Abstract
Pyrimethamine is a potent inhibitor of dihydrofolate reductase and is widely used in the treatment of opportunistic infections caused by the protozoan parasite Toxoplasma gondii. In order to assess the potential role of dhfr sequence polymorphisms in drug treatment failures, we examined the dhfr-ts genes of representative isolates for T. gondii virulence types I, II, and III. These strains exhibit differences in their sensitivities to pyrimethamine but no differences in predicted dhfr-ts protein sequences. To assess the potential for pyrimethamine-resistant dhfr mutants to emerge, three drug-sensitive variants of the T. gondii dhfr-ts gene (the wild-type T. gondii sequence and two mutants engineered to reflect polymorphisms observed in drug-sensitive Plasmodium falciparum) were subjected to random mutagenesis and transfected into either wild-type T. gondii parasites or dhfr-deficient Saccharomyces cerevisiae under pyrimethamine selection. Three resistance mutations were identified, at amino acid residues 25 (Trp→Arg), 98 (Leu→Ser), and 134 (Leu→His).
The protozoan parasite Toxoplasma gondii is a ubiquitous human pathogen, but a robust cellular immune response ordinarily suppresses acute infection by the rapidly dividing tachyzoite stage of the parasite life cycle (9). As a consequence, primary T. gondii infections are generally self-limited and do not require therapeutic intervention, barring the special case of primary infection during pregnancy (50). Once infected, individuals carry a latent, encysted form of the parasite (the bradyzoite) for life. This condition poses a chronic health hazard for patients who are immunocompromised, immunosuppressed, or otherwise unable to respond effectively to the challenge posed by reactivation of latent infection (22). Long-term chemoprophylaxis is generally advised for such individuals (2), even in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy (although recent studies indicate that the duration of treatment for these patients can be shortened considerably) (34, 43).
Antifolates form the basis of chemotherapy against both primary and secondary (recurrent) infection by T. gondii (18, 19, 50; S. Y. Wong and J. S. Remington, Editorial, AIDS 7:299–316, 1993), either pyrimethamine combined with sulfadiazine or the somewhat less potent combination of pyrimethamine and clindamycin (5, 17). Both strategies are effective at disease remediation with short-duration use (10), but the frequency of treatment failures associated with long-term antifolate use is high, particularly among AIDS patients (2, 16, 42, 47; P. Caramello, T. Brancale, B. Forno, A. Lucchini, A. M. Pollono, A. Ullio, P. Gioannini, I. Viano, and E. Tonso, Letter, Antimicrob. Agents Chemother. 39:2371–2372, 1995). In one study, relapse rates over a 2-year period were 11% for pyrimethamine-sulfadiazine and 22% for pyrimethamine-clindamycin (17). Many determinants may contribute to treatment failures, including host factors, such as intolerance to either or both component drugs (22) or malabsorption into infected neural tissues (16, 49). Parasite-specific factors, such as preexisting differences in drug sensitivity between strains or the development of drug resistance mutations during chemotherapy, may also play an important role. Prolonged drug treatment—particularly with drugs such as pyrimethamine that exhibit a long half-life (16, 26)—can elicit a strong selection for drug-resistant pathogens, as has been seen with Mycobacterium tuberculosis (20, 21), human immunodeficiency virus (32, 33), and the malaria parasite Plasmodium falciparum (15). This report examines both the influence of parasite genotype on drug sensitivity and the potential for acquired resistance in disease relapse.
The target for pyrimethamine in T. gondii is the dihydrofolate reductase (DHFR) domain of a bifunctional fusion protein that also harbors thymidylate synthase (TS) activity (31). The primary sequence for the single-copy T. gondii dhfr-ts gene has been determined for strain RH (35). Restriction fragment length polymorphism and isozyme analysis indicate that most T. gondii isolates fall into one of three clonal lineages (designated type I, II, or III) (6, 12). While correlations among the parasite genotype, virulence, and disease presentation have been established (13, 44), less is known about the role of parasite genotypes in cases of failed drug therapy. The first part of this study considers intrinsic differences in pyrimethamine sensitivity between strains of T. gondii and evaluates whether the observed variations in drug susceptibility can be attributed to resident dhfr polymorphisms.
In P. falciparum (a member of the same phylum as T. gondii), resistance to pyrimethamine is commonly associated with dhfr point mutations (4, 29, 45; reviewed in reference 15). To better understand the potential role of dhfr polymorphisms in toxoplasmosis, we developed a method to generate and select pyrimethamine resistance mutations in T. gondii dhfr. Random mutagenesis of the dhfr-ts gene was followed by in vitro drug selection during intracellular replication of T. gondii parasites. Several pyrimethamine-sensitive T. gondii dhfr alleles were mutagenized and subsequently screened for drug resistance in either T. gondii parasites or yeast, yielding novel resistance mutations.
MATERIALS AND METHODS
Pyrimethamine sensitivities of natural T. gondii strains.
Three strains representing the three major virulence groups of T. gondii (12) were assessed for pyrimethamine sensitivity: the type I strain RH (38), the type II strain P(LK) (23), and the type III strain Veg (28). Each strain was allowed to infect confluent host cell monolayers of primary human foreskin fibroblasts grown in modified essential medium (Gibco) containing 0.1% dialyzed fetal bovine serum, as previously described (37). Pyrimethamine was added at various concentrations, and parasite proliferation was measured by following [3H]uracil incorporation in a 4-h uptake assay carried out ∼24 h postinfection (30).
Mutagenesis of T. gondii dhfr alleles.
Two constructs were used as templates for mutagenesis experiments (Fig. 1). In pC3 vectors, cDNA-derived (intronless) T. gondii dhfr-ts minigenes flanked by autologous genomic flanking sequences (8) were introduced into the pBluescript KS(−) plasmid (Stratagene). Two pyrimethamine-sensitive dhfr-ts alleles were employed in pC3 vectors: the wild type and allele T83S, which carries a Thr→Ser substitution at position 83 (31). In pTRP constructs, a 1-kb BamHI to EcoRI fragment encompassing the T. gondii dhfr domain was introduced into the nonintegrative, single-copy Saccharomyces cerevisiae shuttle vector pRS304 (40) under control of the yeast dhfr promoter (51). Both wild-type dhfr and the pyrimethamine-hypersensitive allele A10V (31) were introduced into the pTRP background.
FIG. 1.
Scheme for random mutagenesis of T. gondii dhfr and in vivo selection of pyrimethamine-resistant mutants. The plasmid maps for pC3 and pTRP are drawn to scale, indicating the coding sequence for T. gondii dhfr (shaded boxes) and ts (hatched box; pC3 only), flanking untranslated sequences (heavy black lines; the arrows indicate the promoters and the direction of transcription) derived from the T. gondii dhfr-ts genomic locus (7, 8) (pC3) or S. cerevisiae dhfr (51) (pTRP), and pBluescript KS(−) or pRS304 vector sequences (thin gray lines; miscellaneous features are as marked). YEPD, yeast extract-peptone-dextrose agar.
Mutagenesis of each template was performed by propagation of the plasmids (four in all) in the E. coli “mutator” strain XL1-red (endA1 gyr96 Thi-1 hdsR17 supE44 relA1 lac mutS mutD5 mutT Tn10 Tetr) (Stratagene). The plasmids were introduced into the mutagenic strain by heat shock, and multiple (∼10) ampicillin-resistant E. coli transformants harboring the parental template were cultivated independently in 5-ml cultures of Luria broth for 20 to 36 h. The mutational frequencies are estimated to be ∼1 alteration/2,000 nucleotides/plasmid in an overnight culture (11). Mutated plasmids were recovered by alkaline lysis “miniprep” (24), and plasmids from the same parental template were pooled before purification by phenol-chloroform extraction and polyethylene glycol precipitation (24).
Selection for pyrimethamine-resistant T. gondii and S. cerevisiae transformants.
For direct selection of pyrimethamine-resistant dhfr-ts alleles in transgenic T. gondii, 30 μg of mutated pC3 plasmid DNA (containing mutated wild-type or T83S alleles of dhfr-ts) were transfected into RH strain tachyzoites by electroporation (8). Stably resistant parasites were selected at 0.8 μM pyrimethamine in 25-cm2 T flasks containing confluent monolayers of primary human foreskin fibroblasts and cloned by limiting dilution in microtiter plates, as previously described (37). Genomic DNA was obtained from resistant clones by phenol-chloroform extraction for analysis of the dhfr-ts transgene copy number and genomic organization by Southern hybridization (24).
Because the A10V allele of T. gondii dhfr is hypersensitive to pyrimethamine (31), precluding analysis in T. gondii parasites harboring a wild-type dhfr-ts allele, pyrimethamine-resistant mutants of the A10V allele were selected in a dhfr-deficient strain of S. cerevisiae (YH5; MATa ura3-52 leu2-3, 112 trp1 tup dfr1::URA3) (14, 51). Mutated pTRP templates from both the wild-type and A10V alleles of T. gondii dhfr-ts were introduced into YH5 using lithium acetate (39), and transformants were first selected for their ability to grow at 30°C on yeast extract-peptone-dextrose agar without dTMP (required in the absence of functional dhfr expression) (14). All transformants were then screened for growth on minimal agar containing 0, 5, 10, 15, or 20 μM pyrimethamine. The 50% inhibitory concentrations (IC50s) for putative resistance mutants were assessed from growth rates in unsupplemented minimal liquid medium containing 0 to 6 μM pyrimethamine (0.5-μM increments). Mutated plasmids from resistant yeast clones were recovered by standard methods (39).
Identification of DHFR polymorphisms and confirmation of resistance phenotypes.
The dhfr domain of putative resistance genes was amplified by PCR and sequenced with Sequenase 2.0 (U.S. Biochemicals) to identify polymorphisms. Candidate resistance mutations were then introduced into pC3 plasmids containing various dhfr-ts backgrounds by site-directed mutagenesis (25) and transiently expressed in RH strain parasites under pyrimethamine selection. Parasite proliferation was measured 30 h posttransfection by following the uptake of [3H]uracil (30). IC50s were calculated from percent growth inhibition at 0.3, 0.6, and 1.0 μM pyrimethamine relative to untreated controls, and mutants were assigned to resistance classes based on established categories (31).
RESULTS
Pyrimethamine sensitivity and dhfr sequences in different T. gondii strains.
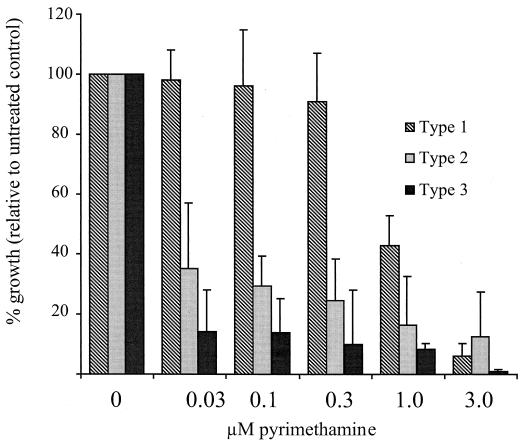
To evaluate whether patterns might be established between treatment failures and the parasite genotype, we examined the susceptibilities of parasites from different virulence lineages to pyrimethamine, as shown in Fig. 2. The three strains evaluated [RH, a type I strain; P(LK), type II; and Veg, type III] represent each of the major parasite lineages (12) and show a marked divergence in drug sensitivity. The type II and type III parasite strains were highly sensitive to pyrimethamine, exhibiting IC50s of ∼0.02 μM pyrimethamine. The type I isolate (RH) was significantly less sensitive to pyrimethamine (IC50, ∼0.9 μM). This strain is highly proliferative and well adapted to tissue culture (37, 38) but appears to be virtually identical to recent type I clinical isolates (12).
FIG. 2.
Pyrimethamine sensitivities of representative isolates from three parasite strains representing the major virulence lineages of T. gondii (12). Inhibition of parasite growth was assessed 24 h postinfection by incorporation of [3H]uracil and is presented as the average of two independent experiments (the error bars indicate the upward extent of the 95% confidence interval for the mean).
We examined the dhfr-ts coding sequences from RH, P(LK), and Veg strain parasites, to determine whether pyrimethamine sensitivity is correlated with the dhfr genotype. As shown in Table 1, three diagnostic sequence polymorphisms serve to distinguish strain RH from P and Veg. The dhfr-ts sequences in the last two strains were identical, in accord with previous observations indicating that type II and III strains are more closely related to each other than either is to type I (6, 12). One polymorphism results in a silent substitution at Leu 156 in the dhfr coding sequence, while the other two affect noncoding sequences in exon III. As none of these polymorphisms affects the predicted dhfr-ts protein sequence, it seems unlikely that sequence differences are responsible for the observed differences in drug sensitivity.
TABLE 1.
Strain-specific polymorphisms in T. gondii dhfr-ts
| Locationa | Sequence
|
||
|---|---|---|---|
| RH (type I) | P(LK) (type II) | Veg (type III) | |
| Exon III (Leu 156) | CGGGACTGTAC | CGGGATTGTAC | CGGGATTGTAC |
| Intron III (nt 74) | AAATGCGGAGA | AAATGTGGAGA | AAATGTGGAGA |
| Intron III (nt 131-133) | TTTTTTTT––AA | TTTTTTTAAAAA | TTTTTTTAAAAA |
nt, nucleotide.
Random mutagenesis of T. gondii dhfr.
Previous studies have demonstrated that dhfr point mutations suspected to be responsible for pyrimethamine resistance in Plasmodium parasites can confer resistance to transgenic Toxoplasma tachyzoites when introduced in the context of the T. gondii dhfr-ts gene (7, 8, 31). On the other hand, direct selection for pyrimethamine-resistant T. gondii mutants in the laboratory has been difficult, and such parasites have thus far failed to yield dhfr-ts mutations (unpublished results). In order to assess the spectrum of T. gondii dhfr mutations with the potential to confer pyrimethamine resistance, we devised a method to generate and select such mutations, as outlined in Fig. 1.
Random mutagenesis was performed by propagation of the shuttle vectors pC3 and pTRP, each carrying a copy of T. gondii dhfr-ts (or dhfr), in a mutator strain of E. coli. Three pyrimethamine-sensitive alleles were employed as the initial background for mutagenesis: the wild-type T. gondii gene, an allele harboring a Thr→Ser substitution at position 83 (T83S), and an allele with an Ala→Val substitution at position 10 (A10V). The two mutant alleles simulate distinct polymorphisms observed in two pyrimethamine-sensitive variants of P. falciparum dhfr (strains 3D7 and FCR3, respectively) (3, 29) and were included to determine the extent to which the dhfr background influences the range of resistance mutations obtained.
In vivo selection for pyrimethamine resistance in transgenic T. gondii.
Plasmid pC3 inserts randomly in the T. gondii genome, generating stable transformants at high frequency (7, 8). Mutagenized pC3 plasmids from both wild-type and T83S backgrounds were introduced into RH strain parasites by electroporation, and drug-resistant parasites were selected through multiple passages in 0.8 μM pyrimethamine. After three passages under pyrimethamine selection, stably resistant parasite clones were isolated by limiting dilution (37). Southern blot analysis of the population transfected with mutated wild-type dhfr-ts indicated the presence of four unique parasite clones, each carrying a single-copy transgene insertion. Only one unique pyrimethamine-resistant parasite clone was obtained from the population of resistant parasites transfected with the T83S dhfr-ts allele (although one variant of this clone contained additional tandem integrations, presumably of the same transgene).
The results from sequence analysis of two putative resistance mutants generated in the pC3 dhfr wild-type background and the single mutant in the T83S background are shown in Table 2. A transgene derived from the wild-type dhfr parent contained two mutations, a silent substitution (GTC→GTT, encoding valine), and the substitution of arginine for tryptophan at position 25. Mutation of Trp to Arg at the analogous position in murine DHFR is known to produce methotrexate resistance (46). The second transgene derived from the wild-type parental pC3 harbored a transversion mutation resulting in the substitution of histidine for leucine at position 134. The transgene recovered from pyrimethamine-resistant T. gondii transfected with mutagenized T83S dhfr harbored a mutation resulting in the substitution of serine for leucine at position 98. All three predicted amino acid substitutions occur at residues which are either invariant (Trp 25) or highly conserved (Leu 98 and Leu 134) in multiple sequence alignments of dhfr genes from various species (35).
TABLE 2.
Mutations identified in pyrimethamine-resistant T. gondii dhfr
| Clone | Parental allelea | Mutationb | Conservationc | Location in 3D structuree |
|---|---|---|---|---|
| 1 | Wild type | V9V (silent) (GTC→GTT) | Semiconserved | Substrate binding pocket |
| W25R (TGG→AGG) | Invariant | Substrate binding pocket | ||
| 2 | Wild type | L134H (CTC→CAC) | Semiconserved | Solvent-exposed helix (proximal to aa 98) |
| 3 | T83S | L98S (TTG→TCG) | Semiconserved | Cofactor binding domain |
| 4 | A10V | nt−10d (promoter mutation) | NAf | NA |
Parental dhfr genotype mutagenized in E. coli XL1-red.
Amino acid position, e.g., W25R indicates Trp→Arg at amino acid 25 (or the nucleotide position relative to the ATG initiation codon, i.e., −10 indicates a mutation in the yeast promoter).
Based on a comparison of viral, bacterial, protozoan, plant, and animal dhfr (or dhfr-ts) protein sequences; semiconserved residues indicate conservation in the majority of species considered.
pTRP plasmids recovered from four independent pyrimethamine-resistant YH5 transformants contained a single mutation in the S. cerevisiae promoter.
3D, three dimensional; aa, amino acid. From reference 31.
NA, not applicable.
Selection for pyrimethamine-resistant T. gondii dhfr alleles in yeast.
In vitro assays conducted on protein expressed from the A10V variant of T. gondii DFHR have previously shown that this variant exhibits a pyrimethamine-hypersensitive phenotype (31), precluding analysis in parasites expressing wild-type dhfr. As there are no dhfr-deficient strains of T. gondii, a dhfr-deficient (dTMP-dependent) strain of S. cerevisiae (YH5) was employed for this purpose (14, 51). YH5 transformants harboring (nonmutagenized) pTRP plasmids including only the dhfr domain of wild-type T. gondii dhfr-ts were insensitive to pyrimethamine inhibition at drug concentrations as high as 10 μM (the highest concentration that could practically be tested). In contrast, transformants harboring the (nonmutagenized) A10V allele were inhibited by 2 μM pyrimethamine. Similar effects were observed in liquid culture: the IC50s were 2.0 μM for transformants carrying the wild-type allele versus 0.75 μM for A10V transformants.
Wild-type and A10V alleles of T. gondii dhfr in the pTRP shuttle vector were mutagenized, transformed into YH5, selected for dTMP independence on rich medium (yeast extract-peptone-dextrose), and finally screened for drug resistance on minimal agar medium containing pyrimethamine, as indicated in Fig. 1. Five pyrimethamine-resistant S. cervisiae clones (harboring mutant T. gondii dhfr alleles derived from the A10V allele) were isolated and quantitatively assayed for drug sensitivity. All five clones exhibited identical IC50s of ∼3.5 μM, five times that of the A10V parent, suggesting that they may have been siblings. The (nonintegrative) pTRP plasmid was recovered from two clones, and secondary transformants confirmed that these plasmids were responsible for the resistance phenotype. The only mutation identified in these plasmids was located within the S. cerevisiae promoter but 10 nucleotides upstream of the ATG initiation codon (1). Thus, altered regulation of dhfr expression (rather than the expression of a mutant dhfr gene) may account for resistance in these yeast clones.
Expression of mutant dhfr genes in transgenic parasites.
To determine the role of the observed mutations in generating resistance, and to assess the levels of resistance associated with each, we engineered mutant dhfr-ts genes for expression in wild-type T. gondii parasites. The mutations Trp 25→Arg (W25R), Leu 98→Ser (L98S), and Leu 134→His (L134H) were reintroduced by site-directed mutagenesis into three T. gondii dhfr-ts backgrounds in the pC3 vector: (i) wild-type (pyrimethamine-sensitive), (ii) a moderately-resistant allele carrying the Thr 83→Asn substitution (analogous to the S108N mutation in P. falciparum), and (iii) the pyrimethamine-hypersensitive A10V allele.
Mutant dhfr-ts alleles were transiently expressed in RH strain parasites, and IC50s were obtained for pyrimethamine. All three mutations (W25R, L98S, and L134H) confer low-level pyrimethamine resistance in the wild-type background: the W25R mutation provides only a modest advance over the level of resistance produced by overexpression of the wild-type allele alone, but the L98S and L134H mutants exhibit IC50s comparable to that of the moderately resistant allele F245S (8, 31). (Note that the IC50s generated by transient expression of mutant dhfr-ts alleles cannot be directly compared with those of the untransfected parasites shown in Fig. 2, as transfection of pC3 plasmid into parasites has a transient negative impact on parasite growth.) Neither L98S nor L134H produced an enhanced resistance phenotype when combined with the Asn 83 mutation; indeed, the Asn 83 mutation may detract slightly from the resistance capacities of these mutations (compared with the same mutations in the wild-type background). Finally, all combinations with A10V behaved as null alleles in transient-expression assays, supporting the notion that A10V (analogous to A16V in P. falciparum) is intrinsically incompatible with many pyrimethamine resistance substitutions (31).
DISCUSSION
This study provides a demonstration of intrinsic heterogeneity in pyrimethamine sensitivity among different stains of T. gondii parasites and outlines methods suitable for identifying new resistance mutations in the DHFR-TS target of pyrimethamine. We anticipate that the procedures reported here will also be useful in screening for mutations in other drug targets (e.g., DHPS, the target of sulfonamides). Preliminary observations from this study also provide the basis for considering the nature and evolution of pyrimethamine resistance mechanisms in T. gondii and related pathogens.
We find no evidence to suggest that differential strain sensitivity to pyrimethamine is governed by preexisting polymorphisms in the dhfr-ts gene, but it is interesting to note that significant differences in drug sensitivity were observed among the three parasite strains tested, representing the major T. gondii lineages (6, 12). A representative of type I parasites (which are characterized by extreme virulence in mice) was relatively insensitive to pyrimethamine (IC50, ∼0.9 μM). In contrast, type II parasites (which account for most human congenital and AIDS-related infections) and type III parasites (common in veterinary infections) displayed IC50s of ∼0.02 μM. These concentrations all fall below the ∼2-μM serum levels for pyrimethamine typically achieved during low-dosage therapy (100 mg/week) (18), but drug accumulation in the cerebrospinal fluid can be as low as ∼0.4 to 1.8 μM, even with doses of up to 175 mg/week (48). At the lower end of this range, treatment for toxoplasmic encephalitis might be problematic for parasites exhibiting drug sensitivity comparable to that of the RH strain (type I). A more thorough sampling of strains to assess the correlation between drug sensitivity and parasite genotype is clearly warranted.
Using in vitro mutagenesis, we identified three T. gondii dhfr-ts mutations capable of producing pyrimethamine resistance (W25R, L98S, and L134H). These mutations are distinct from the canonical mutations found in drug-resistant malaria, perhaps due to the small number of mutants isolated (there is no reason to suspect that these experiments saturated the range of possible mutations). It is also possible that differences in T. gondii and P. falciparum codon usage (27) or dhfr-ts enzyme structure may play a role. All three mutations affect conserved or semiconserved amino acids in the dhfr domain that are likely to affect substrate and/or cofactor binding (Table 2), and at least one of these mutations (W25R) has previously been observed to cause antifolate resistance in mice (46).
All three mutations produce relatively low-level resistance to pyrimethamine, comparable to the levels observed for the mutations T83N and F245S (Table 3)—T. gondii homologs of two single-point mutations known to produce pyrimethamine resistance in P. falciparum (4, 15, 29, 45). It is possible that no single-point mutation is capable of yielding a highly pyrimethamine-resistant parasite (31). In P. falciparum, high-level resistance to pyrimethamine is associated with multiply mutated dhfr-ts alleles, presumably derived by either successive addition of epistatically favorable mutations (31, 41) or genetic recombination between two low-level resistance alleles. The recombination scenario may be less likely, as mutations such as S36R (which confers high-level resistance in combination with T83N, equivalent to the Arg 59 and Asn 108 mutations in P. falciparum) typically confer no resistance on their own (31) and combining two low-level drug resistance mutations (such as L98S or L134H and T83N) does not necessarily enhance resistance (Table 3).
TABLE 3.
Pyrimethamine sensitivities of T. gondii dhfr allelesa
| Parental alleleb | Mutationc | IC50 for pyrimethamine (μM ± SD)d | Resistance class in vivoe |
|---|---|---|---|
| Null | (M1L) | 0.73 ± 0.14 | − |
| Wild type | None | 0.87 ± 0.16 | +/− |
| W25R | 0.94 ± 0.03 | +/− | |
| L98S | 1.15 ± 0.84 | + | |
| L134H | 1.17 ± 0.13 | + | |
| A10V | None | +/− | |
| W25R | 0.71 ± 0.05 | − | |
| L98S | 0.84 ± 0.06 | − | |
| L134H | 0.71 ± 0.05 | − | |
| T83N | None | + | |
| L98S | 1.01 ± 0.16 | +/− | |
| L134H | 0.95 ± 0.14 | +/− | |
| F245S | 1.12 ± 0.13 | + | |
| S36R + T83N | 1.45 ± 0.16 | ++ |
Determined by transient expression in parasite tachyzoites.
T. gondii dhfr allele into which mutations were introduced by site-directed mutagenesis (31). Null, wild-type, F245S, and S36R + T83N alleles serve as experimental controls for the determination of resistance phenotype.
See Table 2, note b.
Note that these values cannot be directly compared with those of untransfected parasites shown in Fig. 2.
Resistance phenotypes were classified into four groups based on their ability to protect parasites from pyrimethamine inhibition in transient-expression assays. +/−, inhibition comparable to that of parasites transfected with wild-type dhfr (<50% inhibition at 0.6 μM pyrimethamine); −, ≥50% inhibition (comparable to that in parasites transfected with a null allele); +, <35% inhibition (moderate resistance); ++ ≤25% inhibition (high-level resistance) (31).
P. falciparum and T. gondii share many aspects of biology and pathogenesis (obligate intracellular lifestyle in vertebrate cells, common mechanisms of host cell invasion, similar ultrastructure and biosynthetic pathways, etc.) (36), but these parasites also differ in ways which are likely to influence the evolution of drug resistance. Because P. falciparum spreads from one human host to another via mosquito vectors, drug pressure can be maintained for many parasite generations, increasing the probability of acquiring multiple resistance mutations. The obligatory sexual cycle within the mosquito may also enhance the potential for recombination. In contrast, toxoplasmosis in humans is usually acquired by ingestion of water or soil contaminated with T. gondii oocysts (shed in cat feces) or by eating undercooked meat containing T. gondii tissue cysts (bradyzoites). Because humans are effectively dead-end hosts for T. gondii, it seems unlikely that high-level resistance will evolve through a succession of epistatically favorable mutations (the probability of obtaining multiple fortuitous resistance mutations within a single host is low). Since antifolates are not commonly used for treatment or chemoprophylaxis in domestic animals, there is little opportunity for drug resistance to develop in the field or for sexual recombination between individual drug-resistant mutants. Overall, the possibilities for mutation and selection beyond an initial host are considerably diminished for T. gondii relative to P. falciparum. Should clinical resistance to antifolates arise in T. gondii, it is most likely to develop via single, moderately potent mutations (such as those identified in this study), enhanced dhfr-ts expression (cf. the yeast experiments noted above and low-level resistance produced by transient transfection of wild-type DHFR-TS [Table 3]), or other attributes (such as the unidentified factors responsible for observed differences between T. gondii strain RH versus P(LK) and Veg [Fig. 2]).
In conclusion, little or no clinical data are presently available to address the extent to which drug resistance or strain sensitivity contributes to treatment failures for toxoplasmosis. However, long-term use of antibiotics is expected to impose strong selection for resistant organisms, and this study demonstrates that single-point mutations in T. gondii dhfr-ts (e.g., W25R, L98S, and L134H) can produce moderate levels of drug resistance in RH strain parasites. We also show that pyrimethamine sensitivity can vary markedly among different strains of T. gondii. These results do not answer the question of whether resistance is a clinical problem in toxoplasmosis, but they do provide new insights into potential sources of treatment failures and possible drug resistance mechanisms.
ACKNOWLEDGMENTS
We thank Jason Wooden and Carol Sibley for providing host strains and vectors suitable for yeast expression and members of the Roos and Levin laboratories for critical appraisal of the manuscript.
This work was supported by National Institute of Health grants AI-28724 and AI-31808. D.S.R. is a Burroughs Wellcome Scholar in Molecular Parasitology, and M.G.R. was supported by an NIH Training Grant in Cell and Molecular Biology.
REFERENCES
- 1.Barclay B J, Huang T, Nagel M G, Misener V L, Game J C, Wahl G M. Mapping and sequencing of the dihydrofolate reductase gene (DFR1) of Saccharomyces cerevisiae. Gene. 1988;63:175–185. doi: 10.1016/0378-1119(88)90523-9. [DOI] [PubMed] [Google Scholar]
- 2.Bossi P, Caumes E, Astagneau P, Li T S, Paris L, Mengual X, Katlama C, Bricaire F. Epidemiologic characteristics of cerebral toxoplasmosis in 399 HIV-infected patients followed between 1983 and 1994. Rev Med Interne. 1998;19:313–317. doi: 10.1016/s0248-8663(98)80100-8. . (In French.) [DOI] [PubMed] [Google Scholar]
- 3.Bzik D J, Li W B, Horii T, Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase thymidylate synthase gene. Proc Natl Acad Sci USA. 1987;84:8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowman A F, Morry M J, Biggs B A, Cross G A M, Foote S J. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci USA. 1988;85:9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannemann B, McCutchan J A, Israelski D, Antoniskis D, Leport C, Luft B, Nussbaum J, Clumeck N, Morlat P, Chiu J, et al. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med. 1992;116:33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- 6.Darde M L, Bouteille B, Pestre-Alexandre M. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J Parasitol. 1992;78:786–794. [PubMed] [Google Scholar]
- 7.Donald R G, Roos D S. Homologous recombination and gene replacement at the dihydrofolate reductase-thymidylate synthase locus in Toxoplasma gondii. Mol Biochem Parasitol. 1994;63:243–253. doi: 10.1016/0166-6851(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 8.Donald R G, Roos D S. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc Natl Acad Sci USA. 1993;90:11703–11707. doi: 10.1073/pnas.90.24.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzinelli R T, Denkers E Y, Sher A. Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect Agents Dis. 1993;2:139–149. [PubMed] [Google Scholar]
- 10.Georgiev V S. Management of toxoplasmosis. Drugs. 1994;48:179–188. doi: 10.2165/00003495-199448020-00005. [DOI] [PubMed] [Google Scholar]
- 11.Greener A, Callahan M, Jerpseth B. An efficient random mutagenesis technique using an E. coli mutator strain. Mol Biotechnol. 1997;7:189–195. doi: 10.1007/BF02761755. [DOI] [PubMed] [Google Scholar]
- 12.Howe D K, Sibley L D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 13.Howe D K, Summers B C, Sibley L D. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii. Infect Immun. 1996;64:5193–5198. doi: 10.1128/iai.64.12.5193-5198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang T, Barclay B J, Kalman T I, von Borstel R C, Hastings P J. The phenotype of a dihydrofolate reductase mutant of Saccharomyces cerevisiae. Gene. 1992;121:167–171. doi: 10.1016/0378-1119(92)90177-q. [DOI] [PubMed] [Google Scholar]
- 15.Hyde J E. The dihydrofolate reductase-thymidylate synthetase gene in the drug resistance of malaria parasites. Pharmacol Ther. 1990;48:45–59. doi: 10.1016/0163-7258(90)90017-v. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson J M, Davidian M, Rainey P M, Hafner R, Raasch R H, Luft B J. Pyrimethamine pharmacokinetics in human immunodeficiency virus-positive patients seropositive for Toxoplasma gondii. Antimicrob Agents Chemother. 1996;40:1360–1365. doi: 10.1128/aac.40.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katlama C, De Wit S, O'Doherty E, Van Glabeke M, Clumeck N. Pyrimethamine-clindamycin vs. pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis. 1996;22:268–275. doi: 10.1093/clinids/22.2.268. [DOI] [PubMed] [Google Scholar]
- 18.Klinker H, Langmann P, Richter E. Plasma pyrimethamine concentrations during long-term treatment for cerebral toxoplasmosis in patients with AIDS. Antimicrob Agents Chemother. 1996;40:1623–1627. doi: 10.1128/aac.40.7.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinker H, Langmann P, Richter E. Pyrimethamine alone as prophylaxis for cerebral toxoplasmosis in patients with advanced HIV infection. Infection. 1996;24:324–327. doi: 10.1007/BF01743370. [DOI] [PubMed] [Google Scholar]
- 20.Lipsitch M, Levin B R. Population dynamics of tuberculosis treatment: mathematical models of the roles of non-compliance and bacterial heterogeneity in the evolution of drug resistance. Int J Tuberc Lung Dis. 1998;2:187–199. [PubMed] [Google Scholar]
- 21.Lipsitch M, Levin B R. The within-host population dynamics of antibacterial chemotherapy: conditions for the evolution of resistance. Ciba Found Symp. 1997;207:112–127. doi: 10.1002/9780470515358.ch8. [DOI] [PubMed] [Google Scholar]
- 22.Luft B J, Remington J S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 23.Lunde M N, Jacobs L. Antigenic differences between endozoites and cystozoites of Toxoplasma gondii. J Parasitol. 1983;69:806–808. [PubMed] [Google Scholar]
- 24.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1982. [Google Scholar]
- 25.McClary J A, Whitner F, Geisselsoder J. Efficient site-directed mutagenesis using phagemid vectors. BioTechniques. 1989;7:282–289. [PubMed] [Google Scholar]
- 26.Mitchison D A. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis. 1998;2:10–15. [PubMed] [Google Scholar]
- 27.Musto H, Rodriguez-Maseda H, Bernardi G. Compositional properties of nuclear genes from Plasmodium falciparum. Gene. 1995;152:127–132. doi: 10.1016/0378-1119(94)00708-z. [DOI] [PubMed] [Google Scholar]
- 28.Parmley S F, Gross U, Sucharczuk A, Windeck T, Sgarlato G D, Remington J S. Two alleles of the gene encoding surface antigen P22 in 25 strains of Toxoplasma gondii. J Parasitol. 1994;80:293–301. [PubMed] [Google Scholar]
- 29.Peterson D S, Walliker D, Wellems T E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci USA. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfefferkorn E R, Pfefferkorn L C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977;24:449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds M G, Roos D S. A biochemical and genetic model for parasite resistance to antifolates. Toxoplasma gondii provides insights into pyrimethamine and cycloguanil resistance in Plasmodium falciparum. J Biol Chem. 1998;273:3461–3469. doi: 10.1074/jbc.273.6.3461. [DOI] [PubMed] [Google Scholar]
- 32.Richman D D. Antiretroviral drug resistance. AIDS. 1991;5(Suppl. 2):S189–S194. doi: 10.1097/00002030-199101001-00027. [DOI] [PubMed] [Google Scholar]
- 33.Richman D D. Resistance of clinical isolates of human immunodeficiency virus to antiretroviral agents. Antimicrob Agents Chemother. 1993;37:1207–1213. doi: 10.1128/aac.37.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Rosado R, Soriano V, Dona C, Gonzalez-Lahoz J. Opportunistic infections shortly after beginning highly active antiretroviral therapy. Antivir Ther. 1998;3:229–231. [PubMed] [Google Scholar]
- 35.Roos D S. Primary structure of the fused dihydrofolate reductase/thymidylate synthase gene from Toxoplasma gondii. J Biol Chem. 1993;268:6269–6280. [PubMed] [Google Scholar]
- 36.Roos D S, Crawford M J, Donald R G K, Fohl L M, Hager K M, Kissinger J C, Reynolds M G, Striepen B, Sullivan W J., Jr Transport and trafficking: Toxoplasma as a model for Plasmodium. Novartis Found Symp. 1999;226:176–198. doi: 10.1002/9780470515730.ch13. [DOI] [PubMed] [Google Scholar]
- 37.Roos D S, Donald R G, Morrissette N S, Moulton A L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 38.Sabin A B. Toxoplasmic encephalitis in children. JAMA. 1941;116:801–807. [Google Scholar]
- 39.Sherman F, Fink G R, Lawrence C W. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1979. [Google Scholar]
- 40.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sirawaraporn W, Sathitkul T, Sirawaraporn R, Yuthavong Y, Santi D V. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc Natl Acad Sci USA. 1997;94:1124–1129. doi: 10.1073/pnas.94.4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skolasky R L, Dal Pan G J, Olivi A, Lenz F A, Abrams R A, McArthur J C. HIV-associated primary CNS morbidity and utility of brain biopsy. J Neurol Sci. 1999;163:32–38. doi: 10.1016/s0022-510x(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 43.Soriano V, Dona C, Rodriguez-Rosado R, Barreiro P, Gonzalez-Lahoz J. Discontinuation of secondary prophylaxis for opportunistic infections in HIV-infected patients receiving highly active antiretroviral therapy. AIDS. 2000;14:383–386. doi: 10.1097/00002030-200003100-00011. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki Y, Yang Q, Remington J S. Genetic resistance against acute toxoplasmosis depends on the strain of Toxoplasma gondii. J Parasitol. 1995;81:1032–1034. [PubMed] [Google Scholar]
- 45.Tanaka M, Gu H M, Bzik D J, Li W B, Inselburg J. Mutant dihydrofolate reductase-thymidylate synthase genes in pyrimethamine-resistant Plasmodium falciparum with polymorphic chromosome duplications. Mol Biochem Parasitol. 1990;42:83–91. doi: 10.1016/0166-6851(90)90115-3. [DOI] [PubMed] [Google Scholar]
- 46.Thillet J, Absil J, Stone S R, Pictet R. Site-directed mutagenesis of mouse dihydrofolate reductase. Mutants with increased resistance to methotrexate and trimethoprim. J Biol Chem. 1988;263:12500–12508. [PubMed] [Google Scholar]
- 47.Tuazon C U. Toxoplasmosis in AIDS patients. J Antimicrob Chemother. 1989;23(Suppl. A):77–82. doi: 10.1093/jac/23.suppl_a.77. [DOI] [PubMed] [Google Scholar]
- 48.Weiss L M, Harris C, Berger M, Tanowitz H B, Wittner M. Pyrimethamine concentrations in serum and cerebrospinal fluid during treatment of acute Toxoplasma encephalitis in patients with AIDS. J Infect Dis. 1988;157:580–583. doi: 10.1093/infdis/157.3.580. [DOI] [PubMed] [Google Scholar]
- 49.Winstanley P, Khoo S, Szwandt S, Edwards G, Wilkins E, Tija J, Coker R, McKane W, Beeching N, Watkin S. Marked variation in pyrimethamine disposition in AIDS patients treated for cerebral toxoplasmosis. J Antimicrob Chemother. 1995;36:435–439. doi: 10.1093/jac/36.2.435. [DOI] [PubMed] [Google Scholar]
- 50.Wong S Y, Remington J S. Toxoplasmosis in pregnancy. Clin Infect Dis. 1994;18:853–861. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]
- 51.Wooden J M, Hartwell L H, Vasquez B, Sibley C H. Analysis in yeast of antimalaria drugs that target the dihydrofolate reductase of Plasmodium falciparum. Mol Biochem Parasitol. 1997;85:25–40. doi: 10.1016/s0166-6851(96)02808-3. [DOI] [PubMed] [Google Scholar]