Abstract
Background
Epilepsy is a neurological disorder affecting both children and adults. Epileptic seizures are the result of excessive and abnormal cortical cell electrical activity in the brain. In response to criticism that epilepsy care for children has little impact on long‐term outcomes, healthcare professionals and administrators have developed various service models and strategies to address perceived inadequacies.
This is an updated version of a Cochrane Review previously published in 2018.
Objectives
To assess the effects of any specialised or dedicated intervention for epilepsy versus usual care in children and adolescents with epilepsy and their families.
Search methods
We searched the following databases on 14 January 2020: the Cochrane Register of Studies (CRS Web), MEDLINE (Ovid, 1946 to 13 January 2020), PsycINFO (1887 to 14 January 2020), CINAHL Plus (1937 to 14 January 2020), ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform. The Cochrane Register of Studies (CRS Web) includes the Cochrane Epilepsy Group Specialised Register and the Cochrane Central Register of Controlled Trials (CENTRAL). We also contacted experts in the field seeking information on unpublished and ongoing studies and checked the websites of epilepsy organisations and the reference lists of included studies.
Selection criteria
We included randomised controlled trials recruiting children and adolescents with epilepsy.
Data collection and analysis
Two review authors independently selected trials for inclusion and extracted the relevant data. We assessed the following outcomes: 1. Seizure frequency and severity; 2. Appropriateness and volume of medication prescribed (including evidence of drug toxicity); 3. Participants' reported knowledge of information and advice received from professionals; 4. Participants' reports of health and quality of life; 5. Objective measures of general health status; 6. Objective measures of social or psychological functioning (including the number of days spent on sick leave/absence from school or work, and employment status); and 7. Costs of care or treatment. The results of the data extraction and quality assessment for each study were presented in structured tables and as a narrative summary. All summary statistics were extracted for each outcome.
Main results
We included nine studies of eight interventions in the review, reporting on seven distinct self‐management programmes for educating or counselling children with epilepsy and their parents, and one new model of care. Based largely on self‐reported outcomes, each programme showed some benefits for the well‐being of children with epilepsy; however, all of the included studies had methodological flaws. No single programme was evaluated with different study samples, and in no instance was the same outcome measured and reported in the same way across studies, precluding any possible meta‐analysis, even if the interventions were considered sufficiently similar to include in meta‐analysis.
We chose the outcomes for which data might be important for decisions about the interventions as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions. We found moderate certainty evidence that one of the educational interventions reduced seizure frequency. There was low certainty evidence that two other educational interventions reduced seizure severity, seizure control, and seizure cure rates. The evidence for all other outcomes (drug adherence, knowledge, self‐efficacy and self‐perception of epilepsy on quality of life) was mixed.
Authors' conclusions
Whilst each of the programmes evaluated in this review showed some benefit to children with epilepsy, their impact was extremely variable. No programme showed benefits across the full range of outcomes, and all studies had methodological problems. There is currently insufficient evidence in favour of any single programme. Further evidence from randomised controlled trials using validated measures and considering clinical meaningfulness as well as statistical significance of results is required.
Keywords: Adolescent, Adult, Child, Humans, Epilepsy, Epilepsy/psychology, Epilepsy/therapy, Medication Adherence, Quality of Life, Seizures, Self Care, Self-Management, Systematic Reviews as Topic
Plain language summary
Care delivery and self‐management strategies for children with epilepsy
Background
Epilepsy is a disorder that affects the nervous system of children and adults. Epileptic seizures (fits) are the result of excessive and abnormal activity in the brain, which are unpredictable in frequency. Most seizures are well controlled with medicines and other types of treatments, although epilepsy can cause problems in social, school, and work situations, making independent living difficult. People with seizures tend to have physical problems (e.g. fractures, bruising, and a slightly increased risk of sudden death) as well as social problems because of the stigma attached to the illness. People with epilepsy and their families may lack social support or experience social isolation, embarrassment, fear, and discrimination, and some parents of children with epilepsy may also feel guilty. Self‐management of epilepsy refers to a wide range of health behaviours and activities that a person can learn and adapt to control their seizures and improve their well‐being. This approach requires a partnership between the person and the providers of services (e.g. specialist epilepsy outpatient clinics, nurse‐based liaison services between family doctors and specialist hospital doctors, specialist epilepsy community teams), as well as targeted services for specific groups (e.g. children, teenagers, and families).
Study characteristics
We searched scientific databases for studies in children and adolescents with epilepsy that looked at the effects of self‐management of epilepsy. We wanted to look at several outcomes to see how well people with epilepsy and their families generally cope with the condition.
Search date
We included evidence published up to January 2020.
Key results
This review compared seven education‐ or counselling‐based self‐management programmes for children and adolescents with epilepsy, plus one new way of delivering nursing care. Each strategy appeared to improve some of the outcomes studied, although no intervention improved all the outcomes that were measured. The studies also had problems with their methods, which made their results less reliable. Whilst none of the interventions caused any harm, their impact was limited. There is insufficient evidence to support any single strategy as the best one for children with epilepsy.
Quality of the evidence
The quality of the evidence was poor because all of the included studies had problems in how they were run.
Summary of findings
Summary of findings 1. Care delivery and self‐management strategies compared to usual care for children/adolescents with epilepsy and/or their parents.
| Care delivery and self‐management strategies compared to usual care for children/adolescents with epilepsy and/or their parents | ||||||
| Patient or population: children/adolescents with epilepsy and/or their parents Setting: outpatients Intervention: care delivery and self‐management strategies Comparison: usual care, waiting list control, or no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% Cl) |
No. of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Estimated risk | Corresponding risk | |||||
| Without care delivery and self‐management strategies | With care delivery and self‐management strategies | |||||
| Number of seizures at 12 months | The mean number of seizures at 12 months without care delivery and self‐management strategies was 1.11. | The mean number of seizures at 12 months with care delivery and self‐management strategies was 0.34. | MD −0.77 (−1.47 to ‐0.07) | 167 (1 study) | ⊕⊕⊕⊝ MODERATEa | Intervention studied was a non‐epilepsy‐specific model for self‐management training for children with chronic conditions and their parents known as ACINDES (Tieffenberg 2000). The control group received usual care. |
| Seizure severity (frequency and duration of seizures) at 3 months | 25 per 100 | 37.2 per 100 (24.6 to 52.1) |
OR 1.78 (0.98 to 3.26) | 214 (1 study) | ⊕⊕⊝⊝ LOWa |
Intervention studied was clinician advice plus an 8.52‐minute video animation for children, adolescents, and their parents (Saengow 2018). The control group only received clinician advice. It is not clear how the seizures were recorded, whether self‐reported or medical records. |
| Seizure control rate at 12 months | 71.7 per 100 | 86.7 per 100 (71.9 to 94.3) |
OR 2.57
(1.01 to 6.53) |
120 (1 study) | ⊕⊕⊝⊝ LOWa,b |
Intervention studied was a nursing process strategy for children and their parents (Jia 2018). The control group received usual care. Seizure control rate was defined as a binary outcome (yes/no) based on medical records. Seizure control was considered to have been achieved if seizure frequency was reduced by ≥ 50% and the duration of epilepsy reduced. |
| Seizure cure rate at 12 months | 38.3 per 100 | 58.3 per 100 (40.2 to 74.4) |
OR 2.25 (1.08 to 4.68) | 120 (1 study) | ⊕⊕⊝⊝ LOWa,b |
Intervention studied was a nursing process strategy for children and their parents (Jia 2018). The control group received usual care. Seizure cure was considered to have been achieved if seizure frequency was reduced by ≥ 90% and the duration of epilepsy reduced by ≥ 80%. |
| Drug adherence (MMAS‐8) at 3 months | 15.9 per 100 The mean score across control group was from 5.50 to 5.57.a |
42.8 per 100 (27.7 to 59.5) The mean score in the intervention group was 1.12 points lower (0.47 lower to 1.77 higher). |
OR 3.96
(2.03 to 7.76) MD −1.12 (−0.47 to 1.77) |
274 (2 studies) | ⊕⊕⊕⊝ MODERATEc |
Interventions studied were the implementation of a self‐care education programme for adolescents delivered via SMS, Kazemi Majd 2017, and clinician advice (i.e. usual care) plus an 8.52‐minute video animation for children, adolescents, and their parents (Saengow 2018). The control groups received routine education and clinical advice, respectively. |
| Epilepsy Knowledge Test for Children (EKTC) at 3 months | The mean score across control group was 4.054.a | The mean score in the intervention group was 3.83 points lower (3.08 lower to 4.59 higher). | MD −3.83 (−3.08 to 4.59) | 92 (1 study) | ⊕⊕⊕⊝ MODERATEa |
Intervention studied was a Modular Education Program for children aged 7 to 18 years with epilepsy and their parents (Gürhopur 2018). The control group did not receive the intervention. |
|
Seizure Self‐Efficacy Scale for Children (SSES‐C) at 3 months |
Not estimable | Not estimable | Not estimable |
168 (2 studies) | ⊕⊕⊝⊝ LOWc |
Interventions studied were a Modular Education Program for children aged 7 to 18 years with epilepsy and their parents, Gürhopur 2018, and a manual‐based brief psychosocial group intervention for adolescents and their parents known as PIE (Dorris 2017). Compared with the control groups (no intervention and waiting list control, respectively), the former significantly improved self‐efficacy about seizures, whereas there was no statistically significant difference between groups in the latter. |
| Self‐perception of epilepsy on quality of life (PedsQL) at 3 months | The mean score across control group was 69.19.d | The mean score in the intervention group was −1.40 points lower (−8.21 lower to 5.41 higher). | MD −1.40 (−8.21 to 5.41) | 76 (1 study) | ⊕⊕⊝⊝ LOWa |
Intervention studied was a manual‐based brief psychosocial group intervention known as PIE (Dorris 2017). The control group was a waiting list control. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; MMAS‐8: 8‐Item Morisky Medication Adherence Scale; OR: odds ratio; PedsQL: Pediatric Quality of Life Inventory; SMS: Short Message Service | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aResults were based on one study with a small sample side and wide 95% confidence interval. bDowngraded once due to lack of clarity as to how seizures were measured. cDowngraded once due to risk of bias: unclear methodological information provided for some studies. dControl groups results measured at the same point as was used in the meta‐analysis were used to calculate mean scores across the included studies.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Lindsay 2010), which was updated in 2018 (Fleeman 2018).
Description of the condition
Epilepsy is a spectrum of disorders in which a person may experience seizures that are unpredictable in frequency (England 2012). Researchers have identified at least 40 different seizure types (Berg 2010). Whilst most people with epilepsy can control seizures with medications and other treatment options, the condition can pose challenges in social, school, and work situations and for independent living. Not only do people with seizures tend to have more physical problems (such as fractures, bruising, and a slightly increased risk of sudden death), they also face significant challenges due to how the condition is perceived (or indeed misperceived), which can lead to people with epilepsy being stigmatised (Bandstra 2008). As a result, both people with epilepsy and their families may lack social support and experience social isolation, embarrassment, fear, and discrimination, with some parents also reporting feelings of parental guilt (England 2012). Epilepsy affects around 50 million people worldwide, with around 80% of all cases in low‐ and middle‐income countries (WHO 2013). Epilepsy is most common in children and older adults (Betts 1992; Sander 1990).
Description of the intervention
The 'self‐management of epilepsy' refers to a wide range of health behaviours and activities that a person can learn and adapt in order to promote seizure control and enhance well‐being (Austin 1997). Self‐management of any condition typically entails a partnership between users and service providers (Clark 2008). Various dedicated models of service provision exist to improve care networks and self‐education (Clark 2010; Fitzsimons 2012; SIGN 2003; SIGN 2005). Services may include specialist epilepsy outpatient clinics, nurse‐based liaison services between primary (general practitioner; GP) and secondary/tertiary (hospital‐based) care, and specialist epilepsy multidisciplinary community teams (Clark 2010; Fitzsimons 2012; SIGN 2003; SIGN 2005). Services may also include input from social care or the voluntary sector and be targeted at specific groups, such as children, teenagers, and the families of people with epilepsy (Clark 2010; SIGN 2003; SIGN 2005).
How the intervention might work
Specialist or dedicated models of care, care networks, or self‐education and self‐management may improve the quality of care, promote more systematic multidisciplinary follow‐up, and enhance communication amongst professionals, patients, and other services (Fitzsimons 2012). Importantly, it should enable people with epilepsy (and their families) to cope with all aspects of the disease through improved self‐education and self‐management (Clark 2008; Fitzsimons 2012).
Why it is important to do this review
Different authors have criticised epilepsy care for its limited impact on the range of health and social needs of people with epilepsy (Betts 1992; Chappell 1992; Elwyn 2003; Thapar 1996). In order to improve the quality of care for people with epilepsy, we aimed to produce a systematic review of the evidence from studies investigating the effects of these service models compared to non‐specialist services.
Objectives
To assess the effects of any specialised or dedicated intervention for epilepsy versus usual care in children and adolescents with epilepsy and their families.
Methods
Criteria for considering studies for this review
Types of studies
In this updated review, we included randomised controlled trials (RCTs) and excluded non‐RCTs. Non‐RCTs were included in previous versions of the review (Fleeman 2015; Fleeman 2018; Lindsay 2006; Lindsay 2010), since the number of relevant RCTs was originally anticipated to be low. However, as the number of RCTs has increased, and as RCTs minimise the possibility of bias from confounding factors compared to non‐RCTs, it was agreed that only RCTs would be included in this update.
We planned to assess studies reported only as abstracts and those described in trial registries as having an expected study completion date prior to 14 January 2020 as studies awaiting classification.
Types of participants
As specified in the review protocol (Lindsay 2006), studies that included children aged under 18 years were eligible for inclusion in the review.
We excluded studies including a mixture of children and adults, unless analyses were presented for children separately.
We included studies incorporating epilepsy with other long‐term conditions if results were reported separately for each condition.
Types of interventions
In accordance with the protocol for this review (Lindsay 2006), we included any intervention involving a specialised or dedicated team or person for the care of children or adolescents with epilepsy, whether based:
in hospital (e.g. a specialist epilepsy clinic);
in the community (e.g. a specialist pharmacist);
in general practice (e.g. a specialist epilepsy nurse);
elsewhere (e.g. social worker, the voluntary sector);
as a care network combining any of these elements;
on education or counselling for improved self‐management.
Types of outcome measures
We considered the following outcomes in this update:
seizure frequency and severity;
appropriateness and volume of medication prescribed (including evidence of drug toxicity);
child or family's reported knowledge of information and advice received from professionals;
child or family's reports of health and quality of life (including adverse effects of medication);
objective measures of general health status;
objective measures of social or psychological functioning (including the number of days spent on sick leave/absence from school and employment status);
costs of care or treatment.
We assessed all outcome measures for reliability and validity (i.e. for clinical relevance or whether validated tools were used for outcome measurement). If measures were misused (e.g. adults scales used on children), we would investigate their effect on study results using a sensitivity analysis.
Primary outcomes
No outcomes were specified as primary outcomes in the review protocol (Lindsay 2006).
Secondary outcomes
No outcomes were specified as secondary outcomes in the review protocol (Lindsay 2006).
Search methods for identification of studies
Electronic searches
The searches for the original review were run in May 2006. Subsequent searches were run in March 2010, February 2011, July 2012, December 2013, November 2015, September 2016, and May 2018. For the latest update, we searched the following databases on 14 January 2020.
Cochrane Register of Studies (CRS Web) (Appendix 1).
MEDLINE via Ovid (1946 to 13 January 2020) (Appendix 2).
Embase (1974 to 1 November 2016) (Appendix 3).
PsycINFO via EBSCOhost (1887 to 14 January 2020) (Appendix 4).
CINAHL Plus via EBSCOhost (1937 to 14 January 2020) (Appendix 5).
ClinicalTrials.gov (clinicaltrials.gov/) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) (Appendix 7).
CRS Web includes randomised controlled trials (RCTs) and quasi‐RCTs from PubMed, Embase, ClinicalTrials.gov, the WHO ICTRP, the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, and the Specialised Registers of Cochrane Review Groups, including the Epilepsy Group. We did not update the Embase searches for this update.
We applied no language restrictions.
Searching other resources
We checked the reference lists of retrieved studies for additional reports of relevant studies. We contacted experts in the field seeking information on unpublished and ongoing studies, and checked the websites of epilepsy organisations. We identified duplicate studies by screening reports according to title, study author names, location, and medical institute. We omitted any duplicate studies.
Our search strategy was the same as for a parallel review of care delivery and self‐management strategies for adults with epilepsy (Bradley 2016)
Data collection and analysis
Selection of studies
We screened papers in two stages. At stage one, two review authors independently screened all titles and abstracts of papers identified by the searches for relevance. We only excluded papers that were clearly irrelevant at this stage. At stage two, two review authors independently screened the full papers, identified relevant studies, and assessed the eligibility of studies for inclusion. Any disagreements were resolved by discussion.
Data extraction and management
The same review authors extracted the following types of data.
Study characteristics: place of publication, date of publication, population characteristics, setting, detailed nature of intervention, detailed nature of comparator, and detailed nature of outcomes. A key purpose of these data was to define unexpected clinical heterogeneity in included studies independently of analysis of results.
Results of included studies with respect to each of the main outcomes indicated in the review question, including data on outcomes not considered, and considering the possibility of selective reporting of results on particular outcomes.
Any disagreements that arose during data extraction were resolved by discussion. In the case of insufficient information, we contacted study authors for further information.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study using the suggested risk of bias criteria for RCTs in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). In accordance with the review protocol (Lindsay 2006), we resolved any disagreements during risk of bias assessment by discussion. In the case of insufficient information, we contacted study authors for further information.
Measures of treatment effect
We presented the measures of treatment effect as reported in the published papers. Where P values were presented in the published papers, we reported P values exactly as presented in the papers (including the reporting of P values for non‐statistically significant findings where the authors reported these P values). As specified in the protocol for this review (Lindsay 2006), had it been possible to combine results in a meta‐analysis, we would have measured treatment effects using (standardised) weighted mean differences for continuous variables and risk ratios (including Mantel‐Haenszel analysis) for dichotomous variables.
Unit of analysis issues
Where studies included multiple treatment arms, we reported data from all treatment arms. Had it been possible to combine results in a meta‐analysis, where only one experimental arm was considered sufficiently similar to the experimental arm of other included studies, we would have included only the relevant experimental arm. Where more than one experimental arm were considered sufficiently similar to the experimental arm of other included studies, we would have either: 1) combined experimental groups to make a simple pair‐wise comparison; or 2) split the control group to include more than one comparison.
Dealing with missing data
Had we discovered that important data were missing precluding us from reporting the results of a study or conducting a meta‐analysis, or both, we attempted to obtain the relevant data from study authors.
Assessment of heterogeneity
We assessed clinical heterogeneity between studies by reviewing the differences across studies. As there was considerable methodological and clinical heterogeneity amongst the studies, we did not consider meta‐analysis to be appropriate. Had we decided to combine the results of any studies in a meta‐analysis, we would have investigated statistical heterogeneity using the Chi2 test for homogeneity and the I2 statistic (Higgins 2003). Had the results shown heterogeneity, we would have investigated the cause (Higgins 2021).
Assessment of reporting biases
We checked whether the outcomes intended to be measured (reported in the methods sections) were reported in the findings sections of the included studies. We attempted to contact study authors for any missing data. Had we included 10 studies or more in a meta‐analysis, we would have assessed the risk of publication bias by constructing a funnel plot and conducting a simple test of asymmetry to test for possible bias (Egger 1997).
Data synthesis
We presented the results of the data extraction and quality assessment for each study in structured tables and as a narrative summary. We extracted all summary statistics for each outcome. Had studies been of a suitable quality and sufficiently homogeneous, we would have pooled the results in a meta‐analysis. We planned to use a fixed‐effect model in the case of minimal heterogeneity, and a random‐effects model in the case of substantial heterogeneity.
Subgroup analysis and investigation of heterogeneity
We planned no subgroup analyses a priori, with no subgroups prespecified in the review protocol (Lindsay 2006). Had we decided to combine the results of any studies in a meta‐analysis and found evidence of statistical heterogeneity, we would have considered conducting post hoc subgroup analyses where appropriate and where the data allowed (Higgins 2021).
Sensitivity analysis
For future updates of this review, if the data permit the conduct of meta‐analysis, we will consider sensitivity analyses based on risk of bias. Where studies with multiple experimental arms are included, we will consider sensitivity analyses by including different experimental arms from a particular study; we will also consider different approaches to meta‐analysis (e.g. combining experimental groups to make a simple pair‐wise comparison and/or splitting the control group to include more than one comparison) if the data permit.
Summary of findings and assessment of the certainty of the evidence
Given the variability of interventions and control groups amongst the included studies, we presented a single summary of findings table for the most important comparison considered within the review (see Table 1) (Schünemann 2013). We chose the outcomes for which data might be important for decisions about the interventions (Higgins 2021). We determined the certainty of the evidence using the GRADE approach (GRADEpro GDT), downgrading certainty in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, and high probability of publication bias. We downgraded the certainty of the evidence by one level if we considered the limitation to be serious, and by two levels if very serious.
Results
Description of studies
We searched for RCTs that investigated the effects of care delivery and self‐management strategies for children or adolescents with epilepsy.
Results of the search
In the original review, our initial searches identified over 4000 papers, including duplicates, of which four studies were included in the review (Glueckauf 2002; Lewis 1991; Rau 2006; Tieffenberg 2000). We identified a fifth paper, Lewis 1990, from the reference list of Lewis 1991; both papers reported on the same intervention, but Lewis 1990 focused on the impact on children, whilst Lewis 1991 assessed parental outcomes.
The search in the 2015 update, Fleeman 2015, yielded 2438 additional papers, including duplicates, plus two studies from the original review that were awaiting classification (Jantzen 2009; Shore 2008). We included one of these, a controlled before‐and‐after study, in the review update (Jantzen 2009). We also included Pfäfflin 2012, which was published after the original review. The study report by Pfäfflin 2012 evaluated the same intervention as a previously included controlled before‐and‐after study published in German (Rau 2006); however, it also provided additional information. The additional information was obtained from the same participants and at the same point in time. Whilst we previously included both study reports in the review as separate studies, we classified these study reports as the same study in the 2015 update, with Pfäfflin 2012 cited as the primary reference.
For the 2016 update, the searches yielded 1680 additional papers including duplicates. We included only one additional study (Modi 2016).
In the current update (January 2020), we included five new RCTs (Dorris 2017; Gürhopur 2018; Jia 2018; Kazemi Majd 2017; Saengow 2018), and excluded three studies we included in previous updates (Glueckauf 2002; Jantzen 2009; Pfäfflin 2012): two controlled before‐and‐after studies (Jantzen 2009; Pfäfflin 2012), and a study designed as an RCT but for which randomisation failed (Glueckauf 2002).
We included a total of nine reports of trials designed as RCTs in the current update (Dorris 2017; Gürhopur 2018; Jia 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Saengow 2018; Tieffenberg 2000). The reports were of eight separate interventions, one of the interventions being reported in two separate publications with a focus on the impact from the child perspective, Lewis 1990, and the adult perspective, Lewis 1991. A study flow chart is presented in Figure 1. For details on study characteristics, see Characteristics of included studies.
1.
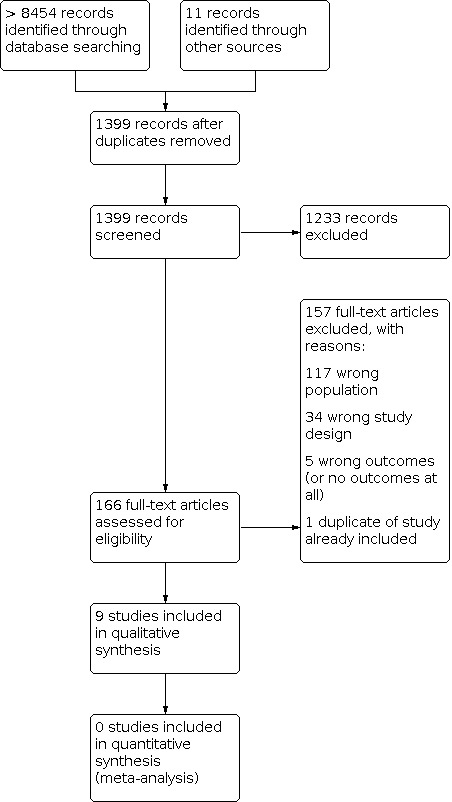
Study flow diagram (illustrating results from previous versions of the review as well as this update).
Included studies
All of the included studies investigated interventions for improved self‐management (see Characteristics of included studies). Eight of these interventions consisted of education, counselling, or training (Dorris 2017; Gürhopur 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Saengow 2018; Tieffenberg 2000). Jia 2018 was the only study to evaluate a modified model of care delivery.
The interventions can broadly be described as being:
strategies for children and parents: four interventions reported in five study reports (Jia 2018; Lewis 1990; Lewis 1991; Modi 2016; Tieffenberg 2000);
strategies for adolescents (aged 12 and over) and parents: one intervention (Dorris 2017).
strategies for children, adolescents (aged 12 and over), and their parents: two interventions (Gürhopur 2018; Saengow 2018);
strategies for adolescents: one intervention (Kazemi Majd 2017).
Details provided about the specifics of the interventions varied amongst the included studies (see Appendix 8). Five studies reported they had previously piloted their interventions (Dorris 2017; Gürhopur 2018; Lewis 1990; Lewis 1991; Modi 2016), but only the pilot feasibility study for Modi 2016 had been previously published (Modi 2013); we identified this pilot feasibility study in our searches but excluded it from the review (see Excluded studies).
Strategies for children and parents
Jia 2018 evaluated "a modified clinical nursing procedure applied in pediatric epilepsy treatment" which included inpatient care and posthospitalisation follow‐up (see Appendix 8 for details). The aim of the intervention was to improve epilepsy control and cure rates and focus more on areas of care traditionally neglected by conventional care. The study included 120 children aged 2 to 13 years diagnosed with epilepsy for the first time at the Xuzhou Children’s Hospital in China, who were then randomised to receive conventional care (n = 60) or the modified delivery of care (n = 60). Study outcomes were measured at baseline and 12 months.
Lewis 1990 and Lewis 1991 evaluated the Children's Epilepsy Program (CEP), a child‐centred, family‐focused educational programme developed at the Medical Center of the University of California in Los Angeles (UCLA) for children and their parents (see Appendix 8 for details). Following the completion of a pilot study, the researchers could not recruit a suitable sample from the UCLA Medical Center because of an insufficient number of referrals of children with epilepsy in the Los Angeles area, so the evaluation of the CEP took place in Santiago, Chile. This required translating the programme into Spanish for the trial. Lewis 1990 reported on the impact of CEP on children, and Lewis 1991 reported on the impact of CEP on parents. The study recruited 252 children aged 7 to 14 years and 294 parents selected from 1000 families belonging to the Liga Contra Epilepsia. Families were randomly allocated in groups of 20 to the intervention and control groups. All participants were tested immediately prior to the first session and five months after the end of the CEP. The intervention groups of children (n = 123) and parents (n = 185) undertook CEP separately, whereas the control groups of children (n = 113) and parents (n = 109) jointly attended three two‐hour sessions consisting of lectures and question‐and‐answer discussions. The authors described the control intervention as "passive learning" in contrast to the "active learning" of the intervention. Only 78.6% of children in the intervention group and 52% of children in the control group attended all of the required sessions (Lewis 1990); 73.2% of mothers and 59% of fathers attended all four sessions in the intervention group, and 62% of mothers and 49% of fathers attended all three sessions in the control group (Lewis 1991).
Modi 2016 evaluated the Supporting Treatment Adherence Regimen (STAR). The aim of this family‐tailored problem‐solving intervention was to improve adherence to antiepileptic drugs (see Appendix 8 for details). The study was conducted in a children’s hospital in the Midwestern United States. Of 50 children aged 2 to 12 years and their caregivers who agreed to participate in the study, 45 families were reported as eligible for randomisation and were followed up over three months. However, families were only randomised if investigators assessed adherence to antiepileptic drugs as less than 95% over the previous seven months; those with adherence of 95% or more were allocated to a maintenance "high adherence" group. In total, 22 families were not randomised; 11 were allocated to STAR; and 12 were allocated to the treatment‐as‐usual group.
Tieffenberg 2000 reported on the effects of ACINDES, a non‐epilepsy‐specific model for self‐management training for children with chronic conditions (children with asthma were also included) based on play techniques. The model was developed by the researchers specifically for Spanish‐speaking children aged 6 to 15 years. It was delivered outside the hospital environment (by teachers, in schools, with physicians providing guidance, acting as counsellors) and included sessions held simultaneously for parents. These sessions were meant to enable parents to learn to recognise and accept their children's autonomy and become "facilitators" rather than "managers" in their children's disease self‐management (see Appendix 8 for details). ACINDES was evaluated by a cluster‐RCT of 355 children (167 with epilepsy) in Buenos Aires, Argentina. Both children and parents were interviewed before the programme and at six and 12 months after its completion. In addition, medical and school records were monitored for emergency and routine visits, hospitalisations, and school absenteeism. The intervention group received the ACINDES programme (n = 103), whilst children and parents in the control group received routine care without additional training (n = 64).
Strategies for adolescents (aged 12 and over) and parents
Dorris 2017 evaluated a manual‐based brief psychosocial group intervention for young people with epilepsy (PIE) which aimed to improve epilepsy knowledge, self‐management skills, mood, and quality of life. The PIE intervention ran over six weeks and consisted of two‐hourly weekly group sessions (see Appendix 8 for details). This study was identified in our previous update as a study awaiting classification (Fleeman 2018), and included adolescents aged 12 to 17 years with a diagnosis of epilepsy (controlled or refractory) of at least six months, who attended mainstream schooling and who were treated at seven neuroscience centres across the UK. According to the trial registry (NCT02349529), the study planned to recruit 200 participants. However, the published trial report states that a total of 83 adolescents were included, randomised to the intervention (n = 43) or waiting‐list control group (n = 40). Study outcomes were reported after three months follow‐up.
Strategies for children, adolescents (aged 12 and over), and their parents
Gürhopur 2018 evaluated an eight‐module programme consisting of four modules for children and adolescents and four modules for their parents. The sessions consisted of brainstorming, discussion, role‐play, drawing, videos, slides, question‐and‐answer sessions, and being provided with the Guide to Living With Epilepsy for Children and Parents (see Appendix 8 for details). The study included 92 children and adolescents aged 7 to 18 years attending the Akdeniz University Hospital Paediatric Neurology Polyclinic in Antalya (Turkey) and 92 parents. Participants were randomised to the intervention (n = 42) or control group (n = 50), who did not receive the intervention, and were followed up for three months.
Saengow 2018 evaluated an intervention consisting of a short video animation supervised by paediatric neurologists who also provided counselling. The intervention included information on six areas from Thai guidelines (diagnosis of epilepsy, aetiology of epilepsy, treatment of epilepsy, first aid seizure care, prognosis of epilepsy, and safe activity for epilepsy; see Appendix 8 for details). A total of 214 children and adolescents aged between 1 month and 15 years who were patients of the paediatric neurology clinic at Maharat Nakhon Ratchasima Hospital, Thailand, were included in the study. Participants were either randomised to receive the intervention (n = 126) or clinician advice only (n = 88) and followed up for three months.
Strategies for children, adolescents (aged 12 and over)
We had identified the study by Kazemi Majd 2017 as an ongoing study (IRCT2015060122514N1) in the previous review (Fleeman 2018). The study had been registered as three‐arm trial, with two interventions: 1) self‐care education based on Short Message Service (SMS) and workshop on self‐efficacy and adherence to the medication regimen, accompanied by five self‐care education pamphlets given at regular intervals over three months; and 2) a self‐care education programme delivered via SMS. The published study report only evaluated the second of these interventions (see Appendix 8 for details). In the trial registry, it was reported that 90 participants who were members of the Iranian Epilepsy Association were to be enrolled. The published trial report stated that 60 participants were randomly assigned to the intervention (n = 30) or control (n = 30), which consisted of routine education normally provided by the Iranian Epilepsy Association. It is not reported how this information was provided. Study outcomes were reported after three months follow‐up.
Excluded studies
We excluded one study awaiting classification from the 2010 version of this review because it lacked a control group (Shore 2008). It reported a feasibility study of the Seizures and Epilepsy Education (SEE) programme. Similarly, we excluded Austin 2002 for being a pre‐ and post‐test feasibility study lacking a control group. We excluded three other studies for having the wrong study design (Mar 2005; Price 2004; Snead 2004). We excluded four studies because they included a mix of adults and children with a mean age of participants over 18 years (Bahrani 2017; Dash 2015; Ibinda 2014; Li 2013). As described above, a small feasibility study (n = 8), Modi 2013, previously evaluated the intervention assessed in Modi 2016, and so this small study was also excluded. In the current update (January 2020) we excluded two previously included controlled before‐and‐after trials (Jantzen 2009; Pfäfflin 2012). We also excluded a paper by Hallfahrt 2007 which we had previously established in our original review, Bradley 2009, did not include any new data to that reported (in English) by Jantzen 2009. Finally, we excluded another study, Glueckauf 2002, that had been previously included in the review because although it was designed as a RCT, randomisation failed. For further information on the excluded studies, see Characteristics of excluded studies.
Ongoing studies and studies awaiting classification
We did not identify any ongoing studies or studies awaiting classification.
Risk of bias in included studies
We include details of our risk of bias judgements and the rationale for them in the Characteristics of included studies table, and risk of bias summaries are displayed in Figure 2 and Figure 3. A summary of our risk of bias judgements is provided below.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered the risk of bias as high for one study (Saengow 2018), due to the method employed for recruiting participants (experimental group participants were recruited every Tuesday, and control group participants were recruited every Thursday). The cluster‐RCT evaluating ACINDES by Tieffenberg 2000 did not report the details of randomisation (including the 'clustering techniques'), so we judged it to be at unclear risk of bias for this domain. We considered all other studies to be at low risk of bias for this domain (Dorris 2017; Gürhopur 2018; Jia 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016).
Allocation concealment
Only two RCTs adequately reported on allocation concealment, and were therefore considered to be at low risk of bias for this domain (Dorris 2017; Kazemi Majd 2017). This was inadequately reported by six studies (Gürhopur 2018; Jia 2018; Lewis 1990; Lewis 1991; Modi 2016; Tieffenberg 2000), which were considered as at unclear risk of bias. We considered the risk of bias to be high for one study (Saengow 2018), due to the allocation concealment method employed, whereby participants were allocated to the experimental or control group depending on the day they attended their routine service paediatric neurology clinic (Saengow 2018).
Performance bias
No studies reported blinding for participants, clinicians, or assessors and nine of these studies were therefore considered to be at high risk of performance bias (Dorris 2017; Gürhopur 2018; Jia 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Tieffenberg 2000). Saengow 2018 stated that in order to avoid contamination, participants were recruited to their study arms on different days (experimental group who received clinician advice and an animated video on every Tuesday, and control group on every Thursday). However, as the same centre was used for recruiting participants, we assessed the risk of bias as unclear.
Detection bias
Because most outcomes for the interventions were derived from self‐report, we considered that the lack of blinding introduced a high risk of detection bias in five studies (Gürhopur 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Saengow 2018). The evaluations of STAR and ACINDES and the evaluation of the new model of clinical nursing care were considered to have unclear risk of bias because some outcomes reported were less susceptible to subjective interpretation (i.e. analysis of hospital and school records) (Jia 2018; Modi 2016; Tieffenberg 2000). The evaluation of PIE was considered to be at low risk of bias because the second author inputting the data remained blinded until study completion (Dorris 2017).
Incomplete outcome data
Loss to follow‐up was relatively low (less than 10%) in five studies (Dorris 2017; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Saengow 2018), hence we judged these studies to be at low risk of attrition bias. In two studies (Modi 2016; Tieffenberg 2000), loss to follow‐up was relatively high (over 10%) in at least one of the study arms, meriting a judgement of high risk of bias. The risk of bias was unclear in two studies (Gürhopur 2018; Jia 2018). In Gürhopur 2018, all participants completed all the questionnaires immediately after the module and at the one‐ and three‐month follow‐ups. However, participants were not followed up for six months as initially suggested in the study hypothesis, as it was reported that there was a significant decrease in the number of cases. Furthermore, the number of participants who completed the questionnaires at three months is not reported. In Jia 2018, participant dropout rates were not reported in the trial. However, for the outcomes of seizure frequency and comparison of course of treatment and complications, data were reported as a proportion of all participants enrolled into the trial. It is not clear if any intention‐to‐treat analysis or sensitivity analysis was used to account for participant dropout for other outcomes during the analysis.
Selective reporting
All studies reported findings for the outcomes described in the methods sections (although Modi 2016 reported some outcomes in detail only in a supplementary appendix). Hence, all nine studies were at low risk of bias for selective reporting (Dorris 2017; Gürhopur 2018; Jia 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Saengow 2018; Tieffenberg 2000).
Other potential sources of bias
We identified other potential sources of bias in only one study (Kazemi Majd 2017). This trial was registered as a three‐arm trial (IRCT2015060122514N1); however, no reference is made in the published paper to the third arm. As this was a different intervention (i.e. self‐care education based on SMS, workshop on self‐efficacy and adherence to the medication regimen, and self‐care education pamphlets) to that being evaluated (SMS only), we considered the risk of bias to be unclear.
Effects of interventions
See: Table 1
The effects of interventions on identified outcomes can be found in Table 1; the results by study are described in Table 2; Table 3; Table 4; Table 5; Table 6; Table 7. The types of outcomes reported varied considerably between studies, even within apparently similar types of outcomes. The only findings presented are those that we considered to match the predefined outcomes of our review. We only presented outcomes in forest plots that reported objective outcomes (such as seizures) or that used validated measures (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9; Analysis 4.10; Analysis 4.11; Analysis 5.1; Analysis 5.2; Analysis 6.1). Only two outcomes were reported by more than one study: the 8‐item Morisky Medication Adherence Scale (MMAS‐8) and the Seizure Self‐Efficacy Scale for Children (SSES‐C).
1. Seizure frequency and severity .
| Study | Population | Experimental | Control | Outcome | Follow‐up | Results |
|
Dorris 2017 |
Adolescents |
Manual‐based brief psychosocial group intervention for young people with epilepsy (PIE) |
Waiting list control |
Self‐reported frequency of seizures (bespoke questionnaire) | 3 months | No statistically significant differences in change of median seizure frequency between the experimental group (−1) and the control group (0) over time (P = 0.135) |
| Self‐reported severity of seizures (bespoke questionnaire) | 3 months | No statistically significant differences in change of median seizure severity between the experimental group (0) and the control group (0.5) over time (P = 0.619) | ||||
|
Jia 2018 |
Children and their parents |
New model of clinical nursing care |
Conventional treatment |
Control rate (reduction in seizures by ≥ 50% and the duration of treatment shortened; information from medical records) | 12 months | Experimental, 52 (86.7%) Control, 43 (71.7%) P = 0.043 |
| Cure rate (reduction in seizures by ≥ 90% and the duration of treatment shortened; information from medical records) | 12 months | Experimental, 35 (58.3%) Control, 23 (38.3%) P = 0.028 |
||||
| Saengow 2018 | Children, adolescents, and their parents |
Clinician advice and an 8.52‐minute video animation | Clinician advice | Seizure severity (defined as frequency and duration of seizures; unclear how measured) | 3 months |
Experimental, n (%) Same, 72 (57.1) Worsened, 7 (5.6) Improved, 47 (37.3) Control, n (%) Same, 62 (70.5) Worsened, 4 (4.5) Improved, 22 (25.0) The difference between groups was reported to be statistically significant regarding those who had the same severity and those who had improved seizure severity (P < 0.05). |
|
Tieffenberg 2000 |
Children and their parents | ACINDES: a child‐centred training programme | Routine care | Mean (SD) number of epileptic seizures (from medical records) | 12 months | Experimental Baseline: 0.80 (1.46) 12 months: 0.34 (0.98) Control Baseline: 0.49 (1.15) 12 months: 1.11 (2.77) P = 0.026 |
SD: standard deviation
2. Appropriateness and volume of medication prescribed (including evidence of drug toxicity) .
| Study | Population | Experimental | Control | Outcome | Follow‐up | Results |
| Jia 2018 | Children and their parents | New model of clinical nursing care | Conventional treatment | Compliance (investigated as a questionnaire composite measure including medication regularity and follow‐up visit inspection) | 12 months | Experimental, mean (SD) 79.8 (12.2) Control, mean (SD) 68.5 (11.4) P = 0.010 |
| Kazemi Majd 2017 | Adolescents |
Self‐care education programme delivered via SMS | Routine education | Drug adherence measured by MMAS‐8 | Measured before intervention and at 3 months |
Experimental, mean (SD) 6.69 (1.23) Control, mean (SD) 5.57 (1.33) P < 0.001 |
| Modi 2016 | Children and their parents | Supporting Treatment Adherence Regimen (STAR), problem‐solving sessions | Treatment as usual | Antiepileptic drug adherence (Medication Event Monitoring System (MEMS‐6) track cap) | 3 months | Statistically significant differences were reported during the intervention period after sessions 2 (P = 0.053), 3 (P = 0.002), and 4 (P = 0.021), but there were no statistically significant differences between groups during the 3‐month follow‐up period (P value not reported). Adherence rates (presented graphically): Experimental 8/11 (72.7%) Control 9/12 (75.0%) |
| Saengow 2018 | Children, adolescents, and their parents |
Clinician advice and an 8.52‐minute video animation | Clinician advice | Drug adherence measured by MMAS‐8 | Measured before intervention and at 3 months |
Experimental, n (%) Same, 69 (54.8) Worsened, 3 (2.3) Improved, 54 (42.9) Control, n (%) Same, 67 (76.2) Worsened, 7 (7.9) Improved, 14 (15.9) The difference between groups was reported to be statistically significant regarding those who had the same adherence and those who had improved adherence (P < 0.05). |
MMAS‐8: 8‐item Morisky Medication Adherence Scale SD: standard deviation SMS: Short Message Service
3. Knowledge of information and advice received from professionals .
| Study | Population | Experimental | Control | Outcome | Follow‐up | Results |
| Dorris 2017 | Adolescents and their parents | Manual‐based brief psychosocial group intervention for young PIE | Waiting list control | Medical and social knowledge (EKP‐G) | 3 months | Experimental, mean (SD) Baseline: 39.15 (5.28) 3 months: 43.36 (3.24) Control, mean (SD) Baseline: 39.87 (4.69) 3 months: 41.10 (4.41) After 3 months, difference between groups, P = 0.02 |
|
Gürhopur 2018 |
Children, adolescents, and parents: child perspective |
Modular Education Program for Children with Epilepsy and Their Parents |
No training |
Epilepsy Self Knowledge Test for Children (EKTC) |
3 months | Change in mean (SD) Experimental 7.885 (2.167) Control 4.054 (1.357) P = 0.001 |
| Parents' knowledge of epilepsy as measured by the Epilepsy Knowledge Scale for Parents (EKSP) | 3 months | Change in mean (SD) Experimental 14.321 (2.992) Control 9.552 (1.140) P = 0.001 |
||||
|
Lewis 1990 |
Children and their parents: child perspective |
Children's Epilepsy Program (CEP), a counselling model based on Rogerian principles (child‐centred, family‐focused (active learning) programme) |
Traditional educational format (passive learning) |
“What were the important things that you learned?" (from questionnaire) | 5 months | Children in the intervention group were more likely to report generic gain in knowledge than those in the control group (mean: 64% with intervention versus 47% with control; P < 0.01) |
| Importance of taking medicines exactly as prescribed (from questionnaire) | 5 months | Differences “not significant” between groups in the percentage of children responding correctly (92.7% with intervention versus 85.8% with control; P value not reported) | ||||
| Inappropriate to have objects in mouth during seizure (from questionnaire) | 5 months | Statistically significant differences between groups in the percentage of children responding correctly (71.5% with intervention versus 52.2% with control; P = 0.002) | ||||
| Inappropriate to restrain during seizure (from questionnaire) | 5 months | Statistically significant differences between groups in the percentage of children responding correctly (79.7% with intervention versus 46.0% with control; P = 0.001) | ||||
| Knowledge that seizures start in the brain (from questionnaire) | 5 months | Differences “not significant” between groups in the percentage of parents responding correctly (82.9% with intervention versus 81.4% with control; P value not reported) | ||||
| Loss of sleep can trigger seizures (from questionnaire) | 5 months | Differences “not significant” between groups in the percentage of parents responding correctly (49.6% with intervention versus 42.5% with control; P value not reported) | ||||
| Not required to visit emergency department after seizure (from questionnaire) | 5 months | Statistically significant differences between groups in the percentage of children responding correctly (78.1% with intervention versus 52.2% with control; P = 0.001) | ||||
| Positive effects of participation in sports (from questionnaire) | 5 months | Differences “not significant” between groups in the percentage of parents responding correctly (78.1% with intervention versus 74.3% with control; P value not reported) | ||||
| Purpose of drug blood levels to monitor dosage (from questionnaire) | 5 months | Differences “not significant” between groups in the percentage of parents responding correctly (51.6% with intervention versus 53.9% with control; P value not reported) | ||||
| Purpose of electroencephalogram (EEG) (from questionnaire) | 5 months | Statistically significant differences between groups in the percentage of children responding correctly (mean 82.1% with intervention versus 69.0% with control; P = 0.02) | ||||
| Restriction of activities should be minimal (from questionnaire) | 5 months | Statistically significant differences between groups in the percentage of children responding correctly (mean 86.2% with intervention versus 68.1% with control; P = 0.001) | ||||
|
Lewis 1991 |
Children and their parents: parental perspective |
Children's Epilepsy Program (CEP), a counselling model based on Rogerian principles (child‐centred, family‐focused (active learning) programme) |
Traditional educational format (passive learning) |
“What were the important things that you learned?" (from questionnaire) | 5 months | Parents in the intervention group were more likely to report generic gain in knowledge (59% with intervention versus 48% with control; P < 0.05). |
| Importance of medicines (from questionnaire)* | 5 months | Statistically significant differences between groups in the percentage of parents responding correctly (19% with intervention versus 9% with control; P < 0.01) | ||||
| Importance of taking medicines exactly as prescribed (from questionnaire) | 5 months | Differences "not significant" between groups in the percentage of parents responding correctly (97.3% with intervention versus 99.0% with control; P value not reported) | ||||
| Inappropriate to have objects in mouth during seizure (from questionnaire) | 5 months | Differences "not significant" between groups in the percentage of parents responding correctly (78.8% with intervention versus 76.1% with control; P value not reported) | ||||
| Inappropriate to restrain during seizure (from questionnaire) | 5 months | Differences "not significant" between groups in the percentage of parents responding correctly (76.3% with intervention versus 81.1% with control; P value not reported) | ||||
| Knowledge that seizures start in the brain (from questionnaire) | 5 months | Differences "not significant" between groups in the percentage of parents responding correctly (93.5% with intervention versus 90.0% with control; P value not reported) | ||||
| Loss of sleep can trigger seizures (from questionnaire) | 5 months | Statistically significant differences between groups in the percentage of parents responding correctly (mean 50.3% with intervention versus 65.2% with control; P = 0.005) | ||||
| Not required to visit emergency department after seizure (from questionnaire) | 5 months | Differences "not significant" between groups in the percentage of parents responding correctly (93.0% with intervention versus 88.3% with control; P value not reported) | ||||
| Positive effects of participation in sports (from questionnaire) | 5 months | Differences "not significant" between groups in the percentage of parents responding correctly (95.1% with intervention versus 90.0% with control; P value not reported) | ||||
| Purpose of drug blood levels to monitor dosage (from questionnaire) | 5 months | Statistically significant differences between groups in the percentage of parents responding correctly (mean baseline to 5 months: 79.6% with intervention versus 87.8% with control; P = 0.04) | ||||
| Purpose of electroencephalogram (EEG) (from questionnaire) | 5 months | Statistically significant differences between groups in the percentage of parents responding correctly (mean 90.3% with intervention versus 83.3% with control; P = 0.05) | ||||
| Restriction of activities should be minimal (from questionnaire) | 5 months | Differences "not significant" between groups in the percentage of parents responding correctly (96.7% with intervention versus 97.2% with control; P value not reported) | ||||
|
Modi 2016 |
Children and their parents |
Supporting Treatment Adherence Regimen (STAR) problem‐solving sessions |
Treatment as usual |
Parents’ knowledge measured by Epilepsy Knowledge Questionnaire (EKQ) | 3 months | Experimental, mean (SD) Baseline, 83.6 (6.1) 3 months, 90.2 (5.0) Control, mean (SD) Baseline, 84.1 (7.3) 3 months, 82.0 (8.4) After 3 months, difference between groups, P < 0.01 |
| Parents’ disease and treatment knowledge measured by Pediatric Epilepsy Medication Self‐Management Questionnaire (PEMSQ): Epilepsy Disease and Treatment Knowledge | 3 months | Experimental, mean (SD) Baseline, 36.6 (3.4) 3 months, 39.8 (1.7) Control, mean (SD) Baseline, 36.8 (4.7) 3 months, 37.1 (3.8) After 3 months, difference between groups, P < 0.01 |
||||
| Saengow 2018 | Children, adolescents, and their parents |
Clinician advice and an 8.52‐minute video animation | Clinician advice | Knowledge and understanding of epilepsy using questionnaire with 10‐item questions | 3 months |
Experimental, mean Baseline, 6.73 3 months, 7.47 Control, mean (SD) Baseline, 7.48 3 months, 7.44 After 3 months, difference between groups, P > 0.05 |
|
Tieffenberg 2000 |
Children and their parents |
ACINDES: a child‐centred training programme |
Routine care |
Parents’ knowledge of epilepsy (from questionnaire via interviews) | 12 months | Improved in the experimental group at 12 months (from 22% to 56%) compared to control group (from 8% to 15%, probability of gain = 0.62, variance = 0.0026) |
| Parents’ fears and anxieties (from questionnaire via interviews) | 12 months | Improved in the experimental group at 12 months (from 69% to 30% for fear of child's death) compared to no change in the control group (from 74% to 65%, probability of gain = 0.63, variance = 0.0026) |
*Not asked of children in Lewis 1991.
EKP‐G: Epilepsy Knowledge Profile‐General SD: standard deviation
4. Health and quality of life (including side effects of medication).
| Study | Population | Experimental | Control | Outcome | Follow‐up | Results |
|
Dorris 2017 |
Adolescents and their parents |
Manual‐based brief psychosocial group intervention for young people with epilepsy (PIE) |
Waiting list control |
Self‐perception of epilepsy on quality of life (Pediatric Quality of Life Inventory (PedsQL)) | 3 months | Experimental, mean (SD) Baseline: 70.93 (15.41) 3 months: 67.79 (11.74) Control, mean (SD) Baseline: 69.36 (19.42) 3 months: 69.19 (17.79) After 3 months, difference between groups, P > 0.05 |
| Seizure Self‐Efficacy Scale for Children (SSES‐C) | 3 months | Experimental, mean (SD) Baseline: 57.15 (14.72) 3 months: 60.69 (8.23) Control, mean (SD) Baseline: 59.26 (12.80) 3 months: 60.55 (10.45) After 3 months, difference between groups, P > 0.05 |
||||
| Self‐perception of physical, emotional, social and school functioning (Glasgow Epilepsy Outcome Scale for Young Persons (GEOS‐YP)) | 3 months | Experimental, mean (SD) Baseline: 62.61 (14.85) 3 months: 65.83 (11.62) Control, mean (SD) Baseline: 66.20 (13.95) 3 months: 66.16 (12.13) After 3 months, difference between groups, P > 0.05 |
||||
| Gürhopur 2018 | Children, adolescents, and parents | Modular Education Program for Children with Epilepsy and Their Parents | No training | Children’s quality of life as measured by Quality of Life in Epilepsy Inventory (QOLIE)‐48 questionnaire | 3 months |
Change in mean (SD) Experimental 2.540 (0.238) Control 2.261 (0.254) P < 0.001 |
| Parent’s anxiety about their child’s epilepsy as measured by Parents' Anxiety About Seizures Scale (PAAS) | 3 months | Change in mean (SD) Experimental 19.962 (2.340) Control 14.114 (2.089) P < 0.001 |
||||
|
Lewis 1990 |
Children and their parents: child perspective |
Children's Epilepsy Program (CEP), a counselling model based on Rogerian principles (child‐centred, family‐focused (active learning) programme) |
Traditional educational format (passive learning) consisting of 3 x 2‐hour sessions conducted by a physician who gave traditional lectures followed by question‐and‐answer sessions to present the same information related to epilepsy that the experimental group received |
Scholastic competency (from Harter’s self‐competency scale)* | 5 months | Adjusted 5‐month scores Experimental 2.63 (0.6) Control 2.50 (0.6) P > 0.05 |
| Social competency (from Harter’s self‐competency scale)* | 5 months | Adjusted 5‐month scores Experimental 2.91 (0.5) Control 2.76 (0.5) P < 0.05 |
||||
| Athletic competency (from Harter’s self‐competency scale)* | 5 months | Adjusted 5‐month scores Experimental 2.83 (0.6) Control 2.79 (0.6) P > 0.05 |
||||
| Appearance competency (from Harter’s self‐competency scale)* | 5 months | Adjusted 5‐month scores Experimental 3.03 (0.6) Control 3.01 (0.6) P > 0.05 |
||||
| Behaviour competency (from Harter’s self‐competency scale)* | 5 months | Adjusted 5‐month scores Experimental 2.78 (0.5) Control 2.64 (0.5) P > 0.05 |
||||
| Self‐esteem competency (from Harter’s self‐competency scale)* | 5 months | Adjusted 5‐month scores Experimental 3.00 (0.5) Control 3.10 (0.5) P > 0.05 |
||||
| Gain in social skills (from bespoke questionnaire) | 5 months | Children in the intervention group were more likely to report gain in social skills (9% with intervention versus 2% with control; P < 0.02). | ||||
| Participation in normal activities (from bespoke questionnaire) | 5 months | Children in the intervention group were more likely to report participation in normal activities (11% with intervention versus 3.5% with control; P < 0.03). | ||||
| Children's self‐care skills (from bespoke questionnaire) | 5 months | "No differences" between children in the intervention and control groups (proportions and P value not reported) | ||||
| Children's reports of parents' behaviours (from bespoke questionnaire) | 5 months | "No differences" between children in the intervention and control groups (proportions and P value not reported) | ||||
| Children's disclosure of epileptic status (from bespoke questionnaire) | 5 months | "No impact" between children in the intervention and control groups (proportions and P value not reported) | ||||
|
Lewis 1991 |
Children and their parents: child perspective |
Children's Epilepsy Program (CEP), a counselling model based on Rogerian principles (child‐centred, family‐focused (active learning) programme) |
Traditional educational format (passive learning) consisting of 3 x 2‐hour sessions conducted by a physician who gave traditional lectures followed by question‐and‐answer sessions to present the same information related to epilepsy that the experimental group received |
Parental anxiety ‐ feeling less anxious (from bespoke questionnaire) | 5 months | Statistically significant difference in the proportion of parents who reported feeling less anxious and fearful after the sessions (31% with intervention versus 10% with control; P < 0.001) |
| Parental anxiety score (from Taylor Manifest Anxiety Scale) | 5 months | Mothers Experimental Before 56.0 After 50.7 Control Before 54.0 After 52.6 Difference between groups at 5 months, P = 0.01 Fathers Experimental Before 46.5 After 43.1 Control Before 44.1 After 42.6 Difference between groups at 5 months, P > 0.05 Both parents Experimental Before 52.5 After 47.9 Control Before 50.2 After 48.7 Difference between groups at 5 months, P < 0.01 |
||||
| Kazemi Majd 2017 | Adolescents |
Self‐care education programme delivered via SMS | Routine education | Epilepsy Self‐Efficacy Scale (ESES) | Measured before intervention and at 3 months | Control, mean (SD) Before training 4.77 (1.47) After training 4.75 (1.46) Paired t‐test result P = 0.167 Intervention, mean (SD) Before training 4.51 (1.26) After training 7.35 (1.03) Paired t‐test result P < 0.001 The results of independent t‐test showed a statistically significant difference between the 2 groups in terms of self‐efficacy score after training (P < 0.001). |
|
Modi 2016 |
Children and their parents |
Supporting Treatment Adherence Regimen (STAR) problem‐solving sessions |
Treatment as usual |
Parents’ self‐management (measured by total Pediatric Epilepsy Medication Self‐Management Questionnaire (PEMSQ) score) | 3 months | Experimental, mean (SD) Baseline 127.2 (7.8) 3 months 129.4 (5.3) Control, mean (SD) Baseline 125.2 (12.6) 3 months 123.3 (11.5) P < 0.01 |
| Epilepsy management (measured by Parent Response to Child Illness (PRCI)) | 3 months | Experimental, mean (SD) Baseline 4.5 (0.4) 3 months 4.8 (0.3) Control, mean (SD) Baseline 4.4 (0.5) 3 months 4.3 (0.5) P > 0.05 |
||||
| Child support (measured by PRCI) | 3 months | Experimental, mean (SD) Baseline 4.4 (0.5) 3 months 4.5 (0.4) Control, mean (SD) Baseline 4.2 (0.4) 3 months 4.3 (0.4) P > 0.05 |
||||
| Family life and leisure (measured by PRCI) | 3 months | Experimental, mean (SD) Baseline 4.2 (0.9) 3 months 4.1 (0.9) Control, mean (SD) Baseline 3.6 (1.0) 3 months 4.1 (0.9) P > 0.05 |
||||
| Child autonomy (measured by PRCI) | 3 months | Experimental, mean (SD) Baseline 3.2 (0.5) 3 months 3.0 (0.8) Control, mean (SD) Baseline 3.0 (0.6) 3 months 3.2 (0.5) |
||||
| Child discipline (measured by PRCI) | 3 months | Experimental, mean (SD) Baseline 4.2 (0.4) 3 months 4.1 (0.4) Control, mean (SD) Baseline 3.9 (0.7) 3 months 3.9 (0.8) P > 0.05 |
||||
| Social problem‐solving measured by total Social Problem‐Solving Inventory‐Revised (SPSI‐R): Short Form | 3 months | Experimental, mean (SD) Baseline 117.7 (17.1) 3 months 114.4 (12.8) Control, mean (SD) Baseline 110.6 (9.6) 3 months 107.1 (10.9) P > 0.05 |
||||
|
Tieffenberg 2000 |
Children and their parents | ACINDES: a child‐centred training programme | Routine care | Allowed child to sleep at friends' homes more often (from questionnaire via interviews) | 12 months | After participating in the groups, the parents of children with epilepsy allowed them to sleep at friends' homes more often (proportions not reported, probability of gain = 0.59, variance = 0.0026). |
SD: standard deviation SMS: Short Message Service
*Harter’s self‐competency scores adjusted by analysis of covariance for entry value of corresponding variable, age, and sex. Because this instrument is only appropriate for children aged ≥ 8 years, the data on 7‐year‐olds were eliminated from all analyses using this scale. Hence, n = 106 for experimental and n = 92 for control for these analyses.
5. Objective measures of general health status.
| Study | Population | Experimental | Control | Outcome | Follow‐up | Results |
|
Jia 2018 |
Children and their parents |
New model of clinical nursing care |
Conventional treatment |
Mean (SD) duration of treatment (months) from medical records | 12 months | Experimental 4.7 (1.2) Control 6.5 (1.7) P = 0.027 |
| Complication rate from medical records (complications = cerebral injury, growth and intelligence development disorders, disability, lethal outcome) | 12 months | Experimental 8.3% Control 21.7% P = 0.041 |
||||
|
Tieffenberg 2000 |
Children and their parents |
ACINDES: a child‐centred training programme |
Routine care |
Emergency visits (from medical records) per 12 months | 12 months | Experimental, mean (SD) Baseline: 0.90 (0.95) 12 months: 0.22 (0.58) Control, mean (SD) Baseline: 0.83 (0.95) 12 months: 0.46 (0.66) P = 0.046 |
| Regular medical visits (from medical records) per 12 months | 12 months | Experimental, mean (SD) Baseline: 3.64 (3.01) 12 months: 3.06 (2.57) Control, mean (SD) Baseline: 3.89 (4.47) 12 months: 2.91 (3.19) P > 0.05 |
SD: standard deviation
6. Objective measures of social or psychological functioning (including the number of days spent on sick leave/absent from school and work, and employment status).
| Study | Population | Experimental | Control | Outcome | Follow‐up | Results |
|
Tieffenberg 2000 |
Children and their parents | ACINDES: a child‐centred training programme | Routine care | School absenteeism (from school records) | 12 months | Experimental, mean absences per 100 school days Baseline: 10.31 12 months: 6.85 Control, mean absences per 100 school days Baseline: 9.32 12 months: 9.21 P = 0.011 Note: SDs not reported. |
SD: standard deviation
1.1. Analysis.

Comparison 1: Seizure frequency and severity, Outcome 1: Number of seizures at 12 months
1.2. Analysis.

Comparison 1: Seizure frequency and severity, Outcome 2: Seizure severity (frequency and duration of seizures) improved at 12 months
1.3. Analysis.

Comparison 1: Seizure frequency and severity, Outcome 3: Seizure control rate at 12 months
1.4. Analysis.

Comparison 1: Seizure frequency and severity, Outcome 4: Seizure cure rate at 12 months
2.1. Analysis.

Comparison 2: Appropriateness and volume of medication prescribed, Outcome 1: Drug adherence improved using the 8‐item Morisky Medication Adherence Scale (MMAS‐8) at 3 months
2.2. Analysis.

Comparison 2: Appropriateness and volume of medication prescribed, Outcome 2: Mean adherence to medication using the 8‐item Morisky Medication Adherence Scale (MMAS‐8) at 3 months
2.3. Analysis.

Comparison 2: Appropriateness and volume of medication prescribed, Outcome 3: Antiepileptic drug adherence measured using the Medication Event Monitoring System (MEMS) 6 Cap at 3 months
3.1. Analysis.

Comparison 3: Knowledge of information and advice received from professionals, Outcome 1: Medical and social knowledge (Epilepsy Knowledge Profile‐General (EKP‐G)) at 3 months
3.2. Analysis.

Comparison 3: Knowledge of information and advice received from professionals, Outcome 2: Knowledge of epilepsy (Epilepsy Self Knowledge Test for Children (EKTC)) at 3 months
3.3. Analysis.

Comparison 3: Knowledge of information and advice received from professionals, Outcome 3: Parents’ disease and treatment knowledge (Pediatric Epilepsy Medication Self‐Management Questionnaire (PEMSQ) ‐ Epilepsy and Treatment Knowledge and Expectations) at 3 months
3.4. Analysis.

Comparison 3: Knowledge of information and advice received from professionals, Outcome 4: Parents’ knowledge (Epilepsy Knowledge Questionnaire (EKQ)) at 3 months
3.5. Analysis.

Comparison 3: Knowledge of information and advice received from professionals, Outcome 5: Epilepsy Knowledge Scale for Parents (EKSP) at 3 months
4.1. Analysis.

Comparison 4: Health and quality of life, Outcome 1: Mean Seizure Self‐Efficacy Scale for Children (SSES‐C) score at 3 months
4.2. Analysis.

Comparison 4: Health and quality of life, Outcome 2: Self‐perception of epilepsy on quality of life (Pediatric Quality of Life Inventory (PedsQL)) at 3 months
4.3. Analysis.

Comparison 4: Health and quality of life, Outcome 3: Quality of Life in Epilepsy Inventory (QOLIE‐48) at 3 months
4.4. Analysis.

Comparison 4: Health and quality of life, Outcome 4: Mean self‐efficacy (Epilepsy Self‐Efficacy Scale (ESES)) at 3 months
4.5. Analysis.

Comparison 4: Health and quality of life, Outcome 5: Social problem‐solving (Social Problem‐Solving Inventory‐Revised (SPSI‐R)) at 3 months
4.6. Analysis.

Comparison 4: Health and quality of life, Outcome 6: Self‐perception of physical, emotional, social and school functioning (Glasgow Epilepsy Outcome Scale for Young Persons (GEOS‐YP)) at 3 months
4.7. Analysis.

Comparison 4: Health and quality of life, Outcome 7: Perceived competencies for control: Harter's adjusted 5‐month scores
4.8. Analysis.

Comparison 4: Health and quality of life, Outcome 8: Parents’ Anxiety About Seizures Scale (PAASS) at 3 months
4.9. Analysis.

Comparison 4: Health and quality of life, Outcome 9: Parents’ self‐management (Pediatric Epilepsy Medication Self‐Management Questionnaire (PEMSQ) ‐ Total) at 3 months
4.10. Analysis.

Comparison 4: Health and quality of life, Outcome 10: Parent Response to Child Illness (PRCI) at 3 months
4.11. Analysis.

Comparison 4: Health and quality of life, Outcome 11: Taylor Manifest Anxiety Scale
5.1. Analysis.

Comparison 5: Objective measures of general health status, Outcome 1: Emergency visits at 12 months
5.2. Analysis.

Comparison 5: Objective measures of general health status, Outcome 2: Regular medical visits at 12 months
6.1. Analysis.

Comparison 6: Objective measures of social or psychological functioning, Outcome 1: Mean number of absences per 100 school days
Seizure frequency and severity
Four studies reported outcomes relating to seizure frequency and severity (Dorris 2017; Jia 2018; Saengow 2018; Tieffenberg 2000). The results are presented in forest plots where possible (Analysis 1.1 to Analysis 1.4), and are summarised in Table 2. We could not include data from Dorris 2017 regarding the evaluation of PIE in a forest plot because the data showed the difference of change in median between intervention and control group for all categorical outcome measures completed by caregivers between baseline and postintervention (six weeks), and between baseline and three months follow‐up. This was the only one of the four studies reporting this outcome to not report a statistically significant improvement for the experimental versus control group (difference in median frequency, experimental −1, control 0, P = 0.135; difference in median severity, experimental 0, control +0.5, P = 0.619). After three months, Saengow 2018 found a statistically higher number of participants who reported their seizure severity (defined as frequency and duration of seizures) to have "improved" in the experimental group receiving an animated video alongside clinician advice versus the control group, who received clinician advice only (experimental 37.3%, control 25.0%, P < 0.05). However, it is not clear how the seizures were recorded, that is whether self‐reported or medical records. Furthermore, the odds ratio (OR) we found in Analysis 1.2 (OR 1.78, 95% confidence interval (CI) 0.98 to 3.26) suggests, contrary to the authors' reported statistical significance testing, that the result is not statistically significant in relation to improved severity. Two studies evaluated outcomes after 12 months (Jia 2018; Tieffenberg 2000). Jia 2018 found that, compared to conventional treatment, their new care model of clinical nursing resulted in improvements in both cure rate (58.3% versus 38.3%, P = 0.028) and control rate (86.7% versus 71.7%, P = 0.043) of seizures after 12 months. Cure and control were defined as binary outcomes, in which cure/control was considered to have been achieved based on seizure frequency and duration of epilepsy. However, it is not clear what was meant by duration of epilepsy, and how it was determined whether this had been reduced. Tieffenberg 2000 reported a statistically significant difference (P = 0.026) in the number of epileptic seizures between groups over time in favour of the experimental group, who received the ACINDES child‐centred training programme (in which the number of seizures decreased from 0.80 to 0.34) versus the control group, who received routine care (seizures increased from 0.49 to 1.11).
Appropriateness and volume of medication prescribed
Four studies reported outcomes relating to the appropriateness and volume of medication prescribed (Jia 2018; Kazemi Majd 2017; Modi 2016; Saengow 2018). The results are presented in forest plots (Analysis 2.1 to Analysis 2.3) and also summarised in Table 3. After 12 months, compliance investigated as a questionnaire composite measure including medication regularity and follow‐up visit inspection was statistically significantly better in the experimental group in the study by Jia 2018, that is the modified model of clinical nursing care increased compliance compared with conventional care, mean (standard deviation (SD)) 79.8 (12.2) versus 68.5 (11.4), P = 0.010. The MMAS‐8 was used to measure adherence at three months in two studies (Kazemi Majd 2017; Saengow 2018). Both studies reported findings favouring the interventions (Kazemi Majd 2017: mean (SD) 6.69 (1.23) in the experimental group versus 5.57 (1.33) in the control group, P < 0.001; Saengow 2018: proportion improved in the experimental group 42.9% versus 15.9% in the control group, P < 0.05). The evaluation of STAR problem‐solving sessions by Modi 2016 found no statistically significant differences in antiepileptic drug adherence, as measured using the Medication Event Monitoring System track cap (MEMS‐6)) between the intervention and treatment as usual groups after three months (72.7% in the experimental group and 75.0% in the control group, P not reported).
Knowledge of information and advice received from professionals
Seven studies of six interventions reported outcomes relating to knowledge of information and advice received from professionals (Dorris 2017; Gürhopur 2018; Lewis 1990; Lewis 1991; Modi 2016; Saengow 2018; Tieffenberg 2000). The length of follow‐up varied from three months, Dorris 2017; Modi 2016; Saengow 2018, to 12 months (Tieffenberg 2000). Four studies of three interventions used bespoke as opposed to standardised instruments (Lewis 1990; Lewis 1991; Saengow 2018; Tieffenberg 2000). As summarised in Table 4, these favoured the ACINDES intervention in Tieffenberg 2000 after 12 months, but no differences were found between intervention and control after three months in the evaluation by Saengow 2018 of the animated video alongside clinician advice. After five months, results were mixed in the evaluation of CEP by Lewis 1990 and Lewis 1991. This was the case from both the perspective of children, in which 11 outcomes that measured knowledge were reported (Lewis 1990), and adults where 12 outcomes that measured knowledge were reported (Lewis 1991). Results using standardised measures (Epilepsy Knowledge Profile‐General (EKP‐G), Epilepsy Self Knowledge Test for Children (EKTC), Epilepsy Knowledge Questionnaire (EKQ), Pediatric Epilepsy Medication Self‐Management Questionnaire (PEMSQ): Epilepsy Disease and Treatment Knowledge, Epilepsy Knowledge Scale for Parents (EKSP)) are summarised in Analysis 3.1 to Analysis 3.5; all were statistically significant favouring interventions versus controls (Dorris 2017: mean (SD) EKP‐G 43.36 (3.24) versus 41.10 (4.41), P = 0.02; Gürhopur 2018: change in mean (SD) EKTC 7.885 (2.167) versus 4.054 (1.357), P < 0.001 and change in mean (SD) EKSP 14.321 (2.992) versus 9.552 (1.140), P < 0.001; Modi 2016: mean (SD) EKQ 90.2 (5.0) versus 82.0 (8.4), P < 0.01).
Health and quality of life
Eight studies of seven interventions reported outcomes relating to health, Dorris 2017; Gürhopur 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Tieffenberg 2000, and quality of life (Lewis 1991). A mixture of standardised measures (Pediatric Quality of Life Inventory (PedsQL), Glasgow Epilepsy Outcome Scale for Young Persons (GEOS‐YP), SSES‐C, Quality of Life in Epilepsy Inventory (QOLIE)‐48, Harter's self‐competency scale, Taylor Manifest Anxiety Scale, Epilepsy Self‐Efficacy Scale (ESES), PEMSQ, Parent Response to Child Illness (PRCI), Social Problem‐Solving Inventory‐Revised (SPSI‐R): Short Form, Parents' Anxiety about Seizures Scale (PAAS)); Analysis 4.1 to Analysis 4.11 and Table 5) and bespoke questionnaires and interviews were utilised (Table 5). Only the SSES‐C was used by more than one study (Dorris 2017; Gürhopur 2018). The findings employing this measure were similar between study arms in Dorris 2017 (mean (SD) 60.69 (8.23) versus 60.55 (10.45), P > 0.05), and were only reported to be statistically significant in the evaluation of a Modular Education Program for Children with Epilepsy and Their Parents by Gürhopur 2018. However, the findings reported for this outcome are presented in a table and a graph in the published paper, but the data did not match and appear to be incorrectly reported in the table. We were therefore unable present the data in the review. Gürhopur 2018 also reported statistically significant differences favouring the experimental group for QOLIE‐48 and PAAS outcomes (change in mean (SD) 2.540 (0.238) versus 2.261 (0.254), P < 0.001 and 19.962 (2.340) versus 14.114 (2.089), P < 0.001, respectively). Kazemi Majd 2017 reported statistically significant improvements in ESES in their evaluation of a self‐care education programme delivered via SMS (mean (SD) 4.75 (1.46) versus 7.35 (1.03), P < 0.001). In the evaluation of CEP by Lewis 1990 and Lewis 1991, results were mixed depending on the aspect of health and quality of life being measured and the instrument used. The only statistically significant difference between groups of children using a validated measure was the adjusted five‐month Harter's self‐competency scale score, favouring the intervention (mean (SD) 2.91 (0.5) versus 2.76 (0.5), P < 0.05). For parents, the only statistically significant difference between groups using a validated measure was the reduced anxiety scores using the Taylor Manifest Scale amongst parents (47.9 in the experimental group versus 48.7 in the control group, P < 0.01). The difference was statistically different only for mothers (50.7 versus 52.6, P = 0.01), not for fathers (43.1 versus 42.6, P > 0.05). Similarly, in the evaluation of the STAR intervention, Modi 2016 reported mixed results: the total PEMSQ score favoured the experimental group (129.4 (5.3) versus 123.3 (11.5) in the control group, P < 0.01), but there were no differences between groups in PRCI or SPSI‐R scores. Dorris 2017 found no statistically significant differences between experimental and control groups using the PedsQL or GEOS‐YP scales in their evaluation of PIE (mean (SD) in experimental versus control: 67.79 (11.74) versus 69.19 (17.79) and 65.83 (11.62) versus 66.16 (12.13), respectively). The study authors reported that parents of children with epilepsy who participated in ACINDES allowed their children to sleep at friends' homes more often than did those in the routine care group; however, very few data were provided to support this (Tieffenberg 2000).
Objective measures of general health status
Objective measures of general health status were rarely reported. Only two studies included such measures (Jia 2018; Tieffenberg 2000; Analysis 5.1 to Analysis 5.2 and Table 6). Jia 2018 found that after 12 months, compared to conventional treatment, their new care model of clinical nursing resulted in a statistically significant decrease in both the time participants required treatment (mean (SD) months: 4.7 (1.2) versus 6.5 (1.7), P = 0.027) and complications arising from epilepsy (a composite of cerebral injury, growth and intelligence development disorders, disability and death; no deaths were reported: 8.3% versus 21.7%, P = 0.041). There were statistically significantly fewer emergency visits over the last 12 months in children who received the ACINDES programme compared to the control group (mean (SD) 0.22 (0.58) versus 0.46 (0.66), P = 0.046) (Tieffenberg 2000). The number of regular medical visits also decreased over time in each group, but the differences between groups were not statistically significant (mean (SD) 3.06 (2.57) versus 2.91 (3.19), P > 0.05).
Objective measures of social or psychological functioning
Only one study reported an outcome that objectively measured social or psychological functioning (Tieffenberg 2000). The evaluation of ACINDES showed statistically significant improvement in school absenteeism in the last 12 months when compared with routine care: 6.8 versus 9.2 mean absences per 100 school days, P = 0.011 (Analysis 6.1, Table 7).
Costs of care or treatment
No studies reported on the costs of care or treatment.
Discussion
This review included nine reports of studies that were all designed as RCTs (Dorris 2017; Gürhopur 2018; Jia 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Saengow 2018; Tieffenberg 2000). The reports evaluated eight separate interventions, as one of the interventions was reported in two separate publications with a focus on the impact from the child perspective, Lewis 1990, and the parent perspective, Lewis 1991.
The interventions may broadly be classified as consisting of educational interventions (Gürhopur 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Saengow 2018; Tieffenberg 2000), counselling interventions (Dorris 2017), and a new model of clinical nursing care delivery (Jia 2018). The studies took place in diverse locations and investigated the use of a range of innovative interventions with children, adolescents, and parents.
Each study used a unique combination of outcome measures, most of which were subjective in nature. However, in no instance was the same outcome measured and reported in the same way across studies, precluding meta‐analysis even if we had considered the interventions to be sufficiently similar to include in meta‐analysis. One study of the CEP from the child perspective included only bespoke measures (Lewis 1990). All of the other studies included at least one objective or at least one validated outcome measure. The focus of this discussion is on the outcomes that were objectively measured or those that used validated instruments and were analysed comparing the interventions versus control groups at the end of study follow‐up.
Summary of main results
Half of the studies included analyses of outcomes from sample sizes of fewer than 100 children or adolescents, or both, ranging from 50 (of whom only 23 were randomised), Modi 2016, to 234, Lewis 1990. In the latter study, the number of parents included in the analysis of outcomes from the parent perspective by Lewis 1991 was 365, the largest sample size of all the included studies. The length of follow‐up of studies was three to five months in seven studies, Dorris 2017; Gürhopur 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Saengow 2018, and 12 months in only two studies (Jia 2018; Tieffenberg 2000).
Two educational interventions reduced seizure frequency at 12 months, Tieffenberg 2000, or seizure severity at three months, Saengow 2018. However, it was unclear how the seizures were recorded in this latter study, that is whether they were self‐reported or from medical records. The new model of clinical care was found to result in reduced seizure control and cure rates (Jia 2018). However, this was based on a definition that included a reduced "duration of epilepsy", which is not a clearly understood term.
Two educational interventions had a positive impact on medication adherence as measured using the MMAS‐8 at three months (Kazemi Majd 2017; Saengow 2018), but resulted in no difference versus control in another study as measured by the MEMS‐6 (Modi 2016).
Most educational interventions improved participants' knowledge of epilepsy at three months when this was measured using standardised instruments (Gürhopur 2018; Modi 2016), the exception being the study by Saengow 2018, where there were no statistically significant differences between groups at three months. The counselling intervention studied by Dorris 2017 also resulted in statistically significant improvements for the intervention versus control at three months.
The most commonly evaluated outcome measure was health‐related quality of life at three months, albeit using a variety of different standardised instruments. Three educational interventions reported statistically significantly improved results for the intervention versus control (Gürhopur 2018; Kazemi Majd 2017; Modi 2016). However, only the PRCI epilepsy management score was statistically significantly improved for the experimental group in Modi 2016, there being no statistically significant differences between groups for all other domains of the PRCI or social problem‐solving (measured by SPSI‐R: Short Form Total). Only one of the six scales of the Harter's self‐consistency instrument resulted in differences between groups in Lewis 1990, that of scholastic competency. Parents in the experimental group showed greater reductions in anxiety using the Taylor Manifest Anxiety Scale than parents in the control group; however, subgroup analyses found that this was true only for mothers, not fathers. The counselling intervention evaluated by Dorris 2017 did not find any statistically significant differences in quality of life measures between experimental and control groups at three months.
Only two studies included objective measures of general health status, both reported after 12 months follow‐up. The educational intervention evaluated by Tieffenberg 2000 found statistically significantly fewer emergency visits over time in children who received the ACINDES programme compared to the control group, but no statistically significant differences in terms of regular medical visits. Jia 2018 found that their new care model of clinical nursing care resulted in a statistically significant decrease in both the time participants required treatment and in complications arising from epilepsy.
Only one study reported an outcome that objectively measured social or psychological functioning (Tieffenberg 2000). The evaluation of ACINDES showed a statistically significant improvement in school absenteeism after 12 months when compared with routine care.
No studies considered the costs or cost‐effectiveness of care or treatment.
Overall completeness and applicability of evidence
Overall, whilst in general the educational interventions appeared to have a positive impact, there were differences in how outcomes were collected. It is therefore unclear which intervention, if any, may be considered the best at improving these outcomes.
Whilst studies reported statistically significant results for some of the outcomes measured, the included studies did not provide evidence for the clinical meaningfulness of these results. Furthermore, as only two studies measured outcomes after 12 months follow‐up (Jia 2018; Tieffenberg 2000), it was impossible to elucidate the impact of the interventions on the long‐term self‐management of epilepsy.
Although all of the included studies investigated self‐management improvement strategies, no individual strategy has been investigated with different study samples. The generalisability of the interventions is therefore unclear.
Quality of the evidence
Overall, the quality of evidence was generally poor, with all reports containing methodological problems (Dorris 2017; Gürhopur 2018; Jia 2018; Kazemi Majd 2017; Lewis 1990; Lewis 1991; Modi 2016; Saengow 2018; Tieffenberg 2000). Even the study with an overall low risk of bias, the evaluation of the PIE manual‐based brief psychosocial group intervention by Dorris 2017, intended to recruit 200 participants, but only managed to recruit 83 adolescents aged 12 to 17 years, and therefore yielded results with wide confidence intervals.
Potential biases in the review process
We did not identify any biases in the review process.
Agreements and disagreements with other studies or reviews
Other systematic reviews have examined psychosocial treatment programmes in epilepsy (Mittan 2009), evidence‐based models of care for people with epilepsy (Fitzsimons 2012), care delivery and self‐management strategies for adults with epilepsy (Bradley 2009; Bradley 2016), strategies for improving adherence to antiepileptic drug treatment in people with epilepsy (Al‐Aqeel 2020), and psychological treatments for people with epilepsy (Michaelis 2020). However, our review is the only review we are aware of that has focused solely on interventions for children or adolescents, or both.
Authors' conclusions
Implications for practice.
The evidence from this review suggests that innovative models of service delivery may improve some outcomes relating to epilepsy in children and their parents. However, no single intervention was consistently effective across the full range of reported outcomes, most of which were self‐reported, and given the methodological deficiencies within each study, the results must be interpreted with caution. Furthermore, no single programme was evaluated with different study samples, and no interventions were sufficiently similar to be included in a meta‐analysis, even if outcomes had been consistently reported across studies to permit this. Whilst no programme showed negative impacts on children with epilepsy or their parents, no single programme can be recommended as being more effective than any other. Healthcare professionals and families need to be aware of this when considering any of these strategies for implementation.
Implications for research.
We identified seven distinct self‐management programmes for educating or counselling children with epilepsy and their parents and a new model of care. However, no intervention has been evaluated with different study samples, and the studies show methodological flaws, utilised different outcome measures, and had inconsistent results. As a result, further studies are needed that:
offer an improved quality of study design and reporting;
improve generalisability (e.g. include a full description of the intervention, a process evaluation, and a multicentred assessment of the benefits for more than one population and service provider);
evaluate the effects of interventions for those subgroups most likely to benefit (e.g. children with newly diagnosed epilepsy, children with learning disabilities);
consider objective outcomes using validated measures, preferably with consideration of the clinical meaningfulness as well as statistical significance of results;
consider the cost‐effectiveness of interventions.
To maximise the potential of future studies for generalisability and to ensure study quality, we would recommend that studies be designed as randomised controlled trials. Studies should also ensure that the interventions are adequately defined and described and that investigators take into account contextual factors in the study design. Where socially complex interventions such as these are under study, enough service providers must be included to ensure that individual characteristics do not bias the results.
What's new
| Date | Event | Description |
|---|---|---|
| 14 January 2020 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 14 January 2020 | New search has been performed | Updated searches and modified study eligibility criteria to randomised controlled trials only: five new included studies. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 27 September 2016 | New search has been performed | Searches updated 27 September 2016; one new included study. |
| 27 September 2016 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 9 December 2013 | New search has been performed | Searches updated 9 December 2013; two new studies have been included, and the review has been extensively rewritten by one of the original authors (Peter Bradley) and a new author (Nigel Fleeman). |
| 9 December 2013 | New citation required but conclusions have not changed | Conclusions remain the same. |
Acknowledgements
This review update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service (NHS), or the Department of Health and Social Care.
Cochrane Epilepsy supported the authors in the development of this review update.
The following people conducted the editorial process for this update.
Sign‐off Editor (final editorial decision): Professor Tony Marson
Managing Editor (provided editorial guidance to authors, edited the update, conducted editorial policy checks): Rachael Kelly
Copy Editor (copy‐editing and production): Lisa Winer
We would like to acknowledge Bruce Lindsay for his valuable contribution to the original protocol and previous versions of this review.
We would also like to thank Laura Burfoot, who helped us summarise the results from the studies in the original review.
We would like to acknowledge all the Information Specialists who conducted the searches for the original and updated reviews.
We would also like to thank Luma Haj Kasem who translated the text of one of the included studies from Arabic to English.
Appendices
Appendix 1. Cochrane Register of Studies (CRS Web) search strategy
1. MeSH DESCRIPTOR Program Evaluation Explode All AND INSEGMENT
2. MeSH DESCRIPTOR Delivery of Health Care Explode All AND INSEGMENT
3. MeSH DESCRIPTOR Ambulatory Care Explode All AND INSEGMENT
4. MESH DESCRIPTOR Outcome and Process Assessment, Health Care EXPLODE ALL AND INSEGMENT
5. program* NEAR2 evaluat* AND INSEGMENT
6. epilep* NEAR3 specialist* AND INSEGMENT
7. epilep* NEAR2 nurs* AND INSEGMENT
8. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 AND INSEGMENT
9. #8 AND >14/05/2018:CRSCREATED AND INSEGMENT
10. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
11. epilep*:AB,KW,MC,MH,TI AND CENTRAL:TARGET
12. #10 OR #11 AND CENTRAL:TARGET
13. MeSH DESCRIPTOR Program Evaluation Explode All AND CENTRAL:TARGET
14. MeSH DESCRIPTOR Delivery of Health Care Explode All AND CENTRAL:TARGET
15. MeSH DESCRIPTOR Ambulatory Care Explode All AND CENTRAL:TARGET
16. MESH DESCRIPTOR Outcome and Process Assessment, Health Care EXPLODE ALL AND CENTRAL:TARGET
17. program* NEAR2 evaluat* AND CENTRAL:TARGET
18. #13 OR #14 OR #15 OR #16 OR #17 AND CENTRAL:TARGET
19. #12 AND #18 AND CENTRAL:TARGET
20. (epilep* NEAR3 specialist*):AB,KW,MC,MH,TI AND CENTRAL:TARGET
21. (epilep* NEAR2 nurs*):AB,KW,MC,MH,TI AND CENTRAL:TARGET
22. #19 OR #20 OR #21 AND CENTRAL:TARGET
23. #22 AND >14/05/2018:CRSINCENTRAL AND CENTRAL:TARGET
24. #9 OR #23
Appendix 2. MEDLINE search strategy
Original review
#1 exp EPILEPSY/ #2 epilep$.tw. #3 1 or 2 #4 exp Program Evaluation/ #5 exp "Delivery of Health Care"/ #6 4 or 5 #7 3 and 6 #8 exp Ambulatory Care/ #9 3 and 8 #10 (epilep$ adj4 centre$).ab,ti. #11 (epilep$ adj4 center$).ab,ti. #12 (epilep$ adj3 specialist$).ab,ti. #13 (epilep$ adj2 nurs$).ab,ti. #14 exp "Outcome Assessment (Health Care)"/ #15 14 and 3 #16 7 or 9 or 10 or 11 or 12 or 13 or 15
Latest review update
1. exp Epilepsy/
2. epilep$.tw.
3. 1 or 2
4. exp Program Evaluation/
5. exp "Delivery of Health Care"/
6. exp Ambulatory Care/
7. exp *"Outcome and Process Assessment, Health Care"/
8. (program$ adj2 evaluat$).tw.
9. 4 or 5 or 6 or 7 or 8
10. 3 and 9
11. (epilep$ adj3 nurs$).tw.
12. (epilep$ adj3 specialist$).tw.
13. 11 or 12
14. 10 or 13
15. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
16. clinical trials as topic.sh.
17. trial.ti.
18. 15 or 16 or 17
19. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv or comparative study or evaluation studies or multicenter study or observational study or pragmatic clinical trial or validation studies).pt.
20. ((clinical or comparative or evaluation or multicenter or multi‐center or multicentre or multi‐centre or validation) adj2 (study or studies or trial?)).tw,hw.
21. epidemiologic studies/ or exp case‐control studies/ or exp cohort studies/ or exp controlled before‐after studies/ or exp cross‐sectional studies/ or exp historically controlled study/ or exp interrupted time series analysis/
22. (cohort$ or (case$ adj2 control$) or (case$ adj2 series)).tw,hw.
23. epidemiologic methods/
24. limit 23 to yr=1966‐1989
25. (("before and after" or "before‐and‐after" or case$ or cross?section$ or "cross section$" or "follow up" or "follow‐up" or longitudinal or observation$ or prospective or "record‐linkage" or "record linkage" or retrospective or "time‐series" or "time series") adj2 (analy$ or method or procedure or study or studies or trial?)).tw,hw.
26. ("quasi‐experiment$" or quasiexperiment$ or "quasi experiment$" or "quasi random$" or "quasi‐random$" or quasirandom$ or "quasi control$" or "quasi‐control$" or quasicontrol$).ti,ab,hw.
27. (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab.
28. (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. not (controlled clinical trial or randomized controlled trial).pt.
29. (control year? or experimental year? or control period? or experimental period?).ti,ab.
30. ((strategy or strategies) adj2 (improv$ or education$)).ti,ab.
31. 19 or 20 or 21 or 22 or 24 or 25 or 26 or 27 or 28 or 29 or 30
32. 18 or 31
33. exp animals/ not humans.sh.
34. 32 not 33
35. 34 not case reports.pt.
36. 14 and 35
37. limit 36 to ed=20180514‐20200114
38. 36 not (1$ or 2$).ed.
39. 38 and (2018$ or 2019$ or 2020$).dt.
40. 37 or 39
41. remove duplicates from 40
Appendix 3. Embase search strategy
Original review
#1 exp Epilepsy/ #2 epilep$ #3 1 or 2 #4 exp Ambulatory Care/ #5 exp Institutional Care/ #6 exp Community Care/ #7 exp Health Care Delivery/ #8 *Outcomes Research/ #9 (program$ adj2 evaluat$) #10 4 or 5 or 6 or 7 or 8 or 9 #11 3 and 10 #12 (center$ or centre$) #13 nurs$ #14 specialist$ #15 (epilep$ adj4 (centre$ or center$)) #16 (epilep$ adj3 nurs$) #17 (epilep$ adj3 specialist$) #18 11 or 15 or 16 or 17
Latest review update
#1 exp epilepsy/ #2 epilep$.mp. #3 1 or 2 #4 exp ambulatory care/ #5 exp institutional care/ #6 exp community care/ #7 exp health care delivery/ #8 *outcomes research/ #9 (program$ adj2 evaluat$).mp. #10 4 or 5 or 6 or 7 or 8 or 9 #11 3 and 10 #12 (epilep$ adj4 (centre$ or center$)).mp. #13 (epilep$ adj3 nurs$).mp. #14 (epilep$ adj3 specialist$).mp. #15 12 or 13 or 14 #16 11 and 15 #17 limit 16 to yr="2012 ‐Current"
Appendix 4. PsycINFO search strategy
Original review
This search was carried out in two phases. The first search was carried out in May 2006 using the following strategy: #10 #1 and #9 #9 #2 or #3 or #4 or #5 or #6 or #7 or #8 #8 specialist* #7 nurs* #6 centre* or center* #5 treatment effectiveness evaluation #4 treatment outcome* #3 health care delivery #2 ambulatory care #1 epilep* The second search was carried out in March 2010 using the EBSCO host platform for PsycINFO, and the following strategy: S12 S8 or S9 or S10 or S11 S11 S3 and S7 S10 epilep* N3 specialist* S9 epilep* N3 nurs* S8 epilep* N4 center* or epilep* N4 centre* S7 S4 or S5 or S6 S6 MM “Program Evaluation” S5 MM “Health Care Delivery” S4 MM “Outpatient Treatment” S3 S1 or S2 S2 epilep* S1 MM “Epilepsy” or DE “Epileptic Seizures” or DE “Grand Mal Seizures” or DE “Petit Mal Seizures”
Latest review update
S12 S9 OR S10 OR S11
Publication Year: 2018‐
S11 TI epilep* N3 specialist* OR AB epilep* N3 specialist* OR SU epilep* N3 specialist*
S10 TI epilep* N3 nurs* OR AB epilep* N3 nurs* OR SU epilep* N3 nurs*
S9 S3 AND S8
S8 S4 OR S5 OR S6 OR S7
S7 TI program* N2 evaluat* OR AB program* N2 evaluat* OR SU program* N2 evaluat*
S6 MM "Program Evaluation"
S5 MM "Health Care Delivery"
S4 MM "Outpatient Treatment"
S3 S1 OR S2
S2 epilep*
S1 MM "Epilepsy" OR DE "Epileptic Seizures" OR DE "Grand Mal Seizures" OR DE "Petit Mal Seizures"
Appendix 5. CINAHL search strategy
Original review
This search was carried out in two phases. The first search was carried out in May 2006 using the Ovid platform for CINAHL, and the following strategy: #1 exp EPILEPSY/ #2 epilep$.tw. #3 1 or 2 #4 exp Ambulatory Care/ #5 exp Health Care Delivery/ #6 exp Program Evaluation/ #7 exp “Outcomes (Health Care)”/ #8 (epilep$ adj4 (centre$ or center$)).tw. #9 (epilep$ adj3 nurs$).tw. #10 (epilep$ adj3 specialist$).tw. #11 4 or 5 or 6 or 7 #12 3 and 11 #13 8 or 9 or 10 or 12 The second search was carried out in March 2010 using the EBSCO host platform for CINAHL, and the following strategy: S13 S9 or S10 or S11 or S12 S12 S3 and S8 S11 epilep* N3 specialist* S10 epilep* N3 nurs* S9 epilep* N4 centre* or epilep* N4 center* S8 S4 or S5 or S6 or S7 S7 (MM “Outcomes (Health Care)”) S6 (MM “Program Evaluation”) S5 (MM “Health Care Delivery”) S4 (MM “Ambulatory Care”) S3 S1 or S2 S2 epilep* S1 (MH “Epilepsy+”)
Latest review update
S13 S10 OR S11 OR S12
Published: 20180101‐
S12 epilep* N3 specialist*
S11 epilep* N3 nurs*
S10 S3 AND S9
S9 S4 OR S5 OR S6 OR S7 OR S8
S8 program* N2 evaluat*
S7 (MM "Outcomes (Health Care)")
S6 (MM "Program Evaluation")
S5 (MM "Health Care Delivery")
S4 (MM "Ambulatory Care")
S3 S1 OR S2
S2 epilep*
S1 (MH "Epilepsy+")
Appendix 6. ClinicalTrials.gov search strategy
Latest review update
Care delivery OR Care management | Epilepsy | First posted on or after 05/14/2018
Appendix 7. WHO ICTRP search strategy
Latest review update
Care AND epilepsy NOT NCT*
Appendix 8. Additional detail about the interventions evaluated
Strategies for children and parents
ACINDES
ACINDES was a child‐centred training model based on play techniques designed to be applied in institutions such as schools or community centres for children with moderate to severe conditions (asthma and epilepsy in Tieffenberg 2000). Group activities of the model included games, drawings, stories, videos, and role‐play.
The model was based on the idea of children's autonomy in which children are trained to assume a leading role in the management of their own health. The programme aimed to provide children with self‐management skills and help them to achieve self‐reliance and to use appropriate preventive strategies, thereby improving their social functioning and quality of life. ACINDES also aimed to enable parents to learn to recognise and accept their children's autonomy and become 'facilitators' rather than 'managers' in their child’s self‐management of their condition.
Teachers co‐ordinated group activities which were supervised by programme physicians. These teachers were required to be trained in promoting children's self‐regulation or autonomy as well as certain specific aspects of chronic conditions (asthma, epilepsy) and their clinical management. Groups of children were arranged according to age (6 to 8 years, 9 to 12 years, 13 to 15 years) with no more than 10 children per teacher. Parent groups were co‐ordinated by one or two teachers but were not arranged according to the ages of the children. The child's physician participated in ACINDES by being invited to sessions, at which he or she met separately with children and parents and answered their questions.
The programme consisted of five weekly two‐hour meetings, followed by a reinforcement meeting two to six months later. The aims of the sessions were to enable children and parents to:
learn about the child's condition (asthma, epilepsy) and identify body signals and early warning signs;
recognise the elements of equilibrium (maintaining balance, avoiding imbalance) and identify their own triggers;
understand treatment, therapeutic alternatives, and the usefulness of a direct patient‐physician relationship;
handle specific risk situations (identify risks and learn strategies to handle them, including emergency home treatments);
develop appropriate decision‐making strategies based on the child's own expected values.
Children's Epilepsy Program (CEP)
The CEP was initially developed and piloted with 40 children with epilepsy (aged 7 to 12 years) at the Medical Center of the University of California, Los Angeles (UCLA) (Lewis 1990; Lewis 1991). It consisted of four sessions, each of which lasted 1.5 hours, delivered at weekly intervals. Children and parents were taught separately, meeting to share experiences at the end of each session.
Each session had a specific theme, as follows.
Session 1, understanding body messages: this used electronic toys and cartoon drawings to teach children about seizures and to help them identify seizure‐related emotions and feelings.
Session 2, controlling seizures with medication: this focused on seizure‐related information, using a card‐sorting exercise to separate facts and fictions about seizures. It also taught seizure management and decision‐making skills.
Session 3, telling others in a matter‐of‐fact way: children were encouraged to share personal experiences, especially experiences with friends or peers, whether related to epilepsy or not. Children learned how to tell others about their epilepsy.
Session 4, coping and adapting to balance my life: various exercises were used to develop coping skills, including ways of dealing with bullying or taunting or with negative attitudes.
The parental group of the CEP followed the same basic structure as the child‐focused group, but was based on a Rogerian model of counselling and enabled parents to review the children's sessions as described above. The parental sessions for the intervention group were as follows (it was not reported who delivered these sessions).
Session 1, telling a story: parents introduced themselves to other group members and shared their experiences of their child with epilepsy. A card‐sorting exercise to dispel false perceptions or myths was undertaken.
Session 2, making decisions: a decision‐making process was used to develop decision‐making skills.
Session 3, working as a family system: the group developed their understanding of how a child's epilepsy can impact on family life, and parents discussed their parenting styles.
Session 4, coping and adapting: in this final session parents discussed how to be more open about their child's epilepsy and how to acknowledge the pain and grief that may arise when parenting a child with a chronic condition.
Supporting Treatment Adherence Regimen (STAR)
This family‐tailored antiepileptic drug adherence intervention was developed by two paediatric psychologists specialising in epilepsy (Modi 2016). It consisted of four educational and problem‐solving sessions over two months. In particular, the first session of the intervention focused on addressing deficits in epilepsy knowledge and providing education about the importance of antiepileptic drug adherence. This included:
education on epilepsy treatment and antiepileptic drug adherence;
review of patient’s prescribed treatment regimen;
review of Epilepsy Knowledge Questionnaire.
The goals of the problem‐solving approach were introduced during the second session and were as follows.
Problem definition: family identified an important adherence barrier.
Generating alternative solutions: family taught to generate several creative solutions.
Family decision‐making: family writes down solutions and systematically evaluate.
Implementation of new solution: family selects one solution to implement (action plan).
Evaluation and renegotiation: a detailed solution was written out with specifics regarding when, where, and how the new solution will be attempted, and a behavioural contract was signed by all participants of the problem‐solving session; telephone follow‐ups were conducted one week after the problem‐solving session to assist the family in either fine‐tuning the solution or renegotiating a new solution.
A review of the participant’s prescribed treatment regimen with feedback on antiepileptic drug adherence over the past two weeks was provided at all the sessions. As described in Modi 2013, during sessions 2 to 4, families identified a specific antiepileptic drug adherence barrier, and then a problem‐solving exercise was conducted. Families generated potential solutions to overcome the barrier. Each family member participating in the session was required to rate each solution, and a final solution was agreed on by the family. The written action plan provided a detailed solution with specifics regarding when, where, and how the new solution should be implemented. A behavioural contract outlining the action plan was signed by all participants of the problem‐solving session. Between intervention sessions, the family was instructed to use the plan, and the interventionist contacted the family in between via telephone or email to provide continued guidance and support as the family implemented the action plan. This provided families the opportunity to fine‐tune the solution or renegotiate a new solution if the solution identified in sessions 2 to 3 was not working well. A similar problem‐solving format was used for sessions 3 to 4, with a follow‐up phone contact in between visits.
Modi 2016 notes that even young children were able to participate in problem‐solving sessions, although the extent of the engagement did vary depending on age. For example, the authors note that toddlers and preschool children could provide examples of rewards/reinforcers they liked and whether they liked possible solutions. In contrast, older children were more likely to provide viable solutions that families could choose and would often be involved in helping write these down and in choosing the solution.
New model of clinical nursing care delivery
The intervention evaluated by Jia 2018 was a new model of clinical nursing care delivery with the aim of improving epilepsy control and cure rates and focusing more on areas of care traditionally neglected by conventional care. These areas included the importance of environment, psychology and parental involvement, and continuing education posthospitalisation. In accordance with epilepsy guidelines, children and patients received conventional care which included medication and other associated treatment based on epilepsy type, seizure frequency and duration and severity of accompanying clinical symptoms. The main differences between the intervention and conventional care related to the content of the treatment programme and the extent of the supporting nursing operations. The new model of care delivery was established by following four steps:
establishing the strict standards of quality control for nursing care (by employing four people as head nurses);
designing and standardising the nursing care procedure (and summarising the procedure as concise tables);
adjusting nursing intensity according to epilepsy type (e.g. focusing on triggers for patients with partial seizures, building up confidence and strengthening preventive measures for patients with generalised seizures);
extending nursing care posthospitalisation.
Strategies for adolescents (aged 12 and over) and parents
Psychosocial group intervention for young people with epilepsy (PIE)
The PIE intervention evaluated by Dorris 2017 consisted of six weekly group sessions facilitated by an epilepsy nurse and a clinical psychologist delivered using a mixture of facilitator‐led didactic psycho‐education along with open group discussion, paired work, role‐plays, and educational videos/audio clips.
Each week a particular theme was focused on in each two‐hour session. The first three sessions of the PIE intervention focused on sharing experiences of having epilepsy, increasing epilepsy knowledge, and improving self‐management of the condition. The last three sessions focused on strategies such as problem‐solving and techniques based on cognitive behavioural therapy and mindfulness in order to increase resilience and develop coping strategies for anxiety or low mood.
During the sessions, caregivers were invited to wait in a separate room and provided with work sheets for the corresponding group session. Participants were given homework tasks most weeks and sent reminder appointment letters in between group sessions.
Strategies for children, adolescents (aged 12 and over), and their parents
Modular Education Program
The Modular Education Program for Children with Epilepsy and Their Parents evaluated by Gürhopur 2018 was developed by the researchers and consisted of eight modules, four for parents of children with epilepsy and four for children with epilepsy.
The following modules were provided for children.
Module 1: Knowledge about Epilepsy
Module 2: Epilepsy and I
Module 3: Seizure Management
Module 4: Epilepsy and Social Life
The following modules were provided for parents.
Module 1: Knowledge about Epilepsy
Module 2: Epilepsy and My Child
Module 3: Seizure Management
Module 4: Epilepsy and Social Life
These sessions consisted of brainstorming, discussion, role‐play, drawing, videos, slides, question‐and‐answer sessions, and the guide to living with epilepsy for children and parents. Each session was 2 to 3 hours long; the total educational programme took 16 hours to deliver. The aims of the sessions were to increase the child’s knowledge about epilepsy, increase the child’s self‐efficacy regarding epilepsy, improve the child’s quality of life, and decrease parental anxiety.
Video animation and clinician advice
Saengow 2018 evaluated the effectiveness of an 8.52‐minute video animation which was created based on a Thai epilepsy guideline. The video animation included information about diagnosis of epilepsy, aetiology of epilepsy, treatment of epilepsy, first aid seizure care, prognosis of epilepsy, and safe activity for epilepsy. The video was supervised by paediatric neurologists who also provided counselling.
Strategies for adolescents (aged 12 and over)
Implementation of a self‐care education programme via SMS text messaging
Kazemi Majd 2017 evaluated an intervention which included the implementation of a self‐care education programme via Short Message Service (SMS). The text messages were sent to participants in the SMS group in a concise manner and included content on the following lifestyle and treatment topics: diet information, avoidance of stimuli and stress, exercise and activity sleep and rest, taking the exact dose of drugs, knowing the exact time of taking drugs, side effects of drugs, not stopping drugs, not taking other drugs without informing the treating physician, the need to continue treatment despite stopping medication, and observance of safety. A total of four educational messages were sent every week, and 48 messages were sent overall over three months.
Data and analyses
Comparison 1. Seizure frequency and severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Number of seizures at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Seizure severity (frequency and duration of seizures) improved at 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Seizure control rate at 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Seizure cure rate at 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Appropriateness and volume of medication prescribed.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Drug adherence improved using the 8‐item Morisky Medication Adherence Scale (MMAS‐8) at 3 months | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Mean adherence to medication using the 8‐item Morisky Medication Adherence Scale (MMAS‐8) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 Antiepileptic drug adherence measured using the Medication Event Monitoring System (MEMS) 6 Cap at 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Knowledge of information and advice received from professionals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Medical and social knowledge (Epilepsy Knowledge Profile‐General (EKP‐G)) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Knowledge of epilepsy (Epilepsy Self Knowledge Test for Children (EKTC)) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Parents’ disease and treatment knowledge (Pediatric Epilepsy Medication Self‐Management Questionnaire (PEMSQ) ‐ Epilepsy and Treatment Knowledge and Expectations) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4 Parents’ knowledge (Epilepsy Knowledge Questionnaire (EKQ)) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5 Epilepsy Knowledge Scale for Parents (EKSP) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Health and quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mean Seizure Self‐Efficacy Scale for Children (SSES‐C) score at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Self‐perception of epilepsy on quality of life (Pediatric Quality of Life Inventory (PedsQL)) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3 Quality of Life in Epilepsy Inventory (QOLIE‐48) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4 Mean self‐efficacy (Epilepsy Self‐Efficacy Scale (ESES)) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.5 Social problem‐solving (Social Problem‐Solving Inventory‐Revised (SPSI‐R)) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.6 Self‐perception of physical, emotional, social and school functioning (Glasgow Epilepsy Outcome Scale for Young Persons (GEOS‐YP)) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7 Perceived competencies for control: Harter's adjusted 5‐month scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7.1 Scholastic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7.2 Social | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7.3 Athletic | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7.4 Appearance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7.5 Behaviour | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7.6 Self‐esteem | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.8 Parents’ Anxiety About Seizures Scale (PAASS) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9 Parents’ self‐management (Pediatric Epilepsy Medication Self‐Management Questionnaire (PEMSQ) ‐ Total) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10 Parent Response to Child Illness (PRCI) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10.1 PRCI: Epilepsy management | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10.2 PRCI: Child support | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10.3 PRCI: Family life and leisure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10.4 PRCI: Child autonomy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10.5 PRCI: Child discipline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.11 Taylor Manifest Anxiety Scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.11.1 Both parents | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.11.2 Mothers | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.11.3 Fathers | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Objective measures of general health status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Emergency visits at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2 Regular medical visits at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Objective measures of social or psychological functioning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mean number of absences per 100 school days | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dorris 2017.
| Study characteristics | ||
| Methods | Study type: 2‐arm randomised controlled trial Recruitment period: 4‐month period from April to July 2015 Duration of study: 3 months |
|
| Participants | Population type: adolescents (aged 12 to 17 years) with a diagnosis of epilepsy (controlled or refractory) of at least 6 months who attended mainstream schooling Setting: 7 paediatric neurosciences centres across the UK Number enrolled: a total of 85 participants were screened for eligibility (2 participants withdrew before randomisation). All adolescents, n = 83 (experimental, n = 43; control, n = 40) Number of participants with baseline characteristics reported: all adolescents, n = 83 (experimental, n = 43; control, n = 40) Number evaluated: all adolescents, n = 83 (experimental, n = 43; control, n = 40) Proportion of males at baseline: 33/83 (39.8%) (experimental 14/40 (35.0%); control 19/43 (44.2%)) Age at baseline, mean (SD) years: experimental 14.4 (1.5), range 12 to 17; control 14.3 (1.4), range 12 to 17 Disease duration at baseline, mean (SD) years: experimental 7.4 (3.9), range 2 to 16; control 5.6 (3.5), range 1 to 16 Seizure frequency at baseline: not reported Type of seizures at baseline: most adolescents had:
Comorbidities at baseline: not reported, but adolescents were excluded if they had a formal diagnosis of learning disability, attended a school for children with special educational needs, reported suicidal ideation and/or scored ≥ 40 on the Beck Depression Inventory for Youth (BDI‐Y) and Beck Anxiety Inventory for Youth (BAI‐Y) |
|
| Interventions | Experimental: manual‐based brief psychosocial group intervention for young people with epilepsy (PIE), which aimed to improve epilepsy knowledge, self‐management skills, mood and quality of life Control: waiting list control |
|
| Outcomes | Knowledge and self‐management (functioning) was measured using the Epilepsy Knowledge Profile‐General (EKP‐G), Seizure Self Efficacy Scale for Children (SSEC‐C), and Brief ‐ Illness Representations Questionnaire (B‐IPQ). Quality of life was measured using the Pediatric Quality of Life Inventory (PedsQL) version 4.0, Glasgow Epilepsy Outcome Scale for Young Persons (GEOS‐YP), and a bespoke questionnaire. This bespoke questionnaire was also used to measure self‐reported functioning and seizure control. Outcomes relating to intervention feasibility were also measured. All outcomes were measured at baseline, immediately following completion of the intervention, and at 3‐month follow‐up. |
|
| Notes | Investigator‐initiated study supported by UCB Pharma and Yorkhill Children's Foundation Published data, no unpublished data sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to the intervention or control conditions using a stratified (block) randomisation protocol based on age, gender, and type of mental health support. |
| Allocation concealment (selection bias) | Low risk | Participants were assigned to either the experimental or control group using an Excel random number generator. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants or personnel delivering the intervention/control as the control group was a waiting list control. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The second author inputting the data remained blinded until study completion. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods section were reported in the Results section. |
| Other bias | Low risk | No other biases identified. |
Gürhopur 2018.
| Study characteristics | ||
| Methods | Study type: 2‐arm randomised controlled trial Recruitment period: January to June 2014 Duration of study: 8 months data collection (1 and 3 months follow‐up) |
|
| Participants | Population type: literate children aged 7 to 18 years with at least a 6‐month history of epilepsy and no mental deficiencies who consented to the study and their parents. The parents were also literate. Setting: Akdeniz University Hospital Paediatric Neurology Polyclinic in Antalya, Turkey Number enrolled: 184 individuals; children, n = 92; parents, n = 92 Number of participants with baseline characteristics reported: children, n = 92; parents, n = 92 Number evaluated: children, n = 92; parents, n = 92 Proportion of males at baseline: children, experimental 19/42 (45.3%); control 30/50 (60%) Age at baseline, mean (SD) years: experimental 11.54 (3.15); control 12.54 (3.33) Disease duration at baseline:
Seizure frequency at baseline:
Type of seizures at baseline:
Comorbidities at baseline: not reported Level of education at baseline: not reported |
|
| Interventions | Experimental: Modular Education Program for Children with Epilepsy and Their Parents. This was an 8‐module programme consisting of 4 modules for children and 4 modules for their parents; this was followed by an immediate and 1‐ and 3‐month follow‐up. Control: no training was given to the control groups, but they participated in a 1‐ and 3‐month follow‐up. |
|
| Outcomes | Epilepsy‐specific knowledge of children and parents, self‐efficacy regarding seizures for children, quality of life of children with epilepsy, parental anxiety about seizures | |
| Notes | Research supported by the Akdeniz University Scientific Research Projects Management Unit Published data, no unpublished data sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbers from 1 to 100 were put in envelopes, and the participants picked an envelope. Children and their parents who picked a number from 1 to 50 were in the experimental group. Children and their parents who picked a number from 51 to 100 were in the control group. |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes were used for allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor assessors appear to have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors do not appear to have been blinded, and as information was collected through questionnaires, this may have introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed all the questionnaires immediately after the module and at the 1‐ and 3‐month follow‐up. However, the authors did not do a 6‐month follow‐up as was initially suggested in their hypothesis, as there was a significant decrease in the number of cases. Furthermore, the number of participants who completed the questionnaires at 3 months is not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods section were reported in the Results section. |
| Other bias | Low risk | No other potential sources of risk of bias identified. |
Jia 2018.
| Study characteristics | ||
| Methods | Study type: 2‐arm randomised controlled trial Recruitment period: between January 2012 and January 2016 Setting: Xuzhou Children’s Hospital Duration of study: 12 months |
|
| Participants | Population type: children diagnosed with epilepsy for the first time were selected Number enrolled: children, N = 120 (experimental, N = 60; control, N = 60); no details given regarding the number of parents Number of participants with baseline characteristics reported: no details given, but the text implies that they were measured for all participants Number evaluated: n = 120 in total; n = 60 control, n = 60 intervention Proportion of males at baseline: children, experimental 35/60 (58.3%); control 32/60 (53.3%) Age at baseline, mean (SD) years: experimental 6.5 (2.7), range 2.5 to 13; control 6.6 (2.4), range 3 to 12 Disease duration at baseline: newly diagnosed Seizure frequency at baseline: not reported Type of seizures at baseline: partial seizures (experimental 31/60 (51.7%); control 33/60 (55.0%)); generalised seizures (experimental 29/60 (48.3%); control 27/60 (45.0%)) Comorbidities at baseline: children possessing brain developmental abnormalities, dementia, and/or genetic metabolic diseases were excluded. No others reported. Experimental: in addition to conventional care, nursing process which followed four steps: (1) establishing the strict standards of quality control for nursing care; (2) designing and standardising the nursing care procedure and summarising as concise tables; (3) adjusting nursing intensity according to epilepsy type; (4) extending nursing care posthospitalisation Control: conventional care included monitoring of vital signs, making emergency preparations for epileptic seizures, imparting correct knowledge of epilepsy prevention and first aid to children and parents, giving diet and psychological guidance, conducting medication supervision, and providing support for various treatments |
|
| Interventions | Experimental: conventional care with additional emphasis on the importance of environment, psychology and parental involvement, and continuing education posthospitalisation by 4 steps: (1) establishing the strict standards of quality control for nursing care; (2) designing and standardising the nursing care procedure and summarising as concise tables; (3) adjusting nursing intensity according to epilepsy type; (4) extending nursing care posthospitalisation Control: conventional care included monitoring of vital signs, making emergency preparations for epileptic seizures, imparting correct knowledge of epilepsy prevention and first aid to children and parents, giving diet and psychological guidance, conducting medication supervision, and providing support for various treatments |
|
| Outcomes | Epilepsy control and cure rates, course of treatment and complications, nursing satisfaction and patient compliance All outcomes were measured at baseline and at 12‐month follow‐up. |
|
| Notes | Source of study funding not reported. Published data, no unpublished data sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were evenly divided into control and observation groups by a computerised randomised method. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None of the participants nor personnel appeared to have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The assessors do not appear to have been blinded. The subjective nature of the outcomes measured (all by self‐reported questionnaire) means that this may have introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant dropout rates were not reported in the trial. However, for the outcomes of seizure frequency and comparison of course of treatment and complications, data are reported as a proportion of all participants enrolled into the trial. It is not clear if any intention‐to‐treat analysis or sensitivity analysis was used to account for participant dropout for other outcomes during the analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods section were reported in the Results section. |
| Other bias | Low risk | No other potential sources of risk of bias identified. |
Kazemi Majd 2017.
| Study characteristics | ||
| Methods | Study type: 2‐arm randomised controlled trial Recruitment period: July 2015 to October 2015 Duration of study: 3 months |
|
| Participants | Population type: adolescents aged 12 to 18 years with a definitive diagnosis of epilepsy by a doctor being treated with at least 1 anticonvulsant drug for at least 6 months (based on medical records) Setting: adolescents registered as members of the Iranian Association for Epilepsy Number enrolled: adolescents n = 64 (before randomisation, 60 adolescents were randomised to the experimental or control group, as 4 adolescents refused to participate) Number of participants with baseline characteristics reported: adolescents n = 60 (experimental n = 60, control n = 60) Proportion of males at baseline: 32/50 (53.3%); experimental 18/30 (60%), control 14/30 (43.3%) Age at baseline, mean (SD) years: experimental 15.83 (1.98), control 14.97 (2.20) Disease duration at baseline, mean (SD) years: experimental 10.73 (4.94), control 9.87 (5.19) Seizure frequency at baseline: not reported Type seizures at baseline: not reported Comorbidities at baseline: adolescents with epilepsy not suffering from other physical and mental illnesses |
|
| Interventions | Experimental: implementation of a self‐care education programme delivered via SMS (4 educational SMS were sent every week, 48 SMS over 3 months) Control: routine education normally provided by the Iranian Epilepsy Association |
|
| Outcomes | Self‐efficacy as measured by the Epilepsy Self‐Efficacy Scale‐33 Items questionnaire and drug adherence (using MMAS‐8). Questionnaires were completed at baseline and after 3 months. | |
| Notes | Published data, no unpublished data sought According to the entry for this trial in the Iranian Registry of ClinicalTrials (IRCT2015060122514N17), the trial was intended to be a 3‐arm trial with a second experimental group for whom the educational intervention was to be delivered via pamphlets. However, no information regarding this second experimental group is reported in the published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned a number from 1 to 6 and drawn at random to the experimental or control group. |
| Allocation concealment (selection bias) | Low risk | The unit of allocation was by patient number drawn randomly. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None of the participants nor personnel appeared to have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The assessors do not appear to have been blinded. The subjective nature of the outcomes measured (all by self‐reported questionnaire) means that this may have introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It is reported that only 4 participants did not complete questionnaires, and so were removed from the analysis. |
| Selective reporting (reporting bias) | Low risk | The results from the 2 outcomes are reported. |
| Other bias | Unclear risk | This trial was registered (IRCT2015060122514N1) as a 3‐arm trial. No reference is made to the third arm in the published paper. |
Lewis 1990.
| Study characteristics | ||
| Methods | Study type: 2‐arm randomised controlled trial Recruitment period: not reported Duration of study: 5 months follow‐up |
|
| Participants | Population type: children (aged 7 to 14 years) with epilepsy Setting: clinic‐based educational programme in Santiago, Chile Number enrolled: n = 252 (experimental, n = 126; control, n = 126) Number of participants with baseline characteristics reported: n = 234 (experimental, n = 123; control, n = 113) Number evaluated: n = 234 (experimental, n = 123; control, n = 113) for questionnaires; n = 234 (experimental, n = 106; control, n = 92) for Hartinger’s Self‐Competency Scale Proportion of males at baseline: experimental 54/123 (48.0%); control 54/123 (52.0%) Age at baseline, mean (SD) years: children, experimental 10.1 (2.2); control 9.9 (2.1) Disease duration at baseline: not reported Seizure frequency at baseline: not reported Type of seizures at baseline: where determined, 59/132 (44.7%) of children had generalised tonic‐clonic seizure disorders (calculated from following: medical records from physicians providing care could be obtained for only 56% of children, of whom 45% had generalised tonic‐clonic seizure disorders) Comorbidities at baseline: where information was available from school records (65% of experimental group and 77% of control group), “slightly more than half” of all children had behavioural problems |
|
| Interventions | Experimental group: Children's Epilepsy Program, a counselling model based on Rogerian principles (child‐centred family‐focused (active learning) programme) Control: traditional educational format (passive learning) consisting of 3 x 2‐hour sessions conducted by a physician who gave traditional lectures followed by question‐and‐answer sessions to present the same information related to epilepsy that the experimental group received |
|
| Outcomes | Children's knowledge about seizures, self‐perceived of self competency, behaviour All outcomes were measured at baseline and at 5‐month follow‐up. |
|
| Notes | Evaluation of same intervention as Lewis 1991, but focuses on the children’s perspective Supported by PHS Grant N01‐NS‐0‐2332. Funds for the data analysis were made available by a grant from the William T. Grant Foundation, New York, NY. Published data, no unpublished data sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From a master list of children aged 7 to 14 years, groups of 20 families were selected and assigned numbers and randomly selected for the control and intervention groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is unclear if the trained interviewers were blinded. The subjective nature of the outcomes measured (by self‐reported questionnaire) means that this may have introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 78.6% of children in the intervention group and 52% of children in the control group attended all the required sessions. However, pre‐ and post‐test data were available for almost 95% of children. No intention‐to‐treat analysis was explicitly reported; however, data were reported for each participant in the final analysis for some, but not all, outcomes despite attendance at the educational programme being incomplete. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods section were reported in the Results section. |
| Other bias | Low risk | No other potential sources of risk of bias identified. |
Lewis 1991.
| Study characteristics | ||
| Methods | Study type: 2‐arm randomised controlled trial Recruitment period: not reported Duration of study: 5 months follow‐up |
|
| Participants | Population type: parents of children with epilepsy aged 7 to 14 years Setting: clinic‐based educational programme in Santiago, Chile Number enrolled: not reported Number of participants with baseline characteristics reported: n = 365 (experimental, n = 185; control, n = 180) Number evaluated: n = 365; fathers, n = 136; mothers, n = 229 Proportion of males (fathers) at baseline: experimental 65/185 (35.1%); control 71/180 (39.4%) (see Lewis 1990 for proportion of boys at baseline) Age at baseline, mean (SD) years: not reported for parents (see Lewis 1990 for age of children at baseline) Disease duration at baseline: not applicable, as this study reports intervention from the perspective of parents (not reported in accompanying study of children by Lewis 1990) Seizure frequency at baseline: not applicable, as this study reports intervention from the perspective of parents (not reported in accompanying study of children by Lewis 1990) Type of seizures at baseline: where determined, 59/132 (44.7%) of children had generalised tonic‐clonic seizure disorders (calculated from following: medical records from physicians providing care could be obtained for only 56% of the children, of whom 45% had generalised tonic‐clonic seizure disorders) Comorbidities at baseline: not applicable, as this study reports intervention from the perspective of parents (see Lewis 1990 for comorbidities reported for children at baseline) |
|
| Interventions | Experimental group: Children's Epilepsy Program, a counselling model based on Rogerian principles (child‐centred family‐focused active learning programme) Control: traditional educational format (passive learning) consisting of 3 x 2‐hour sessions conducted by a physician who gave traditional lectures followed by question‐and‐answer sessions to present the same information related to epilepsy that the experimental group received |
|
| Outcomes | Parent's knowledge; parent's anxiety (Taylor Manifest Anxiety Scale); perceptions of the programme's efficacy including parental reactions to child's seizures All outcomes measured at baseline and at 6‐month follow‐up. |
|
| Notes | Evaluation of same intervention as Lewis 1990, but focuses on the parents' perspective Supported by PHS Grant N01‐NS‐0‐2332. Funds for the data analysis were made available by a grant from the William T. Grant Foundation, New York, NY. Published data, no unpublished data sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From a master list of children aged 7 to 14 years, groups of 20 families were selected and assigned numbers and randomly selected for the control and intervention groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinicians were not blinded. The subjective nature of the outcomes measured (by self‐reported questionnaire) means that this may have introduced bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is unclear if the trained interviewers were blinded. The subjective nature of the outcomes measured (by self‐reported questionnaire) means that this may have introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 73.2% of mothers and 59% of fathers attended all 4 sessions in the intervention group, and 62% of mothers and 49% of fathers attended all 3 sessions in the control group. However, pre‐ and post‐test data were available for almost all parents. No intention‐to‐treat analysis was explicitly reported; however, data were reported for each participant in the final analysis for some, but not all, outcomes despite attendance at the educational programme being incomplete. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods section were reported in the Results section. |
| Other bias | Low risk | No other potential sources of risk of bias identified. |
Modi 2016.
| Study characteristics | ||
| Methods | Study type: 3‐arm controlled trial (participants were only randomised to 2 arms – see notes below for more detail) Recruitment period: January 2011 to October 2012 Duration of study: 3 months follow‐up |
|
| Participants | Population type: children aged 2 to 12 years and their caregivers Setting: new‐onset seizure clinic in a children’s hospital in the Midwestern USA Number enrolled: n = 50 (non‐randomised maintenance, n = 22; randomised experimental group, n = 11; randomised control, n = 12; withdrew prior to randomisation, n = 5) Number of participants with baseline characteristics reported: total, n = 50; non‐randomised maintenance, n = 22; randomised experimental group, n = 11; randomised control, n = 12; baseline characteristics not reported separately for either 5 participants who withdrew prior to randomisation (n = 5) or aggregated for all the participants in the 3 treatment groups (n = 45) Number evaluated: n = 40 (non‐randomised maintenance, n = 20; randomised experimental group, n = 11; randomised control, n = 8; lost to follow‐up, n = 3) Proportion of males at baseline: children in total sample 33/50 (66.0%); non‐randomised maintenance, n = 16/22 (72.7%); randomised experimental group, n = 6/11 (54.5%); randomised control, n = 9/12 (75.0%) Age at baseline, mean (SD) years: children 7.6 (3.0%); non‐randomised maintenance, n = 7.4 (2.9); randomised experimental group, n = 6.4 (3.5); randomised control, n = 8.4 (3.1) Disease duration at baseline: not reported Seizure frequency at baseline: all children had new‐onset epilepsy. 18/50 (36.0%) of children had had a seizure 3 months prior to assessment; non‐randomised maintenance, n = 13/22 (59.1%); randomised experimental group, n = 2/11 (18.2%); randomised control, n = 2/12 (16.7%) Type of seizures at baseline: not reported Comorbidities at baseline: not reported |
|
| Interventions | Non‐randomised maintenance "high adherence" group: participants with high (≥ 95%) antiepileptic drug adherence were not randomised but received 5 study visits (see Notes below) (n = 22) Randomised experimental group: Supporting Treatment Adherence Regimen (STAR), which included 4 face‐to‐face and 2 telephone problem‐solving sessions over 8 weeks (n = 12) Randomised control: treatment as usual (TAU) (n = 11) |
|
| Outcomes | Seizure activity (absence/presence), seizure type, antiepileptic drug prescription and changes to the regimen over time (medical chart review); daily antiepileptic drug adherence (Medication Event Monitoring Systems Track Cap) and adherence rates calculated from these data; knowledge about the medical and social aspects of epilepsy (Epilepsy Knowledge Questionnaire); medication management by caregivers of children with epilepsy (Pediatric Epilepsy Medication Self‐Management Questionnaire); parental responses and perceptions related to seizures (Parent Response to Child Illness Questionnaire); caregiver feasibility and acceptability of the STAR intervention (study‐specific questionnaire) Data were recorded at baseline and then across 2‐week intervals (coinciding with intervention sessions): 2 to 4 weeks, 4 to 6 weeks, 6 to 8 weeks, 8 to 10 weeks, 10 to 12 weeks, and 3‐month follow‐up postintervention. |
|
| Notes | At study entry, participants were assessed for antiepileptic drug adherence. After a 30‐day monitoring period, participants were either followed due to good adherence (≥ 95%; high adherence group) or randomised into 1 of 2 groups (< 95%; STAR intervention or TAU). In addition to the 30‐day monitoring period, participants with good adherence had 2 additional opportunities to be randomised over the next 6 months. Similar to their 1‐month run‐in period, if adherence fell below 95% at the 3‐ or 6‐month assessment period, the family was randomised to TAU or STAR. Research funded by a grant from the US National Institutes of Health (NIH) awarded to the first author. Published data, no unpublished data sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used, and participants stratified according to their treatment compliance in the preceding months. |
| Allocation concealment (selection bias) | Unclear risk | The allocation process was not described. It is noted that the randomisation list was generated by the first author and held by a research assistant independent of the study to reduce bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the participants nor clinicians appeared to have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The assessors do not appear to have been blinded. However, some of the outcomes reported were derived from medical chart reviews, and are therefore less likely to be prone to bias from a lack of blinding; other outcomes measured via questionnaire were prone to bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study withdrawal and loss to follow‐up occurred in 3/11 (27.2%) of instances in the STAR group. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods section were reported in the Results section, although some outcomes were only reported in detail in a supplementary appendix. |
| Other bias | Low risk | No other potential sources of risk of bias identified. |
Saengow 2018.
| Study characteristics | ||
| Methods | Study type: 2‐arm randomised controlled trial Recruitment period: 1 June 2016 to 30 September 2016 Duration of study: 3 months data collection |
|
| Participants | Population type: children and adolescents aged between 1 month and 15 years who were diagnosed with epilepsy according to ILAE 2014, who have visited routine service paediatric neurology clinic (every Tuesday and Thursday) Setting: service paediatric neurology clinic at Maharat Nakhon Ratchasima Hospital, Thailand Number enrolled: 214 epilepsy patients Number of participants with baseline characteristics reported: 214 participants (experimental 126; control 88) Number evaluated: 214 participants (experimental 126; control 88) Proportion of males at baseline: 125/214 (58.4%) (experimental 73/126 (57.9%); control 52/88 (59.1%)) Age at baseline, mean (SD) age, years: experimental 7.6 (4.5); control 7.6 (4.8) Disease duration at baseline: not reported Seizure frequency at baseline:
Type of seizures at baseline (aetiology):
Comorbidities at baseline: not reported Level of education at baseline: not reported |
|
| Interventions | Experimental: clinician advice and an 8.52‐minute video animation, including information on 6 areas from Thai guideline (diagnosis of epilepsy, aetiology of epilepsy, treatment of epilepsy, first aid seizure care, prognosis of epilepsy, and safe activity for epilepsy) Control: clinician advice |
|
| Outcomes | Epilepsy‐specific knowledge of children and parents, drug adherence (using MMAS‐8), severity of seizure (frequency and duration of seizures). The questionnaires were applied to caregivers in the case of epilepsy patients aged less than 9 years, before intervention and at 3‐month follow‐up. | |
| Notes | Source of study funding not reported. Published data, no unpublished data sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants in the 2 groups were recruited on different days (experimental group every Tuesday, control group every Thursday). |
| Allocation concealment (selection bias) | High risk | Participants in the 2 groups were recruited on different days (experimental group every Tuesday, control group every Thursday). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither participants nor assessors appear to have been blinded. To prevent contamination, participants in the 2 groups were recruited on different days (experimental group every Tuesday, control group every Thursday). However, as the same centre was used for recruiting participants, the risk of bias is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors do not appear to have been blinded, and as information was collected through questionnaires, this may have introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed all the questionnaires before the intervention and at 3‐month follow‐up. The 10‐item questionnaire to evaluate the knowledge of epilepsy was applied before intervention, immediately after, and at 3‐month follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in the Methods section were reported in the Results section. |
| Other bias | Low risk | No other potential sources of risk of bias identified. |
Tieffenberg 2000.
| Study characteristics | ||
| Methods | Study type: 2‐arm randomised controlled trial Recruitment period: not reported Duration of study: 12 months follow‐up |
|
| Participants | Population type: Spanish‐speaking children (aged 6 to 15 years) with asthma or epilepsy and their parents; only children whose condition was considered moderate or severe were included (those requiring medication the whole year, one or more times per month, or permanently) Setting: community‐based programme in Buenos Aires, Argentina Number enrolled: children with asthma and their parents, n = 188; children with epilepsy and their parents, n = 167 Number of participants with baseline characteristics reported: baseline characteristics not reported Number evaluated: children with asthma and their parents, n = 188; children with epilepsy and their parents, n = 167 (experimental, n = 103; control, n = 64) Proportion of males at baseline: not reported Age at baseline, mean (SD) years: not reported Disease duration at baseline: not reported Seizure frequency at baseline: of participants with epilepsy, the mean (SD) number of seizures within the last 12 months was 0.80 (1.46) in the experimental group and 0.49 (1.15) in the control group Type of seizures at baseline: not reported Comorbidities at baseline: not reported |
|
| Interventions | Experimental group: ACINDES, a child‐centred training programme Control: routine care without additional training received by experimental arm participants |
|
| Outcomes | Knowledge, beliefs, attitudes and behaviours of the children; parental knowledge, fear of child death; clinical outcomes including seizure frequency and clinic attendance Children and parents were interviewed before the programme and 6 and 12 months after the programme. |
|
| Notes | Supported in part by grant 86109186 from the W. T. Grant Foundation Published data, no unpublished data sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The details of randomisation including the 'clustering techniques' used were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details regarding how participants were allocated were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel appeared to have been blinded. There was the possibility of contamination in both groups, as randomisation was not conducted by an independent centre. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Neither participants nor personnel appeared to have been blinded. However, some of the outcomes reported were derived from medical and school records and were therefore less likely to be prone to bias due to lack of blinding; other outcomes measured via questionnaire were prone to bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For children with epilepsy, 13.6% of children in the intervention group and 29.7% in the control group were lost to follow‐up. No details were provided regarding families lost to follow‐up, but reasons for non‐attendance were provided. No details were given regarding how dropouts were accounted for. No intention‐to‐treat analysis was reported. |
| Selective reporting (reporting bias) | Low risk | All broad outcome types detailed in the Methods section were reported in the Results section. However, it is not clear if all the outcomes relating to knowledge, beliefs, attitudes, and behaviours were reported. For example, in the group of children with asthma, results from an outcome relating to communication between physicians and children with asthma is reported. It is unclear if a similar outcome was measured for the epilepsy group. |
| Other bias | Unclear risk | No other potential sources of risk of bias identified. |
ILAE: International League Against Epilepsy MMAS‐8: 8‐Item Morisky Medication Adherence Scale SD: standard deviation SMS: Short Message Service
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Austin 2002 | Pre‐ and post‐test feasibility study lacking a control group |
| Bahrani 2017 | Included participants aged > 10 years, mostly adults; mean (SD) age: 24.78 (11.36) years in intervention group and 24.22 (10.89) years in control group |
| Dash 2015 | Included participants aged ≥ 15 years, mostly adults; mean (SD) age: 34.18 (10.65) years in intervention group and 35.81 (11.61) years in control group |
| Glueckauf 2002 | Randomisation failed. |
| Hallfahrt 2007 | Duplicate of included study containing no new data (Jantzen 2009) |
| Ibinda 2014 | Included participants of all ages, mostly adults; mean (SD) age: 19.2 (17.4) years in intervention group and 19.5 (15.6) years in control group |
| Jantzen 2009 | Not an RCT |
| Li 2013 | Included participants aged ≥ 13 years, mostly adults; mean (SD) age: 36.6 (38) years in intervention group and 39.4 (40) years in control group |
| Mar 2005 | Audit of documentation and data recording |
| Modi 2013 | Pilot feasibility study, small sample size (N = 8), and intervention has now been fully evaluated by Modi 2016, which is included in the current review |
| Pfäfflin 2012 | Not an RCT |
| Price 2004 | Before‐and‐after (pre‐ and post‐test) design. Study measured knowledge and skills of educators related to seizure management. No participant‐related outcomes |
| Shore 2008 | No control group |
| Snead 2004 | Before‐and‐after (pre‐ and post‐test) design. No control group |
RCT: randomised controlled trial SD: standard deviation
Differences between protocol and review
PB was lead author on the protocol. Review methodology was unchanged from that included in the protocol.
For the current update, we have only included randomised controlled trials, now excluding the previously included before‐and‐after studies.
We stated in the protocol that we would search Embase. In the original review (and previous updates), we did search Embase. For the current update, we have not searched Embase.
Contributions of authors
PB and Bruce Lindsay (BL) developed the protocol for this review and developed the final systematic review.
NF, PB, AS, MP, and BL independently reviewed papers for inclusion using Cochrane Effective Practice and Organisation of Care Group criteria.
NF, PB, AS, and MP conducted the data extraction and quality assessment of the included papers.
PB led the analysis of the included papers.
BL wrote the original review, and NF wrote the updated reviews.
PB commented on and contributed to the write‐up of the original review, and AS and MP contributed to the write‐up of the updated reviews, including producing the summary of findings table.
Sources of support
Internal sources
No sources of support provided
External sources
National Institute of Health Research (NIHR), UK
Declarations of interest
NF: none known.
PB: none known.
MP: none known.
AS: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dorris 2017 {published and unpublished data}
- Dorris L, Broome H, Wilson M, Grant C, Young D, Baker G, et al.A randomized controlled trial of a manual-based psychosocial group intervention for young people with epilepsy [PIE]. Epilepsy & Behavior 2017;72:89-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02349529.An exploratory RCT of a psychosocial group intervention for epilepsy [An exploratory randomised controlled trial of a manualised psychosocial group intervention for young people with epilepsy (PIE)]. clinicaltrials.gov/ct2/show/NCT02349529 (first received 29 January 2015).
Gürhopur 2018 {published data only}
- Gürhopur FDT, Dalgiç AL.The effect of a modular education program for children with epilepsy and their parents on disease management. Epilepsy & Behavior 2018;78:210-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jia 2018 {published data only}
- Jia LB, Tang L, Cai Y.Comparison of nursing methods of pediatric epilepsy. Journal of Biological Regulators & Homeostatic Agents 2018;32(4):869-74. [PMID: ] [PubMed] [Google Scholar]
Kazemi Majd 2017 {published and unpublished data}
- IRCT2015060122514N1.The effect of self-care education based on short message service in adolescents with epilepsy [Comparison of self-care education based on short message service and pamphlet on self-efficacy and adherence to the medication regimen in adolescents with epilepsy]. en.irct.ir/trial/19406 (first received 27 October 2015).
- Kazemi Majd R, Hosseini M, Safi MH, Norouzi K, Hosseinzadeh S.The effect of self-care education based on short message service on self-efficacy and adherence to the medication regimen in adolescents with epilepsy referred to Iran Epilepsy Association of in 2016. Journal of Nursing Education 2017;6(4):48-55. [URL: www.sid.ir/en/journal/ViewPaper.aspx?ID=600496] [Google Scholar]
Lewis 1990 {published data only}
- Lewis MA, Salas I, la Sota A, Chiofalo N, Leake B.Randomized trial of a program to enhance the competencies of children with epilepsy. Epilepsia 1990;31(1):101-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lewis 1991 {published data only}
- Lewis MA, Hatton CL, Salas I, Leake B, Chiofalo N.Impact of the Children's Epilepsy Program on parents. Epilepsia 1991;32(3):365-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Modi 2016 {published data only}
- Modi AC, Guilfoyle SM, Mann KA, Rausch JR.A pilot randomized controlled clinical trial to improve antiepileptic drug adherence in young children with epilepsy. Epilepsia 2016;57(3):e69-75. [DOI: 10.1111/epi.13289] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saengow 2018 {published data only}
- Saengow VE, Chancharoenchai P, Saartying W, Pimpa W, Chotichanon N, Lewsirirat T, et al.Epilepsy video animation: Impact on knowledge and drug adherence in pediatric epilepsy patients and caregivers. Clinical Neurology and Neurosurgery 2018;172:59-61. [DOI: 10.1016/j.clineuro.2018.06.031] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tieffenberg 2000 {published data only}
- Tieffenberg JA, Wood EI, Alonso A, Tossutti MS, Vicente MF.A randomized field trial of ACINDES: a child-centered training model for children with chronic illnesses (asthma and epilepsy). Journal of Urban Health: Bulletin of the New York Academy of Medicine 2000;77(2):280-97. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Austin 2002 {published data only}
- Austin JK, McNelis AM, Shore CP, Dunn DW, Musick B.A feasibility study of a family seizure management program: "Be Seizure Smart". Journal of Neuroscience Nursing 2002;34(1):30-7. [DOI: 10.1097/01376517-200202000-00007] [DOI] [Google Scholar]
Bahrani 2017 {published data only}
- Bahrani K, Singh MB, Bhatia R, Prasad K, Vibha D, Shukla G, et al.Telephonic review for outpatients with epilepsy - a prospective randomized, parallel group study. Seizure 2017;53:55-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dash 2015 {published data only}
- Dash D, Sebastian TM, Aggarwal M, Tripathi M.Impact of health education on drug adherence and self-care in people with epilepsy with low education. Epilepsy & Behavior 2015;44:213-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glueckauf 2002 {published data only}
- Glueckauf RL, Fritz SP, Ecklund-Johnson EP, Liss HJ, Dages P, Carney P.Videoconferencing-based family counseling for rural teenagers with epilepsy: phase 1 findings. Rehabilitation Psychology 2002;47(1):49-72. [DOI: 10.1037/0090-5550.47.1.49] [DOI] [Google Scholar]
Hallfahrt 2007 {published data only}
- Hallfahrt T.FLIP&FLAP educational program in epilepsy in childhood and adolescence. Kinderkrankenschwester 2007;26(12):516-21. [PMID: ] [PubMed] [Google Scholar]
Ibinda 2014 {published data only}35680481
- Ibinda F, Mbuba CK, Kariuki SM, Chengo E, Ngugi AK, Odhiambo R, et al.Evaluation of Kilifi epilepsy education programme: a randomized controlled trial. Epilepsia 2014;55(2):344-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jantzen 2009 {published data only}
Li 2013 {published data only}
- Li J, Si Y, Hu J, Liu L, Deng Y, He J, et al.Enhancing medical compliance of patients with convulsive epilepsy in rural community: a randomized intervention trial. Epilepsia 2013;54(11):1988-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mar 2005 {published data only}
- Mar S, Dunkley C, Al-Ansari I, Whitehouse WP.Comparison of a dedicated children's seizure clinic to mixed general paediatric clinics. Child: Care, Health and Development 2005;31(5):597-602. [PMID: ] [DOI] [PubMed] [Google Scholar]
Modi 2013 {published data only}
- Modi AC, Guilfoyle SM, Rausch J.Preliminary feasibility, acceptability, and efficacy of an innovative adherence intervention for children with newly diagnosed epilepsy. Journal of Pediatric Psychology 2013;38(6):605-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfäfflin 2012 {published data only}
- Pfäfflin M, Petermann F, Rau J, May TW.The psychoeducational program for children with epilepsy and their parents (FAMOSES): results of a controlled pilot study and a survey of parent satisfaction over a five-year period. Epilepsy & Behavior 2012;25(1):11-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rau J, May TW, Pfäfflin M, Heubrock D, Petermann F.Education of children with epilepsy and their parents by the Modular Education Program Epilepsy for Families (FAMOSES) - results of an evaluation study [Schulung von Kinderen mit Epilepsie und deren Eltern mit dem Modularen Schulungsprogramm Epilepsie für Familien (FAMOSES) - Ergebnisse einer Evaluationsstudie]. Die Rehabilitation 2006;45(1):27-39. [PMID: ] [DOI] [PubMed] [Google Scholar]
Price 2004 {published data only}
- Price V, Murphy SO, Cureton VY.Increasing self-efficacy and knowledge through a seizure education programme for special education teachers. Journal of School Nursing 2004;20(1):43-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shore 2008 {published data only}
Snead 2004 {published data only}
- Snead K, Ackerson J, Bailey K, Schmitt MM, Madan-Swain A, Martin RC.Taking charge of epilepsy: the development of a structured psychoeducational group intervention for adolescents with epilepsy and their parents. Epilepsy & Behavior 2004;5(4):547-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Aqeel 2020
- Al-Aqeel S, Gershuni O, Al-Sabhan J, Hiligsmann M.Strategies for improving adherence to antiepileptic drug treatment in people with epilepsy. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD008312. [DOI: 10.1002/14651858.CD008312.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Austin 1997
- Austin JK, Boer H.Disruption in social functioning and services facilitating adjustment for the child and adult with epilepsy. In: Engel J, Pedley T, editors(s). Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven, 1997:191-201. [Google Scholar]
Bandstra 2008
- Bandstra NF, Camfield CS, Camfield PR.Stigma of epilepsy. Canadian Journal of Neurological Sciences 2008;35(4):436-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Berg 2010
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Emde Boas W, et al.Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 2010;51(4):676-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Betts 1992
- Betts T.Epilepsy services. What people need, what they want, what they get. Acta Neurologica Scandinavica 1992;S140:95-100. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bradley 2009
- Bradley PM, Lindsay B.Care delivery and self-management strategies for adults with epilepsy. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD006244. [DOI: 10.1002/14651858.CD006244.pub2] [DOI] [PubMed] [Google Scholar]
Bradley 2016
- Bradley PM, Lindsay B, Fleeman N.Care delivery and self management strategies for adults with epilepsy. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD006244. [DOI: 10.1002/14651858.CD006244.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chappell 1992
- Chappell B.Epilepsy: patient views on their condition and treatment. Seizure 1992;1(2):103-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2008
- Clark NM, Cabana MD, Nan B, Gong ZM, Slish KK, Birk NA, et al.The clinician-patient partnership paradigm: outcomes associated with physician communication behavior. Clinical Pediatrics 2008;47(1):49-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2010
- Clark NM, Stoll S, Youatt EJ, Sweetman M, Derry R, Gorelick A.Fostering epilepsy self management: the perspectives of professionals. Epilepsy & Behavior 2010;19(3):255-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C.Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2003
- Elwyn G, Todd S, Hibbs R, Thapar A, Edwards P, Webb A, et al.A 'real puzzle': the views of patients with epilepsy about the organisation of care. BMC Family Practice 2003;4:4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
England 2012
- England MJ, Liverman CT, Schultz AM, Strawbridge LM.Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy & Behavior 2012;25(2):266-76. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzsimons 2012
- Fitzsimons M, Normand C, Varley J, Delanty N.Evidence-based models of care for people with epilepsy. Epilepsy & Behavior 2012;23(1):1-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Version accessed 9 May 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Michaelis 2020
- Michaelis R, Tang V, Nevitt SJ, Wagner JL, Modi AC, LaFrance WC Jr, et al.Psychological treatments for people with epilepsy. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD012081. [DOI: 10.1002/14651858.CD012081.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mittan 2009
- Mittan RJ.Psychosocial treatment programs in epilepsy: a review. Epilepsy & Behavior 2009;16(3):371-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rau 2006
- Rau J, May TW, Pfäfflin M, Heubrock D, Petermann F.Education of children with epilepsy and their parents by the modular education program epilepsy for families (FAMOSES) - results of an evaluation study [Schulung von Kindern mit Epilepsie und deren Eltern mit dem Modularen Schulungsprogramm Epilepsie für Familien (FAMOSES) - Ergebnisse einer Evaluationsstudie]. Die Rehabilitation 2006;45(1):27-39. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sander 1990
- Sander JW, Hart YM, Johnson AL, Shorvon SD.National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet 1990;336(8726):1267-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
SIGN 2003
- Scottish Intercollegiate Guidelines Network (SIGN).Diagnosis and Management of Epilepsy in Adults: a National Clinical Guideline (No. 70). Edinburgh: Scottish Intercollegiate Guidelines Network, Royal College of Physicians, 2003. [Google Scholar]
SIGN 2005
- Scottish Intercollegiate Guidelines Network (SIGN).Diagnosis and Management of Epilepsies in Children and Young People: a National Clinical Guideline (No. 81). Edinburgh: Scottish Intercollegiate Guidelines Network, 2005. [Google Scholar]
Thapar 1996
- Thapar AK.Care of patients with epilepsy in the community: will new initiatives address old problems? British Journal of General Practice 1996;46(402):37-42. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization.Epilepsy. www.who.int/news-room/fact-sheets/detail/epilepsy (accessed 16 December 2013).
References to other published versions of this review
Fleeman 2015
- Fleeman N, Bradley PM, Lindsay B.Care delivery and self management strategies for children with epilepsy. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD006245. [DOI: 10.1002/14651858.CD006245] [DOI] [PubMed] [Google Scholar]
Fleeman 2018
- Fleeman N, Bradley P.Care delivery and self-management strategies for children with epilepsy. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD006245. [DOI: 10.1002/14651858.CD006245.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindsay 2006
- Lindsay B, Bradley PM.Care delivery and self-management strategies for children with epilepsy. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD006245. [DOI: 10.1002/14651858.CD006245] [DOI] [PubMed] [Google Scholar]
Lindsay 2010
- Lindsay B, Bradley P.Care delivery and self-management strategies for children with epilepsy. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD006245. [DOI: 10.1002/14651858.CD006245.pub2] [DOI] [PubMed] [Google Scholar]


