed via dust. Finally, we accounted for delays in diagnosis by introducing lagged effects because there is a delay between infection and symptom onset. These covariates were put into models and evaluated for importance for model performance; the highest-performing model was used for the final analysis.
We hypothesize that (i) seasonal temperature will impact fungal growth and development, which may be observed by increased cases with higher temperature in the summer months, and (ii) precipitation will impact soil moisture and fungal growth, and thus, greater total precipitation in the winter and during the monsoon season may lead to increased infections, but this effect could be delayed until the soil dries out and conidia can be more efficiently dispersed by wind.
RESULTS
Human cases of Valley fever in Maricopa, Pima, and Pinal counties, Arizona, from 2013 to 2018 demonstrate seasonality. There is a pattern of bimodal seasonality in the three sampled Arizona counties (see Table S1 in the supplemental material). In other words, Valley fever cases fluctuate throughout the year based on climate. Specifically, cases tend to rise during the summer months and winter months and decrease during the spring but show a slight increase in the fall months.
The Lagged Model that included monthly precipitation, mean maximum monthly temperature, average monthly wind speed, mean monthly PM10, lagged precipitation, and lagged PM10 was the most supported model that best explained seasonal patterns of Valley fever (Tables 1 and 2). The effectiveness of the model to predict the future was assessed using 2019 Valley fever case data (Fig S2). This model has high within-sample predictive ability, with an R2 of 0.93 and explained deviance of 96.2%. Specifically, the model suggests that there are significant seasonal effects of PM10, mean maximum temperature, lagged precipitation, and lagged PM10.
TABLE 1.
Predictive performance statistics of different models used to predict seasonal Valley fever casesa
| Model name | RMSE | R-sq. (adj) | Deviance explained (%) | AIC | Δ AIC |
|---|---|---|---|---|---|
| A. No seasonality | 144.32 | 0.30 | 31.10 | 2,683.43 | 740.7 |
| B. Precipitation | 63.72 | 0.85 | 93.70 | 2,073.62 | 130.89 |
| C. Precipitation/temp | 63.81 | 0.85 | 93.80 | 2,072.88 | 130.15 |
| D. All variables | 47.90 | 0.92 | 95.40 | 1,985.71 | 42.98 |
| E. Laggedb | 42.72 | 0.93 | 96.20 | 1,942.73 | 0.00 |
Model performance is evaluated on four different metrics. The best-fit model should have the lowest error (root mean squared error [RMSE]), best fit {measured in adjusted R-squared [R-sq. (adj)]}, highest explained deviance, and lowest AIC. Random effects of county and year are included in all models.
This is the model with the best fit.
TABLE 2.
Covariate effects of the lagged modela
| Covariate | EDF | P value |
|---|---|---|
| Max temp | 2.215 | 0.0214 |
| PM10 | 7.24 | <0.0001 |
| Lagged precipitation | 2.80 | 0.0003 |
| Lagged PM10 | 3.05 | 0.0095 |
| Wind speed | 0.00013 | 0.67 |
| Precipitation | 1.0003 | 0.89 |
Effective degrees of freedom (EDF) of each covariate represents the complexity of each smooth term; the greater values are more complex (1.00 = linear). P value represents overall significance of the smooth term.
Average monthly PM10.
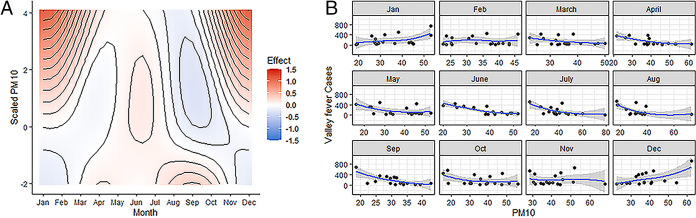
Of the climate variables used in our study, the inhalable particulate matter 10 μm and below (PM10) shows the strongest effects on Valley fever cases. Specifically, high PM10 in the winter (November to January) is associated with higher detection of human cases in the winter (Fig. 1). The effect is particularly strong in December and January. However, during the rest of the year the effect of PM10 is minimal (Fig. 1). Figure 1A explores the relationship between month and PM10 and how the combination affects case numbers. The model is predictive across a range of PM10 values. The contour lines are identifying combinations of PM10 values and month and the relationship to Valley fever cases (26).
FIG 1.
The effects of PM10 on the number of Valley fever cases. (A) Contour plot of predicted Valley fever cases across the entire range of PM10 values (micrograms per cubic meter). Red means that there is a strong positive effect of PM10 on cases (i.e., increased infections), and blue means that there is a weak to negative effect of PM10 on cases (i.e., reduced infections). (B) Raw monthly PM10 data plotted against monthly Valley fever data added together from all three counties. Locally estimated scatterplot smoothing (LOESS) smoother line used in order to visualize relationships.
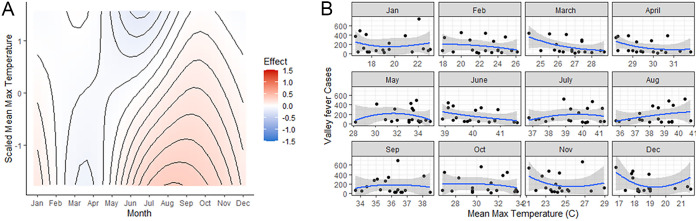
Mean monthly maximum temperature.
In January and February lower maximum temperatures are associated with increased infections, although the effect is weaker than the effect of PM10. Starting in the early summer months, there is a stronger effect, such that lower temperatures are associated with increased cases (Fig. 2). In other words, during the summer to late fall, when temperatures are cooler than average, we tend to see more Valley fever cases.
FIG 2.
The effects of temperature on the number of Valley fever cases. A 2-month lag time before the month where cases were reported was employed. (A) Simulation of predicted Valley fever cases across the entire range of mean maximum temperatures. Red means that there is a strong effect of maximum temperatures and Valley fever infections, and blue indicates a weak effect of maximum temperature on Valley fever infections. (B) Raw mean maximum monthly temperature data plotted against monthly Valley fever data added together from all three counties. LOESS smoother line used in order to visualize relationships.
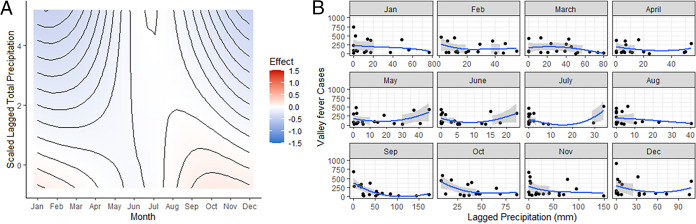
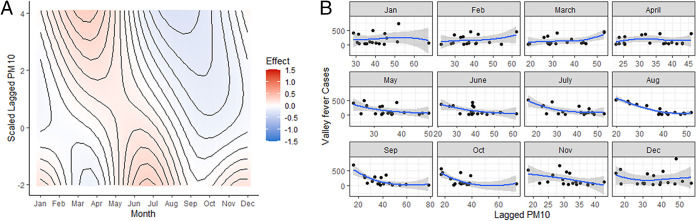
Lagged precipitation and PM10.
Precipitation and PM10 each have significant lagged effects. In other words, the values of these variables 2 months prior to disease reporting have effects on the number of Valley fever cases detected in the current month. First, we tend to see more cases in the late fall and winter months when there was lower precipitation 2 months before (Fig. 3), likely indicating lower overall moisture for the preceding 2 months. Second, lagged PM10 showed more complex effects, although these effects are generally weaker than current PM10 (above). In the winter and summer months, low lagged PM10 has a weakly positive effect, associated with higher cases; however, this effect is reversed in the spring months, such that high PM10 in the late winter is associated with higher cases in the spring months (Fig. 4).
FIG 3.
The effects of lagged precipitation on the number of Valley fever cases. A 2-month lag time before the month where cases were reported was employed. (A) Simulation of predicted Valley fever cases across the entire range of lagged precipitation. Red means that there is a strong effect of maximum temperatures and Valley fever infections, and blue indicates a weak effect of maximum temperature on Valley fever infections. (B) Raw lagged precipitation data plotted against monthly Valley fever data added together from all three counties. LOESS smoother line used in order to visualize relationships.
FIG 4.
The effects of lagged PM10 and temperature on the number of Valley fever cases. A 2-month lag time before the month where cases were reported was employed. (A) Simulation of predicted Valley fever cases across the entire range of lagged PM10. Red means that there is a strong effect of maximum temperatures and Valley fever infections, and blue indicates a weak effect of PM10 on Valley fever infections. (B) Raw lagged PM10 data plotted against monthly Valley fever data added together from all three counties. LOESS smoother line used in order to visualize relationships.
DISCUSSION
Seasonal effects on Valley fever incidence in Arizona.
This study found significant monthly effects of climate on cases of coccidioidomycosis. PM10 shows the strongest effect on the number of cases when PM10 was higher during the winter months leading to increased infections. Interestingly, PM10 during the rest of the year does not appear to have a strong effect on infections. Other variables were also significant in predicting Valley fever cases, although not as strong as PM10. Decreased mean maximum temperatures in January and February are associated with increased infections; however, the effect becomes weaker in early summer when there is an even stronger effect of cooler maximum temperatures associated with increased cases. These contemporary results coincide with previous studies that examined the effect of PM10 on Valley fever cases. Comrie in 2005 as well as others showed that PM10 is positively associated with Valley fever rates in the winter months but is not a useful predictor during monsoon season in Arizona (15, 27). This study shows results contradictory to previous studies done in California where negative relationships were found between dust and Valley fever and no significance in the winter months (23, 28). These differences highlight the dissimilarities between the two species of Coccidioides and the subsequent disease dynamics that follow infection.
We also detected significant lagged effects on both PM10 and precipitation. First, there appears to be a trend of increased infections in the fall and winter months when precipitation was low 2 months prior. Second, in the winter and summer months, low lagged PM10 has a weakly positive effect, associated with higher cases; however, this effect is reversed in the spring, such that high PM10 in the late winter months is associated with higher cases in the spring months. Thus, temperature and precipitation do not have as much of an effect on cases as we hypothesized, but rather, PM10 is the more important predictor. Lagged effects were also observed to be important by Kolivras and Comrie in 2003; however, the significant predictor variables were most sensitive in the winter months and lagged 1 year prior (20).
The effects of climate on the incidence of Valley fever in southern Arizona are quite complex with no obvious pattern when considering only the raw data. The effect of each climatic variable fluctuates throughout the year, and the effect is often nonlinear. The influence of lagged effects also appears to be important. Without the implementation of sophisticated statistical models, such as generalized additive models, quantifying these effects would be challenging. Binning case counts and climate variables by month disentangles the complexity of these relationships, and the effects are more pronounced. This may explain why historically it has been difficult to associate fungal infections with climate, and it emphasizes the complexity of the biology that underlies these climate associations.
Influence of climate on the Coccidioides life cycle.
PM10 is a strong predictor of Valley fever cases because this particulate matter fraction contains fungal spores that cause infection. Particulate matter is an important gauge of air quality, and this metric quantifies the amount of pollutants and biological material such as allergens and potentially pathogenic fungi in the air (29). These small particles are associated with bacterial and fungal spores and are shown to have the most human health impacts (29, 30). These particles have the potential to be airborne for an extended period of time and can travel great distances (29). Several studies have shown that PM10 is an effective predictor of certain infectious diseases that are environmentally acquired (31–33). Our current study shows that PM10 in southern Arizona is a significant predictor of Valley fever cases but only during certain seasons. Previous studies have shown a relationship between soil-disturbing activities releasing a large quantity of airborne dust and Valley fever outbreaks (34–44), but this might not account for most cases that are caused by higher levels of fungi in the air column.
The climatic data were taken from NOAA land-based climate monitoring systems located in each of the representative counties in southern Arizona. There could be subtle differences between rural and urban locations that could skew the data and influence the results of the model. For future analysis, multiple locations from each county should be used to get an accurate representation of that location.
We hypothesize that the effect of wetting and drying on fungal growth and conidiation could explain why the 2-month lag in precipitation shows a strong effect on number of infections. The initial moisture leads to increased fungal biomass with the subsequent drying leading to increased airborne spores, which then have the potential for increased human infection. Precipitation is essential for growth and metabolism for all soil fungi, but with potentially even greater effects on a desert fungus such as Coccidioides species. Water is essential for the transport of nutrients from the soil substrate into fungal cells as well as the hydrolytic process of breaking down macromolecules from the substrate to be used in metabolism (45). In semiarid and arid ecosystems, such as the Sonoran Desert, the soil experiences drought followed by rapid rewetting during the monsoon. The first 24 h after a wetting event has the greatest concentration of nutrient availability from the substrate as well as highest microbial activity followed by a drastic reduction in microbial activity and biomass as the soil dries (46). There is also a shift in the microbial soil community, which goes from being fungal dominant to Gram-positive bacterial dominant (46–48). The increase in moisture from infrequent desert rain may influence mycelium growth of Coccidioides spp., followed by a proliferation of conidial production with the prolonged dry periods leading to less nutrient availability. This increase in conidial production leads to an increase in infectious conidia in the environment and increased mammalian infections.
Understanding when a human population is at most risk of being exposed to Coccidioides has tremendous public health and economic implications. This model and others that come after it will allow public health departments to make more accurate public health announcements regarding risk for exposure. We have shown that we can use climatic variables (PM10, precipitation, and temperature) to accurately predict increases in Valley fever infections. By utilizing complex analytical methods developed for unrelated biological systems, such as generalized additive models, we demonstrate the utility in understanding the seasonal relationship of climate and Valley fever to predict risk.
MATERIALS AND METHODS
Arizona case data.
Monthly Valley fever case data were collected from the Arizona Department of Health Safety and the CDC (7). We focused on cases reported in Pima, Pinal, and Maricopa counties from 2013 to 2018. These counties are located in the region of hyperendemicity and have the highest reported cases of human disease in the state of Arizona (7). Reporting of Valley fever was mandatory in Arizona during this time frame, and an increase in cases was observed.
Climatic data.
Climate data were obtained from the National Oceanic and Atmospheric Administration land-based stations from Maricopa, Pima, and Pinal counties in Arizona (49). Data were collected from one monitor system per county. Climate variables used for the Valley fever case model were taken from the month the cases were reported. The variables used in our analysis are wind speed (miles per hour [mph]), mean maximum temperature (°C), and total monthly precipitation (millimeters). Lagging variables simultaneously account for delays in diagnosis of disease (i.e., delay between exposure and case detection), as well as time for the fungal development. We implemented a 2-month lag period for covariates to account for delays in diagnosis and reporting. We treated the concurrent variables as different variables than lagged variables and tested for correlation via Pearson correlation.
For each covariate, we centered and scaled the climate variables by subtracting the mean of each data set from each individual data point and dividing all data points by the standard deviation of the data set.
Air quality particulate matter 10 μm and smaller (PM10) is commonly used to quantify biological particles in ambient air that are generally deposited in the upper respiratory tract and linked to certain infectious diseases (31, 50, 51). We are using PM10 as a proxy for airborne Coccidioides spores in the ambient atmosphere (15). This is measured in micrograms per cubic meter.
Statistical analysis.
The effects of climate variables on case counts may change over time, and these effects may be nonlinear. For instance, thermal performance curves for microbial growth are often nonlinear (52). Therefore, we used generalized additive modeling (GAM). Specifically, GAMs use smoothed functional relationships (splines) to explain the potential nonlinear effects of covariates, and these splines can be two dimensional, allowing us to account for time-varying effects (26). Moreover, because the Valley fever case data are integer values, we assumed a negative-binomial error distribution for the model.
Model structure.
We used the mgcv R package (53, 54) to perform our analyses (see Supplemental data file 1 in the supplemental material [R code] for details), and the full model used the following structure:
(i) Full model including lagged variables. Human Cases∼te(Month, Precipitation)+te(Month, Temperature)+te(Month, Wind Speed)+te(Month, PM10)+te(Month, Lagged Precipitation)+te(Month, Lagged PM10)+s(County)+s(Year).
The above model structure represents the influence of each climatic covariate on the response variable (human cases of Valley fever). Each continuous covariate employs a cyclic cubic regression spline [i.e., the function, te()] to estimate the marginal effects of the covariate and month of the year. We chose this spline because of the cyclical nature of seasonally recurrent disease transmission. To account for nonindependence of the case data within years and counties, the effects of county and year were added as random effect splines [i.e., the function s()]. We reduced overfitting and limited the degrees of freedom by running an iterative model to get the optimal smoothing parameter (k = 5). The gam.check function in the R package mgcv allows the user to determine how the selected K (smoothing parameter) fits the predictors (overfit check).
Model selection.
Each model was compared to a basic model in which no splines were used. This was a type of null model or “strawman,” which we refer to as the “No Seasonality” model. In other words, no cyclic cubic regression splines were used in this simple “no seasonality” model. More complex models (models B, C, and D) included cyclic cubic regression splines and random effect splines along with the climate covariates (Table 1). Model E has lagged precipitation and lagged PM10 as covariates. We assessed model performance using multiple metrics: Akaike information criterion (AIC), explained deviance, adjusted R-squared, and root mean squared error, as well as examined for variable autocorrelation by Pearson correlation test. A summary of all covariates and splines of each test model can be found in Table S1 in the supplemental material.
(i) Null model. Human cases∼Precipitation+Temperature+Wind Speed+PM10+Month+County.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by an Arizona Biomedical Research Center grant (ABRC 16-162415) to B.M.B.
Footnotes
Supplemental material is available online only.
Contributor Information
Bridget M. Barker, Email: Bridget.Barker@nau.edu.
Christina A. Cuomo, Broad Institute
REFERENCES
- 1.Nucci M, Marr KA. 2005. Emerging fungal diseases. Clin Infect Dis 41:521–526. doi: 10.1086/432060. [DOI] [PubMed] [Google Scholar]
- 2.Dukik K, Muñoz JF, Jiang Y, Feng P, Sigler L, Stielow JB, Freeke J, Jamalian A, Gerrits van den Ende B, McEwen JG, Clay OK, Schwartz IS, Govender NP, Maphanga TG, Cuomo CA, Moreno LF, Kenyon C, Borman AM, de Hoog S. 2017. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 60:296–309. doi: 10.1111/myc.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maddy KT. 1958. The geographic distribution of coccidioides immitis and possible ecologic implications. Ariz Med 15:178–188. [PubMed] [Google Scholar]
- 4.Pappagianis D. 1980. Epidemiology of coccidioidomycosis, p 63–85. In Stevens DA (ed), Coccidioidomycosis. Springer, Boston, MA. [Google Scholar]
- 5.Mead HL, Teixeira MM, Galgiani JN, Barker BM. 2019. Characterizing in vitro spherule morphogenesis of multiple strains of both species of Coccidioides. Med Mycol 57:478–488. doi: 10.1093/mmy/myy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saubolle MA, McKellar PP, Sussland D. 2007. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol 45:26–30. doi: 10.1128/JCM.02230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedict K, McCotter OZ, Brady S, Komatsu K, Sondermeyer Cooksey GL, Nguyen A, Jain S, Vugia DJ, Jackson BR. 2019. Surveillance for coccidioidomycosis - United States, 2011–2017. MMWR Surveill Summ 68:1–15. doi: 10.15585/mmwr.ss6807a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCotter OZ, Benedict K, Engelthaler DM, Komatsu K, Lucas KD, Mohle-Boetani JC, Oltean H, Vugia D, Chiller TM, Sondermeyer Cooksey GL, Nguyen A, Roe CC, Wheeler C, Sunenshine R. 2019. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol 57:S30–S40. doi: 10.1093/mmy/myy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker BM, Tabor JA, Shubitz LF, Perrill R, Orbach MJ. 2012. Detection and phylogenetic analysis of Coccidioides posadasii in Arizona soil samples. Fungal Ecol 5:163–176. doi: 10.1016/j.funeco.2011.07.010. [DOI] [Google Scholar]
- 10.Fisher FS, Bultman MW, Johnson SM, Pappagianis D, Zaborsky E. 2007. Coccidioides niches and habitat parameters in the southwestern United States: a matter of scale. Ann N Y Acad Sci 1111:47–72. doi: 10.1196/annals.1406.031. [DOI] [PubMed] [Google Scholar]
- 11.Greene DR, Koenig G, Fisher MC, Taylor JW. 2000. Soil isolation and molecular identification of Coccidioides immitis. Mycologia 92:406–410. doi: 10.2307/3761498. [DOI] [Google Scholar]
- 12.Kollath DR, Teixeira MM, Funke A, Miller KJ, Barker BM. 2020. Investigating the role of animal burrows on the ecology and distribution of Coccidioides spp. in Arizona soils. Mycopathologia 185:145–159. doi: 10.1007/s11046-019-00391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swatek FE. 1970. Ecology of Coccidioides immitis. Mycopathol Mycol Appl 41:3–12. doi: 10.1007/BF02051479. [DOI] [PubMed] [Google Scholar]
- 14.Chow NA, Kangiser D, Gade L, McCotter OZ, Hurst S, Salamone A, Wohrle R, Clifford W, Kim S, Salah Z, Oltean HN, Plumlee GS, Litvintseva AP. 2021. Factors influencing distribution of Coccidioides immitis in soil, Washington State, 2016. mSphere 6:e00598-21. doi: 10.1128/mSphere.00598-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comrie AC. 2005. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect 113:688–692. doi: 10.1289/ehp.7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comrie AC. 2021. No consistent link between dust storms and Valley fever (coccidioidomycosis). Geohealth 5:e2021GH000504. doi: 10.1029/2021GH000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talamantes J, Behseta S, Zender CS. 2007. Fluctuations in climate and incidence of coccidioidomycosis in Kern County, California: a review. Ann N Y Acad Sci 1111:73–82. doi: 10.1196/annals.1406.028. [DOI] [PubMed] [Google Scholar]
- 18.Lee CM. 2020. Spatiotemporal association between valley fever and PM10: a case study of Arizona. Master’s thesis. Texas Tech University, Lubbock, TX. [Google Scholar]
- 19.Gorris M, Cat L, Zender C, Treseder K, Randerson J. 2018. Coccidioidomycosis dynamics in relation to climate in the southwestern United States. Geohealth 2:6–24. doi: 10.1002/2017GH000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolivras KN, Comrie AC. 2003. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int J Biometeorol 47:87–101. doi: 10.1007/s00484-002-0155-x. [DOI] [PubMed] [Google Scholar]
- 21.Kolivras KN, Johnson PS, Comrie AC, Yool SR. 2001. Environmental variability and coccidioidomycosis (valley fever). Aerobiologia 17:31–42. doi: 10.1023/A:1007619813435. [DOI] [Google Scholar]
- 22.Alexander R, Nugent C, Nugent K. 2018. The Dust Bowl in the US: an analysis based on current environmental and clinical studies. Am J Med Sci 356:90–96. doi: 10.1016/j.amjms.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Tong DQ, Wang JX, Gill TE, Lei H, Wang B. 2017. Intensified dust storm activity and Valley fever infection in the southwestern United States. Geophys Res Lett 44:4304–4312. doi: 10.1002/2017GL073524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown HE, Comrie AC, Tamerius J. 1984. Climate, wind storms, and the risk of valley fever (coccidioidomycosis). Biometrics 40:961–971.6534418 [Google Scholar]
- 25.Guevara RE, Motala T, Terashita D. 2015. The changing epidemiology of coccidioidomycosis in Los Angeles (LA) County, California, 1973–2011. PLoS One 10:e0136753. doi: 10.1371/journal.pone.0136753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen EJ, Miller DL, Simpson GL, Ross N. 2019. Hierarchical generalized additive models in ecology: an introduction with mgcv. PeerJ 7:e6876. doi: 10.7717/peerj.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park BJ, Sigel K, Vaz V, Komatsu K, McRill C, Phelan M, Colman T, Comrie AC, Warnock DW, Galgiani JN, Hajjeh RA. 2005. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998–2001. J Infect Dis 191:1981–1987. doi: 10.1086/430092. [DOI] [PubMed] [Google Scholar]
- 28.Weaver EA, Kolivras KN. 2018. Investigating the relationship between climate and Valley fever (coccidioidomycosis). Ecohealth 15:840–852. doi: 10.1007/s10393-018-1375-9. [DOI] [PubMed] [Google Scholar]
- 29.Kim K-H, Kabir E, Kabir S. 2015. A review on the human health impact of airborne particulate matter. Environ Int 74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Garaga R, Avinash C, Kota SH. 2019. Seasonal variation of airborne allergenic fungal spores in ambient PM 10—a study in Guwahati, the largest city of north-east India. Air Qual Atmos Health 12:11–20. doi: 10.1007/s11869-018-0624-y. [DOI] [Google Scholar]
- 31.Mishra R, Krishnamoorthy P, Gangamma S, Raut AA, Kumar H. 2020. Particulate matter (PM10) enhances RNA virus infection through modulation of innate immune responses. Environ Pollut 266:115148. doi: 10.1016/j.envpol.2020.115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olaniyan T, Dalvie MA, Röösli M, Naidoo RN, Künzli N, de Hoogh K, Berman D, Parker B, Leaner J, Jeebhay MF. 2020. Short term seasonal effects of airborne fungal spores on lung function in a panel study of schoolchildren residing in informal settlements of the Western Cape of South Africa. Environ Pollut 260:114023. doi: 10.1016/j.envpol.2020.114023. [DOI] [PubMed] [Google Scholar]
- 33.Yin F, Ma Y, Zhao X, Lv Q, Liu Y, Li X, Zhang T. 2019. Analysis of the effect of PM10 on hand, foot and mouth disease in a basin terrain city. Sci Rep 9:3233. doi: 10.1038/s41598-018-35814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colson AJ, Vredenburgh L, Guevara RE, Rangel NP, Kloock CT, Lauer A. 2017. Large-scale land development, fugitive dust, and increased coccidioidomycosis incidence in the Antelope Valley of California, 1999–2014. Mycopathologia 182:439–458. doi: 10.1007/s11046-016-0105-5. [DOI] [PubMed] [Google Scholar]
- 35.de Perio MA, Niemeier RT, Burr GA. 2015. Coccidioides exposure and coccidioidomycosis among prison employees, California, United States. Emerg Infect Dis 21:1031–1033. doi: 10.3201/eid2106.141201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freedman M, Jackson BR, McCotter O, Benedict K. 2018. Coccidioidomycosis outbreaks, United States and worldwide, 1940–2015. Emerg Infect Dis 24:417–423. doi: 10.3201/eid2403.170623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laws RL, Cooksey GS, Jain S, Wilken J, McNary J, Moreno E, Michie K, Mulkerin C, McDowell A, Vugia D, Materna B. 2018. Coccidioidomycosis outbreak among workers constructing a solar power farm - Monterey County, California, 2016–2017. MMWR Morb Mortal Wkly Rep 67:931–934. doi: 10.15585/mmwr.mm6733a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson D, Ebisu K, Wu X, Basu R. 2019. A review of coccidioidomycosis in California: exploring the intersection of land use, population movement, and climate change. Epidemiol Rev 41:145–157. doi: 10.1093/epirev/mxz004. [DOI] [PubMed] [Google Scholar]
- 39.Perera P, Stone S. 2002. Coccidioidomycosis in workers at an archeologic site-Dinosaur National Monument, Utah, June-July 2001. Ann Emerg Med 39:566–569. doi: 10.1067/mem.2002.123550. [DOI] [PubMed] [Google Scholar]
- 40.Petersen LR, Marshall SL, Barton-Dickson C, Hajjeh RA, Lindsley MD, Warnock DW, Panackal AA, Shaffer JB, Haddad MB, Fisher FS, Dennis DT, Morgan J. 2004. Coccidioidomycosis among workers at an archeological site, northeastern Utah. Emerg Infect Dis 10:637–642. doi: 10.3201/eid1004.030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sondermeyer Cooksey GL, Wilken JA, McNary J, Gilliss D, Shusterman D, Materna BL, Vugia DJ. 2017. Dust exposure and coccidioidomycosis prevention among solar power farm construction workers in California. Am J Public Health 107:1296–1303. doi: 10.2105/AJPH.2017.303820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamerius JD, Comrie AC. 2011. Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PLoS One 6:e21009. doi: 10.1371/journal.pone.0021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner SB, Pappagianis D. 1973. Coccidioidomycosis in Northern California. An outbreak among archeology students near Red Bluff. Calif Med 119:16–20. [PMC free article] [PubMed] [Google Scholar]
- 44.Wilken JA, Sondermeyer G, Shusterman D, McNary J, Vugia DJ, McDowell A, Borenstein P, Gilliss D, Ancock B, Prudhomme J, Gold D, Windham GC, Lee L, Materna BL. 2015. Coccidioidomycosis among workers constructing solar power farms, California, USA, 2011–2014. Emerg Infect Dis 21:1997–2005. doi: 10.3201/eid2111.150129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul K, Polglase P, O’Connell A, Carlyle J, Smethurst P, Khanna P. 2003. Defining the relation between soil water content and net nitrogen mineralization. Eur J Soil Sci 54:39–48. doi: 10.1046/j.1365-2389.2003.00502.x. [DOI] [Google Scholar]
- 46.Butterly C, Bünemann E, McNeill A, Baldock J, Marschner P. 2009. Carbon pulses but not phosphorus pulses are related to decreases in microbial biomass during repeated drying and rewetting of soils. Soil Biol Biochem 41:1406–1416. doi: 10.1016/j.soilbio.2009.03.018. [DOI] [Google Scholar]
- 47.Fierer N, Schimel JP. 2003. A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci Soc Am J 67:798–805. doi: 10.2136/sssaj2003.0798. [DOI] [Google Scholar]
- 48.Van Gestel M, Merckx R, Vlassak K. 1993. Microbial biomass responses to soil drying and rewetting: the fate of fast-and slow-growing microorganisms in soils from different climates. Soil Biol Biochem 25:109–123. doi: 10.1016/0038-0717(93)90249-B. [DOI] [Google Scholar]
- 49.Young A, Knapp K, Inamdar A, Hankins W, Rossow W. 2018. The International Satellite Cloud Climatology Project H-Series climate data record product. Earth Syst Sci Data 10:583–593. doi: 10.5194/essd-10-583-2018. [DOI] [Google Scholar]
- 50.Dastoorpoor M, Riahi A, Yazdaninejhad H, Borsi SH, Khanjani N, Khodadadi N, Mohammadi MJ, Aghababaeian H. 2021. Exposure to particulate matter and carbon monoxide and cause-specific cardiovascular-respiratory disease mortality in Ahvaz. Toxin Rev 40:1362–1311. doi: 10.1080/15569543.2020.1716256. [DOI] [Google Scholar]
- 51.Romano S, Fragola M, Alifano P, Perrone MR, Talà A. 2021. Potential human and plant pathogenic species in airborne PM10 samples and relationships with chemical components and meteorological parameters. Atmosphere 12:654. doi: 10.3390/atmos12050654. [DOI] [Google Scholar]
- 52.Peleg M, Corradini MG. 2011. Microbial growth curves: what the models tell us and what they cannot. Crit Rev Food Sci Nutr 51:917–945. doi: 10.1080/10408398.2011.570463. [DOI] [PubMed] [Google Scholar]
- 53.R Core Team. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 54.Wood S, Wood MS. 2015. Package ‘mgcv’. R package version 1:29.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM01483-21_Supp_1_seq7.pdf, PDF file, 1.8 MB (1.8MB, pdf)