Abstract
Background
Early recognition of xeroderma pigmentosum is important to minimize the complications arising from the harmful effects of exposure to ultraviolet radiation. This narrative review aims to familiarize physicians with the clinical features, diagnosis and management of xeroderma pigmentosum.
Methods
A search was conducted in December 2021 in PubMed Clinical Queries using the key term “xeroderma pigmentosum”. The search strategy included all clinical trials, observational studies and reviews published within the past 10 years. The information retrieved from the search was used in the compilation of this article.
Results
Xeroderma pigmentosum is a condition of abnormal DNA repair of ultraviolet radiation-induced and oxidative DNA damage, which leads to increased skin cancer susceptibility. Approximately 50% of patients with xeroderma pigmentosum have increased photosensitivity and certain types of xeroderma pigmentosum are more prone to ocular disease and progressive neurodegeneration depending on the causative mutation. The diagnosis should be suspected in patients with increased photosensitivity and characteristic cutaneous, ophthalmological and neurological findings. A definite diagnosis can be made by the identification of biallelic mutation in one of the causative genes. Strict and consistent sun avoidance and protection and early detection and treatment of premalignant and malignant skin lesions are the mainstays of management. Treatment options for actinic keratosis include cryotherapy, topical imiquimod, topical 5-fluorouracil, chemical peeling, excision, CO2 laser resurfacing, fractional/pulsed laser therapy, and photodynamic therapy. Cutaneous malignancy can be treated by photodynamic therapy, curettage and electrodesiccation, or surgical excision. Oral isotretinoin, oral niacinamide, topical imiquimod and topical fluorouracil can be used for the prevention of skin malignancy. Treatment options for poikiloderma include chemical peeling, dermabrasion and laser resurfacing. Methylcellulose eyedrops and soft ultraviolet-protective contact lenses may be used to keep the cornea moist and protect against the harmful effects of keratitis sicca. Investigational therapies include the use of T4 endonuclease-V liposome lotion and oral nicotinamide to reduce the rate of actinic keratoses and non-melanoma skin cancers and gene therapy for radical cure of this condition.
Conclusion
Although currently there is no cure for xeroderma pigmentosum, increased awareness and early diagnosis of the condition, followed by rigorous sun avoidance and protection and optimal management, can dramatically improve the quality of life and life expectancy.
Keywords: conjunctival injection, conjunctival neovascularization, neurodegeneration, photophobia, poikiloderma, skin cancers, xeroderma pigmentosum
Introduction
Xeroderma pigmentosum (XP) was first described in 1874 by Moritz Kohn Kaposi, a Hungarian professor of dermatology, who reported two patients with thin, dry skin, skin contraction, checkered pigmentation, dilatation of cutaneous blood vessels, and development of multiple cutaneous tumours at a young age.1 Kaposi coined the term ‘xeroderma pigmentosum’ to denote the characteristic ‘dry and pigmented skin’. XP is an autosomal recessive inherited genodermatosis due to mutations in genes involved in the DNA repair machinery, leading to deficient repair of DNA damaged by ultraviolet radiation (UVR).2,3 The condition can manifest as photosensitivity and increased skin cancer susceptibility. Certain types of XP are more prone to ocular disease and progressive neurodegeneration, dependent on the causative mutation.4,5 Early recognition of XP is important so that avoidance and protection from UVR can be initiated early to minimize the complications arising from the harmful effects of UVR.
Methods
A search was conducted in December 2021 in PubMed Clinical Queries using the key term “xeroderma pigmentosum”. The search strategy included all clinical trials (including open trials, non-randomized controlled trials, and randomized controlled trials), observational studies and reviews (including narrative reviews and meta-analyses) published within the past 10 years. Only papers published in the English literature were included in this review. The information retrieved from the search was used in the compilation of this article.
Review
Aetiopathogenesis
When DNA is exposed to UVR, nucleic acid-based photoproducts (e.g. pyrimidine 6–4 pyrimidone dimers and cyclobutane pyrimidine dimers) result, which are amendable to DNA repair via the nucleotide excision repair (NER) process.6–10 NER is capable of removing these nucleic acid-based photoproducts and replacing damaged DNA with new DNA.11,12 NER is a central pathway safeguarding cells and genomes damaged by UVR.9 Two types of NER are recognized: the transcription-coupled (TC)-NER and the global genome (GG)-NER.6 TC-NER repairs actively transcribed DNA whilst GG-NER repairs DNA not undergoing transcription at that time.13,14 Eight NER proteins (XPA, XPB, XPC, XPD, XPE, XPF, XPG and XPV) and their genes have been identified.2 XPA–XPG are involved in different steps of the NER in the presence of DNA damage whereas XPV is involved in the postreplication repair of damaged DNA.2 Based on the mutation of the specific gene, XP can be divided into seven complementation groups (XPA, XPB, XPC, XPD, XPE, XPF and XPG) and an XP variant (XPV).5,11,15 Generally, patients with XP are unable to clear the UVR-induced photoproducts or employ NER to mend UVR damage to the DNA.6 Those with XPV have normal NER but have mutations in the translational DNA Pol η gene.16 Pol η is involved in DNA synthesis and allows transcription past UVR-damaged DNA that has not been repaired by NER, in a process known as trans-lesion synthesis.5,6,14,16 As such, the ability to replicate DNA after UVR damage is impaired in individuals with XPV.17,18 The cutaneous features of XP, their progression and patients’ propensity to early cancer result from an accumulation of UVR-induced photoproducts and unrepaired DNA damage.2 Oxidative stress and the cumulative oxidative DNA damage in neurons are responsible for neurodegeneration.2
XP is inherited as an autosomal recessive trait with 100% penetrance.4,19 As such, absence of a family history of XP does not preclude the diagnosis.
Incidence
XP affects all races with a worldwide incidence of 1 in 250,000 live births.20,21 The incidence of XP is estimated to be 1, 2.3, 17.5 and 45 per million live births in the United States, Western Europe, Middle East and Japan, respectively.4,6,11,22,23 The incidence is increased in areas where consanguinity is common.24 The sex ratio is approximately equal.5 Worldwide, the subtypes XPA, XPC and XPV account for approximately 75% of all cases of XP whilst XPV alone accounts for approximately 30% of cases.11 XPC is the most common subtype in the United States, Europe and Africa whilst XPA is the most common subtype in China and Japan.6,11,24–26
Histopathology
Histological findings include increased melanin and melanocytes in the basal cell layer, hyperkeratosis, lymphocytic infiltrate in the upper dermis, atrophic and/or elastotic dermis, thinning of the stratum malpighii with atrophy and/or elongation of the rete, telangiectasia and keratinocyte atypia.5,27,28
Clinical manifestations
XP is characterized by increased photosensitivity, early-onset UVR-induced skin pigmentary changes, UVR-induced damage to the eyes, an increased risk of cutaneous tumour development and, in some cases, progressive neurological degeneration.29,30 Many risk factors can exacerbate the cutaneous manifestations, including chronic exposure to UVR, sunny weather, poor protection from sunlight and fair skin.28 Clinical manifestations vary and are influenced by the precise gene mutation and environmental factors such as cumulative exposure to UVR.14,31,32
The appearance of the skin of patients with XP is usually normal at birth.11,33 However, the skin has an extreme sensitivity to UVR and is soon damaged with minimal exposure to UVR.20 This may manifest as severe or exaggerated sun tanning, burning, or blistering upon minimal sun exposure.11 Erythema may persist for weeks in about 60% of cases.34 XP subtypes XPA, XPB, XPD, XPF and XPG are associated with severe and exaggerated sunburning after minimal sun exposure.4,6,35,36 On the other hand, patients with subtypes XPC, XPE and XPV have less severe sunburning and can even tan after minimal sun exposure; yet, affected patients still develop abnormal skin pigmentation, including freckles and lentigos.4,6,35 Marked freckling-like skin changes typically present before the age of 2 years on sun-exposed areas.4,6,11 Over time, the skin undergoes premature aging, with progressive xerosis, atrophy, wrinkling, telangiectasia, early-onset lentigos which increase in size, number and colour, and poikiloderma (Figures 1–7).4,6,31,37,38 The hypopigmented areas may represent mutated melanocytes that have lost their ability to produce melanin.20 Actinic keratoses are observed at an early age, and actinic cheilitis is not uncommon.11,23,39
Figure 1.
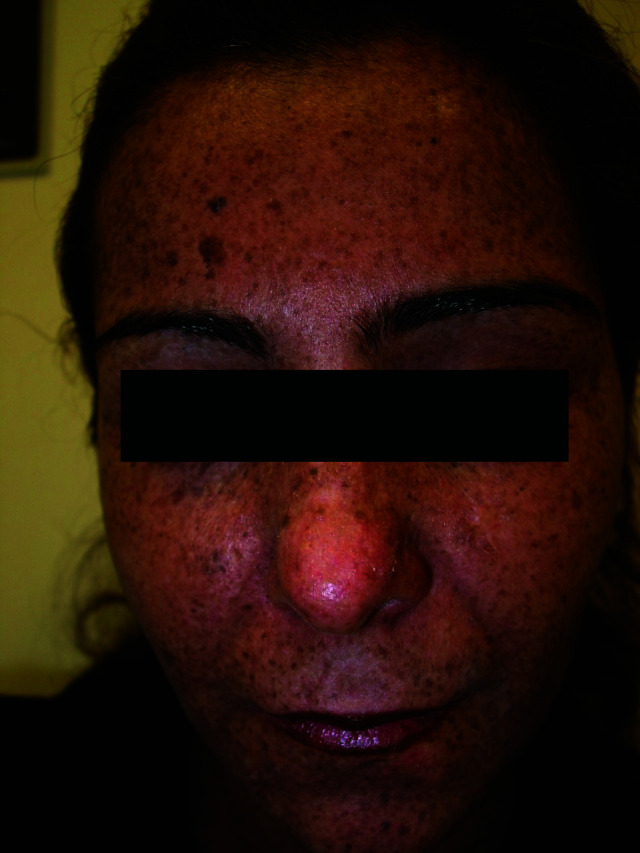
Multiple pigmented freckles, papules, and dryness on the face characteristic of xeroderma pigmentosum.
Figure 2.
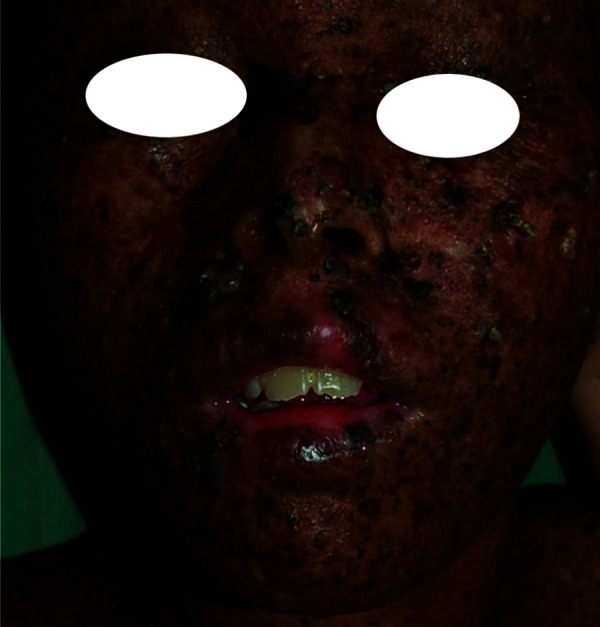
Xeroderma pigmentosum presenting with dry, warty papules and mottled hyperpigmented and hypopigmented macules on the face.
Figure 3.
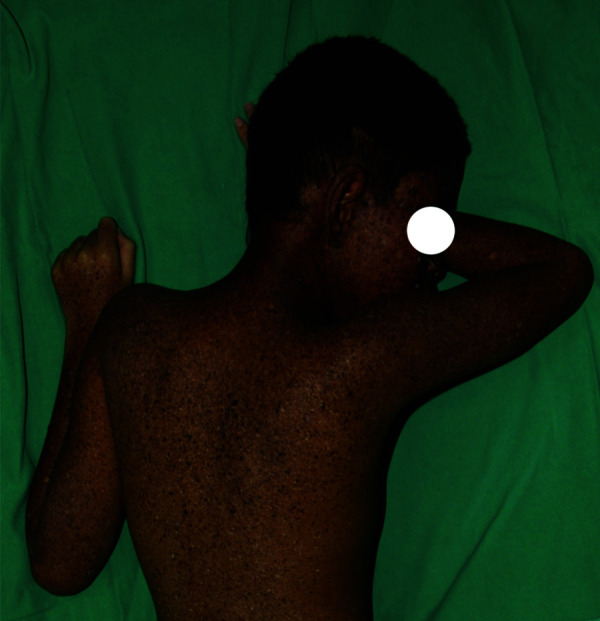
Multiple dry, scaly, mottled hyperpigmented and hypopigmented macules giving rise to the characteristic ‘salt and pepper appearance’ on the neck and upper back.
Figure 4.
Excessive freckling and hypopigmented macules on the forearm.
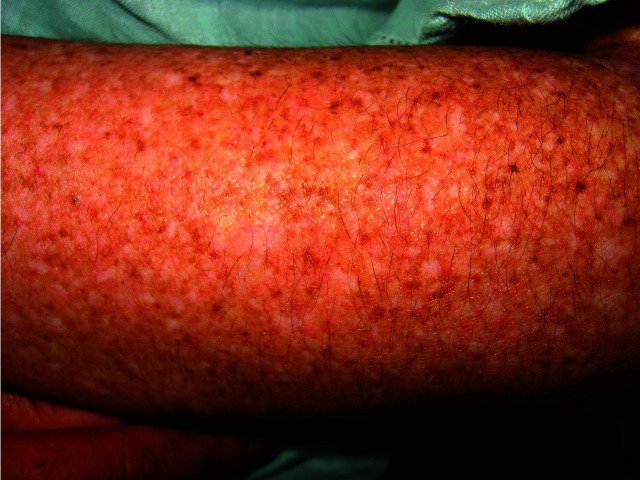
Figure 5.
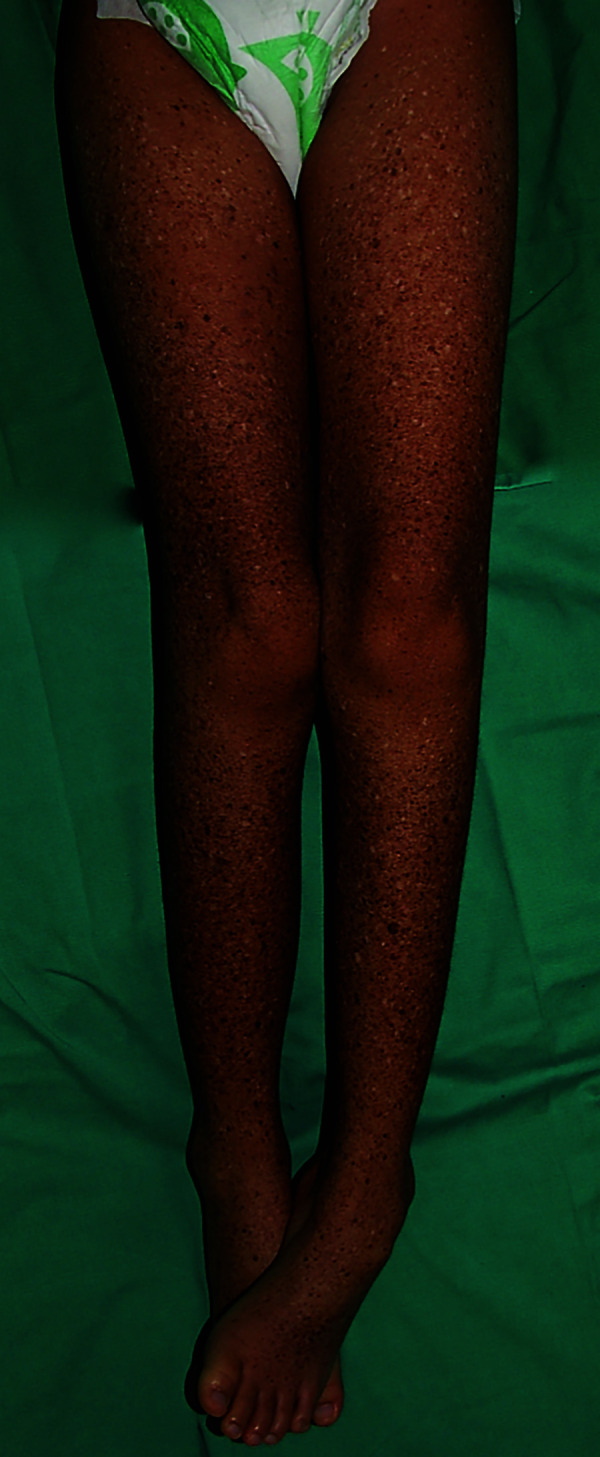
Multiple, dry, scaly, mottled hyperpigmented and hypopigmented macules on the lower limbs.
Figure 6.

Premature aging of the skin with xerosis, atrophy, lentigines, and intermixed hyperpigmented and hypopigmented areas characteristic of xeroderma pigmentosum.
Figure 7.
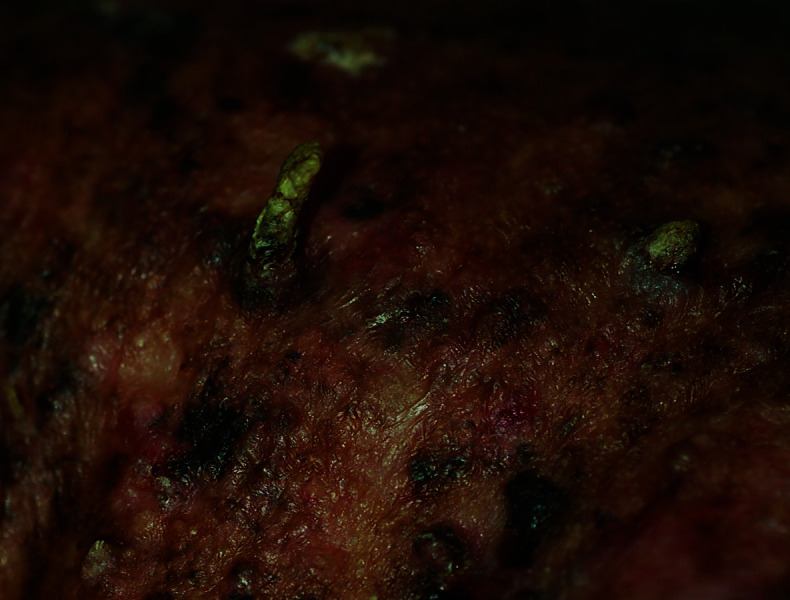
Severe poikiloderma with skin atrophy, xerosis, telangiectasias, cutaneous horns, disfiguring scars, lentigines, and intermixed hyperpigmented and hypopigmented areas.
The anterior parts of the eye (conjunctiva, lens, cornea and eyelids) are particularly susceptible to damaging effects of UVR in patients with XP.6,40 The posterior parts of the eye are protected by the lens which acts as a barrier to UVR.13 Approximately 90% of patients with XP have ocular involvement.14,39 Common ophthalmological manifestations include photophobia, conjunctival xerosis, blepharospasm, prominent conjunctival injection, conjunctival neovascularization (Figure 8) and conjunctival melanosis; findings are usually obvious in the first decade of life.6,14,22,41 Other ophthalmological manifestations include irregularities in eyelid border, increased pigmentation of the eyelids with loss of eyelashes, trichiasis, blepharitis, ectropion, entropion, lagophthalmos, symblepharon, lacrimal point stenosis, blepharitis, keratitis, punctate keratopathy, band keratopathy, keratoconjunctivitis, corneal opacification (Figure 8), corneal ulceration and corneal scarring, pinguecula, pterygium, fibrovascular pannus of the cornea, iritis, ciliary body hamartoma, cataracts, macular oedema, chorioretinal adhesion, retinal degeneration with pigment migration and optic nerve atrophy.11,14,27,34,41–43 Neuro-ophthalmic abnormalities in the form of minimal pupillary constriction to light, strabismus and abnormal extraocular movements have been reported in 25%, 8% and 7% of patients with XP, respectively.14 Interesting, patients with subtypes XPC, XPE and XPV who have preserved TC-NER have significantly more ophthalmological abnormalities than those with subtypes XPA, XPB, XPD, XPF and XPG who have impaired TC-NER, presumably due to lack of aggressive and early initiation of sun-protective measures in patients with preserved TC-NER.4,14
Figure 8.

Excessive neovascularization and corneal opacification in a child with xeroderma pigmentosum.
The accumulation of 6–4 pyrimidine–pyrimidine dimers and cyclobutene–pyrimidine dimers from UVR exposure is crucial in cutaneous carcinogenesis.12,44,45 Patients with XP develop numerous precancerous actinic keratoses early in life.46,47 Affected patients are at risk for skin and mucous membrane cancers in sun-exposed areas.47,48 The most prevalent skin cancers are squamous cell carcinoma followed by basal cell carcinoma and malignant melanoma.49–53 Patients with XP have a 10,000-fold and 2000-fold increased risk over their lives of developing non-melanoma skin cancers (notably squamous cell carcinoma and basal cell carcinoma) and malignant melanoma, respectively.34,54 The mean age for the first appearance of non-melanoma skin cancer and melanoma is 9 and 22 years, respectively, in patients with XP.6,11,55 In contrast, the mean age of onset of UVR-induced non-melanoma skin cancer and melanoma is about 67 and 55 years, respectively, in the general population.31,35,56 Multiple cutaneous malignancies are commonly found on sun-exposed areas in patients with XP.28,57,58 Affected patients with no or minimal protection against UVR may develop hundreds of skin cancers on sun-exposed areas.35 The incidence of intraoral cancers (notably squamous cell carcinoma of the tip of the tongue) is 3000–10,000 higher than in the general population.4,59 Squamous cell carcinoma of the lower lip is also common.60 XP is associated with various ocular surface malignancies, notably squamous cell carcinoma, basal cell carcinoma and melanoma.14,61 The risk of ocular malignancies is increased about 2000-fold.37 Ocular malignancies usually occur in the areas that are exposed to UVR such as the eyelids, conjunctiva and cornea.21 Other skin cancers that occur with increased frequency in patients with XP include keratoacanthoma, epithelioma, sebaceous cell carcinoma, fibrosarcoma and angiosarcoma.27,62,63 Surprisingly, patients without severe sunburn response tend to develop skin cancer earlier than those who have a severe sunburn response, presumably due to less strict sun-protective measures in the former.35,36,64
Progressive neurodegeneration occurs in approximately 25% of patients with XP.6,34,36,65,66 The most common subtypes associated with neurodegeneration are XPA and XPD, followed by XPB, XPG and XPF.2 Neurodegeneration is rarely seen in patients with XPC and XPE.4 Patients with XPV tend to be spared the neurodegeneration seen in other subtypes.67 Neurological manifestations include acquired microcephaly, progressive cognitive impairment, deterioration of neurological status, progressive sensorineural hearing loss, speech delay, dysarthria, ataxia, diminished or absent deep tendon stretch reflexes, loss of ability to walk, spasticity, dyskinesia, chorea, dysphagia, neurogenic bladder, dysuria, supranuclear ophthalmoplegia and seizures.68–75 These manifestations are progressive and irreversible and may be attributed to loss of neurons in the cerebrum and cerebellum, primary axonal degeneration in the peripheral nerves and, occasionally, secondary demyelination.76,77 Oxidative stress may play an important role in damaging the DNA of affected neurons.78
Diagnosis
The diagnosis should be suspected in patients with increased photosensitivity and characteristic cutaneous, ophthalmological and neurological findings (vide supra). A family history of XP or the finding of cutaneous malignancy within the first decade of life further supports the diagnosis. A definite diagnosis can be made by the identification of biallelic pathogenic variants in one of the causative genes.34,79 Molecular testing may include the use of serial single-gene testing, multigene panel and more comprehensive genomic testing.34 In families with a known history of XP, prenatal diagnosis can be made through DNA testing on chorionic villus-derived cells or on amniotic fluid cells from the pregnant mother in the course of an amniocentesis.80
Dermoscopy is a useful non-invasive diagnostic tool to discriminate between benign and malignant lesions in XP.81,82 The presence of an asymmetrical blotch or irregular globules and dots are common dermoscopic features of malignant lesions.82 Malignant melanoma can be differentiated from benign melanocytic nevi by the presence of variegated colours, asymmetry, prominent pigment network, blue-grey areas, blotches, atypical dots/globules, perifollicular pigmentation and follicular obliteration.81,82
Differential diagnosis
The differential diagnosis includes Cockayne syndrome, XP/Cockayne syndrome complex, trichothiodystrophy, XP/trichothiodystrophy complex, Cockayne syndrome/trichothiodystrophy complex, cerebro-oculo-facio-skeletal (COFS) syndrome, UV-sensitive syndrome, Bloom syndrome (also known as Bloom–Torre–Machacek syndrome or congenital telangiectatic erythema), Rothmund–Thomson syndrome, Hartnup disease, Carney complex, De Sanctis–Cacchione syndrome, erythropoietic protoporphyria, cutaneous lupus erythematosus and LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines).83–96 The distinctive features of many of these conditions help to differentiate them from XP.
Complications
Dry parchment-like prematurely aged skin, poikiloderma (constellation of hyperpigmentation, hypopigmentation, atrophy, telangiectasia and keratotic skin lesions) and cutaneous premalignant and malignant lesions in visible areas have been reported by patients as disfiguring and embarrassing.97,98 Affected children are more likely to experience loneliness, sadness, discrimination, shame, ridicule, harassment, teasing, bullying, rejection and abuse.99 The psychological impact can be profound especially those individuals with malignancy and has an adverse effect on the quality of life.45
Patients with XP under 20 years of age have a 50-fold increased risk of developing tumours in the central nervous system, including glioblastoma, medulloblastoma, spinal cord astrocytoma, schwannoma and neurilemoma.6,13,100–102 These malignancies are not UVR-related.13 Haematological malignancies, such as acute lymphoblastic leukaemia, acute myeloid leukaemia, lymphoma and myelodysplastic syndrome, occur with increased frequency in patients with XP.103–106 Patients with XP have a 12-fold increased risk for internal malignancy, such as nasopharyngeal carcinoma, squamous cell carcinoma of the oesophagus, carcinoma of the thyroid, adenocarcinoma of the lung, carcinoma of the breast, carcinoma of the pancreas, leiomyosarcoma of the kidney, cancer of the ovary and cancer of the prostate, suggesting that UVR might not be the only culprit causing malignancy in patients with XP.107–116 Patients with XP are at higher risk of developing smoking-induced cancers.13
Women with XP are at risk for premature menopause.117 In one study of 45 women aged 18 years or older with XP, 14 (31%) had premature menopause (before 40 years of age); the median age of menopause was 49.5 years.117
Patients with subtype XPC are at risk of developing pyogenic granuloma and multinodular thyroid.118
Prognosis
Approximately 60% of patients with XP die before the age of 20 years.12 The median age of death is 32 years.13 Metastatic skin cancer is the leading cause of death, followed by neurodegeneration and internal cancer.34,63,119,120 In one study, the mean age of death in patients with XP with and without neurodegeneration was 29 and 37 years, respectively.65 In general, the prognosis depends on how early sun avoidance and protection are initiated, how appropriate treatment is provided to slow down the progress of disease and its complications, how early malignancy is detected and treated, how severe the disease is, and the mutation of the specific gene. In this regard, patients with XPV typically fare better than patients with other subtypes.11
Management
At present, there is no cure for XP. Therefore, prevention of complications is crucial for this disfiguring and potentially lethal disease. Strict and consistent sun avoidance and protection and early detection and treatment of premalignant and malignant skin lesions are the mainstays of management. These and other treatment options are summarized in Box 1. Regular paediatric, dermatological, ophthalmological and neurological follow-up is essential.
Box 1. Treatment options for xeroderma pigmentosum .
-
Sun avoidance and sun protection
Sun avoidance
Sun protection (regular use of broad-spectrum sunscreens)
-
Chemoprevention of skin cancers
Oral isotretinoin
Topical imiquimod
Topical fluorouracil
-
Treatment of poikiloderma
Chemical peeling
Dermabrasion
Carbon dioxide (CO2) or erbium-YAG laser resurfacing
-
Treatment of actinic keratosis
Cryotherapy
Topical imiquimod
Topical 5-fluorouracil
Chemical peeling
Excision
CO2 or erbium-YAG laser resurfacing
Fractional/pulsed laser therapy
Photodynamic therapy
-
Treatment of skin cancers
Photodynamic therapy
Curettage with electrodessication
Aggressive cryosurgery
Surgical excision
Oral vismodegib, pembrolizumab, nivolumab and cemiplimab
-
Ocular management
Methylcellulose eyedrops
Soft UV-protective contact lens
-
For ocular surface squamous neoplasia
Surgical resection and intraoperative cryotherapy
Subconjunctival injection of IFNα2b with topical cycles of mitomycin C eyedrops
-
Neurological management
May need hearing aids, speech therapy, physical therapy and occupational therapy
No effective treatment for neurodegeneration
-
Investigational therapies
Topical T4 endonuclease-V
Oral vismodegib
Oral nicotinamide
Oral Polypodium leucotomos extract
Oral coenzyme Q10
Gene therapy
Genetic counselling
Psychological counselling
Sun avoidance and sun protection
If possible, outdoor activities should be restricted to either before sunrise or after sunset. If exposure to the sun is inevitable, patients should wear protective wide-brimmed hats, long-sleeved clothing, UV-resistant face masks and UV-absorbing/blocking sunglasses with side shields, regularly use (preferably every 2 hours) broad-spectrum sunscreens with a sun protection factor of 30 or preferably greater to all exposed skin and lip balms to lips and use UV-resistant films on windows in cars and buildings.11,35,121 Patients with XP should also be protected from unfiltered fluorescent light, metal halide lamps and halogen lights that can emit UVR;31,121 such light sources should be shielded.
Sunscreens can be physical or chemical. Physical sunscreens containing microfine particles of zinc oxide, titanium dioxide and red ferric oxide help to reflect and block UVR.5 Chemical sunscreens containing benzophenones mainly block UV-A whilst those containing avobenzone, cinnamates, salicylates and para-amino benzoic acid mainly block UV-B and absorb both UV-A and UV-B.5 Broad-spectrum chemical sunscreens contain a combination of ingredients effective to block both UV-A and UV-B and absorb both UV-A and UV-B that have not been blocked.
Unfortunately, adherence to photoprotection varies widely and is a significant issue.122,123 Indeed, it is difficult to achieve full photoprotection throughout one’s lifetime. For example, the use of broad-spectrum sunscreens on all sun-exposed skin every 2 hours, as some investigators have advised, can be quite expensive. In a cross-sectional survey of patients (n=156) with XP in the United States, United Kingdom, France and Germany, one-third of patients did not achieve optimal face photoprotection.123
As UVR, in particular UV-B radiation, is needed for the production of vitamin D in the skin from 7-dehydrocholesterol, individuals under strict sun protection may have vitamin D deficiency.124,125 Affected patients should be encouraged to consume foods (e.g. eggs, fish and fortified foods) rich in vitamin D.11 Vitamin D supplementation is recommended for individuals with low serum concentration of vitamin D.4,34 Failure to provide vitamin D supplements in patients with XP may result in rickets.126,127
Chemoprevention of skin cancers
High-dose (2 mg/kg/day) oral isotretinoin (13-cis-retinoic acid) can be used to reduce the number of skin tumours.33,128 Adverse events associated with the use of oral isotretinoin include photosensitivity, myalgias, arthralgias, xerostomia, xerophthalmia, palmoplantar desquamation, alopecia, corneal opacities, delayed wound healing, pseudotumour cerebri, bone marrow suppression, hepatotoxicity, hyperlipidaemia, periostitis, hyperostosis and teratogenicity.129 As such, high-dose oral isotretinoin should only be used in severely affected patients with a particularly high number of newly developed skin tumours.31 Some affected patients, however, may respond to an intermediate dose (1 mg/kg/day) or lower dose (0.5 mg/kg/day) of oral isotretinoin with less adverse events.34 Historically, some physicians have used acitretin for chemoprevention. The disadvantage of using acitretin for chemoprevention is its long half-life if esterified to etretinate and is therefore suboptimal for women of childbearing age.
Topical imiquimod and/or fluorouracil have been used with success for the prevention of skin malignancy in patients with XP.128,130 The medications should be used prophylactically at the earliest onset of symptoms such as xerosis, dyspigmentation, actinic keratoses, or at the very early sign of skin malignancy.4 The most common adverse events associated with the use of these two medications are erythema and pain at the site of application.
Treatment of poikiloderma
Treatment options for poikiloderma in patients with XP include chemical peeling (exfoliation), dermabrasion and carbon dioxide (CO2) or erbium-YAG laser resurfacing.128,131 Chemical peeling is preferred to dermabrasion. Topical application of trichloroacetic acid induces superficial coagulation of the proteins in the skin with consequent degradation of the epidermis and upper dermis, leading to activation of regenerative processes that trigger the renewal of damaged skin.5 Nowadays, trichloroacetic acid is the gold standard of chemical peeling.5 The use of a camouflage cream/make up may also be considered for cosmetic purposes.132
Treatment of premalignant skin lesions and skin cancers
Patients with XP should be screened regularly (every 3–6 months) for early detection and treatment of a wide range of cutaneous and ocular surface malignancies.130 In this regard, colour photographs documentation of the entire skin surface and close-up photographs (including a ruler) of individuals lesions are important in early detection and follow-up of cutaneous malignancy. Computerized mole mapping (e.g. Fotofinder) ± artificial intelligence should also be considered.
Premalignant lesions, such as actinic keratosis, should be treated as early as possible to prevent the progression to squamous cell carcinoma. Treatment options for actinic keratosis include cryotherapy using liquid nitrogen, topical imiquimod, topical 5-fluorouracil, chemical peeling (e.g. trichloroacetic acid, phenol-based peeping solution), excision, CO2 or erbium-YAG laser resurfacing, fractional/pulsed laser therapy and photodynamic therapy (e.g. methyl aminolevulinate or 5-aminolevulinic acid as a photosensitizer).22,133,134
Cutaneous malignancy can be treated by photodynamic therapy, curettage with electrodessication, aggressive cryosurgery or surgical excision depending on the type of skin cancer; the latter is the treatment of choice.133,135 Cutaneous malignancies that are recurrent or in places with a high risk of recurrence, such as on the face, are best treated with Mohs micrographic surgery.34,55 Historically IFNα provided modest benefit in the treatment of metastatic melanoma but newer therapies (e.g. kinase inhibitors and immunotherapeutic antibodies) are far more beneficial now.22 Radiation therapy should be avoided in patients with XP. More recently, oral vismodegib (hedgehog pathway inhibitor), pembrolizumab (PD-1 inhibitor), nivolumab (PD-1 inhibitor) and cemiplimab (PD-1 inhibitor) have also been used to treat non-melanoma skin cancers and melanomas and the preliminary results are encouraging.119,120,136–143 Electrochemotherapy may be considered for the treatment of advanced skin cancer.128
Ocular management
Methylcellulose eyedrops and soft UV-protective contact lens may be used to keep the cornea moist and protect against the harmful effects of keratitis sicca.4 In addition, soft UV-protective contact lens may protect against mechanical trauma in patients with deformed eyelids.34 Follow-up by ophthalmologists every 3–6 months is recommended for patients with XP.22 Corneal transplantation can be used to restore vision in patients with severe keratitis with corneal opacity.4,34
Surgical resection and intraoperative cryotherapy are the traditional treatments for ocular surface squamous neoplasia.144 Adverse events associated with surgical resection include conjunctival scarring, stem cell deficiency and recurrence due to inadequate resection of the neoplasm.144,145 Recent studies have shown the successful use of subconjunctival injection of IFNα2b at the site of lesions with topical cycles of mitomycin C eyedrops for the primary or adjuvant treatment of ocular surface squamous neoplasia.144,145 Both topical IFNα2b eyedrops and topical 5-fluorouracil eyedrops have also been used with success for the treatment of ocular surface squamous neoplasia with comparable results.145–147 It is hoped that the previous findings can be confirmed by future studies, thereby circumventing the need for surgical intervention.
Neurological management
Affected patients with neurodegeneration may need hearing aids, speech therapy, physical therapy and occupational therapy. Regular follow-up by neurology and otorhinolaryngology is recommended.
To date, there is no effective treatment for neurodegeneration. In a study of progeroid mice deficient in the DNA NER gene Ercc1, a 30% dietary caloric restriction tripled the lifespan of these mice and delayed numerous aspects of premature aging, including attenuation of neuron loss.148 The authors speculated that caloric restriction might increase the resistance to stress-induced damage to the DNA, raise antioxidant defences and alter metabolic signalling to induce a shift from the production of pro-inflammatory cytokines to anti-inflammatory cytokines.120,148 At present, the relevance of this finding in humans is not known.
Investigational therapies
T4 endonuclease-V, a bacterial prokaryotic DNA repair enzyme, in a topical liposome-containing preparation has been shown to repair cyclobutene–pyrimidine dimers resulting from UVR-induced DNA damage.4,37 The formulation is able to penetrate the stratum corneum to reach the dermis. T4 endonuclease-V is highly efficient in providing temporary ratification of the underlying DNA repair defect in the skin of patients with XP and clinical trials have produced promising results.128 In a multicentre, double-blind study, Yarosh et al. randomly assigned patients (n=20) to receive daily application of topical T4 endonuclease-V liposome lotion or a placebo liposome lotion (n=10) for 1 year.149 At the end of the study period, the annualized rate of new actinic keratoses was 8.2% and 25.9% amongst patients assigned T4 endonuclease-V liposome lotion and a placebo liposome lotion, respectively (95% CI 11.8–26.5; p=0.004). The annualized rate of new basal cell carcinoma was 3.8% and 5.4% amongst patients assigned T4 endonuclease-V liposome lotion and a placebo liposome lotion, respectively. A 2019 phase II clinical trial confirmed the efficacy of T4 endonuclease-V liposome lotion in reducing the rate of actinic keratoses and basal cell carcinoma onset in sun-damaged skin.5,134 Thus, T4 endonuclease-V liposome lotion can be an effective treatment for patients with XP without significant adverse events.5
Oral vismodegib, a hedgehog pathway inhibitor, has been approved by the FDA for the treatment of locally advanced basal cell carcinoma or metastatic basal cell carcinoma.150 The medication can also be used for the treatment of basal cell carcinoma in individuals who are not suitable candidates for topical or surgical treatment.148 The recommended dose of oral vismodegib is 150 mg/day. Adverse effects include alopecia, muscle spasm, arthralgia, dysgeusia, ageusia, fatigue, decreased appetite, weight loss and electrolyte disturbances.150 As vismodegib is a teratogen, its use during pregnancy is contraindicated.4,150 Thus far, there are no published studies on the use and efficacy of oral vismodegib in patients with XP.
Several studies have shown that nicotinamide (vitamin B3) 500 mg twice a day given orally can enhance the repair of UVR-induced DNA damage in human keratinocytes and therefore may have a role in the prevention of actinic keratoses and non-melanoma skin cancers.151–155 Nicotinamide enhances the two pathways of DNA repair, namely, TC-NER and GG-NER.154 It has been shown that patients on oral nicotinamide have a reduced number of actinic keratoses on sun-damaged skin.155,156 Chen et al. randomized 386 immunocompetent patients who had at least two non-melanoma skin cancers in the previous 5 years to receive either 500 mg of nicotinamide or placebo twice per day. At 12-month follow-up, the rate of new non-melanoma skin cancers was lower by 23% in the nicotinamide group than in the placebo group (95% CI 4–38; p=0.02).151 The number of actinic keratoses was 11%, 14% and 20% lower in the nicotinamide group than in the placebo group at 3 months (p=0.01), 6 months (p<0.001) and 9 months (p<0.001), respectively.151 There were no significant adverse effects amongst treated patients. The authors concluded that oral nicotinamide was safe and effective in reducing the rates of new non-melanoma skin cancers and actinic keratoses in patients at high risk.
Studies have shown that oral Polypodium leucotomos extract, derived from a tropical fern of the Polypodiaceae family, has photoprotective properties through its chemoprotective, antioxidative, anti-inflammatory and immunomodulatory effects.157–159 As such, Polypodium leucotomos extract has the potential to be used as an adjunctive treatment to lessen the phototoxic effects of UVR to reduce UVR-induced skin damage and skin cancers. So far, no studies on the use of oral nicotinamide or Polypodium leucotomos extract have been conducted on patients with XP presumably because of the difficulty in recruiting enough numbers of patients for the study.
It has been shown that patients with XP have lower serum concentrations of coenzyme Q10 (COQ10) and the concentrations of COQ10 tend to decrease with age.2,160 In a non-randomized study, daily administration of 0.9–1.5 mg/kg of COQ10 improved the daily activity of a subset of patients with XP.160 It is hoped that future, well-designed, randomized, double-blind, placebo-controlled trials will provide more information on the efficacy and safety profile of COQ10 in patients with XP.
Gene therapy has opened a large opportunity for the treatment of XP. One approach is to use a retroviral or adenoviral vector to transfer functional DNA genes into both keratinocytes and skin fibroblasts of patients with XP to restore their capacity for NER.4,20,130 Another approach for target therapy is based on non-viral carriers such as engineered site-specific nucleases (meganuclease, zinc finger nuclease, transcription activator-like effector-nuclease) or CRISPR–Cas9 to correct skin stem cells.161–163 Although promising, gene therapy has to undergo significant further development and technical implementations before it can be tested clinically for the treatment of patients with XP.
Genetic counselling
Genetic counselling is indicated for all patients with XP. This is especially important in a family with an affected child and if parents are considering more children. The risk of having another affected child and normal child is each 25% whilst 50% of children are asymptomatic carriers. Genetic testing of at-risk siblings allows early diagnosis and sun avoidance/protection from an early age.34
Psychological counselling
Psychosocial issues such as social isolation, peer rejection, discrimination and limited career prospects need to be addressed. Psychological counselling and support should be offered as appropriate.
Conclusion
Although currently there is no radical cure for XP, numerous options are available for the prevention and treatment of skin problems, including malignancies. As such, the diagnosis of XP should be made as early as possible so as to initiate protective measures at an early age. Together with early resection of malignant lesions, this can improve quality of life and increase life expectancy. Further research is necessary to determine the optimal management of XP, in particular the role and implementation of gene therapy for the treatment of this condition.
Acknowledgements
None.
Footnotes
Contributions: AKCL is the principal author. BB, JML, KFL and KLH are coauthors who contributed and helped with the drafting of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: AKCL and KLH are associate editors of Drugs in Context and confirm that this article has no other conflicts of interest otherwise. This manuscript was sent out for independent peer review. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/04/dic.2022-2-5-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2022 Leung AKC, Barankin B, Lam JM, Leong KF, Hon KL. https://doi.org/10.7573/dic.2022-2-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Hebra F, Kaposi M. On diseases of the skin including exanthemata. Volume III. New Sydenham Soc. 1874;61:252–258. [Google Scholar]
- 2.Abeti R, Zeitlberger A, Peelo C, et al. Xeroderma pigmentosum: overview of pharmacology and novel therapeutic strategies for neurological symptoms. Br J Pharmacol. 2019;176(22):4293–4301. doi: 10.1111/bph.14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava G, Srivastava G. Xeroderma pigmentosum. Oxf Med Case Reports. 2021;2021(11):omab107. doi: 10.1093/omcr/omab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenfield LF, Warner CG. Corona R, editor. Xeroderma pigmentosum. UpToDate. [Accessed 30 December 2021]. https://www.uptodate.com/contents/xeroderma-pigmentosum .
- 5.Piccione M, Belloni Fortina A, et al. Xeroderma pigmentosum: general aspects and management. J Pers Med. 2021;11(11):1146. doi: 10.3390/jpm11111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black JO. Xeroderma pigmentosum. Head Neck Pathol. 2016;10(2):139–144. doi: 10.1007/s12105-016-0707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleaver JE. Diagnosis of xeroderma pigmentosum and related DNA repair-deficient cutaneous diseases. Curr Med Lit Dermatol. 2008;13(2):41–48. [PMC free article] [PubMed] [Google Scholar]
- 8.Gratchev A. The nucleotide excision repair of DNA in human cells and its association with xeroderma pigmentosum. Adv Exp Med Biol. 2008;637:113–119. doi: 10.1007/978-0-387-09599-8_12. [DOI] [PubMed] [Google Scholar]
- 9.Koch SC, Simon N, Ebert C, Carell T. Molecular mechanisms of xeroderma pigmentosum (XP) proteins. Q Rev Biophys. 2016;49:e5. doi: 10.1017/S0033583515000268. [DOI] [PubMed] [Google Scholar]
- 10.Martens MC, Emmert S, Boeckmann L. Xeroderma pigmentosum: gene variants and splice variants. Genes. 2021;12(8):1173. doi: 10.3390/genes12081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucero R, Horowitz D. StatPearls. StatPearls Publishing; [Accessed 23 March 2022]. Xeroderma pigmentosum. https://www.ncbi.nlm.nih.gov/books/NBK551563/ [PubMed] [Google Scholar]
- 12.Naik SM, Shenoy AM, Nanjundappa A, et al. Cutaneous malignancies in xeroderma pigmentosum: earlier management improves survival. Indian J Otolaryngol Head Neck Surg. 2013;65(2):162–167. doi: 10.1007/s12070-012-0614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fassihi H. Spotlight on ‘xeroderma pigmentosum’. Photochem Photobiol Sci. 2013;12(1):78–84. doi: 10.1039/c2pp25267h. [DOI] [PubMed] [Google Scholar]
- 14.Lim R, Sethi M, Morley AMS. Ophthalmic manifestations of xeroderma pigmentosum: a perspective from the United Kingdom. Ophthalmology. 2017;124(11):1652–1661. doi: 10.1016/j.ophtha.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Bennett D, Itoh T. The XPE gene of xeroderma pigmentosum, its product and biological roles. Adv Exp Med Biol. 2008;637:57–64. doi: 10.1007/978-0-387-09599-8_7. [DOI] [PubMed] [Google Scholar]
- 16.Masutani C, Hanaoka F, Ahmad SI. Xeroderma pigmentosum variant, XP-V: its product and biological roles. Adv Exp Med Biol. 2008;637:93–102. doi: 10.1007/978-0-387-09599-8_10. [DOI] [PubMed] [Google Scholar]
- 17.Fang X, Sun Y. Whole-exome sequencing enables the diagnosis of variant-type xeroderma pigmentosum. Front Genet. 2019;10:495. doi: 10.3389/fgene.2019.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang N, Fu X, Chen X, Chen L, Wang M. Variant subtype of xeroderma pigmentosum with multiple basal cell carcinomas diagnosed in a Chinese woman. Photodermatol Photoimmunol Photomed. 2021;37(2):161–164. doi: 10.1111/phpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christen-Zaech S, Imoto K, Khan SG, et al. Unexpected occurrence of xeroderma pigmentosum in an uncle and nephew. Arch Dermatol. 2009;145(11):1285–1291. doi: 10.1001/archdermatol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichon V, Khachemoune A. Xeroderma pigmentosum: beyond skin cancer. J Drugs Dermatol. 2007;6(3):281–288. [PubMed] [Google Scholar]
- 21.Webb S. Xeroderma pigmentosum. BMJ. 2008;336(7641):444–446. doi: 10.1136/bmj.39485.698356.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriwaki S, Kanda F, Hayashi M, et al. Xeroderma pigmentosum clinical practice guidelines. J Dermatol. 2017;44(10):1087–1096. doi: 10.1111/1346-8138.13907. [DOI] [PubMed] [Google Scholar]
- 23.Yuniati R, Sihombing NRB, Nauphar D, et al. Clinical manifestation and genetic analysis of familial rare disease genodermatosis xeroderma pigmentosum. Intractable Rare Dis Res. 2021;10(2):114–121. doi: 10.5582/irdr.2020.03143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhutto AM, Kirk SH. Population distribution of xeroderma pigmentosum. Adv Exp Med Biol. 2008;637:138–143. doi: 10.1007/978-0-387-09599-8_15. [DOI] [PubMed] [Google Scholar]
- 25.Yokoi T, Enomoto Y, Uehara T, Kosaki K, Kurosawa K. A Japanese girl with mild xeroderma pigmentosum group D neurological disease diagnosed using whole-exome sequencing. Hum Genome Var. 2020;7:22. doi: 10.1038/s41439-020-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Cheng R, Yu X, Sun Z, Li M, Yao Z. Expansion of the genotypic and phenotypic spectrum of xeroderma pigmentosum in Chinese population. Photodermatol Photoimmunol Photomed. 2017;33(1):58–63. doi: 10.1111/phpp.12283. [DOI] [PubMed] [Google Scholar]
- 27.Norgauer J, Idzko M, Panther E, Hellstern O, Herouy Y. Xeroderma pigmentosum. Eur J Dermatol. 2003;13(1):4–9. [PubMed] [Google Scholar]
- 28.Singh AP, Ansari M, Shukla AK. Basal cell carcinoma with xeroderma pigmentosum in an 8-year-old girl. J Indian Assoc Pediatr Surg. 2019;24(4):314–316. doi: 10.4103/jiaps.JIAPS_162_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feller L, Wood NH, Motswaledi MH, Khammissa RA, Meyer M, Lemmer J. Xeroderma pigmentosum: a case report and review of the literature. J Prev Med Hyg. 2010;51(2):87–91. [PubMed] [Google Scholar]
- 30.Partarrieu-Mejías F, Pérez-Velásquez F. Image gallery: xeroderma pigmentosum. Br J Dermatol. 2016;174(3):e12. doi: 10.1111/bjd.14396. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann AR, McGibbon D, Stefanini M. Xeroderma pigmentosum. Orphanet J Rare Dis. 2011;6:70. doi: 10.1186/1750-1172-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fassihi H, Sethi M, Fawcett H, et al. Deep phenotyping of 89 xeroderma pigmentosum patients reveals unexpected heterogeneity dependent on the precise molecular defect. Proc Natl Acad Sci USA. 2016;113(9):E1236–E1245. doi: 10.1073/pnas.1519444113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butt FM, Moshi JR, Owibingire S, Chindia ML. Xeroderma pigmentosum: a review and case series. J Craniomaxillofac Surg. 2010;38(7):534–537. doi: 10.1016/j.jcms.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Kraemer KH, DiGiovanna JJ. Xeroderma pigmentosum. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A, editors. GeneReviews®. University of Washington; Seattle: [Accessed 23 March 2022]. https://www.ncbi.nlm.nih.gov/books/NBK1397/ [PubMed] [Google Scholar]
- 35.Tamura D, DiGiovanna JJ, Khan SG, Kraemer KH. Living with xeroderma pigmentosum: comprehensive photoprotection for highly photosensitive patients. Photodermatol Photoimmunol Photomed. 2014;30(2–3):146–152. doi: 10.1111/phpp.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sethi M, Lehmann AR, Fawcett H, et al. Patients with xeroderma pigmentosum complementation groups C, E and V do not have abnormal sunburn reactions. Br J Dermatol. 2013;169(6):1279–1287. doi: 10.1111/bjd.12523. [DOI] [PubMed] [Google Scholar]
- 37.Hengge UR, Emmert S. Clinical features of xeroderma pigmentosum. Adv Exp Med Biol. 2008;637:10–18. doi: 10.1007/978-0-387-09599-8_2. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann J, Schubert S, Emmert S. Xeroderma pigmentosum: diagnostic procedures, interdisciplinary patient care, and novel therapeutic approaches. J Dtsch Dermatol Ges. 2014;12(10):867–872. doi: 10.1111/ddg.12419. [DOI] [PubMed] [Google Scholar]
- 39.Lopes-Cardoso C, Paes da Silva Ramos Fernandes LM, Ferreira-Rocha J, et al. Xeroderma pigmentosum - a case report with oral implications. J Clin Exp Dent. 2012;4(4):e248–e251. doi: 10.4317/jced.50727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasanthapuram VH, Kaliki S. Conjunctival melanoma in patients with xeroderma pigmentosum: a series of four cases. Int Ophthalmol. 2020;40(5):1143–1146. doi: 10.1007/s10792-019-01279-2. [DOI] [PubMed] [Google Scholar]
- 41.Schelini MC, Chaves LFOB, Toledo MC, et al. Xeroderma pigmentosum: ocular findings in an isolated Brazilian group with an identified genetic cluster. J Ophthalmol. 2019;2019:4818162. doi: 10.1155/2019/4818162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaurasia S. Differential corneal involvement in xeroderma pigmentosum. Indian J Ophthalmol. 2018;66(11):1623. doi: 10.4103/ijo.IJO_667_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- 44.Awan BA, Alzanbagi H, Samargandi OA, Ammar H. Scalp squamous cell carcinoma in xeroderma pigmentosum. N Am J Med Sci. 2014;6(2):105–106. doi: 10.4103/1947-2714.127754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Probert A, Bailey C, Ahrns H, Seiverling E. Optimizing medical trips to care for those with rare genetic diseases in remote settings: lessons learned from xeroderma pigmentosum. Int J Dermatol. 2017;56(5):e104–e105. doi: 10.1111/ijd.13586. [DOI] [PubMed] [Google Scholar]
- 46.Daya-Grosjean L. Xeroderma pigmentosum and skin cancer. Adv Exp Med Biol. 2008;637:19–27. doi: 10.1007/978-0-387-09599-8_3. [DOI] [PubMed] [Google Scholar]
- 47.Khan MA, Akbar N, Saeed A, Amir A, Ikram A, Saleem Z. Xeroderma pigmentosum associated with squamous and basal cell carcinoma in Pakistan: a case series. Adv Skin Wound Care. 2021;34(11):608–612. doi: 10.1097/01.ASW.0000792924.09969.64. [DOI] [PubMed] [Google Scholar]
- 48.Saleh W, Elansary M. First report of oral angiokeratoma in a xeroderma pigmentosum. Int J Surg Case Rep. 2021;88:106513. doi: 10.1016/j.ijscr.2021.106513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dartaha R, Benmelouka AY, Jobran AWM. A case of lip squamous cell carcinoma with a familial history of xeroderma pigmentosum. Oral Oncol. 2020;111:104896. doi: 10.1016/j.oraloncology.2020.104896. [DOI] [PubMed] [Google Scholar]
- 50.Emir S, Hacısalihoğlu Ş, Özyörük D, Kaçar D, Erdem A, Karakuş E. Squamous cell carcinoma associated with Xeroderma pigmentosum: an unusual presentation with a tremendously huge mass over the face and paraneoplastic hypercalcemia-hyperleukocytosis. Turk J Pediatr. 2017;59(6):711–714. doi: 10.24953/turkjped.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 51.Kaloga M, Dioussé P, Diatta BA, et al. Squamous cell carcinoma in African children with xeroderma pigmentosum: three case reports. Case Rep Dermatol. 2016;8(3):311–318. doi: 10.1159/000452438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shankar R, Qureshi SS, Sugoor P, Kembhavi S, Yadav PS, Mukta R. Colossal squamous cell carcinoma of the face in a child with xeroderma pigmentosum. J Indian Assoc Pediatr Surg. 2014;19(3):185–186. doi: 10.4103/0971-9261.136485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verma P, Shukla P, Singhai A. Facial giant malignant melanoma in xeroderma pigmentosum. Int J Dermatol. 2020;59:110–111. doi: 10.1111/ijd.14561. [DOI] [PubMed] [Google Scholar]
- 54.Baykal C, Atcı T, Yılmaz Z, Büyükbabani N. Skin tumors in xeroderma pigmentosum: evaluation of a large series and a literature review. J Cutan Pathol. 2021;48(7):884–895. doi: 10.1111/cup.13979. [DOI] [PubMed] [Google Scholar]
- 55.de Andrade FAG, Cavalcanti CEO, Isoldi FC, Ferreira LM. Therapeutics of xeroderma pigmentosum: a PRISMA-compliant systematic review. Indian J Dermatol Venereol Leprol. 2021;87(2):176–189. doi: 10.25259/IJDVL_431_19. [DOI] [PubMed] [Google Scholar]
- 56.Min JA, Lee JY, Cho BK, Park HJ. Significance of long-term follow-up in xeroderma pigmentosum. Int J Dermatol. 2010;49(6):720–722. doi: 10.1111/j.1365-4632.2009.04225.x. [DOI] [PubMed] [Google Scholar]
- 57.Mohanty P, Mohanty L, Devi BP. Multiple cutaneous malignancies in xeroderma pigmentosum. Indian J Dermatol Venereol Leprol. 2001;67(2):96–97. [PubMed] [Google Scholar]
- 58.Vora RV, Kota RS, Diwan NG. Multiple cutaneous malignancies in a child with xeroderma pigmentosum: a case report. Indian J Med Paediatr Oncol. 2016;37(4):309–311. doi: 10.4103/0971-5851.195750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahindra P, DiGiovanna JJ, Tamura D, et al. Skin cancers, blindness, and anterior tongue mass in African brothers. J Am Acad Dermatol. 2008;59(5):881–886. doi: 10.1016/j.jaad.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bologna SB, Harumi Nakajima Teshima T, Lourenço SV, Nico MMS. An atrophic, telangiectatic patch at the distal border of the tongue: a mucous membrane manifestation of xeroderma pigmentosum. Pediatr Dermatol. 2014;31(2):e38–e41. doi: 10.1111/pde.12272. [DOI] [PubMed] [Google Scholar]
- 61.Kalamkar C, Radke N, Mukherjee A, Radke S. Xeroderma pigmentosum with bilateral ocular surface squamous neoplasia and review of the literature. BMJ Case Rep. 2016;2016:bcr2016215364. doi: 10.1136/bcr-2016-215364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong WJ, Lee SE, Roh MR, Kim JE, Nishigori C, Kim SC. Angiosarcoma arising on the scalp in a Korean patient with xeroderma pigmentosum variant type. Photodermatol Photoimmunol Photomed. 2018;34(5):343–346. doi: 10.1111/phpp.12391. [DOI] [PubMed] [Google Scholar]
- 63.Onishi M, Tsunoda K, Maeda F, Moriwaki S, Amano H. Angiosarcoma of the auricle in a patient with xeroderma pigmentosum variant. Case Rep Dermatol. 2020;12(2):144–149. doi: 10.1159/000508884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraemer KH, Tamura D, Khan SG, Digiovanna JJ. Burning issues in the diagnosis of xeroderma pigmentosum. Br J Dermatol. 2013;169(6):1176. doi: 10.1111/bjd.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradford PT, Goldstein AM, Tamura D, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48(3):168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mercer D, Hurley A, Tsien F. Detailed audiological evaluation of a patient with xeroderma pigmentosum with neural degeneration. J Am Acad Audiol. 2017;28(1):80–90. doi: 10.3766/jaaa.15112. [DOI] [PubMed] [Google Scholar]
- 67.Armenta AM, Massey PR, Khan SG, et al. Variant subtype of xeroderma pigmentosum diagnosed in a 77-year-old woman. JAAD Case Rep. 2018;4(10):1074–1076. doi: 10.1016/j.jdcr.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi O, Miyahara H, Abe N, et al. Progressive dysautonomia in two patients with xeroderma pigmentosum group A. Pediatr Neurol. 2014;50(6):619–621. doi: 10.1016/j.pediatrneurol.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 69.Lehky TJ, Sackstein P, Tamura D, et al. Differences in peripheral neuropathy in xeroderma pigmentosum complementation groups A and D as evaluated by nerve conduction studies. BMC Neurol. 2021;21(1):393. doi: 10.1186/s12883-021-02414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Moreno H, Fassihi H, Sarkany RPE, et al. Xeroderma pigmentosum is a definite cause of Huntington’s disease-like syndrome. Ann Clin Transl Neurol. 2017;5(1):102–108. doi: 10.1002/acn3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salomão RPA, Pedroso JL, Barsottini OGP. Neurological manifestations of xeroderma pigmentosum due to XPA gene mutation. Pract Neurol. 2018;18(6):489–491. doi: 10.1136/practneurol-2018-001888. [DOI] [PubMed] [Google Scholar]
- 72.Shanbhag NM, Geschwind MD, DiGiovanna JJ, et al. Neurodegeneration as the presenting symptom in 2 adults with xeroderma pigmentosum complementation group F. Neurol Genet. 2018;4(3):e240. doi: 10.1212/NXG.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Totonchy MB, Tamura D, Pantell MS, et al. Auditory analysis of xeroderma pigmentosum 1971–2012: hearing function, sun sensitivity and DNA repair predict neurological degeneration. Brain. 2013;136(Pt 1):194–208. doi: 10.1093/brain/aws317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsuchiyama K, Aoki Y, Ito H, Yoneda M, Yokoyama O. Neurogenic bladder associated with xeroderma pigmentosum type A: a case report and literature review. Urol Case Rep. 2019;27:100996. doi: 10.1016/j.eucr.2019.100996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsuji Y, Ueda T, Sekiguchi K, et al. Progressive length-dependent polyneuropathy in xeroderma pigmentosum group A. Muscle Nerve. 2020;62(4):534–540. doi: 10.1002/mus.27028. [DOI] [PubMed] [Google Scholar]
- 76.Lai JP, Liu YC, Alimchandani M, et al. The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D) Acta Neuropathol Commun. 2013;1:4. doi: 10.1186/2051-5960-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Viana LM, Seyyedi M, Brewer CC, et al. Histopathology of the inner ear in patients with xeroderma pigmentosum and neurologic degeneration. Otol Neurotol. 2013;34(7):1230–1236. doi: 10.1097/MAO.0b013e31829795e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor AM. Neurodegeneration in xeroderma pigmentosum. Brain. 2008;131(Pt 8):1967–1968. doi: 10.1093/brain/awn153. [DOI] [PubMed] [Google Scholar]
- 79.Halpern J, Hopping B, Joshua M, Brostoff JM. Photosensitivity, corneal scarring and developmental delay: xeroderma pigmentosum in a tropical country. Cases J. 2008;1(1):254. doi: 10.1186/1757-1626-1-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kleijer WJ, van der Sterre ML, Garritsen VH, Raams A, Jaspers NG. Prenatal diagnosis of xeroderma pigmentosum and trichothiodystrophy in 76 pregnancies at risk. Prenat Diagn. 2007;27(12):1133–1137. doi: 10.1002/pd.1849. [DOI] [PubMed] [Google Scholar]
- 81.Bang E, Kim YE, Ko JM, et al. Lentigo maligna in a patient with xeroderma pigmentosum, variant type: a case report with dermoscopic findings and review of the literature. Photodermatol Photoimmunol Photomed. 2020;36(5):401–404. doi: 10.1111/phpp.12568. [DOI] [PubMed] [Google Scholar]
- 82.Faria Licarião Rocha LK, Ferreira P, Avancini J, et al. Dermoscopic features of 61 skin lesions in xeroderma pigmentosum patients: a cross-sectional study. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 83.Hafsi W, Badri T. StatPearls. StatPearls Publishing; [Accessed 23 March 2022]. Cockayne syndrome. https://www.ncbi.nlm.nih.gov/books/NBK525998/ [Google Scholar]
- 84.Hafsi W, Badri T, Rice AS. StatPearls. StatPearls Publishing; [Accessed 23 March 2022]. Bloom syndrome. https://www.ncbi.nlm.nih.gov/books/NBK448138/ [PubMed] [Google Scholar]
- 85.Hijazi H, Salih MA, Hamad MH, et al. Pellagra-like condition is xeroderma pigmentosum/Cockayne syndrome complex and niacin confers clinical benefit. Clin Genet. 2015;87(1):56–61. doi: 10.1111/cge.12325. [DOI] [PubMed] [Google Scholar]
- 86.Lala SM, Naik H, Balwani M. Diagnostic delay in erythropoietic protoporphyria. J Pediatr. 2018;202:320–323e2. doi: 10.1016/j.jpeds.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lambert WC, Gagna CE, Lambert MW. Xeroderma pigmentosum: its overlap with trichothiodystrophy, Cockayne syndrome and other progeroid syndromes. Adv Exp Med Biol. 2008;637:128–137. doi: 10.1007/978-0-387-09599-8_14. [DOI] [PubMed] [Google Scholar]
- 88.Manavi S, Mahajan VK. Rothmund-Thomson syndrome. Indian Dermatol Online J. 2014;5(4):518–519. doi: 10.4103/2229-5178.142533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Metlo A, Rehan A, Akmal M, Iqbal U, Jamali M. Xeroderma pigmentosum - Cockayne syndrome complex (XP-CS) - another case. J Pak Med Assoc. 2018;68(10):1531–1534. [PubMed] [Google Scholar]
- 90.Natale V, Raquer H. Xeroderma pigmentosum-Cockayne syndrome complex. Orphanet J Rare Dis. 2017;12(1):65. doi: 10.1186/s13023-017-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel AB, Prabhu AS. Hartnup disease. Indian J Dermatol. 2008;53(1):31–32. doi: 10.4103/0019-5154.39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahbar Z, Naraghi M. De Sanctis-Cacchione syndrome: a case report and literature review. Int J Womens Dermatol. 2015;1(3):136–139. doi: 10.1016/j.ijwd.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandru F, Dumitrascu MC, Petca A, Carsote M, Petca RC, Paun DL. Dermatological and endocrine elements in Carney complex (Review) Exp Ther Med. 2021;22(5):1313. doi: 10.3892/etm.2021.10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spivak G. UV-sensitive syndrome. Mutat Res. 2005;577(1–2):162–169. doi: 10.1016/j.mrfmmm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 95.Tsang V, Leung AKC, Lam JM. Cutaneous lupus erythematosus in children. Curr Pediatr Rev. 2021;17(2):103–110. doi: 10.2174/1573396317666210224144416. [DOI] [PubMed] [Google Scholar]
- 96.Wang LL, Plon SE. Rothmund-Thomson syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al., editors. GeneReviews®. University of Washington; Seattle: [Accessed 23 March 2022]. https://www.ncbi.nlm.nih.gov/books/NBK1237/ [Google Scholar]
- 97.Shams MU, Lail RA, Ullah E, Nagi AH. Xeroderma pigmentosum – a disfiguring disease: single patient with 5 simultaneous tumors on face. Oman Med J. 2014;29(3):e073. doi: 10.5001/omj.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zan T, Huang X, Li Q. Severe facial-disfiguring xeroderma pigmentosum with rapidly progressing malignant tumors. JAMA Otolaryngol Head Neck Surg. 2019;145(2):185–186. doi: 10.1001/jamaoto.2018.3218. [DOI] [PubMed] [Google Scholar]
- 99.Anderson R, Walburn J, Morgan M. Experiences of stigma over the lifetime of people with xeroderma pigmentosum: a qualitative interview study in the United Kingdom. J Health Psychol. 2019;24(14):2031–2041. doi: 10.1177/1359105317714643. [DOI] [PubMed] [Google Scholar]
- 100.DiGiovanna JJ, Kraemer KH. Shining a light on xeroderma pigmentosum. J Invest Dermatol. 2012;132(3 Pt 2):785–796. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karass M, Naguib MM, Elawabdeh N, et al. Xeroderma pigmentosa: three new cases with an in depth review of the genetic and clinical characteristics of the disease. Fetal Pediatr Pathol. 2015;34(2):120–127. doi: 10.3109/15513815.2014.982336. [DOI] [PubMed] [Google Scholar]
- 102.Wang LN, Ma MJ, Shi JT. Malignant neurilemoma with xeroderma pigmentosum. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.06.2008.0127. bcr06.2008.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bencharef H, Lamchahab M, Dassouli D, et al. Xeroderma pigmentosum and acute myeloid leukemia: a case report. J Med Case Rep. 2021;15(1):437. doi: 10.1186/s13256-021-02969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oetjen KA, Levoska MA, Tamura D, et al. Predisposition to hematologic malignancies in patients with xeroderma pigmentosum. Haematologica. 2020;105(4):e144–e146. doi: 10.3324/haematol.2019.223370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pintens S, Pierret L, Keymolen K, Gutermuth J, De Raeve L. Xeroderma pigmentosum and leukaemia in two sisters. J Eur Acad Dermatol Venereol. 2016;30(10):e42–e43. doi: 10.1111/jdv.13288. [DOI] [PubMed] [Google Scholar]
- 106.Sarasin A, Quentin S, Droin N, et al. Familial predisposition to TP53/complex karyotype MDS and leukemia in DNA repair-deficient xeroderma pigmentosum. Blood. 2019;133(25):2718–2724. doi: 10.1182/blood-2019-01-895698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bdioui A, Bchir A, Missaoui N, Hamchi H, Hmissa S, Mokni M. Inhabitual presentation of Sertoli-Leydig cell tumor of the ovary with xeroderma pigmentosum: case report with review of literature. Int J Surg Case Rep. 2021;78:288–291. doi: 10.1016/j.ijscr.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boulma R, Ahmed YB, Oumaya M, et al. Xeroderma pigmentosum and renal leiomyosarcoma: a very rare case report association. Int J Surg Case Rep. 2021;78:310–313. doi: 10.1016/j.ijscr.2020.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coulombe J, Orbach D, Soufir N, Hadj-Rabia S. Primary gingival squamous cell carcinoma in a xeroderma pigmentosum type C patient. J Eur Acad Dermatol Venereol. 2016;30(11):e157–e158. doi: 10.1111/jdv.13464. [DOI] [PubMed] [Google Scholar]
- 110.Khatri ML. Xeroderma pigmentosum in Yemen. Int J Dermatol. 2021;60(3):314–320. doi: 10.1111/ijd.15395. [DOI] [PubMed] [Google Scholar]
- 111.Matsumoto M, Kaneshiro K, Takatsuki K. Lung adenocarcinoma concomitant with xeroderma pigmentosum: a case report. J Med Case Rep. 2021;15(1):160. doi: 10.1186/s13256-021-02754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tian Y, Lin X, Yang F, Zhao J, Yao K, Bian C. Contribution of xeroderma pigmentosum complementation group D gene polymorphisms in breast and ovarian cancer susceptibility: a protocol for systematic review and meta analysis. Medicine. 2020;99(21):e20299. doi: 10.1097/MD.0000000000020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Oliveira Viana F, Cavaleiro LH, Carneiro CM, Bittencourt Mde J, Barros RS, Fonseca DM. Do you know this syndrome? Xeroderma pigmentosum (XP) An Bras Dermatol. 2011;86(5):1029. doi: 10.1590/s0365-05962011000500033. [DOI] [PubMed] [Google Scholar]
- 114.Xu JL, Bai J, Jiao JF, et al. Meta-analysis on the association between xeroderma pigmentosum Group A A23G polymorphism and esophageal cancer in a Chinese population. J Cancer Res Ther. 2018;14(Suppl):S1173–S1177. doi: 10.4103/0973-1482.184517. [DOI] [PubMed] [Google Scholar]
- 115.Yan Y, Xu J, Xu B, et al. Effects of xeroderma pigmentosum group C polymorphism on the likelihood of prostate cancer. J Clin Lab Anal. 2020;34(9):e23403. doi: 10.1002/jcla.23403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang ZH, Liang WB, Jia J, Wei YS, Zhou B, Zhang L. The xeroderma pigmentosum group C gene polymorphisms and genetic susceptibility of nasopharyngeal carcinoma. Acta Oncol. 2008;47(3):379–384. doi: 10.1080/02841860701558815. [DOI] [PubMed] [Google Scholar]
- 117.Merideth M, Tamura D, Angra D, et al. Reproductive health in xeroderma pigmentosum: features of premature aging. Obstet Gynecol. 2019;134(4):814–819. doi: 10.1097/AOG.0000000000003490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hadj-Rabia S, Oriot D, Soufir N, et al. Unexpected extradermatological findings in 31 patients with xeroderma pigmentosum type C. Br J Dermatol. 2013;168(5):1109–1113. doi: 10.1111/bjd.12183. [DOI] [PubMed] [Google Scholar]
- 119.Steineck A, Krumm N, Sarthy JF, et al. Response to pembrolizumab in a patient with xeroderma pigmentosum and advanced squamous cell carcinoma. JCO Precis Oncol. 2019;3:PO1900028. doi: 10.1200/PO.19.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weon JL, Glass DA., 2nd Novel therapeutic approaches to xeroderma pigmentosum. Br J Dermatol. 2019;181(2):249–255. doi: 10.1111/bjd.17253. [DOI] [PubMed] [Google Scholar]
- 121.Banda VR, Banda NR, Reddy R, Banda P. Management of a xeroderma pigmentosum case with oral findings in a dental setup. BMJ Case Rep. 2012;2012:bcr2012007521. doi: 10.1136/bcr-2012-007521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sarkany RPE, Canfield M, Morgan M, et al. Ultraviolet exposure to the face in patients with xeroderma pigmentosum and healthy controls: applying a novel methodology to define photoprotection behaviour. Br J Dermatol. 2021 doi: 10.1111/bjd.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walburn J, Canfield M, Norton S, et al. Psychological correlates of adherence to photoprotection in a rare disease: International survey of people with xeroderma pigmentosum. Br J Health Psychol. 2019;24(3):668–686. doi: 10.1111/bjhp.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ali JT, Mukasa Y, Coulson IH. Xeroderma pigmentosum: early diagnostic features and an adverse consequence of photoprotection. Clin Exp Dermatol. 2009;34(3):442–443. doi: 10.1111/j.1365-2230.2008.02865.x. [DOI] [PubMed] [Google Scholar]
- 125.Martens MC, Emmert S, Boeckmann L. Sunlight, vitamin D, and xeroderma pigmentosum. Adv Exp Med Biol. 2020;1268:319–331. doi: 10.1007/978-3-030-46227-7_16. [DOI] [PubMed] [Google Scholar]
- 126.Mohamed A, Bhargava A, Chaurasia S. Vitamin D supplementation in patients with xeroderma pigmentosum. Indian J Ophthalmol. 2019;67(2):308–309. doi: 10.4103/ijo.IJO_1319_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raza N, Ejaz A, Hussain S. Rickets in xeroderma pigmentosum. J Coll Physicians Surg Pak. 2005;15(12):816–818. [PubMed] [Google Scholar]
- 128.Lambert WC, Lambert MW. Development of effective skin cancer treatment and prevention in xeroderma pigmentosum. Photochem Photobiol. 2015;91(2):475–483. doi: 10.1111/php.12385. [DOI] [PubMed] [Google Scholar]
- 129.Leung AK, Barankin B, Lam JM, Leong KF, Hon KL. Dermatology: how to manage acne vulgaris. Drugs Context. 2021;10:2021-8-6. doi: 10.7573/dic.2021-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sarasin A. Progress and prospects of xeroderma pigmentosum therapy. Adv Exp Med Biol. 2008;637:144–151. doi: 10.1007/978-0-387-09599-8_16. [DOI] [PubMed] [Google Scholar]
- 131.Pyun SH, Shim IS, Ahn GB. Xeroderma pigmentosum treated with advanced phenol-based peeling solution. J Eur Acad Dermatol Venereol. 2008;22(7):879–880. doi: 10.1111/j.1468-3083.2007.02476.x. [DOI] [PubMed] [Google Scholar]
- 132.Krishnaswamy G, Kurian SS, Srinivas CR, Kumar LS. Camouflage in xeroderma pigmentosum. Indian Dermatol Online J. 2016;7(6):553–555. doi: 10.4103/2229-5178.193897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Latour I, Hernández-Martín A, Ged C, Knöpfel N, Taïeb A, Torrelo A. Reversed actinic damage in two children with xeroderma pigmentosum treated with topical imiquimod. J Eur Acad Dermatol Venereol. 2018;32(7):e282–e284. doi: 10.1111/jdv.14818. [DOI] [PubMed] [Google Scholar]
- 134.Lozzi F, Lanna C, Mazzeo M, et al. Investigational drugs currently in phase II clinical trials for actinic keratosis. Expert Opin Investig Drugs. 2019;28(7):629–642. doi: 10.1080/13543784.2019.1636030. [DOI] [PubMed] [Google Scholar]
- 135.Cai H, Yang QQ, Ma C, et al. Photodynamic therapy in the treatment of xeroderma pigmentosum: a case report. Photodiagnosis Photodyn Ther. 2020;30:101761. doi: 10.1016/j.pdpdt.2020.101761. [DOI] [PubMed] [Google Scholar]
- 136.Chambon F, Osdoit S, Bagny K, Moro A, Nguyen J, Réguerre Y. Dramatic response to nivolumab in xeroderma pigmentosum skin tumor. Pediatr Blood Cancer. 2018;65(2) doi: 10.1002/pbc.26837. [DOI] [PubMed] [Google Scholar]
- 137.Fife D, Laitinen MA, Myers DJ, Landsteiner PB. Vismodegib therapy for basal cell carcinoma in an 8-year-old Chinese boy with xeroderma pigmentosum. Pediatr Dermatol. 2017;34(2):163–165. doi: 10.1111/pde.13080. [DOI] [PubMed] [Google Scholar]
- 138.Hauschild A, Eichstaedt J, Möbus L, et al. Regression of melanoma metastases and multiple non-melanoma skin cancers in xeroderma pigmentosum by the PD1-antibody pembrolizumab. Eur J Cancer. 2017;77:84–87. doi: 10.1016/j.ejca.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 139.Kraemer KH, Tamura D, Khan SG. Pembrolizumab treatment of a patient with xeroderma pigmentosum with disseminated melanoma and multiple nonmelanoma skin cancers. Br J Dermatol. 2018;178(5):1009. doi: 10.1111/bjd.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 141.Rischin D, Khushalani NI, Schmults CD, et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer. 2021;9(8):e002757. doi: 10.1136/jitc-2021-002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rubatto M, Merli M, Avallone G, et al. Immunotherapy in xeroderma pigmentosus: a case of advanced cutaneous squamous cell carcinoma treated with cemiplimab and a literature review. Oncotarget. 2021;12(11):1116–1121. doi: 10.18632/oncotarget.27966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Soura E, Plaka M, Dessinioti C, et al. Use of vismodegib for the treatment of multiple basal cell carcinomas in a patient with xeroderma pigmentosum. Pediatr Dermatol. 2018;35(6):e334–e336. doi: 10.1111/pde.13610. [DOI] [PubMed] [Google Scholar]
- 144.Dirar QS, Musalem HM, Al-Hazzaa SAF, Al Zoba AA, Almalki AA. Effect of pegylated interferon and mitomycin C on ocular surface squamous neoplasia in xeroderma pigmentosum: a case series. Am J Case Rep. 2020;21:e921301. doi: 10.12659/AJCR.921301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Al Bayyat G, Arreaza-Kaufman D, Venkateswaran N, Galor A, Karp CL. Update on pharmacotherapy for ocular surface squamous neoplasia. Eye Vis. 2019;6:24. doi: 10.1186/s40662-019-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Joag MG, Sise A, Murillo JC, et al. Topical 5-fluorouracil 1% as primary treatment for ocular surface squamous neoplasia. Ophthalmology. 2016;123(7):1442–1448. doi: 10.1016/j.ophtha.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Venkateswaran N, Mercado C, Galor A, Karp CL. Comparison of topical 5-fluorouracil and interferon alfa-2b as primary treatment modalities for ocular surface squamous neoplasia. Am J Ophthalmol. 2019;199:216–222. doi: 10.1016/j.ajo.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vermeij WP, Dollé ME, Reiling E, et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537(7620):427–431. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yarosh D, Klein J, O’Connor A, Hawk J, Rafal E, Wolf P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Xeroderma Pigmentosum Study Group. Lancet. 2001;357(9260):926–929. doi: 10.1016/s0140-6736(00)04214-8. [DOI] [PubMed] [Google Scholar]
- 150.Zito PM, Nassereddin A, Scharf R. StatPearls. StatPearls Publishing; [Accessed 23 March 2022]. Vismodegib. https://www.ncbi.nlm.nih.gov/books/NBK513360/ [Google Scholar]
- 151.Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373(17):1618–1626. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 152.Chen AC, Martin AJ, Dalziell RA, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol. 2016;175(5):1073–1075. doi: 10.1111/bjd.14662. [DOI] [PubMed] [Google Scholar]
- 153.Malesu R, Martin AJ, Lyons JG, et al. Nicotinamide for skin cancer chemoprevention: effects of nicotinamide on melanoma in vitro and in vivo. Photochem Photobiol Sci. 2020;19(2):1719. doi: 10.1039/c9pp00388f. [DOI] [PubMed] [Google Scholar]
- 154.Surjana D, Halliday GM, Damian DL. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin. Carcinogenesis. 2013;34(5):1144–1149. doi: 10.1093/carcin/bgt017. [DOI] [PubMed] [Google Scholar]
- 155.Thompson BC, Halliday GM, Damian DL. Nicotinamide enhances repair of arsenic and ultraviolet radiation-induced DNA damage in HaCaT keratinocytes and ex vivo human skin. PLoS ONE. 2015;10(2):e0117491. doi: 10.1371/journal.pone.0117491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Surjana D, Halliday GM, Martin AJ, Moloney FJ, Damian DL. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132(5):1497–1500. doi: 10.1038/jid.2011.459. [DOI] [PubMed] [Google Scholar]
- 157.Kohli I, Shafi R, Isedeh P, et al. The impact of oral Polypodium leucotomos extract on ultraviolet B response: a human clinical study. J Am Acad Dermatol. 2017;77(1):33–41e1. doi: 10.1016/j.jaad.2017.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Middelkamp-Hup MA, Pathak MA, Parrado C, et al. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004;51(6):910–918. doi: 10.1016/j.jaad.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 159.Zattra E, Coleman C, Arad S, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol. 2009;175(5):1952–1961. doi: 10.2353/ajpath.2009.090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tanaka J, Nagai T, Okada S. Serum concentration of coenzyme Q in xeroderma pigmentosum. Rinsho Shinkeigaku. 1998;38(1):57–59. [PubMed] [Google Scholar]
- 161.Dupuy A, Valton J, Leduc S, et al. Targeted gene therapy of xeroderma pigmentosum cells using meganuclease and TALEN. PLoS ONE. 2013;8(11):e78678. doi: 10.1371/journal.pone.0078678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dupuy A, Sarasin A. DNA damage and gene therapy of xeroderma pigmentosum, a human DNA repair-deficient disease. Mutat Res. 2015;776:2–8. doi: 10.1016/j.mrfmmm.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 163.Lehmann J, Seebode C, Martens MC, Emmert S. Xeroderma pigmentosum - facts and perspectives. Anticancer Res. 2018;38(2):1159–1164. doi: 10.21873/anticanres.12335. [DOI] [PubMed] [Google Scholar]