Abstract
Scope:
We aimed to investigate the mechanisms by which red raspberry (RR) polyphenolic fractions regulate obesity and inflammation with an emphasis on the crosstalk between adipose tissue macrophages (ATM) and adipocyte progenitors.
Methods and Results:
C57BL/6 male mice were fed either a HF diet or a HF diet supplemented with a RR polyphenolic fraction from whole fruit, pulp, or seed. Supplementation with pulp significantly increased energy expenditure and reduced HF diet-induced obesity and insulin resistance. The pulp, and to a lesser extent, whole polyphenols decreased the recruitment of ATM, activation of the NLRP3 inflammasome and adipocyte hypertrophy, which was associated with epigenetic modulation of adipogenesis (e.g., H3K27Ac, H3K9Ac). Results from an IL-1β reporter assay in J774 macrophages recapitulated the inhibitory role of RR polyphenols on NLRP3 inflammasome activation. Using conditioned media from macrophages, we demonstrated that RR polyphenols reversed the IL-1β-mediated epigenetic suppression of H3K27Ac in adipocyte progenitor cells.
Conclusions:
RR polyphenols from pulp and whole fruit serve as an inhibitor for NLRP3 inflammasome activation and an epigenetic modifier to regulate adipogenesis, which confers resistance against diet-induced obesity and metabolic dysfunction.
Keywords: Adipogenesis, Histone modification, NLRP3 inflammasome, Polyphenols, Red raspberry
Graphical Abstract
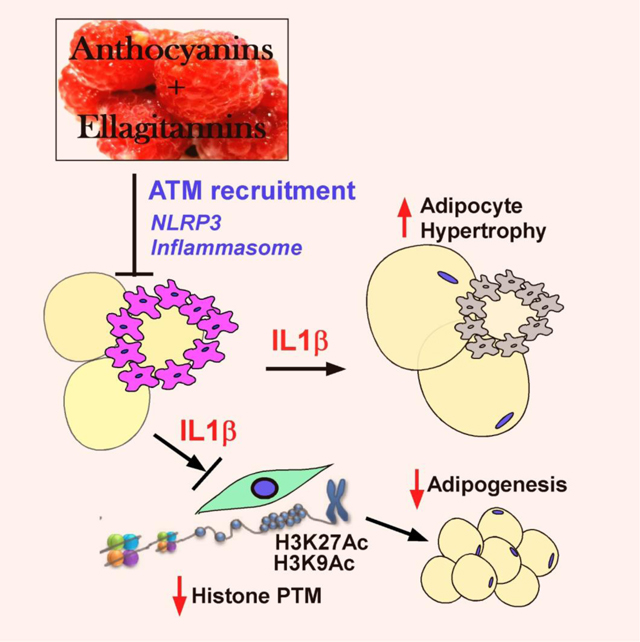
Diet-induced obesity increases the infiltration of adipose tissue macrophage (ATM) and subsequent activation of the NLRP3 inflammasome, an innate immune system for IL-1β secretion. Released IL-1β from ATM alters posttranscriptional modifications (PTM) in adipogenic stem cells, which suppresses the fat cell formation (adipogenesis) while promotes the adipocyte hypertrophy and insulin resistance. Rong et al. demonstrated that polyphenols isolated from red raspberry whole fruit and pulp (i.e., anthocyanins and ellagitannins) suppress ATM infiltration and NLRP3 inflammasome activation, and serve as an epigenetic modifier to regulate adipogenesis, thereby conferring resistance against diet-induced obesity and metabolic dysfunction
1. Introduction
Higher caloric intake than expenditure is a major contributing factor to obesity, which is accompanied by adipose tissue remodeling such as adipocyte enlargement and immune cell infiltration. The recruitment of adipose tissue macrophages (ATM) and their subsequent polarization into a pro-inflammatory M1 status plays a detrimental role in mediating obesity and the manifestation of insulin resistance to type 2 diabetes [1]. Released from cells of the innate immune system, IL-1β is one of the most potent pro-inflammatory cytokines that causes pyroptotic cell death and suppresses insulin signaling through its paracrine actions. IL-1β secretion is tightly regulated by the Nod-like receptor protein 3 (NLRP3) inflammasome, a multiprotein cytosolic receptor for cleavage of caspase-1 and maturation of IL-1β upon sensing damage-associated molecular patterns (DAMPs, e.g., free fatty acids, ATP, free cholesterol) or pathogen-associated molecular patterns (PAMPs, e.g., exotoxins such as lipopolysaccharide) [2]. Accumulating evidence supports the direct association between adipose NLPR3 inflammasome activation and obesity-induced type 2 diabetes [3].
Adipose tissue is comprised of lipid-laden mature adipocytes and the non-adipocyte stromal vascular (SV) fraction that contains mesenchymal stem cells, preadipocytes, endothelial cells, and immune cells. During the early phase of obesity development, differentiation of uncommitted stem cells into new fat cells (hyperplasia) is a natural defense mechanism that expands the lipid-storage capacity without triggering inflammation [4]. It has also been reported that obesity mitigates the adipogenic capacity [5, 6]. Together, the failure to make new fat cells combined with the hypertrophic expansion of existing adipocytes, increased basal lipolysis, and concomitant fatty acid spillover, promotes ectopic fat development. Although the impact of ATM NLRP3 inflammasome activation on existing adipocytes is well-established in suppressing insulin signaling and promoting lipolysis [7], its impact on adipose stem cells in regulating adipogenesis remains unknown.
Several dietary bioactive compounds are reported to suppress the NLRP3 inflammasome including n-3 polyunsaturated fatty acids [8], vitamins [9], and various polyphenolic compounds (e.g., EGCG, resveratrol, curcumin, and quercetins) [10]. A growing body of literature demonstrates the metabolic benefits of red raspberry (RR, Rubus idaeus L) against high fat (HF) diet-induced obesity and inflammation [11–13]. Intriguingly, RR extracts have been implicated in attenuating NLRP3 inflammasome activation in the liver and intestinal epithelial cells [12, 14]. However, it remains uncertain whether the polyphenols from RR are responsible for inhibiting the NLRP3 inflammasome. It is also unknown whether the polyphenols from pulp versus seed differentially affect adipose tissue remodeling and systemic inflammation.
In this study, we hypothesized that RR polyphenols limit HF diet-induced metabolic dysfunction by suppressing NLRP3 inflammasome activation and its accompanying adipose tissue remodeling. To test this, we integrated in vivo RR polyphenol intervention studies along with in vitro experiments to examine the crosstalk between macrophages and adipogenic progenitors.
2. Experimental Section
2.1. Preparation of RR polyphenols and composition analysis
Freshly-frozen ‘WakeField’ red raspberry (RR) fruits were generously donated by Enfield Farms (Lynden, WA). The fractionation and extraction of RR polyphenols from whole fruit, seed, and pulp were conducted according to Gourineni et al. [15]. Briefly, the physical separation of pulp and seed fractions was performed by using a fine sieve. The RR polyphenols from each fraction (i.e., whole fruits, pulp, or seed) were extracted with acidified methanol (0.5% acetic acid). After removing sugar and fibers by macroporous ion-exchange resin column (Ambelite FPX66, Dow Chemical), each polyphenolic fraction was dried, lyophilized, and stored at −20 °C. Approximately, 25 g whole RR polyphenols were obtained from 6 kg of freshly frozen fruit (~410 mg/100g RR). Upon fractionation of 6 kg frozen RR, 15 g of pulp polyphenol and 5 g of seed polyphenols were obtained. The composition of RR polyphenolic compounds was analyzed on an Agilent 1200 HPLC (Agilent Zorbax SB-C18 column) equipped with a binary pump, injector, diode array detector, and fluorescent detector (Agilent Technologies) as we have described previously [16]. Total phenolic content was determined by using Folin-Ciocalteu reagent with gallic acid as a standard (GAE, gallic acid equivalent). The composition of RR polyphenols is summarized in Table 1.
Table 1.
Phenolic composition of RR extracts
| henolic (mg/g dry extract) | Whole fruit | Seeds | Pulp |
|---|---|---|---|
|
| |||
| Quercetin | 2.73 ± 0.02 | 3.13 ± 0.01 | 1.21 ± 0.01 |
| Myricetin | 19.1 ± 0.02b | 14.0 ± 0.01b | 27.7 ± 0.01a |
| Ellagic acid | 66.1 ± 3.92b | 134.8 ± 7.63a | 49.7 ± 1.98c |
| (+)-Catechin | 11.4 ± 1.44b | 20.1 ± 2.68a | 20.4 ±3.41a |
| (−)-Epicatechin | 75.4 ± 0.31b | 117.0 ± 1.58a | 67.4 ± 2.01b |
| Anthocyanin | |||
| Cyanidin 3-O-β-D-glucoside | 8.73 ± 0.01 | 10.3 ± 0.01 | 13.6 ± 0.01 |
| Cyanidin 3-O-β-D-glucoside equivalent | 39.6 ± 0.04b | 31.3 ± 0.02b | 112.0 ± 0.05a |
|
| |||
| Total phenolic content (mg of GAE/g) | 279.0 ± 31 | 330.8 ± 29 | 338.8 ± 45 |
Values represent the mean ± SD of triplicate tests. Columns not sharing a common letter are significantly different (p< 0.05) by one-way ANOVA with Tukey’s post hoc multiple analysis. Ellagitannin is calculated as Ellagic acid after hydrolysis. The Cyanidin-3-sophoroside peak is quantified as Cyanidin 3-O-β-D-glucoside equivalent. GAE=Gallic Acid Equivalent.
2.2. Animals and diet preparation
C57BL/6 male mice (six-week-old, Jackson Laboratory) were randomly assigned to one of five diet groups (n=8/group), low fat (L, 10% calorie from fat), high fat (H, 45% calorie from fat), or HF diet supplemented with polyphenols from whole fruit (H+W, 0.4% w/w), seed (H+S, 0.1% w/w), pulp (H+P, 0.3% w/w). Mice were fed for 16 weeks ad libitum (Fig. 1A). Diet compositions are found in Table S1.
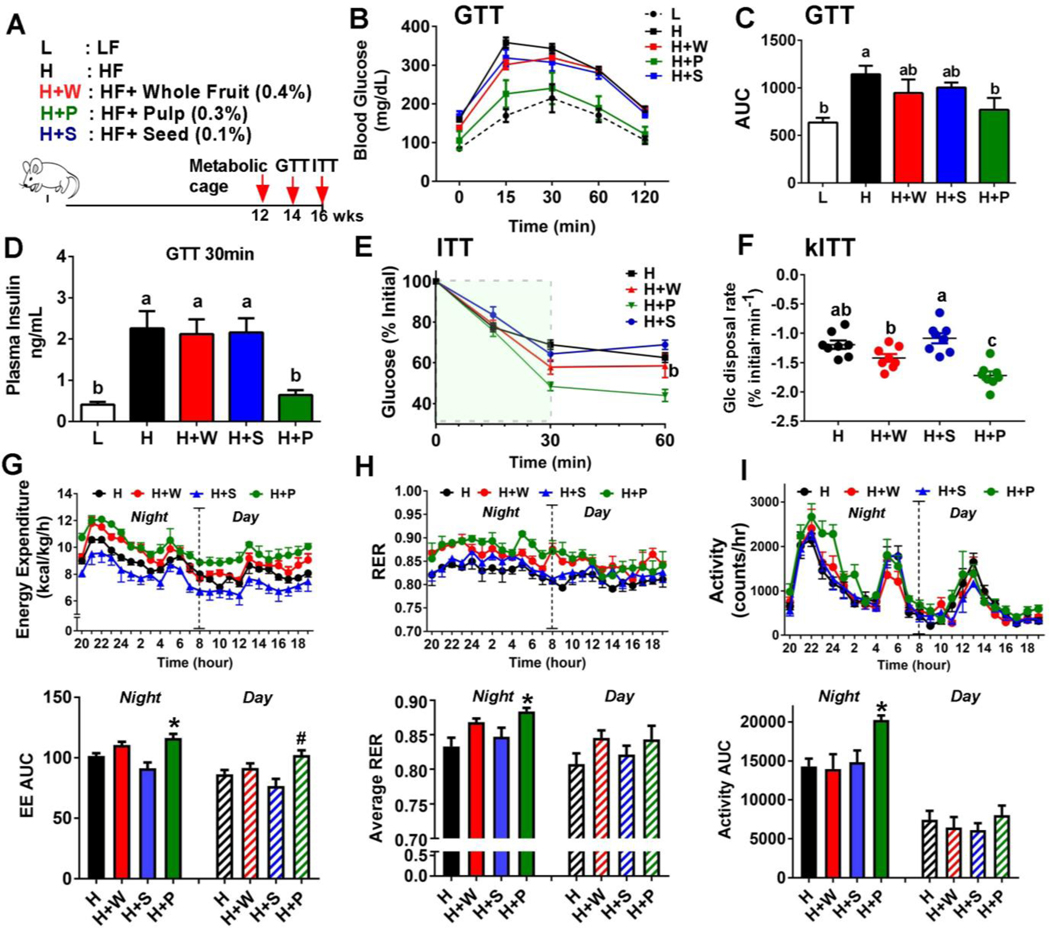
Figure 1. RR pulp polyphenols attenuated HF diet-induced obesity and insulin resistance.
(A) Schematic representation of study design. C57BL/6 mice (n=8) were fed an isocaloric HF diet supplemented with 0.4% whole fruit (H+W), 0.1% seed (H+S), or 0.3% pulp (H+P) polyphenols along with low fat (L) and high fat (H) controls. Red arrows indicated the timeline for assay. (B) Glucose tolerance test (GTT) (C) Area under the curve (AUC) during GTT. (D) Insulin levels measured by ELISA at 30 minutes during GTT. (E) Insulin tolerance test (ITT). (F) Glucose disposal rate in the first 30 minutes during ITT (kITT). (G) Energy expenditure (kcal/kg/h) (H) Respiratory exchange ratio (RER). (I) Activity levels (Counts/hour). All values represented as the mean ± SEM (n=8/group). Treatments with different letters are significantly different from one another (P<0.05) by one-way ANOVA with Tukey’s post hoc multiple analysis. In G-H, *P<0.05 and #P<0.05 by one-way ANOVA compared to corresponding H group as a control.
2.3. Dosage information
The ratio of polyphenols (whole: seed: pulp=4:1:3) was selected based on the yield described in 2.1, since we collected 3 times more polyphenols in the pulp fraction than in the seed fraction starting from the same amount of whole fruits. To evaluate the differential contribution of seed and pulp polyphenols toward the metabolic benefits of whole fruit polyphenols, we prepared the diet to keep the source of polyphenols and ratio equal to the whole fruit (Table S1). We used the 0.4 % of RR polyphenols, which is roughly equivalent to 120 mg/kg mouse/day of RR polyphenols (GAE). When we calculated the human equivalent dose (HED) by applying the scaling factor of 12.3 [17], the estimated value would be 9.6 mg/kg human/day. For an adult human in 60 kg, intake of roughly ~570 mg RR polyphenols is achievable by taking 1.2 cups (~150 g) of RR whole fruits based on our yield. All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln (Approval ID #1469).
2.4. Measurement of energy metabolism by metabolic cages
To measure energy expenditure, a single mouse was placed into an individual metabolic cage (TSE systems) for four days (n=6/group). Indirect oxygen consumption (VO2) and carbon dioxide production (VCO2) were used to calculate respiratory exchange rate (RER=VO2/VCO2).
2.5. Glucose and insulin tolerance test
Glucose (GTT) and insulin tolerance (ITT) tests were performed on the same mice within a two week interval as previously described [18]. Briefly, mice were fasted overnight and intraperitoneally injected with a 10 % D-glucose solution (1g/kg BW) for GTT, and 1U of insulin for ITT (n=8 per group). Glucose disposal from the circulation was measured using a glucometer (Bayer, Contour) at time intervals. During the GTT, blood glucose levels were measured at 0, 15, 30, 60, and 120 minutes post-glucose injection. Blood levels of insulin levels were determined by ELISA (Crystal Chem). For the assessment of insulin sensitivity, glucose disposal rate (kITT) was calculated in the first 30 minutes during ITT.
2.6. Lipid analysis in blood and tissue
To measure plasma triglycerides (TG), a colorimetric assay kit (EzScreen) was purchased from BioVision Inc. For TG determination in the liver and pancreas, lipid was extracted from ~100 mg of tissue (liver and pancreas) (n=8 group) in a chloroform:methanol (2:1) solution and processed as we described previously [19]. Total cholesterol, HDL, and LDL levels were measured using a colorimetric assay (Abcam). Adiponectin and insulin were measured using ELISA kits according to the manufacturer’s protocol (R&D Systems and Crystal Chem, repsctively). NEFA (non-esterified fatty acids) and glycerol analyses were conducted by using commercial kits (HR series NEFA-HR, Wako and Glycerol Assay, Sigma, respectively).
2.7. H&E staining, adipocyte size analysis and adipocyte immunostaining
Tissue samples were fixed in 10 % buffered formalin, embedded in paraffin, cut to 5–10 μm sections, and processed for hematoxylin and eosin (H&E) staining. Adipocyte size was quantified from the H&E stained images using NIH Image J and Adiposoft software [20]. Details of adipocyte immunostaining are found in the Supplemental Information.
2.8. qPCR
To isolate the total RNA from the adipose tissue, ~100 mg of tissue (eWAT, liver and pancreas) were homogenized with Trizol® reagent (Invitrogen) method by following manufacturer’s instruction. To avoid the genomic DNA contamination, extracted total RNA were treated with DNase (Bio-Rad). 2 μg of RNA was converted into cDNA in a total volume of 20 μL (iScript cDNA synthesis kit, Bio-Rad). Relative gene expression was determined by real-time qPCR (QuantStudio 6 Flex, Applied Biosystems) using SYBR green. Relative gene expression was determined based on the 2−ΔΔCT method with normalization of the raw data to 18S rRNA or 36b4 (primer sequences in Table S2).
2.9. Western blot analysis
Tissue lysates (isolated from eWAT, interscapular brown fat, and liver) or cell extracts were prepared in RIPA buffer containing protease and phosphatase inhibitors (Sigma). Proteins were fractionated using 10% SDS-PAGE, transferred to PVDF membranes, and incubated with the relevant antibodies conjugated with horseradish peroxidase (HRP). All antibodies used for experiments were summarized in Table S3. The chemiluminescence from ECL solution was detected using a FluorChem E Imaging System (Cell Biosciences).
2.10. Fractionation of stromal vascular (SV) cells from epididymal adipose tissue
Freshly-isolated eWAT was minced into small pieces (1–2 mm) and subjected to collagenase (0.1%) digestion in a shaking water bath (37 °C) for 1 hour. The released cells were filtered through a 100 μm cell strainer (Corning) and pelleted by centrifugation. After removing reticulocytes by osmotic lysis, cells were washed and centrifuged, and the resulting pellet is defined as SV fraction [21].
2.11. iGLuc NLRP3 inflammasome reporter assay
Primary bone marrow cells were isolated from the femurs of 6- to 10-week-old C57BL/6 mice and differentiated into bone marrow-derived macrophages (BMDM) by using L929-cell conditioned medium [22]. J774 macrophages (Mϕ) stably transfected with pro-IL-1β-Gaussia luciferase (iGLuc) construct were a generous gift from Dr. Veit Hornung at the University of Bonn [23]. Before stimulation, J774 Mϕ were first incubated with whole fruit polyphenols (10 μg/ml) or vehicle for 12 hours. To induce NLRP3 inflammasome activation, Mϕ were first primed with lipopolysaccharide (LPS) (100 ng/ml) for 3 hours followed by ATP (2 mM) stimulation for 30 minutes. The BioLux GLuc assay kit (NEB Inc) and a Synergy H1 plate reader (BioTek) were used to determine Gaussia luciferase (GLuc) activity [9].
2.12. Conditioned medium experiment
To investigate the crosstalk between Mϕ and adipose progenitor cells, conditioned medium from J774 Mϕ was transferred to differentiating C3H10T1/2 cells (ATCC® CCL-226), a murine embryonic cell line that shares many features of mesenchymal stem cells [24] (Fig 5B). To prepare the conditioned medium, J774 Mϕ were cultured in one of three different conditions: I) without any stimulation; II) activation of NLRP3 inflammasome induced by LPS/FFA (i.e., LPS priming followed by FFA stimulation with 400μM palmitate for 12 hours); III) pre-incubation with RR whole polyphenols (10 μg/ml) followed by LPS/FFA stimulation. Differentiation of confluent C3H10T1/2 cells was induced by adding an adipocyte-differentiating cocktail containing insulin (500 nM), isobutylmethylxanthine (0.25 mM), dexamethasone (1 μM), BRL49653 (1 μM) and 2% FBS in DMEM for two days. After the commitment step, C3H10T1/2 cells were treated with one of the three different Mϕ conditioned media (i.e., I, II or III) with a 50% dilution in the presence and absence of whole RR polyphenols (10 μg/ml) for an additional two days (Fig 5B). At day 4 of differentiation, C3H10T1/2 cells were harvested for the analysis of epigenetic marks for adipocyte differentiation.
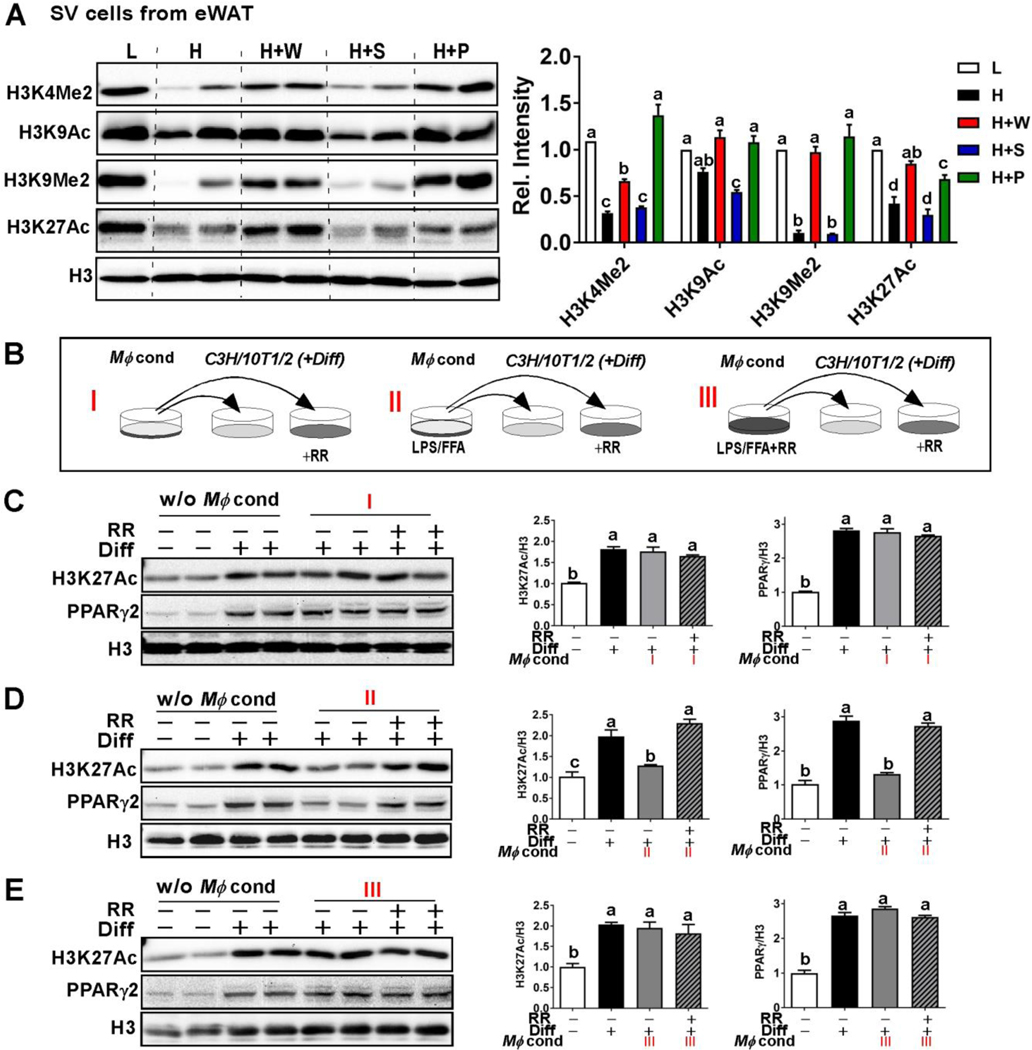
Figure 5. RR polyphenols reversed NLRP3 inflammasome-mediated histone PTM modulation for adipogenesis.
(A) Western blot analysis for histone posttranslational modification (PTM) marks related to adipocyte differentiation including H3K4me2, H3K9Ac, H3K9me2, and H3K27Ac in the SV fraction of eWAT. (B) Study design for conditioned media experiments using J774 macrophages and C3H10T1/2 cells (see details in method section). Western blot analysis of H3K27 acetylation and PPARγ2 in differentiating C3H10T1/2 cells upon receiving conditioned medium I (C), II (D) or III (E) with (+) or without (−) RR polyphenols (10 μg/mL). In A and C-E, relative protein intensities normalized to H3 quantified by Image J. All values represented as the mean ± SEM. **P<0.01 by one-way ANOVA with Tukey’s post hoc multiple analysis.
2.13. Statistical analysis
All data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison tests or Student’s t-test, *p<0.05 and **p<0.01. For the analysis of the statistical significance in GTT, ITT, and metabolic parameters from metabolic cage experiments, the area under the curve was calculated for individual animals and analyzed by one-way ANOVA. For the correlation between adipocyte size and perilipin (a lipid droplet-coating protein), Pearson correlation coefficient (r) was computed. All analyses were performed using Graph Pad Prism (Version 7.02).
3. Results
3.1. RR pulp polyphenols attenuated HF diet-induced obesity and insulin resistance
Consistent with the literature [25], RR polyphenols were found to contain quercetin, myricetin, ellagic acid, cathechin, epicatechins and anthocyanins. The pulp fraction had the highest amount of anthocyanin, while the seed fraction contained the highest amount of ellagic acid and epicatechin (Table 1).
After 16 weeks of feeding, pulp polyphenol supplementation (H+P) significantly reduced the HF diet-induced weight gain compared to the other isocaloric diets, despite no differences in food intake among the treatments. A trend toward limited weight gain resulted in H+W fed mice compared to H or H+S fed mice. Consistent with the reduced body weights, H+P supplementation substantially reduced both visceral (epididymal, mesenteric, and perirenal) and subcutaneous fat mass compared with the other isocaloric diets. Liver and brown adipose tissue (BAT) masses were also significantly reduced in pulp-fed mice (Table 2). Consistent with reduced liver mass, RR pulp polyphenols also significantly decreased plasma triglyceride, total cholesterol, and LDL cholesterol levels in the plasma. There were no differences in plasma non-esterified fatty acid (NEFA) concentrations among all treatments, but glycerol levels were signficantly lower in the H+P fed mice compared to other HF diet-fed animals (Table 3).
Table 2.
Effects of different RR polyphenol fractions on food intake, body weight, and fat mass
| L | H | H+W | H+S | H+P | |
|---|---|---|---|---|---|
|
| |||||
| Food intake (g/day) | ND | 3.58 ± 0.13 | 3.77 ± 0.18 | 3.50 ± 0.23 | 3.42 ± 0.24 |
| Body weight (g) | 33.0 ± 0.7 c | 43.6 ± 1.5 a | 41.86 ± 1.3 a | 45.00 ± 0.7 a | 39.44 ± 0.7 b |
| Liver (g) | 1.40 ± 0.13 b | 2.29 ± 0.26 a | 1.95 ± 0.16 a | 2.37 ± 0.14 a | 1.52 ± 0.11 b |
| Epididymal WAT (g) | 1.30 ± 0.20 b | 1.79 ± 0.14 a | 2.09 ± 0.11 a | 1.90 ± 0.13 a | 1.70 ± 0.25 ab |
| Inguinal WAT (g) | 0.69 ± 0.12 b | 1.61 ± 0.17 a | 1.32 ± 0.06ab | 1.65 ± 0.09 a | 1.08 ± 0.22 b |
| Mesenteric WAT (g) | 0.45 ± 0.07 b | 1.03 ± 0.14 a | 1.13 ± 0.09 a | 1.29 ± 0.07 a | 0.61 ± 0.12 b |
| Perirenal WAT (g) | 0.44 ±0.09 b | 0.98 ±0.11a | 1.03 ± 0.09 a | 1.12 ± 0.07 a | 0.57 ± 0.10 b |
| BAT(g) | 0.17 ± 0.01b | 0.24 ± 0.02 a | 0.29 ± 0.02a | 0.26 ± 0.01 a | 0.16 ± 0.01 b |
Values represent the mean ± SEM (n=8/group), ND=not determined. Columns not sharing a common letter are significantly different (p< 0.05) by one-way ANOVA with Tukey’s post hoc multiple analysis. L: low fat; H: high fat; H+W: high fat with polyphenols from whole fruits (0.4% w/w); H+S: high fat diet with RR polyphenols from seed fraction (0.1% w/w); H+P: high fat with RR polyphenols from pulp fraction (0.3% w/w). WAT=white adipose tissue.
Table 3.
Effects of different RR polyphenol fractions on blood chemistry and lipid profile.
| L | H | H+W | H+S | H+P | |
|---|---|---|---|---|---|
|
| |||||
| Triglyceride (mmol/L) |
0.64 ± 0.10b | 1.14 ± 0.10a | 1.02 ± 0.04 a | 0.89 ± 0.09 ab | 0.77 ± 0.06 b |
| Total cholesterol (mg/dL) |
172.0 ± 37.8 b | 298.2 ± 16.0a | 236.9± 20.8b | 312.9 ± 9.1 a | 217.3 ± 34.6 b |
| HDL-C (mg/dL) |
81.4 ± 18.5c | 141.4 ± 7.1 b | 153.9 ± 15.0ab | 183.1 ± 9.1a | 161.4 ± 13.2ab |
| LDL, VLDL-C (mg/dL) |
24.1 ± 5.4b | 43.7 ± 5.2a | 27.3 ± 3.8b | 35.9 ± 2.3ab | 31.8 ± 4.4ab |
| NEFA (mmol/L) |
0.41 ± 0.03b | 0.51 ± 0.03a | 0.50 ± 0.03ab | 0.44 ± 0.02b | 0.49 ± 0.03ab |
| Glycerol (mmol/L) |
0.20 ± 0.03b | 0.27 ± 0.04a | 0.28 ± 0.02a | 0.27 ± 0.04a | 0.22 ± 0.04b |
| Adiponectin (μg/mL) | 11.2 ± 0.9a | 8.10 ± 0.5b | 8.8 ± 0.4b | 8.7 ± 1.1b | 12.5 ± 1.0a |
| Fasting Glucose (mg/dL) |
70.5 ± 3.4b | 125.0 ± 11.4a | 84.0 ± 10.0b | 123.5 ± 4.9a | 86.3 ± 7.7b |
| Fasting Insulin (ng/mL) |
0.07 ± 0.02c | 0.60 ± 0.21a | 0.46 ± 0.13a | 0.88 ± 0.17a | 0.22 ± 0.02b |
Values represent the mean ± SEM (n=8 per group), Columns not sharing a common letter are significantly different (p< 0.05) by one-way ANOVA with Tukey’s post hoc multiple analysis. L: low fat; H: high fat; H+W: high fat with polyphenols from whole fruits (0.4% w/w); H+S: high fat diet with RR polyphenols from seed fraction (0.1% w/w); H+P: high fat with RR polyphenols from pulp fraction (0.3% w/w); NEFA, non-esterified free fatty acids.
Next, we examined the impact of RR polyphenols on glucose metabolism. Fasting blood glucose levels were significantly lower in H+W and H+P fed mice than HF alone or H+S fed animals. Consistently, fasting insulin levels were in the rank of H+S, H > H+W > H+P. Also, the mice fed the H+P diet had the highest levels of adiponectin, similar to those of LF fed mice (Table 3). H+P fed mice were also the most glucose tolerant among the other isocaloric diet groups during GTT (Fig 1B, C). Also, plasma insulin concentrations at the 30 minute timepoint during GTT were dramatically lower in H+P fed mice (Fig. 1D). During the ITT, H+P fed mice were also able to maintain lower glucose levels than other isocaloric HF fed mice and were comparable to LF fed mice (Fig 1E). The glucose disappearance rate during the ITT (kITT), an indicator of insulin resistance, was significantly faster in the H+P and H+W fed mice compared with H alone or H+S fed mice (Fig 1F).
Additionally, we explored the effect of RR polyphenols on energy expenditure. A metabolic cage experiment revealed that intake of pulp polyphenols significantly increased energy expenditure (Fig 1G), respiratory exchange ratio (RER) (Fig. 1H), and activity levels (Fig 1I) compared with feeding HF alone or seed supplementation. Together, these data suggest that supplementation with RR polyphenols from pulp, and a lesser extend from whole fruit, are effective in preventing HF diet-induced weight gain, insulin resistance, and compromised energy expenditure.
3.2. RR polyphenols from pulp and whole fruit attenuated HF-induced adipocyte hypertrophy and adipose inflammation
Based on our observation that feeding pulp polyphenols reduced fat mass (Table 2), we asked whether RR polyphenol supplementation would reverse HF-diet driven adipose tissue remodeling. A size analysis of epididymal fat revealed that pulp and whole polyphenol supplementation, but not seed, significantly reduced adipocyte hypertrophy compared to feeding a HF diet (Fig 2A). Accordingly, adipocyte size distribution from pulp and whole polyphenol fed mice was shifted toward smaller sizes. Whole and pulp polyphenols feeding also reduced the prevalence of crown-like structures (Fig 2A).
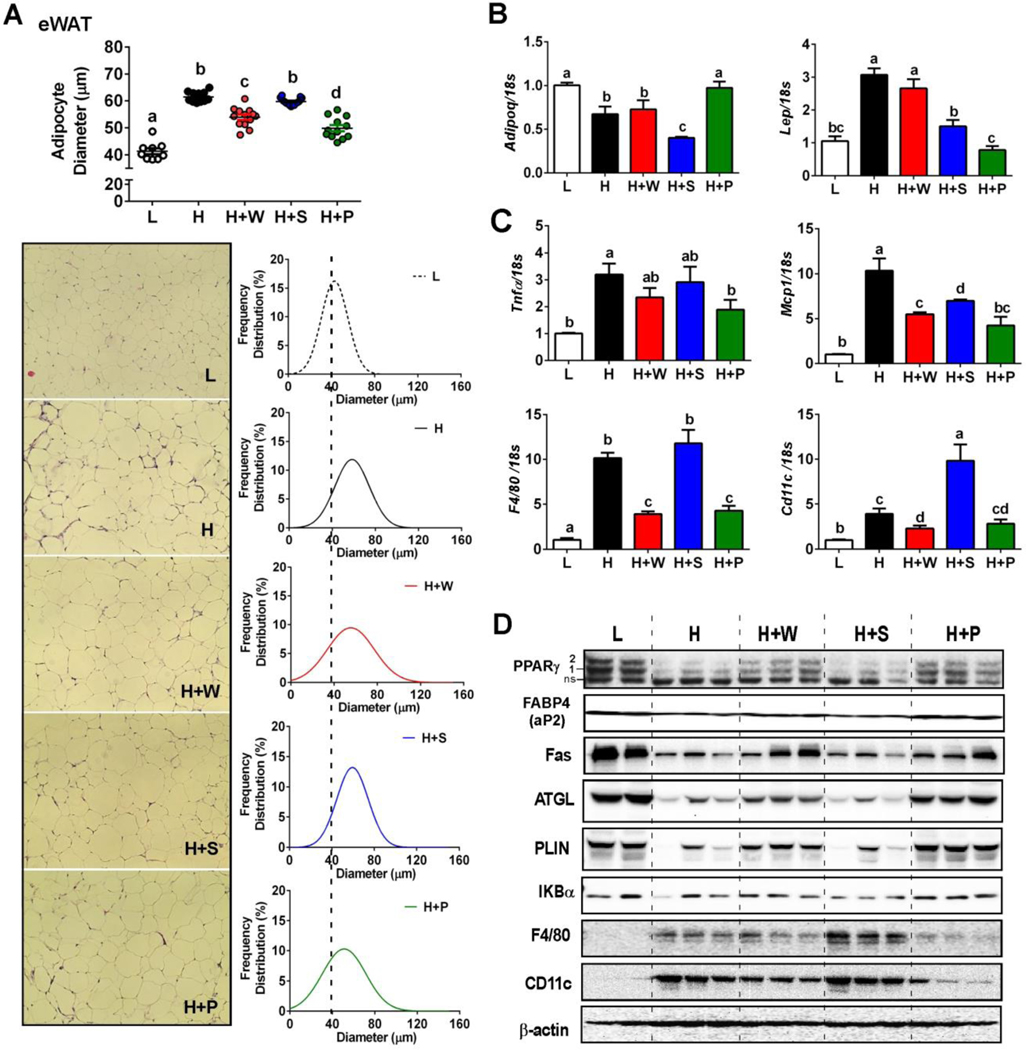
Figure 2. RR pulp and whole polyphenols decreased adipocyte hypertrophy and inflammation.
eWAT was collected (n=8/group) after 16 weeks of dietary intervention (L= low fat, H=high fat, H+W=HF with whole fruit polyphenol, H+S=HF with seed polyphenol, H+P=HF with pulp polyphenol). (A) Average adipocyte diameter (μm), H&E staining, and adipocyte size distribution. The line of best fit is shown (Gaussian curve fitting). (B) mRNA expression levels of adiponectin and leptin. (C) mRNA expression levels of pro-inflammatory genes Tnfa, Mcp1, F4/80, and Cd11c. (B) Protein expression pattern of lipogenesis, lipolysis, lipid droplet coating and inflammation in the eWAT. All values represented as the mean ± SEM. Treatments with different letters are significantly different from one another (P<0.05) by one-way ANOVA with Tukey’s post hoc multiple analysis.
Additional analyses revealed that HF-diet induced adipocyte hypertrophy attenuated protein expression of 1) peroxisome proliferator-activated receptor gamma (PPARγ), a key transcription factor of adipocytes, 2) PPARγ targets, including fatty acid binding protein 4 (FABP4), fatty acid synthetase (FAS) and perilipin 1 (PLIN), a primary lipid droplet coating protein, and 3) lipolysis-related proteins of adipose triglyceride lipase (ATGL) (Fig 2D). Supplementation with pulp and whole polyphenols, but not seed, prevented the loss of PLIN1, showing an inverse correlation with adipocyte size (Pearson’s r = −0.948, r2 = 0.90). HF-diet feeding also promoted IκBα degradation and infiltration of immune cells as evidenced by increased expression of the macrophage marker F4/80; a finding consistent with our observation of increased crown-like structures in these mice. In contrast, supplementation with pulp, and to a lesser extent with whole polyphenols, attenuated these inflammatory responses in the epididymal fat (Fig 2C, D). In addition, pulp polyphenol supplementation was associated with 1) increased adiponectin but decreased leptin gene expression (Fig 2C), and 2) reduced proinflammatory gene expression levels of F4/80, tumor necrosis factor α (Tnfα), monocyte chemoattractant protein 1 (Mcp-1/Ccl2) and Cd11c (M1 polarized macrophage marker) (Fig 2C). Altogether, these results indicate that pulp and whole polyphenols protect adipocytes from HF-mediated hypertrophy and inflammation.
3.3. RR polyphenols reduced ATM recruitment and NLPR3 inflammasome activation.
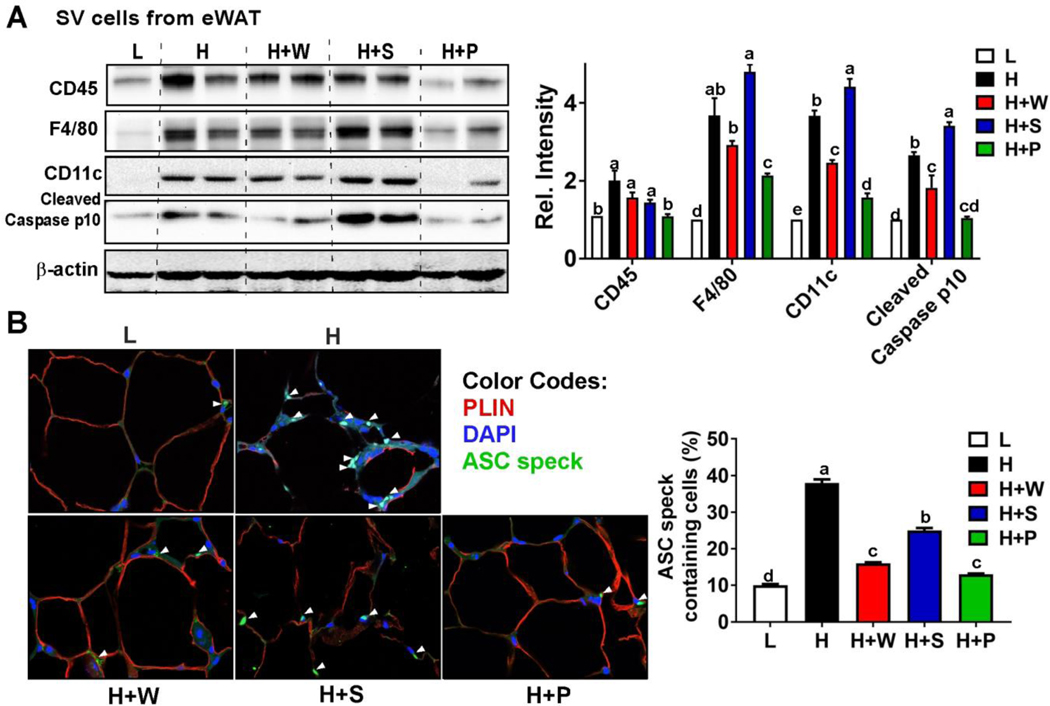
To investigate the effects of RR polyphenols on ATM infiltration, we first fractionated the stromal vascular (SV) cells from eWAT, and examined the signature proteins of macrophages. HF-diet increased expression of signature macrophage proteins (CD45 and F4/80), and proteins associated with classical M1 polarization (CD11C); feeding pulp polyphenols, and to a lesser extent, whole RR polyphenols, prevented these increases (Fig 3A). Expression of cleaved caspase p10, a marker of caspase-1 activation, was reduced in pulp and whole polyphenol supplementation compared to feeding seed polyphenols or a HF diet alone (Fig 3A), indicating that pulp and whole polyphenol supplementation attenuates NLRP3 inflammasome activation in ATM. ASC (apoptosis-associated speck-like protein containing a CARD) is an adaptor protein that polymerizes to form a speck for caspase-1 recruitment, thereby serving as visual evidence for activation of NLRP3 inflammasome. Immunostaining with ASC revealed that HF diet induced massive ASC speck formation within the ATM, which was signficantly reduced in eWAT fed with H+W or H+P feeding (Fig 3B). Consistent with reduced perilipin protein expression, ASC speck formation is correlated with the loss of perilipin around the lipid droplet. These results demonstrate that RR polyphenols isolated from whole fruit and pulp effectively attenuates NLRP3 inflammasome activation in ATM of eWAT.
Figure 3. RR pulp and whole polyphenols decreased NLRP3 inflammasome activation in the eWAT.
(A) Western blot analysis for macrophage markers of CD45, F4/80, CD11c, and cleaved caspase p10 in the SV fraction of eWAT. Relative protein intensities normalized to β-actin quantified by Image J. (B) Immunofluorescence staining for ASC speck formation (green) and perilipin (red) in the eWAT. Average ACS speck formation quantified by image J (n=4/group). Treatments with different letters are significantly different from one another (P<0.05) by one-way ANOVA with Tukey’s post hoc multiple analysis. L= low fat, H=high fat, H+W=HF with whole fruit polyphenol, H+S=HF with seed polyphenol, H+P=HF with pulp polyphenol. eWAT=epididymal WAT.
3.4. RR polyphenols suppressed NLRP3 inflammasome and potentiated histone PTM marks for adipogenesis
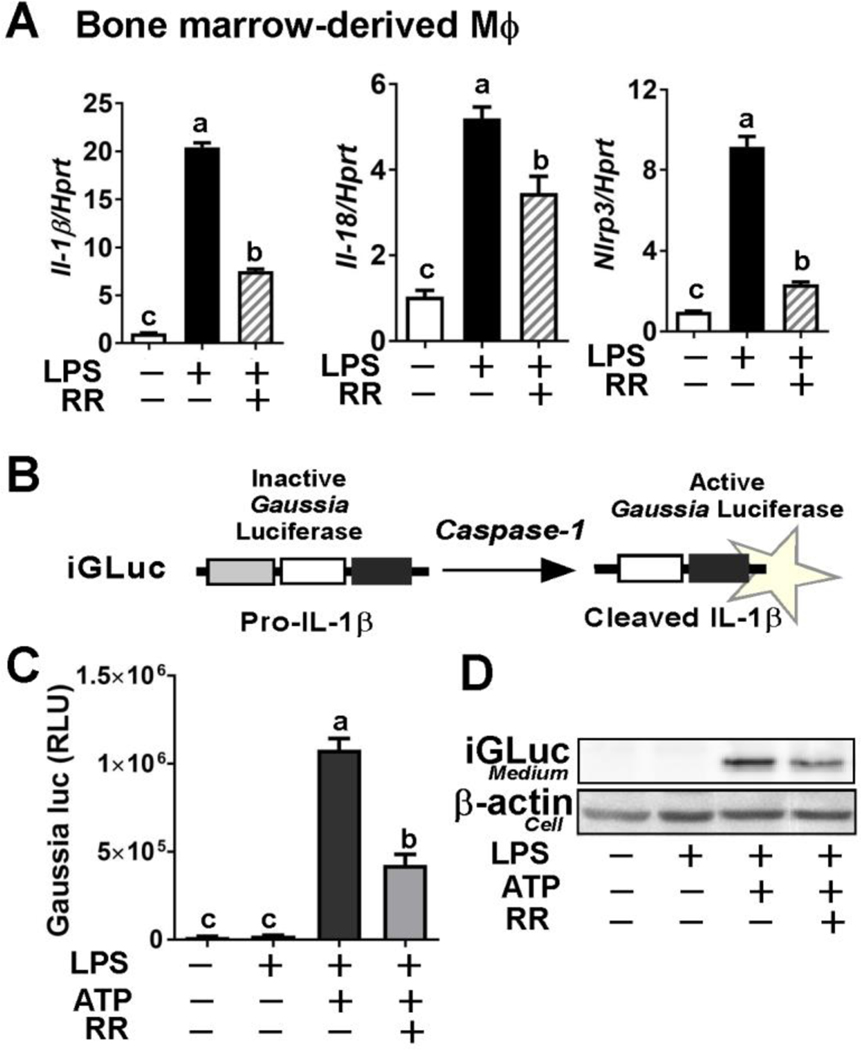
Next, we assessed the ability of whole RR polyphenols to inhibit NLRP3 inflammasome activation in vitro. Incubation with RR polyphenols significantly decreased the effects of LPS priming on NLRP3 inflammasome activation in primary BMDM as evidenced by reduced levels of Il-1β, Il-18, and Nlrp3 expression (Fig 4A). We also conducted an IL-1β reporter assay using iJ774 macrophages that constitutively express a pro-IL-1β-Gaussia luciferase fusion construct (Fig 4B) [23]. The IL-1β reporter assay revealed that RR polyphenol treatment (10 μg/ml) significantly reduced Gaussia luminescence induced by LPS/ATP stimulation to activate the NLRP3 inflammasome (Fig 4C). The cleaved iGLuc fragment in the medium was also reduced in the presence of RR treatment (Fig 4D). These results suggest that RR polyphenols possess the ability to downregulate NLRP3 inflammasome activation in vitro by limiting the effects of PAMP/DAMP priming as well as caspase-1 assembly/activation and IL-1β release.
Figure 4. RR polyphenols suppressed activation of NLRP3 inflammasome in vitro.
(A) LPS-induced mRNA expression of Il-1β, Il-18, and Nlrp3 with (+) or without (−) RR polyphenols (10 μg/mL) in BMDM. (B) Structure of the iGLuc (the NLRP3 inflammasome and caspase-1 activity reporter construct) (upper). (C) Relative Gaussia luciferase activity upon NLRP3 inflammasome activation with (+) or without (−) RR polyphenols (10 μg/mL) in iJ774 cells primed by LPS/ATP stimulation. (D) Detection of cleavage iGluc in the medium by Western blot analysis. All values represented as the mean ± SEM. *P<0.05 and ***P<0.001 by Student’s t-test.
Post-translational modification (PTM) of histones via acetylation (ac) and methylation (me) in adipose stem cells serves as a fundamental mechanism for adipogenesis [26]. The key PTM sites of histone H3 including di-methylation at lysine 4 (H3Kme2) and lysine 9 (H3k9me2) and acetylation of lysine 9 (H3K9Ac) and lysine 27 (H3K9Ac) were consistently reduced by HF diet feeding, suggesting the repression of adipogenic potential (Fig 5A). The suppression of PTM marks by HF diet was reversed by polyphenol supplementation from pulp and whole fruit, but not from seed (Fig 5A), suggesting that pulp and, to a lesser extent, whole polyphenols inhibit HF diet-driven ATM infiltration and alter histone PTM modulation in the adipose tissue SV cell fraction.
Next, we tested the hypothesis that NLRP3 inflammasome activation in ATM prevents adipose stem cells from adipogenic differentiation by decreasing chromatin accessibility through posttranslational modification (PTM). We also hypothesized that RR polyphenols can reverse IL1β-mediated epigenetic PTM marks, thereby sustaining adipogenic potential. To evaluate this paracrine crosstalk, we designed a 3×2 conditioned medium experiment using J774 Mϕ and C3H10T1/2 mesenchymal stem cells (Fig 5B). As expected, stimulation with adipocyte differentiating cocktail promotes H3K27Ac in C3H10T1/2 cells compared to unstimulated cells (Fig 5C in lane 1,2 vs. 3,4). Conditioned medium from unstimulated Mϕ (medium I) had no impact on H3K27Ac of differentiating C3H10T1/2 cells regardless of RR polyphenol presence or absence (Fig 5C, in lane 5,6 vs. 7,8). However, conditioned medium collected from inflammasome-stimulated Mϕ (medium II) significantly reduced H3K27Ac in C3H10T1/2 cells, which was reversed when C3H10T1/2 cells were pre-treated with RR polyphenols (Fig 5D, lane 5,6 vs. 7,8). Lastly, conditioned medium collected from inflammasome-stimulated Mϕ in the presence of RR polyphenols (medium III), showed only a marginal impact on H3K27Ac in the C3H10T1/2 cells irrespective of RR polyphenol exposure (Fig 5E in lane 5,6 vs. 7,8). The expression levels of PPARγ2, a key transcription factor for adipocyte differentiation, were paralleled with the H3K27Ac levels (Fig 5C–E). Taken together, these data show that RR polyphenols not only act on macrophages to suppress NLRP3 inflammasome activation but that they also protect mesenchymal stem cells from the inflammasome-mediated suppression of PTM required for initiation of adipogenesis.
3. Discussion
The red raspberry (RR) has been studied extensively for its ability to limit obesity, inflammation, and insulin resistance [13, 27, 28]. These health benefits are thought to be primarily mediated by the polyphenolic compounds present in this fruit. To obtain a comprehensive understanding of RR polyphenols as a metabolic modifier, we prepared the three polyphenolic fractions from pulp, seed, and whole fruit, and assessed their potential differential impacts on glucose tolerance, energy expenditure, innate immunity, and adipose tissue remodeling. Here, we demonstrated that pulp and whole polyphenols, but not seed, suppressed HF-induced adipose tissue remodeling by blocking NLRP3 inflammasome activation in ATM and its paracrine crosstalk with adipose stem cells. Moreover, our unique study design revealed a novel function of RR polyphenols as an epigenetic modifier in controlling adipocyte size and adipogenesis.
The polyphenolic compounds in RR are key contributors to attenuate the risk of chronic diseases [29]. Based on the thorough analysis by Sójka M et al., the polyphenolic constituents of entire RR fruits include ellagitannins (64.2%), anthocyanins (17.1%), flavanols (16.9%), and flavonols (1.8%) [25]. After pressing, anthocyanins are predominantly found in juice fraction (65%), while most ellagitannins are found in press-cake (seed plus seedless fraction). The seed fraction contains the highest content of ellagitannins, while the seedless fraction retains a substantial amount of ellagitannins and flavanols [25], consistent with our polyphenolic fractions of pulp and seed (Table 1). In vivo and in vitro studies have shown that cyanidin-based anthocyanins and ellagitannins are the primary polyphenolic compounds mediating the health benefits of RR [30]. In terms of obesity control by RR, Luo et al. demonstrated that supplementation (10% of total kcal) of RR juice or puree concentrate significantly reduced HF diet-induced obesity, insulin resistance, and hepatic steatosis. The authors speculated that RR phytochemicals and/or their metabolites might be the significant bioactive molecules that induce metabolic improvement [31]. Recently, Zhao et al. reported metabolic benefits of consuming RR by supplementing with a freeze-dried powder of whole RR fruits containing ~1% of polyphenols in gallic acid equivalent (GAE). Zhao et al. demonstrated that 5% (w/w) RR powder supplementation reduces HF diet-driven insulin resistance and inflammation in muscles in an AMP kinase activation-dependent manner [32]. Despite their careful execution, this work cannot tease out contributions from other confounding factors such as dietary fibers and sugar. To fill this gap, we removed fibers and sugars and concentrated the RR polyphenols from whole fruit, pulp, and seed. All fractions contained roughly 30% of polyphenols in GAE (Table 1). For a fair comparison, the total polyphenolic content in our diet preparation was in the comparable range (0.3–1.2 mg GAE/kg) with the aforementioned work by Zhao et al. (0.5 mg GAE/kg diet).
Among the polyphenolic compounds of RR, we and others have extensively investigated the role of ellagic acid in energy metabolism. We previously reported the metabolic benefits of RR seed flour against HF diet-induced obesity [11]. Hence, it was unexpected that supplementation with seed polyphenol fractions (H+S) failed to exert metabolic benefits despite the similar ellagic acid content in the pulp supplemented diet (H+P) (See Table S1). The following two possibilities could explain these findings: 1) The acid/methanol extraction of RR seed may release unidentified polyphenols that counteract the metabolic benefits of ellagic acids. The existence of these seed polyphenols could provide one potential explanation as to why whole fruit polyphenol supplementation (H+W) was less potent than pulp polyphenol feeding. 2) The reduced ratio of anthocyanins to ellagic acid may decrease the bioavailability of ellagic acid. This possibility is partially supported by our unpublished data showing that pure ellagic acid (synthetic) supplementation showed only minimal to no effects on HF-induced obesity and inflammation.
The RR pulp polyphenol fraction, which contains anthocyanins and ellagic acid in a 2:1 ratio (Table 1), notably attenuated HF diet-driven obesity and inflammation. In particular, we focused on the ability of pulp polyphenols to modulate activation of the NLRP3 inflammasome, a potent innate immune sensor in response to cell-threatening signaling [2]. Insightfully, Zhu et al. demonstrated that 5% RR supplementation attenuated HF diet (60% kcal from fat)-driven hepatic steatosis through suppression of the NLRP3 inflammasome [12]. These authors reported that RR polyphenols decreased caspase-1 cleavage as well as reduced IL-1β and IL-18 gene expression, indicating NLRP3 inflammasome suppression in the liver. In addition to limiting DAMP-associated NLRP3 inflammasome activation, 10–15 μg/mL of ethanol-extracted RR was also potent at suppressing pathogen (E. coli O157:H7)-mediated caspase-1 cleavage and IL-1β maturation in enterocytes [14]. In our current research design (45% kcal from fat, Table S1, Fig 1A), NLRP3 inflammasome activation was only detectable in the adipose tissue (cleaved caspase p10 and ASC speck formation as surrogate markers), which was markedly attenuated by feeding pulp and whole polyphenol fractions (Fig 3A). In this work, we confirmed the ability of RR polyphenols to specifically inhibit both NLRP3 priming (Fig 4A) and assembly for IL-1β secretion (Fig 4C, D). Notably, anthocyanins are the primary constituents of pulp polyphenols. There is insufficient evidence to suggest that anthocyanins pose a direct impact on adiposity control. However, numerous studies have reported that anthocyanins from berries modulate both insulin sensitivity and inflammation (reviewed in [33]). To support our results, cyanidin-3-glucoside (C3G), a major anthocyanin in RR pulp polyphenols, has been shown to attenuate NLRP3 inflammasome activation in the eye epithelium [34].
Unlike the finding by Zhu et al. [12], the caspase-1 cleavage was not detectable in the liver nor pancreas in the present study (data not shown), presumably due to intensity of insult from dietary fat (i.e., 60% vs. 45% fat diet). Nonetheless, pulp and whole polyphenols definitively reduced pro-inflammatory responses, ER stress, and TG accumulation in the liver (Supplement Fig 1) as well as in the BAT and pancreas (Supplement Fig 2). Collectively, our study is the first to reveal that RR polyphenols per se potently suppress NLRP3 inflammasome activation and contribute to metabolic improvement.
Adipocyte size is coordinately regulated by two factors 1) the balance between FA lipogenesis and lipolysis, and 2) the potential for adipogenesis from adipogenic stem cells [4]. Given the reduced adipocyte size in the rank order of H, H+S > H+W > H+P (Fig 2A), we now consider the benefits of RR pulp polyphenols against a HF diet with respect to lipogenesis/lipolysis and adipogenesis. The enlargement of adipocyte is a hallmark of obesity-driven adipose tissue inflammation, which promotes basal lipolysis and ATM recruitment while suppressing new fat cell formation [35]. Conversely, enhanced adipogenesis in adiponectin transgenic mice prevents HF-diet induced adipocyte hypertrophy and inflammation [36]. Moreover, multiple clinical studies have demonstrated an inverse relationship between adipocyte hypertrophy and adipogenesis [37, 38]. The paradoxical reduction of lipogenic proteins in enlarged adipocytes is also linked with augmented basal lipolysis [39, 40]. Supporting this notion, pulp polyphenols normalized the HF diet-driven reduction in the expression of PPARγ and its target proteins including adiponectin (Fig 2D, Table 3). The prevention of perilipin loss by RR polyphenols seems to contain TG within the adipocytes, thus protecting from inflammation-mediated lipolysis and HF-driven lipid spillover. This notion is parallel to our results showing that plasma levels of glycerol were significantly lower in pulp polyphenol-fed mice (Table 3).
Healthy expansion of adipocytes includes new fat cell formation with proper vasculature [41]. Although numerous studies have investigated the effects of individual polyphenolic compounds on adipocyte differentiation in primary adipose stem cells or 3T3L1 preadipocytes [42], in vitro results may not precisely reflect in vivo conditions. Wang et al. reported NLRP3 inflammasome activation upon LPS/palmitate treatment induced the selective differentiation of human mesenchymal stem cells into adipocytes [43], which disagrees with our current view of NLRP3 inflammasome activation as an inhibitory factor for adipogenesis. Taking into consideration that ATM is the primary cellular source for NLRP3 inflammasome and IL-1β secretion in adipose tissue, inclusion of Mϕ may be more reasonable to simulate in vivo conditions. Our strategy was to evaluate the crosstalk between ATM and adipose stem cells in the presence of polyphenols. We also selected the modulation of epigenetic histone marks as an outcome measure for early adipogenesis, because dynamic changes in chromatin accessibility by histone modulation is critical for programming adipose stem cells into adipocytes [44, 45]. We demonstrated that RR polyphenols reverse HF diet-mediated suppression of H3K27Ac in vivo (Fig 5A) as well as in vitro (Fig 5B–E). Intriguingly, incubating adipose stem cells with only RR polyphenols seemed to confer resistance to IL-1β-mediated suppression of H3K27Ac (Fig 5C, lane 5, 6 versus 7, 8), suggesting that RR polyphenol may downregulate the IL-1 receptor (IL1R). However, additional studies are required to evaluate the effectiveness of RR polyphenols in controlling IL1R signaling pathways. Taken together, our results suggest that RR polyphenols not only act to reduce Mϕ inflammation, but also regulate adipocyte size, lipogenesis/lipolysis balance, and adipogenic epigenetic cues.
The metabolic benefits of anthocyanins and ellagic acid are linked with catabolic conversion into bioavailable forms by gut microbes in the colon [46]. Recently, we reported that direct administration of urolithin A, a major gut metabolite from ellagic acid, into the bloodstream is capable of attenuating HF diet-induced obesity and insulin resistance [18], while pure ellagic acid is ineffective in modulating these phenotypes (unpublished). Our in vitro study did not consider any pharmacokinetic or gut microbial catabolism of RR polyphenols, which constitutes potential caveats of the current study. To address this issue, we are currently conducting similar experiments using the germ-free mice in vivo, and measuring the NLRP3 inflammasome suppression activity in vitro by using the gut metabolites of RR polyphenols.
In summary, we demonstrated that RR polyphenols fractionated from pulp, and to a lesser extent, whole fruits, ameliorated HF diet-driven obesity, inflammation, and insulin resistance. We highlighted the novel function of NLRP3 in reversing NLRP3 inflammasome activation in ATM and its paracrine crosstalk with adipogenic stem cells for adipogenic control. The significance of our findings is well-supported by human clinical trials showing that NLRP3 inflammasome activation in visceral fat is a metabolic culprit mediating metainflammation [47], and that a dietary intervention with whole RR is effective in promoting insulin sensitivity in prediabetes and insulin-resistant humans [48]. Our present study provides a novel mechanism by which RR polyphenols per se reverse obesity-mediated adipose tissue dysfunction, thereby supporting the intake of RR or RR-containing agricultural products as an effective dietary strategy to circumvent obesity-mediated metabolic complications.
Supplementary Material
Acknowledgment
This study is supported by a USDA-NIFA grant awarded to S.C. and A.R.T. (2017–67017-26781), a USDA-Hatch grant at the University of Nebraska-Lincoln awarded to S.C., and in part by 1R21HD094273 award to S.C. We acknowledge equipment support from the Biomedical and Obesity Core (BORC) at the University of Nebraska-Lincoln. The BORC in the Nebraska Center for Prevention of Obesity Diseases (NPOD) receives partial support from NIH NIGMS COBRE IDeA award NIH 1P20GM104320.
Abbreviations:
- ASC
apoptosis-associated speck-like protein containing a CARD
- ATM
adipose tissue macrophage
- eWAT
epididymal white adipose tissue
- HF
high fat
- iGLuc
pro-IL-1β-Gaussia luciferase (iGLuc) reporter construct
- Mϕ
macrophage
- GAE
gallic acid equivalent
- NLRP3
Nod-like receptor protein 3
- PTM
posttranscriptional modification
- RR
red raspberry
- SV
stromal vascular
Footnotes
Conflict of interest statement
The authors declared no conflict of interest.
5 References
- [1].Russo L, Lumeng CN, Immunology. 2018, 155, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Elliott EI, Sutterwala FS, Immunol Rev. 2015, 265, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D, Metabolism. 2017, 74, 1. [DOI] [PubMed] [Google Scholar]
- [4].Camp HS, Ren D, Leff T, Trends Mol Med. 2002, 8, 442. [DOI] [PubMed] [Google Scholar]
- [5].Liu LF, Craig CM, Tolentino LL, Choi O, Morton J, Rivas H, Cushman SW, Engleman EG, McLaughlin T, PLoS One. 2017, 12, e0170728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perez LM, Bernal A, de Lucas B, San Martin N, Mastrangelo A, Garcia A, Barbas C, Galvez BG, PLoS One. 2015, 10, e0123397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP, Nat Immunol. 2011, 12, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shen L, Yang Y, Ou T, Key CC, Tong SH, Sequeira RC, Nelson JM, Nie Y, Wang Z, Boudyguina E, Shewale SV, Zhu X, J Lipid Res. 2017, 58, 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim Y, Wang W, Okla M, Kang I, Moreau R, Chung S, J Lipid Res. 2016, 57, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tozser J, Benko S, Mediators Inflamm. 2016, 2016, 5460302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kang I, Espin JC, Carr TP, Tomas-Barberan FA, Chung S, J Nutr Biochem. 2016, 32, 64. [DOI] [PubMed] [Google Scholar]
- [12].Zhu MJ, Kang Y, Xue Y, Liang X, Garcia MPG, Rodgers D, Kagel DR, Du M, J Nutr Biochem. 2018, 53, 96. [DOI] [PubMed] [Google Scholar]
- [13].Noratto GD, Chew BP, Atienza LM, Food Chem. 2017, 227, 305. [DOI] [PubMed] [Google Scholar]
- [14].Xue Y, Du M, Zhu M-J, Journal of Functional Foods. 2019, 56, 224. [Google Scholar]
- [15].Gourineni V, Shay NF, Chung S, Sandhu AK, Gu L, J Agric Food Chem. 2012, 60, 7674. [DOI] [PubMed] [Google Scholar]
- [16].Sandhu AK, Gray DJ, Lu J, Gu L, Food Chemistry. 2011, 126, 982. [Google Scholar]
- [17].Reagan-Shaw S, Nihal M, Ahmad N, Faseb j. 2008, 22, 659. [DOI] [PubMed] [Google Scholar]
- [18].Toney AM, Fan R, Xian Y, Chaidez V, Ramer-Tait AE, Chung S, Obesity (Silver Spring). 2019, 27, 612. [DOI] [PubMed] [Google Scholar]
- [19].Kim Y, Natarajan SK, Chung S, Mol Nutr Food Res. 2018, 62, e1800519. [DOI] [PubMed] [Google Scholar]
- [20].Galarraga M, Campion J, Munoz-Barrutia A, Boque N, Moreno H, Martinez JA, Milagro F, Ortiz-de-Solorzano C, J Lipid Res. 2012, 53, 2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hausman DB, Park HJ, Hausman GJ, Methods Mol Biol. 2008, 456, 201. [DOI] [PubMed] [Google Scholar]
- [22].Zhao L, Kang I, Fang X, Wang W, Lee MA, Hollins RR, Marshall MR, Chung S, Int J Obes (Lond). 2015, 39, 438. [DOI] [PubMed] [Google Scholar]
- [23].Bartok E, Bauernfeind F, Khaminets MG, Jakobs C, Monks B, Fitzgerald KA, Latz E, Hornung V, Nat Methods. 2013, 10, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tang QQ, Otto TC, Lane MD, Proc Natl Acad Sci U S A. 2004, 101, 9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sojka M, Macierzynski J, Zaweracz W, Buczek M, J Agric Food Chem. 2016, 64, 5549. [DOI] [PubMed] [Google Scholar]
- [26].Mortada I, Mortada R, Eur J Med Genet. 2018, 61, 114. [DOI] [PubMed] [Google Scholar]
- [27].Beekwilder J, Jonker H, Meesters P, Hall RD, van der Meer IM, Ric de Vos CH, J Agric Food Chem. 2005, 53, 3313. [DOI] [PubMed] [Google Scholar]
- [28].Noratto G, Chew BP, Ivanov I, Food Funct. 2016, 7, 4944. [DOI] [PubMed] [Google Scholar]
- [29].Burton-Freeman BM, Sandhu AK, Edirisinghe I, Adv Nutr. 2016, 7, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kang I, Buckner T, Shay NF, Gu L, Chung S, Adv Nutr. 2016, 7, 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luo T, Miranda-Garcia O, Sasaki G, Shay NF, Food Funct. 2017, 8, 4081. [DOI] [PubMed] [Google Scholar]
- [32].Zhao L, Zou T, Gomez NA, Wang B, Zhu MJ, Du M, Nutr Diabetes. 2018, 8, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee YM, Yoon Y, Yoon H, Park HM, Song S, Yeum KJ, Nutrients. 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jin X, Wang C, Wu W, Liu T, Ji B, Zhou F, J Immunol Res. 2018, 2018, 5604610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr., J Clin Invest. 2003, 112, 1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE, J Clin Invest. 2007, 117, 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Acosta JR, Douagi I, Andersson DP, Backdahl J, Ryden M, Arner P, Laurencikiene J, Diabetologia. 2016, 59, 560. [DOI] [PubMed] [Google Scholar]
- [38].Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U, Am J Physiol Endocrinol Metab. 2009, 297, E999. [DOI] [PubMed] [Google Scholar]
- [39].Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M, American Journal of Physiology-Endocrinology And Metabolism. 2002, 282, E46. [DOI] [PubMed] [Google Scholar]
- [40].Gao X, van der Veen JN, Hermansson M, Ordoñez M, Gomez-Muñoz A, Vance DE, Jacobs RL, Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2015, 1851, 152. [DOI] [PubMed] [Google Scholar]
- [41].Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD, J Plast Reconstr Aesthet Surg. 2016, 69, 170. [DOI] [PubMed] [Google Scholar]
- [42].Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen C-L, The Journal of nutritional biochemistry. 2014, 25, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang L, Chen K, Wan X, Wang F, Guo Z, Mo Z, Biochem Biophys Res Commun. 2017, 484, 871. [DOI] [PubMed] [Google Scholar]
- [44].Okamura M, Inagaki T, Tanaka T, Sakai J, Organogenesis. 2010, 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kasinska MA, Drzewoski J, Sliwinska A, Arch Med Sci. 2016, 12, 1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ludwig IA, Mena P, Calani L, Borges G, Pereira-Caro G, Bresciani L, Del Rio D, Lean ME, Crozier A, Free Radic Biol Med. 2015, 89, 758. [DOI] [PubMed] [Google Scholar]
- [47].Koenen TB, Stienstra R, van Tits LJ, Joosten LA, van Velzen JF, Hijmans A, Pol JA, van der Vliet JA, Netea MG, Tack CJ, Stalenhoef AF, de Graaf J, Endocrinology. 2011, 152, 3769. [DOI] [PubMed] [Google Scholar]
- [48].Xiao D, Zhu L, Edirisinghe I, Fareed J, Brailovsky Y, Burton-Freeman B, Obesity (Silver Spring). 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.